Oxidative Stress Correlates with More Aggressive Features in Thyroid Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cases
2.2. Cellular Fractioning
2.3. OS Measurement in Tissues
2.4. Genetic Characterization
2.5. Expression Characterization and Thyroid Differentiation Score (TDS) Calculation
2.6. NOX4 Expression
2.7. GPx Activity
2.8. Statistical Analyses
3. Results
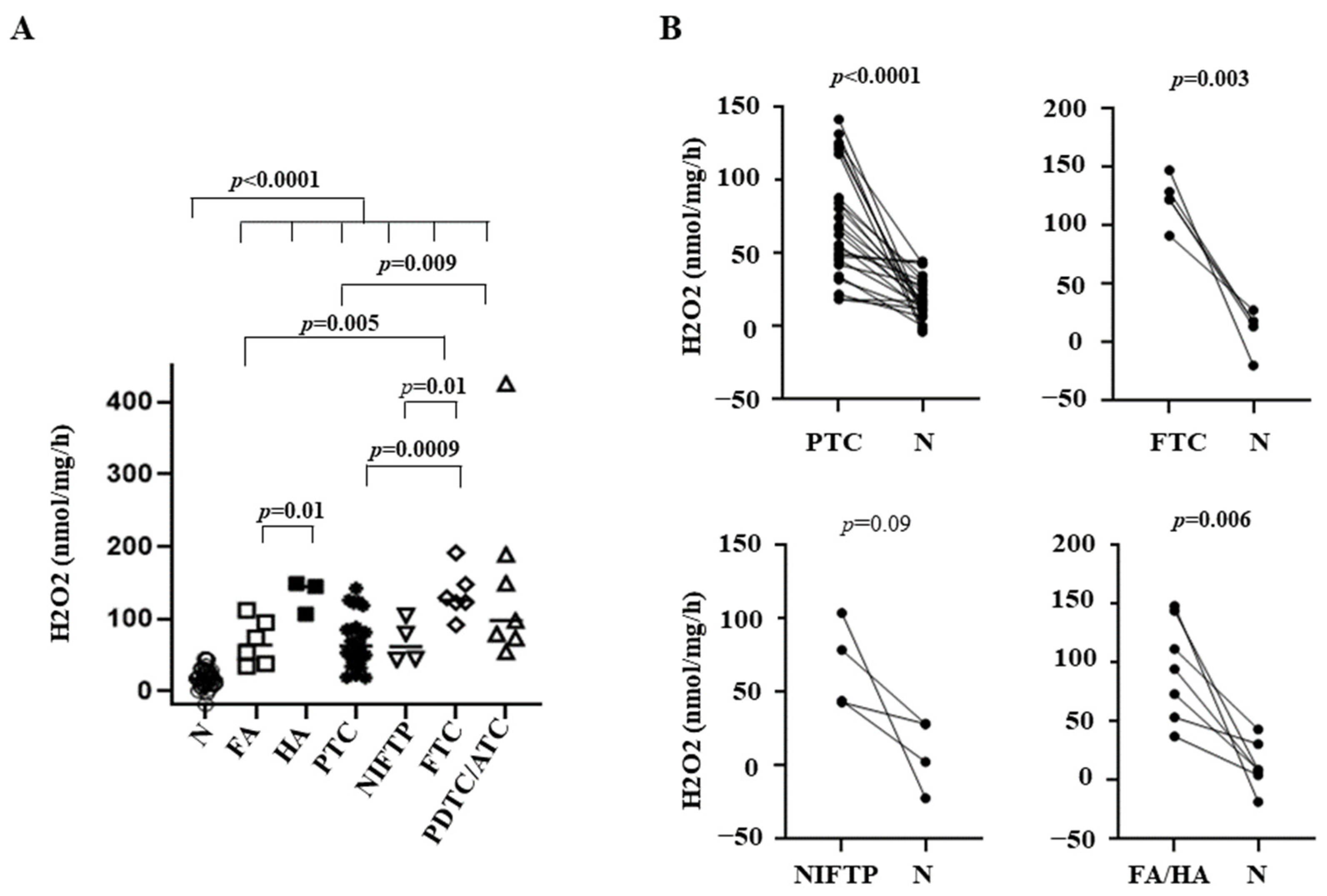
3.1. OS in Thyroid Tissues
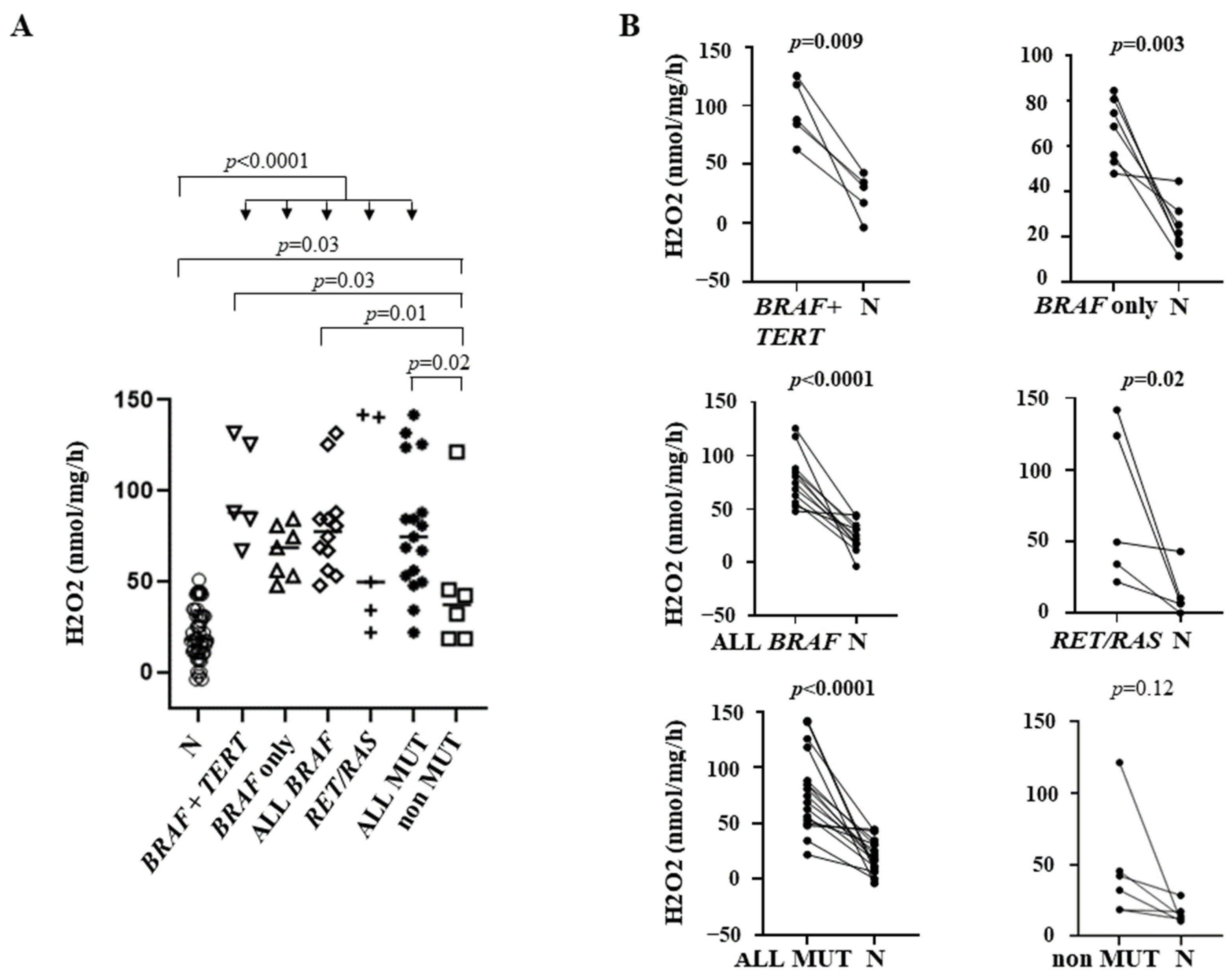
3.2. Correlation between OS and Genetic Profile
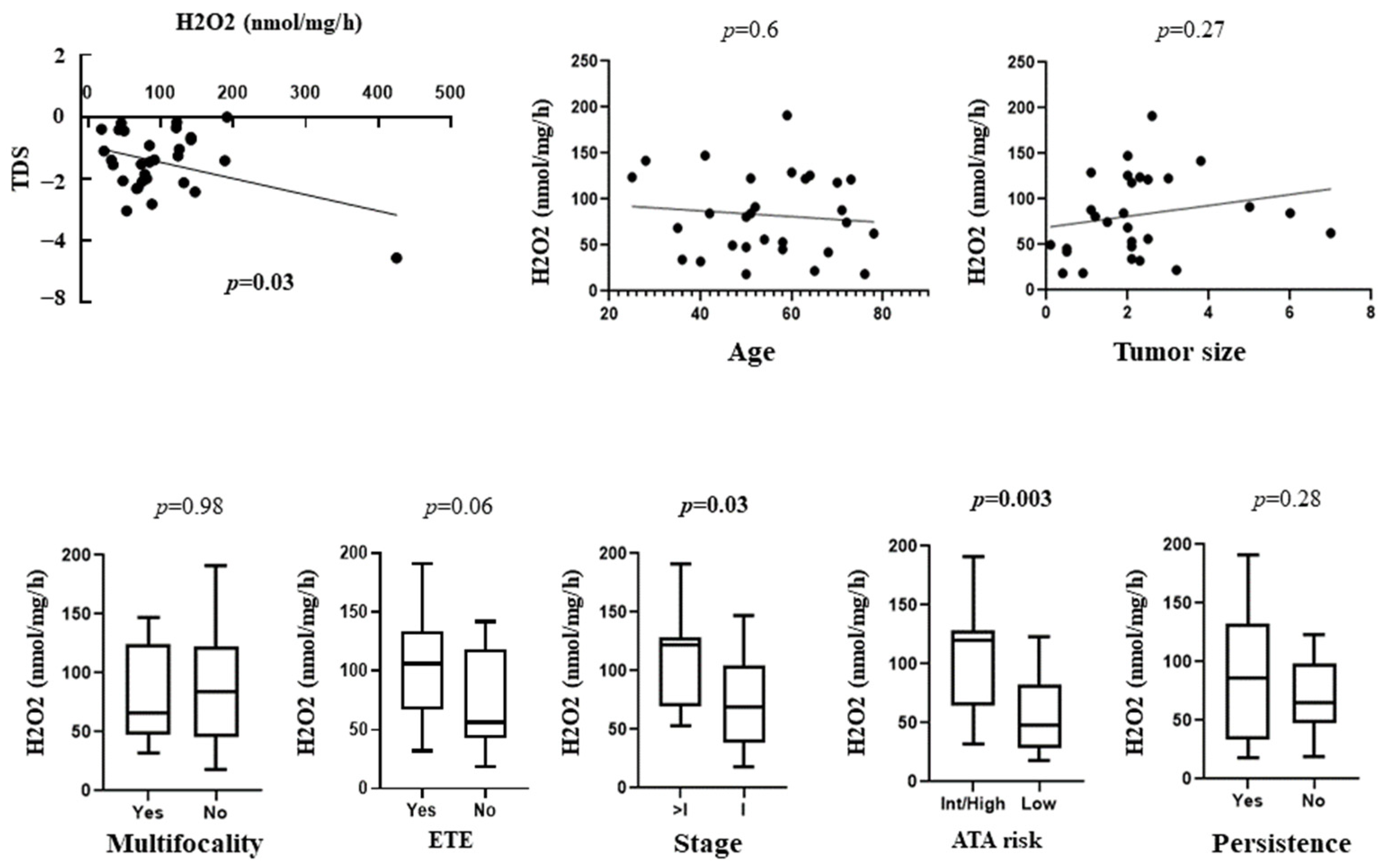
3.3. Correlation between OS with TDS and Clinical and Prognostic Features of Patients
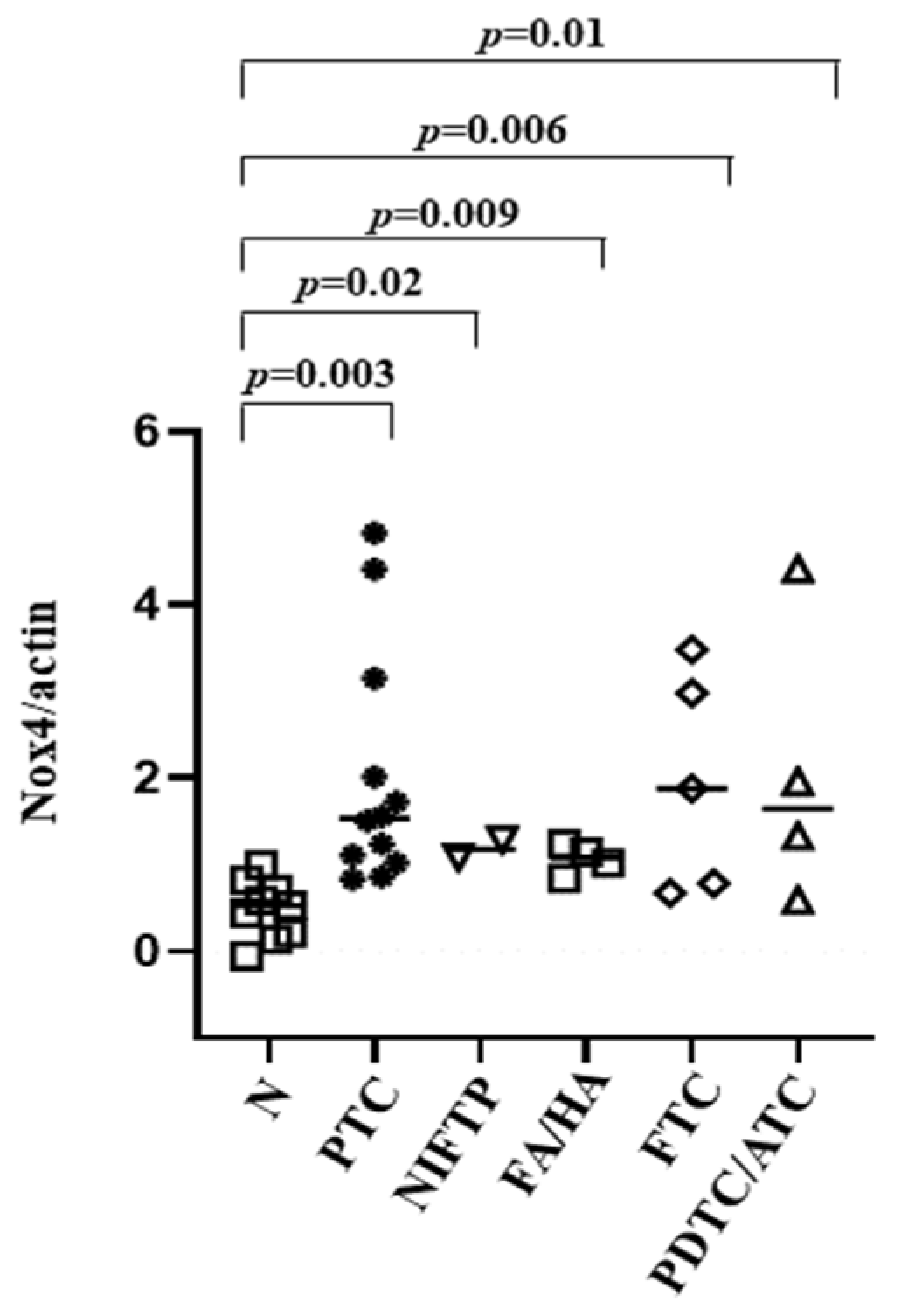
3.4. NOX4 Protein Expression in Tissues
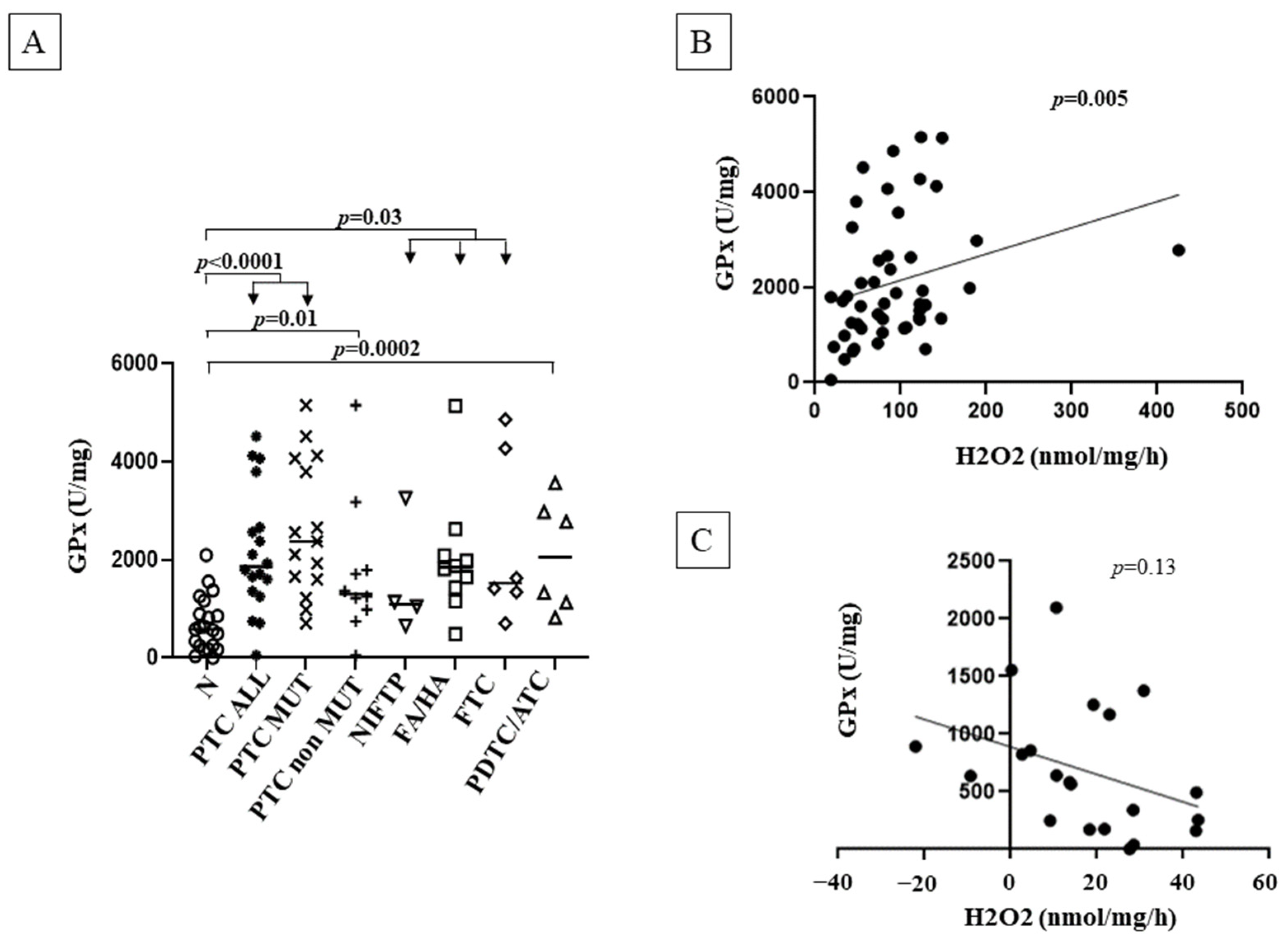
3.5. GPx Activity
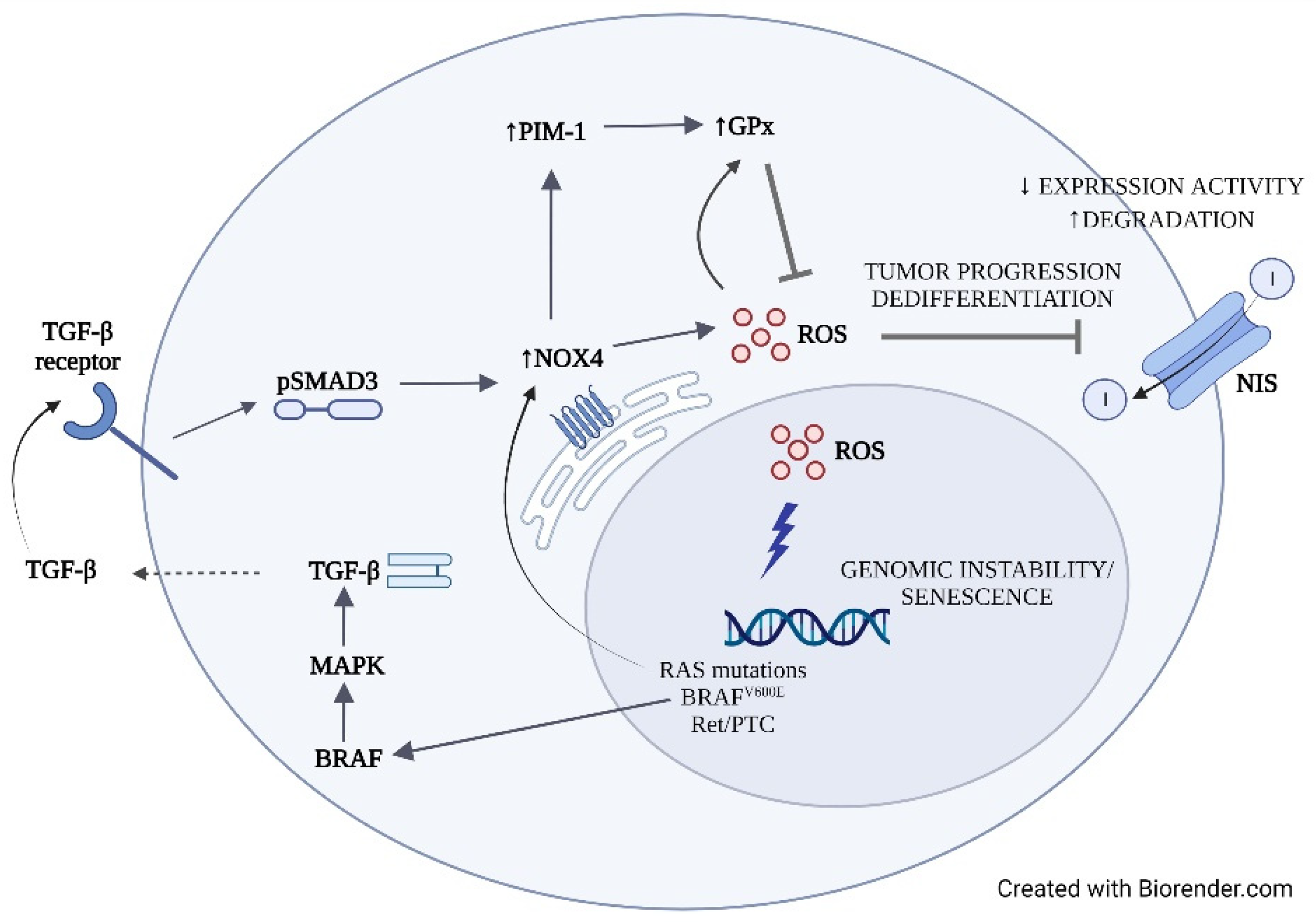
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rossi, E.D.; Pantanowitz, L.; Hornick, J.L. A worldwide journey of thyroid cancer incidence centred on tumour histology. Lancet Diabetes Endocrinol. 2021, 9, 193–194. [Google Scholar] [CrossRef] [PubMed]
- Pizzato, M.; Li, M.; Vignat, J.; Laversanne, M.; Singh, D.; La Vecchia, C.; Vaccarella, S. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol. 2022, 10, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Fugazzola, L.; Elisei, R.; Fuhrer, D.; Jarzab, B.; Leboulleux, S.; Newbold, K.; Smit, J. 2019 European Thyroid Association Guidelines for the Treatment and Follow-up of Advanced Radioiodine-Refractory Thyroid Cancer. Eur. Thyroid. J. 2019, 8, 227–245. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef]
- Xing, M.; Westra, W.H.; Tufano, R.P.; Cohen, Y.; Rosenbaum, E.; Rhoden, K.J.; Carson, K.A.; Vasko, V.; Larin, O.; Tallini, G.; et al. BRAF Mutation Predicts a Poorer Clinical Prognosis for Papillary Thyroid Cancer. J. Clin. Endocrinol. Metab. 2005, 90, 6373–6379. [Google Scholar] [CrossRef]
- Azouzi, N.; Cailloux, J.; Cazarin, J.M.; Knauf, J.A.; Cracchiolo, J.; Al Ghuzlan, A.; Hartl, D.; Polak, M.; Carré, A.; El Mzibri, M.; et al. NADPH Oxidase NOX4 Is a Critical Mediator of BRAFV600E-Induced Downregulation of the Sodium/Iodide Symporter in Papillary Thyroid Carci-nomas. Antioxid Redox Signal 2017, 26, 864–877. [Google Scholar] [CrossRef]
- El Hassani, R.A.; Buffet, C.; Leboulleux, S.; Dupuy, C. Oxidative stress in thyroid carcinomas: Biological and clinical significance. Endocr.-Relat. Cancer 2019, 26, R131–R143. [Google Scholar] [CrossRef]
- Xing, M. Oxidative stress: A new risk factor for thyroid cancer. Endocr.-Relat. Cancer 2011, 19, C7–C11. [Google Scholar] [CrossRef]
- Maier, J.; Van Steeg, H.; Van Oostrom, C.; Karger, S.; Paschke, R.; Krohn, K. Deoxyribonucleic Acid Damage and Spontaneous Mutagenesis in the Thyroid Gland of Rats and Mice. Endocrinology 2006, 147, 3391–3397. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Szanto, I.; Pusztaszeri, M.; Mavromati, M. H2O2 Metabolism in Normal Thyroid Cells and in Thyroid Tumorigenesis: Focus on NADPH Oxidases. Antioxidants 2019, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Weyemi, U.; Caillou, B.; Talbot, M.; Ameziane-El-Hassani, R.; Lacroix, L.; Lagent-Chevallier, O.; Al Ghuzlan, A.; Roos, D.; Bidart, J.-M.; Virion, A.; et al. Intracellular expression of reactive oxygen species-generating NADPH oxidase NOX4 in normal and cancer thyroid tissues. Endocr.-Relat. Cancer 2010, 17, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Muzza, M.; Colombo, C.; Cirello, V.; Perrino, M.; Vicentini, L.; Fugazzola, L. Oxidative stress and the subcellular localization of the telomerase reverse transcriptase (TERT) in papillary thyroid cancer. Mol. Cell. Endocrinol. 2016, 431, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Björkman, U.; Ekholm, R. Hydrogen peroxide degradation and glutathione peroxidase activity in cultures of thyroid cells. Mol. Cell. Endocrinol. 1995, 111, 99–107. [Google Scholar] [CrossRef]
- Nicolussi, A.; D’Inzeo, S.; Capalbo, C.; Giannini, G.; Coppa, A. The role of peroxiredoxins in cancer. Mol. Clin. Oncol. 2017, 6, 139–153. [Google Scholar] [CrossRef]
- Kim, H.; Lee, T.H.; Park, E.S.; Suh, J.M.; Park, S.J.; Chung, H.K.; Kwon, O.Y.; Kim, Y.K.; Ro, H.K.; Shong, M. Role of perox-iredoxins in regulating intracellular hydrogen peroxide and hydrogen peroxide-induced apoptosis in thyroid cells. J. Biol. Chem. 2000, 275, 18266–18270. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Driessens, N.; Costa, M.; De Deken, X.; Detours, V.; Corvilain, B.; Maenhaut, C.; Miot, F.; Van Sande, J.; Many, M.-C.; et al. Roles of Hydrogen Peroxide in Thyroid Physiology and Disease. J. Clin. Endocrinol. Metab. 2007, 92, 3764–3773. [Google Scholar] [CrossRef]
- Köhrle, J.; Gärtner, R. Selenium and thyroid. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 815–827. [Google Scholar] [CrossRef]
- Beckett, G.J.; Arthur, J.R. Selenium and endocrine systems. J. Endocrinol. 2005, 184, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.; Neelamohan, R.; Suthagar, E.; Vengatesh, G.; Jayakumar, J.; Chandrasekaran, M.; Banu, S.; Aruldhas, M. Lipid peroxidation and antioxidants status in human malignant and non-malignant thyroid tumours. Hum. Exp. Toxicol. 2015, 35, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, S.; Vlantis, A.C.; Liu, S.Y.; Ng, E.K.; Chan, A.B.; Wu, J.; Du, J.; Wei, W.; Liu, X.; et al. Expression of Antioxidant Molecules and Heat Shock Protein 27 in Thyroid Tumors. J. Cell. Biochem. 2016, 117, 2473–2481. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Takano, T.; Miyauchi, A.; Matsuzuka, F.; Yoshida, H.; Kuma, K.; Amino, N. Decreased expression of glutathione peroxidase mRNA in thyroid anaplastic carcinoma. Cancer Lett. 2002, 182, 69–74. [Google Scholar] [CrossRef]
- Metere, A.; Frezzotti, F.; Graves, C.E.; Vergine, M.; De Luca, A.; Pietraforte, D.; Giacomelli, L. A possible role for selenoprotein glutathione peroxidase (GPx1) and thioredoxin reductases (TrxR1) in thyroid cancer: Our experience in thyroid surgery. Cancer Cell Int. 2018, 18, 7. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual; 8th ed. Springer: New York, NY, USA, 2017; p. XVII, 1032. [Google Scholar]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Pesenti, C.; Muzza, M.; Colombo, C.; Proverbio, M.C.; Farè, C.; Ferrero, S.; Miozzo, M.; Fugazzola, L.; Tabano, S. MassAR-RAY-based simultaneous detection of hotspot somatic mutations and recurrent fusion genes in papillary thyroid carcinoma: The PTC-MA assay. Endocrine 2018, 61, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Muzza, M.; Pogliaghi, G.; Palazzo, S.; Vannucchi, G.; Vicentini, L.; Persani, L.; Gazzano, G.; Fugazzola, L. The thyroid risk score (TRS) for nodules with indeterminate cytology. Endocr.-Relat. Cancer 2021, 28, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Pogliaghi, G.; Tosi, D.; Muzza, M.; Bulfamante, G.; Persani, L.; Fugazzola, L.; Cirello, V. Thyroid cancer harboring PTEN and TP53 mutations: A peculiar molecular and clinical case report. Front. Oncol. 2022, 12, 949098. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Wen, Q.-L.; Yi, H.-Q.; Yang, K.; Yin, C.-T.; Yin, W.-J.; Xiang, F.-Y.; Bao, M.; Shuai, J.; Song, Y.-W.; Ge, M.-H.; et al. Role of oncogene PIM-1 in the development and progression of papillary thyroid carcinoma: Involvement of oxidative stress. Mol. Cell. Endocrinol. 2021, 523, 111144. [Google Scholar] [CrossRef] [PubMed]
- Weyemi, U.; Lagentechevallier, O.; Boufraqech, M.; Prenois, F.; Courtin, F.; Caillou, B.; Talbot, M.R.; Dardalhon, M.; Al Ghuzlan, A.; Bidart, J.-M.; et al. ROS-generating NADPH oxidase NOX4 is a critical mediator in oncogenic H-Ras-induced DNA damage and subsequent senescence. Oncogene 2011, 31, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Sheng, J.; Peng, X.; Zhang, R.; Xu, T.; Hu, J.; Kang, Y.; Wu, B.; Dang, H. Targeting NOX4 disrupts the resistance of papillary thyroid carcinoma to chemotherapeutic drugs and lenvatinib. Cell Death Discov. 2022, 8, 177. [Google Scholar] [CrossRef] [PubMed]
| Sample | Age at Diagnosis (Years) | Size (cm) | Multifocality | ETE | ATA Risk | Stage | Persistence |
|---|---|---|---|---|---|---|---|
| PTC1 | 64 | 2 | Yes | Yes | Intermediate | II | Yes |
| PTC2 | 51 | 6 | No | No | Low | I | No |
| PTC3 | 71 | 1.1 | No | Yes | High | II | Yes |
| PTC4 | 72 | 1.5 | No | Yes | Low | I | No |
| PTC5 | 50 | 1.2 | No | No | Low | I | No |
| PTC6 | 50 | 2.1 | No | No | Low | I | No |
| PTC7 | 58 | 2.1 | Yes | No | Intermediate | II | No |
| PTC8 | 42 | 1.9 | No | No | Intermediate | I | Yes |
| PTC9 | 54 | 2.5 | No | No | Low | I | No |
| PTC10 | 35 | 2 | Yes | Yes | Intermediate | I | No |
| PTC11 | 36 | 2.1 | No | No | Low | I | Yes |
| PTC12 | 25 | 2.3 | Yes | Yes | Intermediate | I | Yes |
| PTC13 | 47 | 0.1 | Yes | No | Intermediate | I | Yes |
| PTC14 | 65 | 3.2 | No | No | Low | I | Yes |
| PTC15 | 28 | 3.8 | No | No | Intermediate | I | Yes |
| PTC16 | 58 | 0.5 | No | No | Low | I | No |
| PTC17 | 68 | 0.5 | Yes | No | Low | I | No |
| PTC18 | 76 | 0.9 | No | No | Low | I | No |
| PTC19 | 73 | 2.5 | No | No | Intermediate | II | No |
| PTC20 | 50 | 0.4 | No | No | Low | I | Yes |
| PTC21 | 40 | 2.3 | Yes | Yes | Intermediate | I | Yes |
| PTC22 | 70 | 2.1 | No | No | Intermediate | I | No |
| PTC23 | 78 | 7 | Yes | Yes | High | II | Yes |
| FTC1 | 52 | 5 | No | No | Low | I | No |
| FTC2 | 51 | 3 | No | No | Low | I | No |
| FTC3 | 41 | 2 | Yes | Yes | High | I | Yes |
| FTC4 | 63 | 3 | No | No | Int | II | Yes (deceased) |
| FTC5 | 60 | 1.1 | No | Yes | High | Ivb | Yes |
| FTC6 | 59 | 2.6 | No | Yes | High | IVa | Yes (deceased) |
| ATC1 | 76 | 4.5 | Yes | Yes | High | IVb | Yes (deceased) |
| ATC2 | 60 | 5.5 | Yes | Yes | High | IVb | Yes (deceased) |
| ATC3 | 65 | 4 | Yes | Yes | High | IVb | Yes (deceased) |
| PDTC1 | 74 | 3.6 | Yes | Yes | High | IVb | Yes (deceased) |
| PDTC2 | 42 | 3.5 | Yes | Yes | High | IVb | Yes (deceased) |
| PDTC3 | 80 | 2 | Yes | Yes | Int | II | Yes (deceased) |
| PDTC4 | 34 | 3.5 | Yes | Yes | High | II | Yes |
| Sample | Mutation |
|---|---|
| PTC1 | BRAF p.V600E + TERT-124C > T |
| PTC2 | BRAF p.V600E + TERT-124C > T |
| PTC3 | BRAF p.V600E + TERT-124C > T |
| PTC4 | BRAF p.V600E + TERT-124C > T |
| PTC5 | BRAF p.V600E + TERT-124C > T |
| PTC6 | BRAF p.V600E |
| PTC7 | BRAF p.V600E |
| PTC8 | BRAF p.V600E |
| PTC9 | BRAF p.V600E |
| PTC10 | BRAF p.V600E |
| PTC11 | BRAF p.V600E |
| PTC12 | BRAF p.V600E |
| PTC13 | RET/PTC1 |
| PTC14 | RET/PTC3 |
| PTC15 | NRAS p.Q61K |
| PTC16 | NRAS p.Q61R |
| PTC17 | NRAS p.Q61R |
| PTC18 | WT |
| PTC19 | WT |
| PTC20 | WT |
| PTC21 | WT |
| PTC22 | WT |
| PTC23 | WT |
| NIFTP1 | WT |
| NIFTP2 | WT |
| NIFTP3 | NRAS p.Q61R |
| NIFTP4 | WT |
| FA1 | NRAS p.Q61R |
| FA2 | WT |
| FA3 | WT |
| FA4 | WT |
| FA5 | WT |
| FA6 | WT |
| FA7 | WT |
| HA1 | WT |
| HA2 | NRAS p.Q61R |
| HA3 | WT |
| FTC1 | NRAS p.Q61R + TERT-124C > T |
| FTC2 | NRAS p.Q61R |
| FTC3 | NRAS p.Q61R |
| FTC4 | WT |
| FTC5 | NRAS p.Q61R + TERT-124C > T |
| FTC6 | WT |
| ATC1 | TP53 c.722_724delCCT + TERT-124C > T |
| ATC2 | WT |
| ATC3 | TP53 p.R175H |
| PDTC1 | TP53 c.866_867delTC |
| PDTC2 | WT |
| PDTC3 | BRAF p.V600E + TERT-124C > T |
| PDTC4 | TP53 p.C135Y, p.M133R, c.920-2A > G+ PTEN c.741dupA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muzza, M.; Pogliaghi, G.; Colombo, C.; Carbone, E.; Cirello, V.; Palazzo, S.; Frattini, F.; Gentilini, D.; Gazzano, G.; Persani, L.; et al. Oxidative Stress Correlates with More Aggressive Features in Thyroid Cancer. Cancers 2022, 14, 5857. https://doi.org/10.3390/cancers14235857
Muzza M, Pogliaghi G, Colombo C, Carbone E, Cirello V, Palazzo S, Frattini F, Gentilini D, Gazzano G, Persani L, et al. Oxidative Stress Correlates with More Aggressive Features in Thyroid Cancer. Cancers. 2022; 14(23):5857. https://doi.org/10.3390/cancers14235857
Chicago/Turabian StyleMuzza, Marina, Gabriele Pogliaghi, Carla Colombo, Erika Carbone, Valentina Cirello, Sonia Palazzo, Francesco Frattini, Davide Gentilini, Giacomo Gazzano, Luca Persani, and et al. 2022. "Oxidative Stress Correlates with More Aggressive Features in Thyroid Cancer" Cancers 14, no. 23: 5857. https://doi.org/10.3390/cancers14235857
APA StyleMuzza, M., Pogliaghi, G., Colombo, C., Carbone, E., Cirello, V., Palazzo, S., Frattini, F., Gentilini, D., Gazzano, G., Persani, L., & Fugazzola, L. (2022). Oxidative Stress Correlates with More Aggressive Features in Thyroid Cancer. Cancers, 14(23), 5857. https://doi.org/10.3390/cancers14235857






