Design and Experimental Setup of a Robotic Medical Instrument for Brachytherapy in Non-Resectable Liver Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- (1)
- —The overall survival rate is not an average calculation but rather an indicator of detection efficiency, pointing out that some cancers are “silent” ones, being detected only in later stages when the treatment options are limited;
- (2)
- —The incidence points out the potential spread and evolution in numbers.
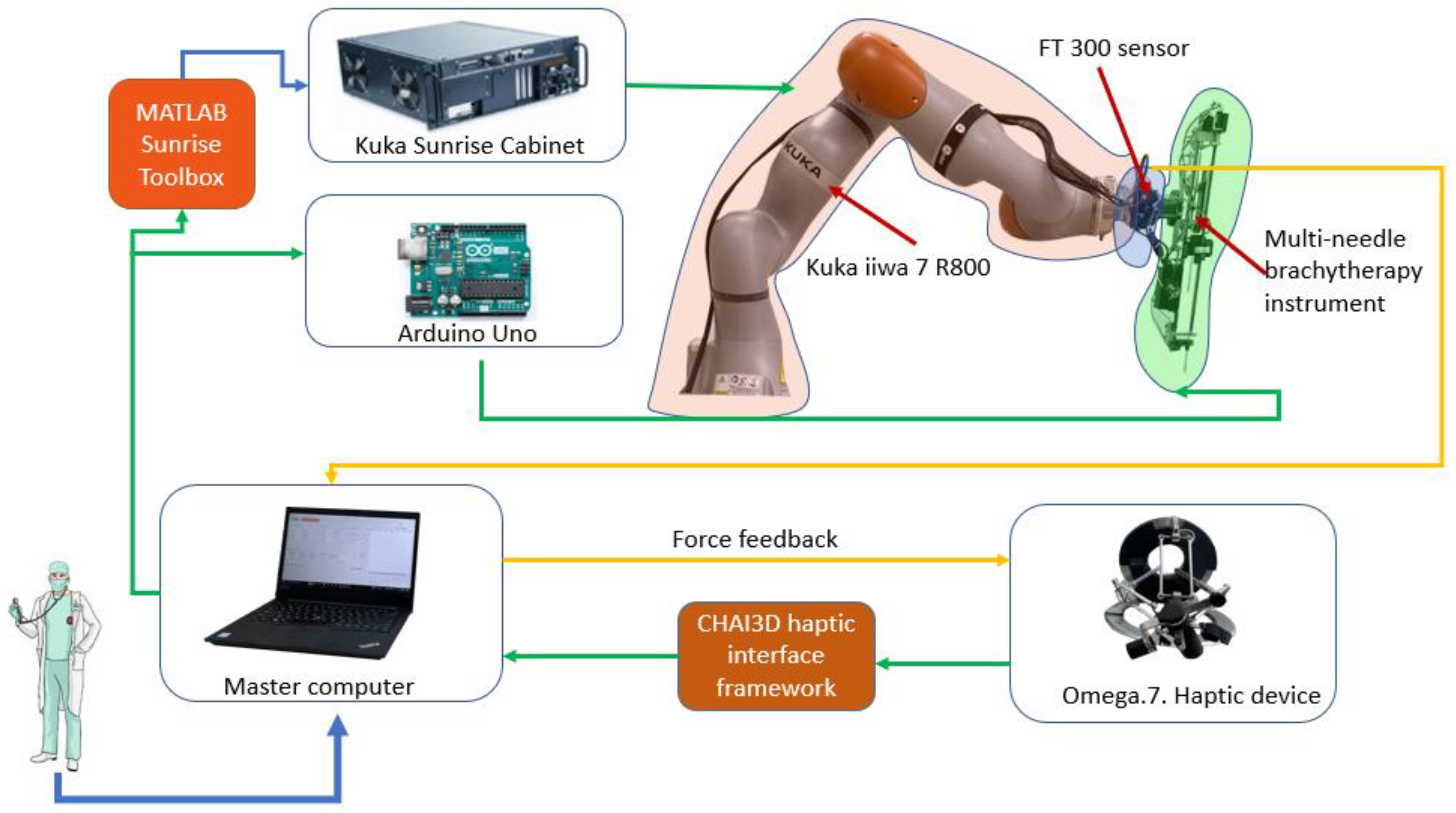
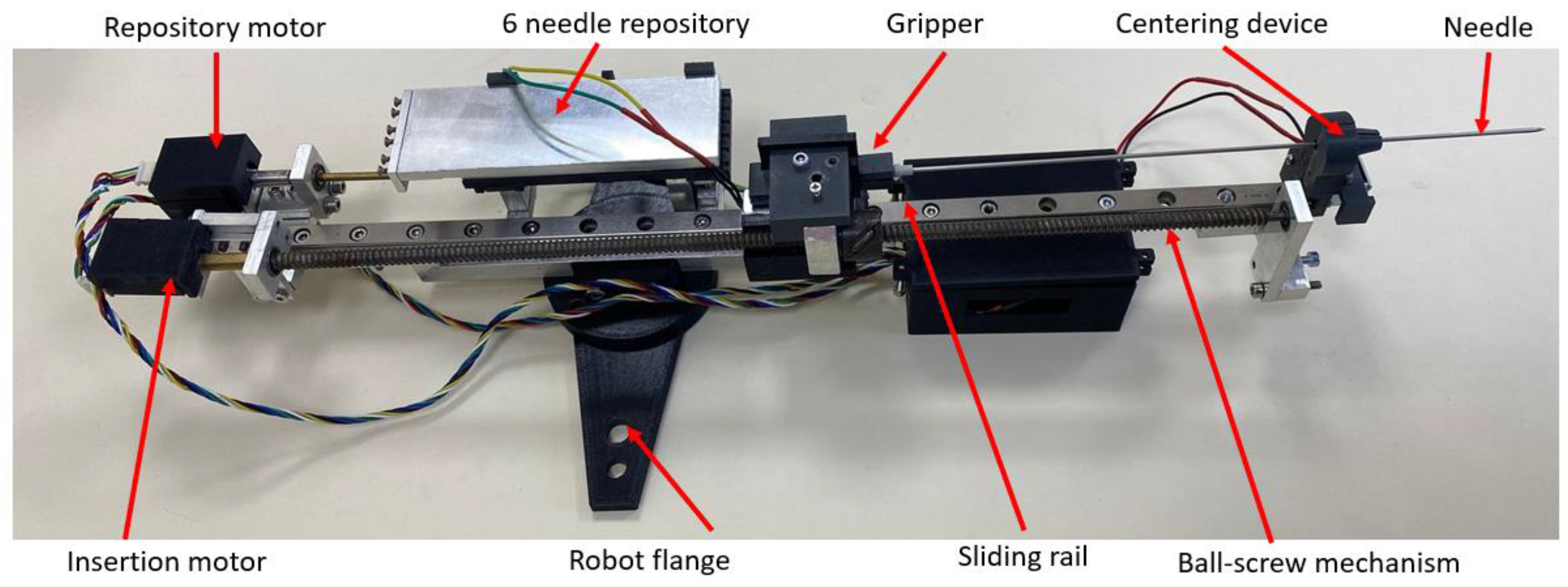
2.1. Robotic System for Brachytherapy
- Payload—the total mass of the brachytherapy instrument is 1.8 kg;
- Repeatability—in order to achieve high accuracy, a high repeatability was required;
- Light weight—the robotic arm is mounted on a table near the CT, and it also needs to be easily removed from the operating workspace;
- Collaborative—in order to be able to use the robot inside the human workspace, the system must have Human Robot Interactivity (HRI).
2.2. The Medical Protocol for Robotic Brachytherapy
2.3. Systematic Analysis of the Robotic System Characteristics
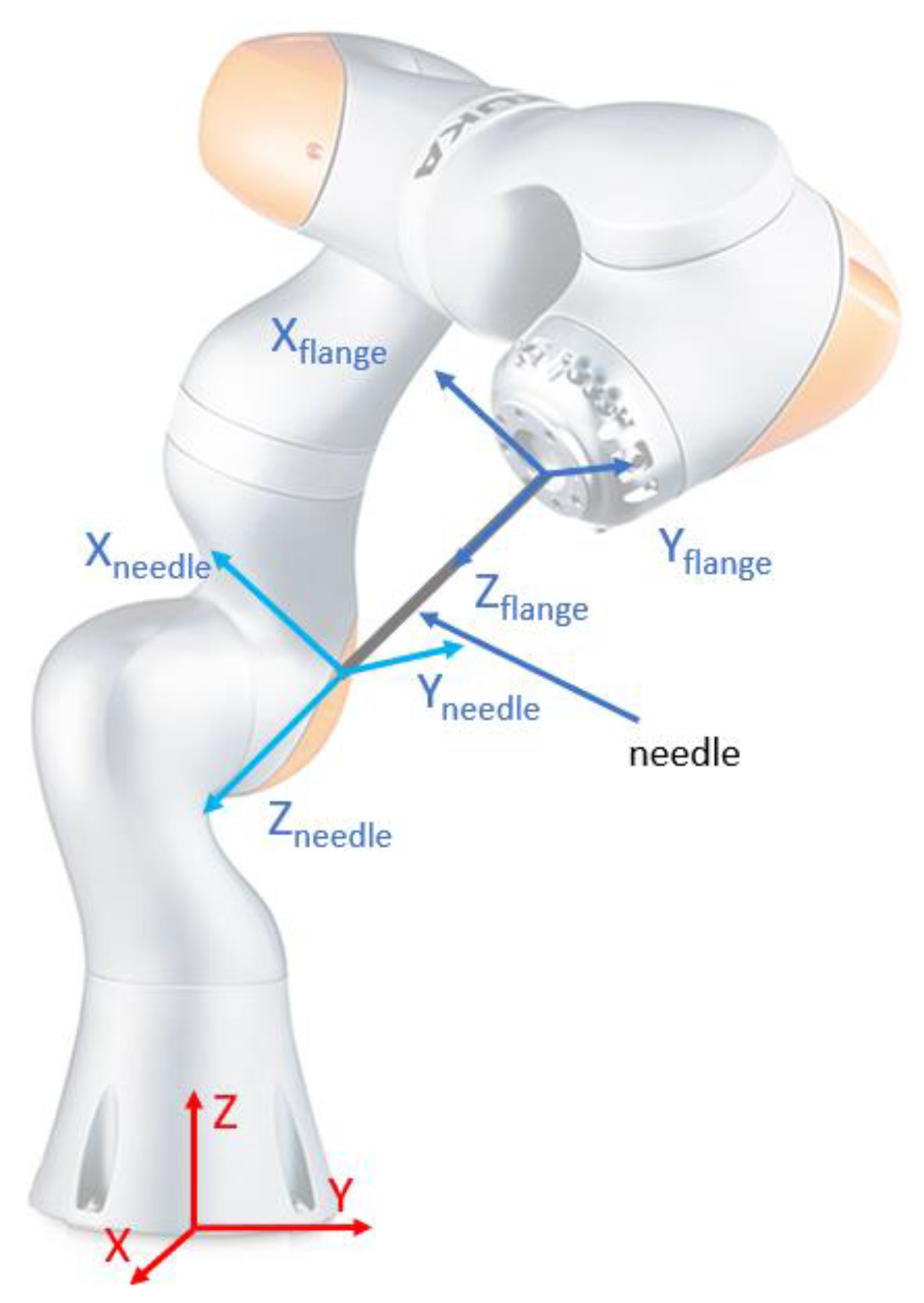
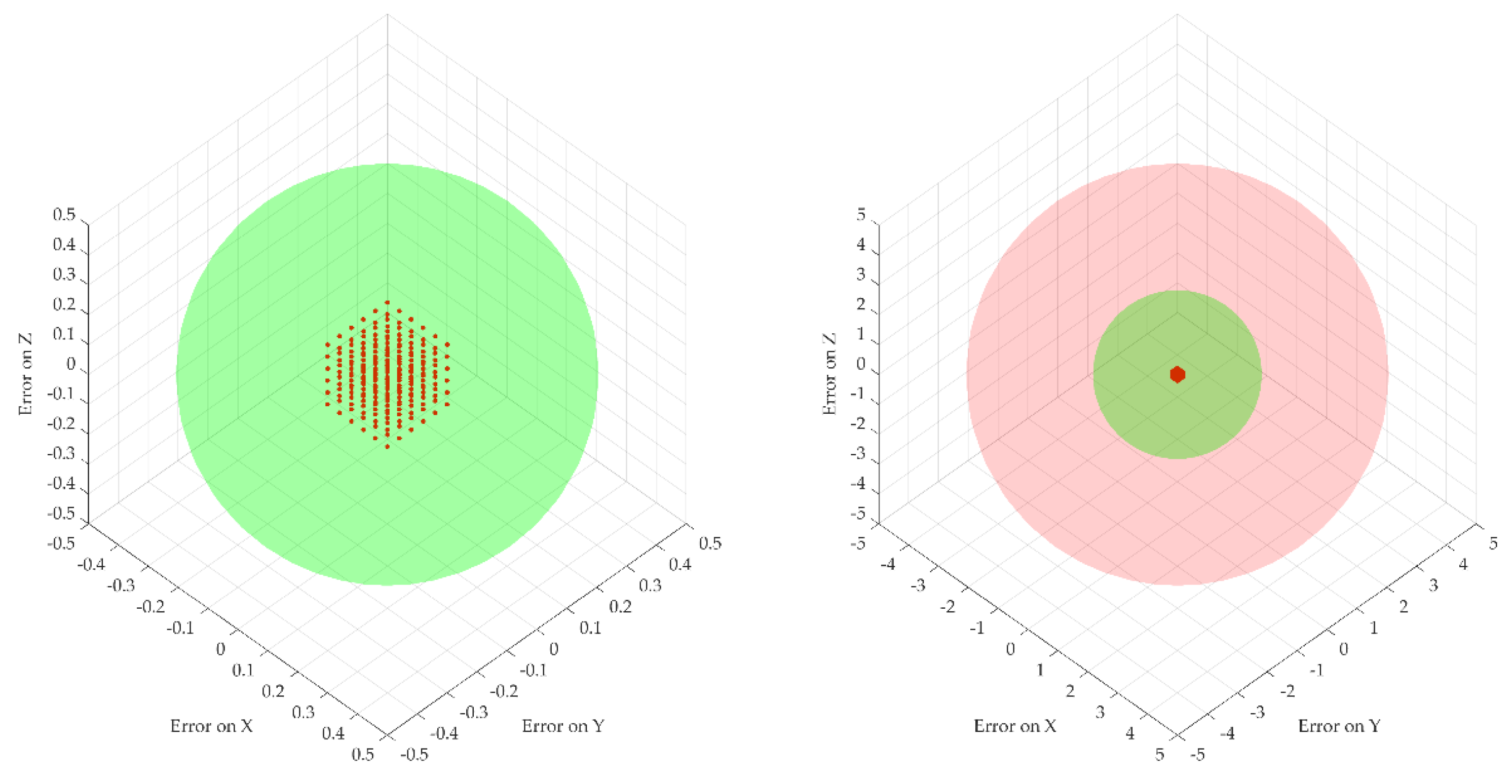
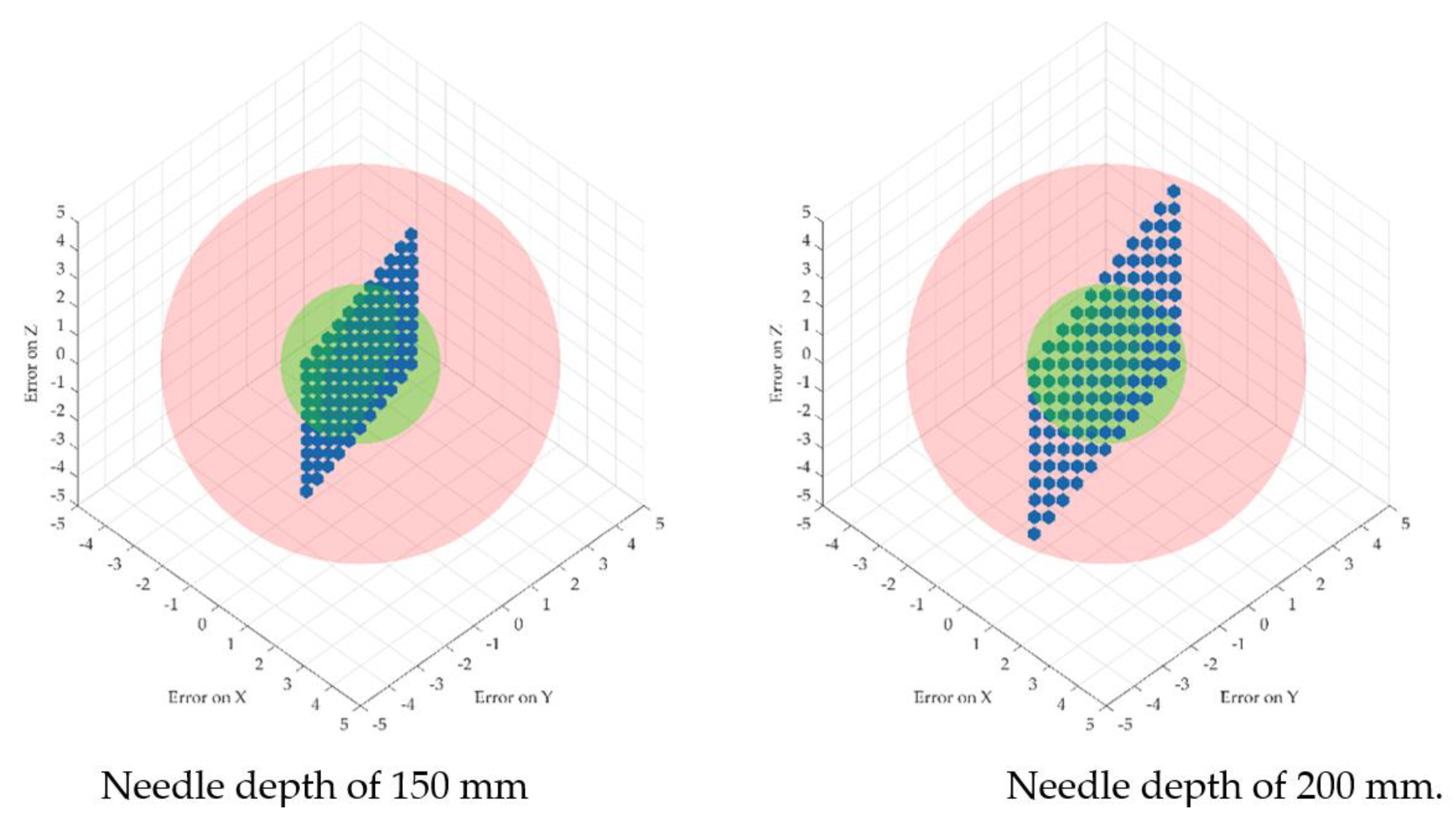
2.4. A Theoretical Study of the Procedure Accuracy
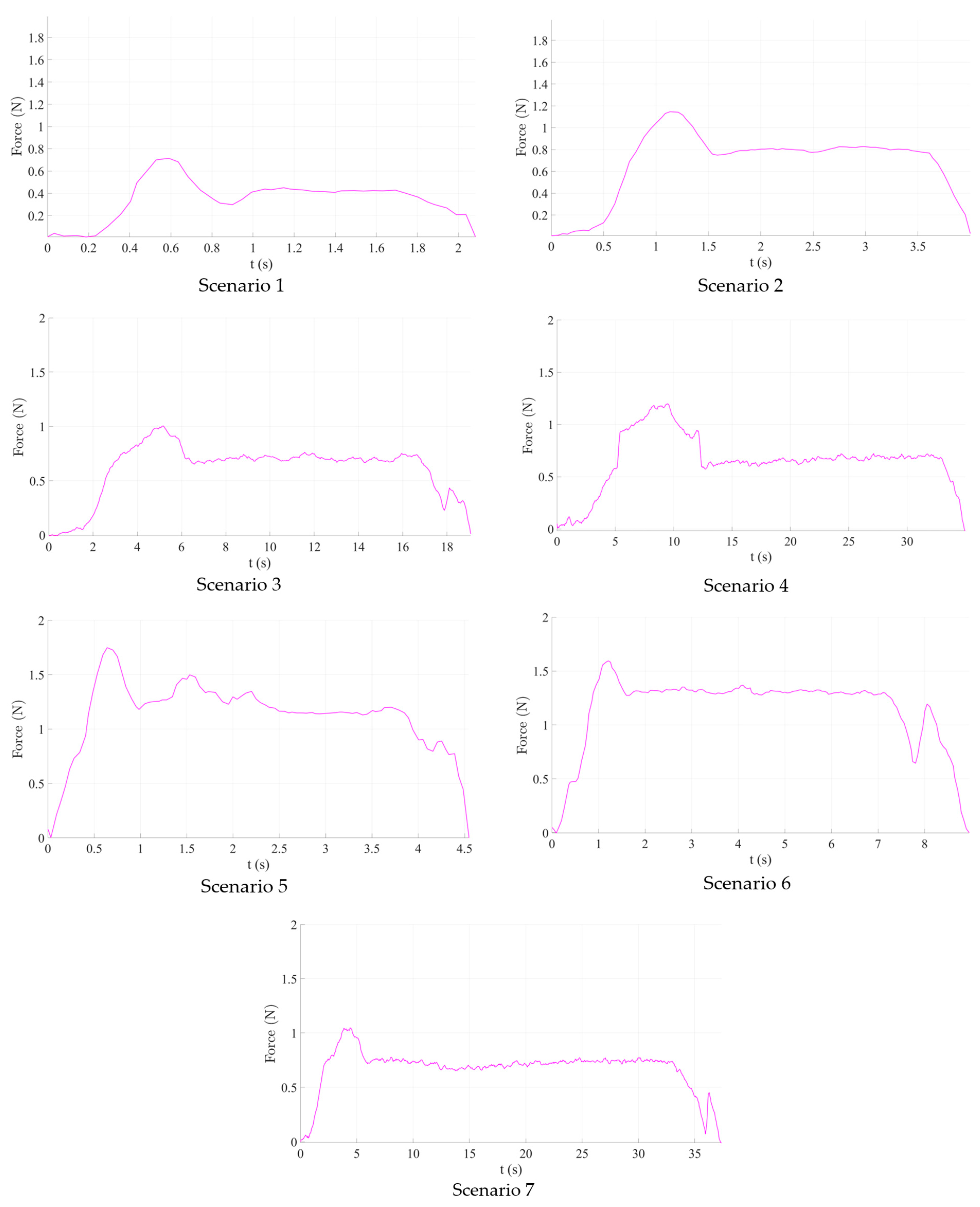
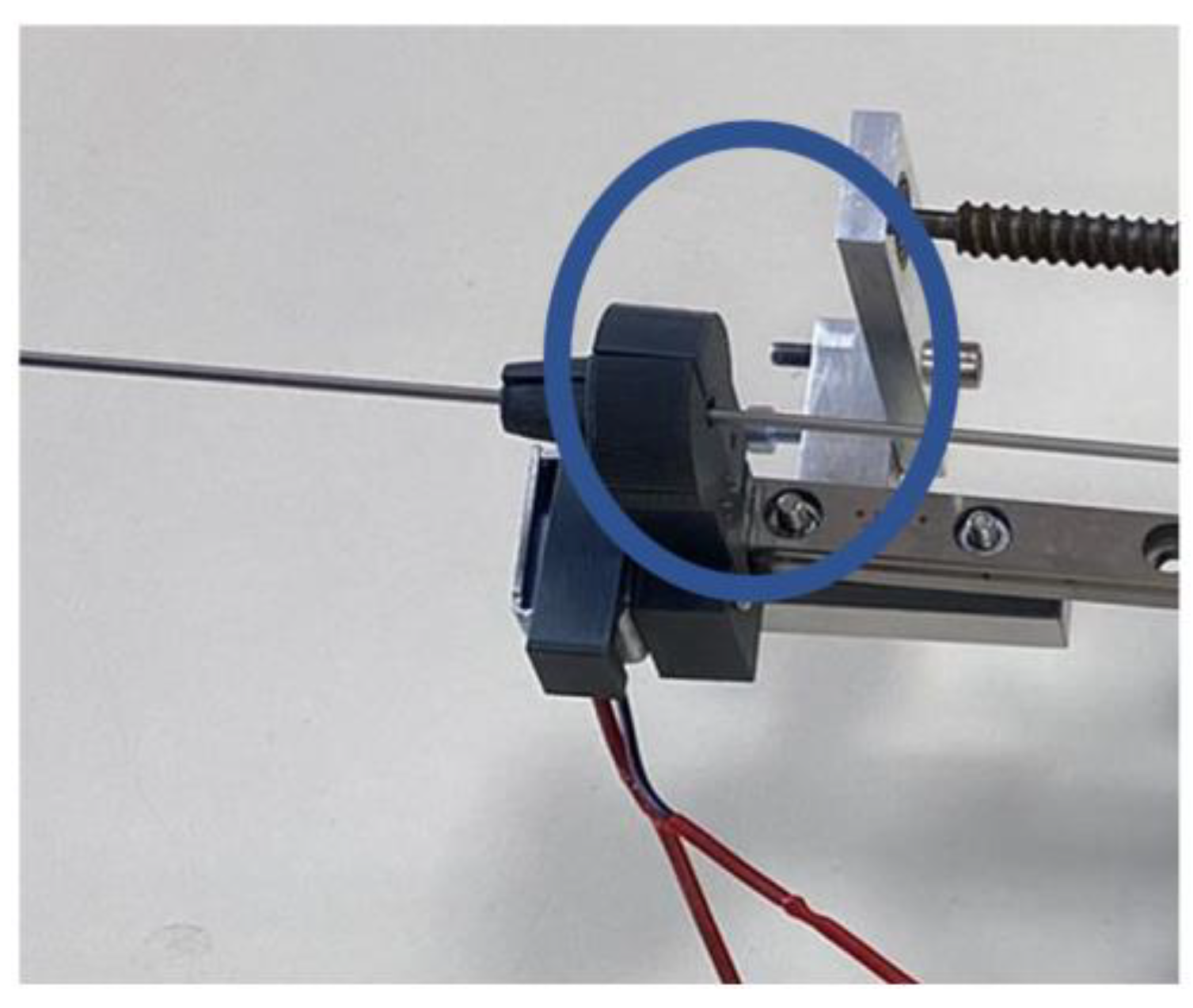
2.5. Experimental Analysis of the Needle Insertion and Retraction Forces
- Scenario no.1: five insertions with the needle perpendicular to the targeted area (vertical) using 250 mm/s speed and 10 mm spacing between the insertions, 60 mm deep.
- Scenario no.2: five insertions with the needle perpendicular to the targeted area (vertical) using 125 mm/s speed and 10 mm spacing between the insertions, 60 mm deep.
- Scenario no.3: five insertions with the needle perpendicular to the targeted area (vertical) using 25 mm/s speed and 10 mm spacing between the insertions, 60 mm deep.
- Scenario no.4: five insertions with the needle perpendicular to the targeted area (vertical) using 12.5 mm/s speed and 10 mm spacing between the insertions, 60 mm deep.
- Scenario no.5: five insertions with the needle perpendicular to the targeted area (vertical) using 250 mm/s speed and 150 degrees rotation between the insertion point and target point, 10 mm spacing between the insertions, 60 mm deep.
- Scenario no.6: five insertions with the needle perpendicular to the targeted area (vertical) using 125 mm/s speed and 150 degrees rotation between the insertion point and target point, 10 mm spacing between the insertions, 60 mm deep.
- Scenario no.7: five insertions with the needle perpendicular to the targeted area (vertical) using 12.5 mm/s speed and 150 degrees rotation between the insertion point and target point, 10 mm spacing between the insertions, 60 mm deep.
3. Results
3.1. Force Analysis
3.2. Normal Forces Decomposition
- Sliding friction, which is generated during the needle insertion between the centering devices’ jaws and the needle and during the stylet extraction, generated between the canula and the stylet;
- Static friction, generated during the stylet extraction between the centering devices’ jaws and the canula (which is kept in place while the stylet is removed).
3.3. Wear Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Agency for Research on Cancer; World Health Organization. Press Release 307. Available online: https://www.iarc.who.int (accessed on 30 August 2022).
- World Health Organization. Available online: https://www.who.int (accessed on 30 August 2022).
- American Cancer Society. Cancer Facts & Figures 2022; American Cancer Society: Atlanta, GA, USA, 2022. [Google Scholar]
- Dalmartelo, M.; La Vechia, C.; Bertucio, P.; Boffetta, P.; Levi, F.; Negri, E.; Malvezzi, M. European cancer mortality prediction for the year 2022 with focus on ovarian cancer. Ann. Oncol. 2022, 33, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Caner statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Chidambaranathan-Reghupaty, S.; Fisher, P.B.; Sarkar, D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv. Cancer Res. 2021, 149, 1–61. [Google Scholar] [PubMed]
- Sparchez, Z.; Radu, P.; Bartos, A.; Nenu, I.; Craciun, R.; Mocan, T.; Horhat, A.; Spârchez, M.; Dufour, J.F. Combined treatments in hepatocellular carcinoma: Time to put them in the guidelines? World J. Gastrointest. Oncol. 2021, 13, 1896–1918. [Google Scholar] [CrossRef] [PubMed]
- Öcal, O.; Rössler, D.; Ricke, J.; Seidensticker, M. Advances in Diagnostic and Interventional Radiology in Hepatocellular Carcinoma. Dig. Dis. 2022, 40, 458–467. [Google Scholar] [CrossRef]
- National Cancer Institute. Radiation Therapy to Treat Cancer. Available online: https://www.cancer.gov (accessed on 1 September 2022).
- Ozhasoglu, C.; Murphy, M.J. Issues in respiratory motion compensation during external-beam radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2021, 52, 1389–1399. [Google Scholar] [CrossRef]
- Hoskin, P.J.; Rojas, A.M.; Ostler, P.J.; Bryant, L.; Lowe, G.J. Randomized trial of external-beam radiotherapy alone or with high-dose-rate brachytherapy for prostate cancer: Mature 12-year results. Radiother. Oncol. 2021, 154, 214–219. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Bahl, A.; Persad, R.; Melhuish, C. A review on the techniques used in prostate brachytherapy. Cogn. Comput. Syst. 2022, 4, 317–328. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, Y.; Jiang, J.; Li, B. Image-guided robots for low dose rate prostate brachytherapy: Perspectives on safety in design and use. Int. J. Med. Robot. Comput. Assist. Surg. 2020, 17, e2239. [Google Scholar] [CrossRef]
- Xiong, J.; Xu, C.; Ibrahim, K.; Deng, H.; Xia, Z. A Mechanism- Image Fusion Approach to Calibration of an Ultrasound-Guided Dual Arm Robotic Brachytherapy System. IEEE/ASME Trans. Mechatron. 2021, 26, 3211–3220. [Google Scholar] [CrossRef]
- Kaya, M.; Senel, E.; Ahmad, A.; Bebek, O. Visual needle tip tracking in 2D US guided robotic interventions. Mechatronics 2019, 57, 129–139. [Google Scholar] [CrossRef]
- Chen, S.H.; Wang, F.; Lin, Y.P.; Shi, Q.S.; Wang, Y.L. Ultrasound-guided needle insertion robotic system for percutaneous puncture. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Fuchtinger, G.; Fiene, J.P.; Kennedy, C.W.; Kronreif, G.; Iordachita, I.; Song, S.Y.; Burdette, E.C.; Kazanzides, P. Robotic assistance for ultrasound-guided prostate brachytherapy. Med. Image Anal. 2008, 12, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Belarouci, A.; Dhaliwal, S.S.; Sanz-Lopez, M.; Verbrugghe, F.; Lakchal, O.; Chettibi, T.; Merzouki, R. Cooperative Brachytherapy Robotic Concept for Localized Cancer Treatment Under Real-Time MRI. IEEE Trans. Med. Robot. Bionics 2022, 4, 667–681. [Google Scholar] [CrossRef]
- Patriciu, A.; Petrisor, D.; Muntener, M.; Azilu, D.; Schar, M.; Stoianovici, D. Automatic Brachytherapy Seed Placement Under MRI Guidance. IEEE Trans. Biomed. Eng. 2007, 54, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Iordachita, I.I.; Yan, X.; Cole, G.A.; Fischer, G.S. Reconfigurable MRI-guided robotic surgical manipulator. Prostate brachytherapy and neurosurgery applications. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 2111–2214. [Google Scholar]
- Dou, H.S.; Jiang, S.; Yang, Z.Y.; Sun, L.Q.; Ma, X.D.; Huo, B. Design and validation of a CT-guided robotic system for lung cancer brachytherapy. Med. Phys. 2017, 44, 4828–4837. [Google Scholar] [CrossRef]
- Jiang, S.; Yoan, W.; Yang, Y.; Zhang, D.; Liu, N.; Wang, W. Modelling and analysis of a novel CT-guided puncture robot for lung brachytherapy. Adv. Robot. 2017, 31, 557–569. [Google Scholar] [CrossRef]
- Pisla, D.; Galdau, B.; Covaciu, F.; Vaida, C.; Popescu, D.; Plitea, N. Safety issues in the development of the experimental model for an innovative medical parallel robot used in brachytherapy. Int. J. Prod. Res. 2017, 55, 684–699. [Google Scholar] [CrossRef]
- Ma, X.; Jiang, S.; Yang, Z.; Zhang, G.; Yu, Z.; Chai, S. A real-time tracking and visualization system for robot -asssited template location method applied to lung cancer brachytherapy. J. Med. Devices 2019, 13, 1–25. [Google Scholar] [CrossRef]
- Schlenker, B.; Apfelbeck, M.; Buchner, A.; Stief, C.; Clavert, D.A. MRI-TRUS fusion biopsy of the prostate: Quality of image fusion in a clinical setting. Clin. Hemorheol. Microcirc. 2018, 70, 433–440. [Google Scholar] [CrossRef]
- Shakeri, S.; Menard, C.; Lopes, R.; Kadoury, S. Deformable MRI-TRUS surface registration from statistical deformation models of the prostate. In Proceedings of the Conference on Medical Imaging-Image Guided Procedures, Robotic Interventions and Modeling, San Diego, CA, USA, 16–21 February 2019; p. 10951. [Google Scholar]
- Hungr, N.; Baumann, M.; Long, J.A.; Troccaz, J. A 3-D ultrasound robotic prostate brachytherapy system with prostate motion tracking. IEEE Trans. Robot. 2013, 28, 1382–1397. [Google Scholar] [CrossRef]
- Pisla, D.; Plitea, N.; Vidrean, A.; Prodan, B.; Gherman, B.; Lese, D. Kinematics and Design of two variants of a reconfigurable parallel robot. In Proceedings of the 2009 ASME/IFToMM International Conference on Reconfigurable Mechanisms and Robots, London, UK, 22–24 June 2009; pp. 624–631. [Google Scholar]
- Lin, X.; Zhou, S.; Wen, T.; Jiang, S.; Chen, J. A novel multi-DoF surgical robotic system for brachytherapy on liver tumor: Design and Control. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Pisla, D.; Caida, C.; Birlescu, I.; Gherman, B.; Plite, N. Risk Management for the reliability of the robotic assisted treatment of non resectable liver tumors. Appl. Sci. 2020, 10, 52. [Google Scholar] [CrossRef]
- Pei, X.; Xie, W.; Fan, X.; Hu, Y.D.; Buzurovic, I. The Miniature Robotic Needling Device in Brachytherapy: Design and modelling -An approach towards smart needle system. In Proceedings of the 2nd WRc Symposium on Advanced Robotics and Automation 2019, Beijing, China, 21–22 August 2019; pp. 68–73. [Google Scholar]
- Varnamkhasti, Z.K.; Konh, B. Design, Fabrication and Testing of a Flexible Three-Dimensional Printed Percutaneous Needle with Embedded Actuators. J. Med. Devices-Trans. ASME 2021, 15, 021007. [Google Scholar] [CrossRef]
- Vaida, C. Medical robotic systems with application in surgery, oncology and rehabilitation. Ph.D. Thesis, Faculty of Physical Education—Palacky University in Olomouc, lomouc-Neředín, Czech Republic, 2018. [Google Scholar]
- Omega 7. Available online: https://www.forcedimension.com (accessed on 10 October 2022).
- FT 300 Robotiq. Available online: https://robotiq.com/products/ft-300-force-torque-sensor (accessed on 10 October 2022).
- Safeea, M.; Neto, P. KUKA Sunrise Toolbox: Interfacing Collaborative Robots With MATLAB. IEEE Robot. Autom. Mag. 2019, 26, 91–96. [Google Scholar] [CrossRef]
- Kuka iiwa 7 R800. Available online: https://www.kuka.com/en-de/products/robot-systems/industrial-robots/lbr-iiwa (accessed on 10 October 2022).
- Arduino Uno. Available online: https://store.arduino.cc/products/arduino-uno-rev3 (accessed on 10 October 2022).
- Pisla, D.; Vaida, C.; Birlescu, I.; Graur, F.; Gherman, B.; Tucan, P.; Plitea, N. Automated Medical Instrument With Multiple Needles for Brachytherapy. A00431/12.09.2017, 12 September 2017. [Google Scholar]
- Vaida, C.; Plitea, N.; Al Hajjar, N.; Burz, A.; Graur, F.; Gherman, B.; Pisla, D. A new robotic system for minimally invasive treatment of liver tumours. Proc. Rom. Acad. Ser. A Math. Phys. Tech. Sci. Inf. Sci. 2020, 21, 271–363. [Google Scholar]
- MATLAB. Available online: https://www.mathworks.com (accessed on 10 October 2022).
- Tucan, P.; Gherman, B.; Major, K.; Vaida, C.; Major, Z.; Plitea, N.; Carbone, G.; Pisla, D. Fuzzy Logic-Based Risk Assessment of a Parallel Robot for Elbow and Wrist Rehabilitation. Int. J. Environ. Res. Public Health 2020, 17, 654. [Google Scholar] [CrossRef]
- Vaida, C.; Pisla, D.; Schadlbauer, J.; Husty, M.; Plitea, N. Kinematic Analysis of an Innovative Medical Parallel Robot Using Study Parameters. In New Trends in Medical and Service Robots. Mechanisms and Machine Science; Wenger, P., Chevallereau, C., Pisla, D., Bleuler, H., Rodić, A., Eds.; Springer: Cham, Switzerland, 2016; Volume 39. [Google Scholar] [CrossRef]
- Birlescu, I.; Graur, F.; Vaida, C.; Radu, C.; Tucan, P.; Gherman, B.; Pisla, A.; Al Hajjar, N.; Pisla, D. Experimental testing and implementation of a force—torque sensor in automated percutaneous needle insertion instruments. In Proceedings of the 2021 International Conference on e-Health and Bioengineering (EHB), Iasi, Romania, 18–19 November 2021; pp. 1–6. [Google Scholar]
- De Cobeli, O.; Terracciano, D.; Tagliabue, E.; Raimondi, S.; Bottero, D.; Cioffi, A.; Jareczek-Fossa, B.; Petralia, G.; Cordima, G.; Almeida, G.L.; et al. Predicting pathological features at radical prostatectomy in patients with prostate cancer eligible for active surveillance by multiparametric magnetic resonance imaging. PLoS ONE 2015, 10, e0139696. [Google Scholar] [CrossRef]
- Qualica QFD. Available online: https://www.qualica.net/ (accessed on 10 October 2022).
- Gherman, B.; Vaida, C.; Pisla, D.; Plitea, N.; Gyurka, B.; Lese, D.; Glogoveanu, M. Singularities and workspace analysis for a parallel robot for minimally invasive surgery. In Proceedings of the 2010 IEEE International Conference on Automation, Quality and Testing, Robotics (AQTR), Cluj-Napoca, Romania, 28–30 May 2010; pp. 1–6. [Google Scholar] [CrossRef]
- Friction Coefficient Tables in Air and Vacuum. Available online: https://www.tribonet.org (accessed on 11 October 2022).
- Flores, P. Modeling and simulation of wear in revolute clearance joints in multibody systems. Mech. Mach. Theory 2009, 44, 1211–1222. [Google Scholar] [CrossRef]
- Archard, J.F. Contact and Rubbing of Flat Surfaces. J. Appl. Phys. 1953, 24, 981. [Google Scholar] [CrossRef]
- Salib, J.; Kligerman, Y.; Etsion, I. A Model for Potential Adhesive Wear Particle at Sliding Inception of a Spherical Contact. Tribol. Lett. 2008, 30, 225–233. [Google Scholar] [CrossRef]
- The Engineering Toolbox. Available online: https://www.engineeringtoolbox.com/bhn-brinell-hardness-number-d_1365.html (accessed on 10 October 2022).





| Cancer Location | 5-Year Survival Rates (%) Related to Detection Time | Incidence (Number) | ||||
|---|---|---|---|---|---|---|
| Overall | Local | Regional | Distant | New Cases | Deaths | |
| Breast | 90 | 99 | 86 | 29 | 290,560 | 43,780 |
| Colon and rectum | 65 | 91 | 72 | 15 | 151,030 | 52,580 |
| Esophagus | 20 | 46 | 26 | 5 | 20,640 | 16,410 |
| Kidney | 76 | 93 | 71 | 14 | 79,000 | 13,920 |
| Liver | 20 | 35 | 12 | 3 | 41,260 | 30,520 |
| Lung and bronchus | 22 | 60 | 33 | 6 | 236,740 | 130,180 |
| Skin melanoma | 93 | 99 | 68 | 30 | 99,780 | 7650 |
| Pancreas | 11 | 42 | 14 | 3 | 62,120 | 49,830 |
| Prostate | 98 | >99 | >99 | 31 | 268,490 | 34,500 |
| Stomach | 32 | 70 | 32 | 6 | 26,380 | 11,090 |
| No. | Diagnosis | Therapy | Evolution |
|---|---|---|---|
| Tumors locate in the prostate | |||
| 1 | Prostate adenocarcinoma T3NoMo, G8,iPSA = 18 ng/mL (2013) | Hormone therapy (HT) + External radiotherapy (RT) 76 Gy | Local recurrence. A complement of brachytherapy seeds in 2013 could have avoided the recurrence. |
| 2 | Prostate adenocarcinoma T2NoMo, G6, iPSA = 11 ng/mL (2011). Right seminal bladder recurrence | Permanent implant of iodine 125 | Local recurrence. A complement of brachytherapy seeds would avoid a mutilating surgery that the patient refused. |
| 3 | Prostate adenocarcinoma T2aNoMo, G6, iPSA = 9 (2012). Prior rectum cancer surgically removed | Hormone therapy (HT) | Death. Robotic brachytherapy would have saved the patient. |
| 4 | Bladder cancer, T2NoMo | Radical cystectomy | Partial cystectomy and robotic brachytherapy would have avoided the mutilating surgery with a better quality of life. |
| Tumours located in the liver | |||
| 1 | Rectosigmoid cancer stage IV (liver and pulmonary metastases), Radio- and Chemotherapy (RCT), surgery | Palliative chemotherapy. Radiofrequency ablation | Focal brachytherapy would have performed better on the liver metastases. |
| 2 | Oesophagus cancer, stage III, RCT. Local recurrence and liver spread. Cirrhosis | Supportive care | Death. Brachytherapy (oesophagus and liver) would have extended the patient survival with good life quality. |
| 3 | Unifocal hepatocellular carcinoma over cirrhosis. Inoperable due to comorbidities | Sorafenib | Death. Local brachytherapy would have extended the patient survival. |
| Tumours located at the rectum level | |||
| 1 | Rectal cancer, RCT, surgery. Local recurrence | Palliative chemotherapy | Local and distant recurrence. Brachytherapy would have improved the prognosis avoiding (or delaying) metastases. |
| 2 | Rectum (stage III) and prostate Synchronous Adenocarcinoma T3NoMo | RCT + surgery | Prostate brachytherapy would have prevented the surgery and all its complications (incontinence, urinary infection). |
| 3 | Epidermoid carcinoma anal canal. T4N2Mo, RCT. Local recurrence. Surgery (rectum amputation) | Surgery | Initial brachytherapy would have avoided the recurrence and thus the amputation. |
| 4 | Inferior rectum adenocarcinoma T2NoMo | Rectum amputation | Local excision and brachytherapy would have avoided the second, mutilating, surgery. |
| Thoracic tumours (lungs and breast) | |||
| 1 | Pulmonary metastases following testicle cancer, multiple recurrences | Chemotherapy, surgeries (testicle, ganglions, lungs) External pulmonary radiotherapy | Death. Brachytherapy would have avoided the recurrences. |
| 2 | Epidermoid pulmonary cancer. Local inoperable recurrence | Chemotherapy, palliative radiotherapy | Death. Brachytherapy would have, at least, extended the survival. |
| 3 | Thoracic sarcoma | Surgery, radiotherapy | Brachytherapy would have provided the same outcome with much lower toxicity. |
| 4 | Retroperitoneal sarcoma, positive margins resection, irradiated | Surgery, RT, CHT | Local recurrence. Brachytherapy could have complemented the external dose of radiation to avoid recurrences, without the increase in intestinal toxicity. |
| Step | (1)—Preplanning |
|---|---|
| 1. | The patient undergoes a complete non-invasive imagistic investigation (CT—compulsory and MRI—if needed) for the exact definition of the tumor(s) location. |
| 2. | The CT images are analyzed and the following parameters are defined:
|
| 3. | Using CT-scan equipment which has an external laser-based fixed coordinate system, the patient position for the procedure is established to enable:
|
| 4. | A set of markers is positioned on the patient to enable:
|
| 5. | A second tomography is performed, using the same CT-scan, to validate the positions of all the markers (if needed fine adjustments are made for the final positions). |
| 6. | The relative robot–patient position is optimized to ensure that all the targeted points can be reached (are located within the robot workspace) and the final trajectories are validated. |
| (2)—CT-guided robotic brachytherapy | |
| 7. | Using the numbered mounting holes the robot is positioned on the mobile CT-scan couch followed by the patient, whose position will be reproduced using the existing markers (step 4). The patient and robot are calibrated with respect to the laser coordinate system and the transformation matrix for the transformation of the patient coordinates into the robot coordinates is introduced. |
| 8. | The robot performs the homing and then positions the needle insertion module in a predefined point, above the patient, but not very far from the area of interest. |
| 9. | The coordinates of the pairs of points (Insertion—Target) are loaded using a secured USB drive to avoid any human errors. |
| 10. | The first needle is positioned from the current (neutral point) into the Insertion point oriented on the linear trajectory defined by the first pair of points. Due to the external markers the doctors will be able to see any misalignments before any invasive action has been performed. |
| 11. | The needle is introduced into the patient at a depth of 40–50 mm followed by a quick scan aimed to validate the trajectory. |
| 12. | If needed, small trajectory corrections are applied. |
| 13. | In case the trajectory has an error higher than 0.2°–0.5° (depending on the target depth) the needle is retracted and introduced again. |
| 14. | The needle is introduced until the targeted point and then released from the insertion module. |
| For each following needle the steps 10–14 are repeated until all the needles are in place. | |
| 15. | The robot is retracted into the home position to allow full access to the inserted needles. |
| 16. | The needles are connected to dedicated equipment that will deliver the radioactive seeds based on the treatment protocol. |
| 17. | After the treatment the needles are extracted from the patient body and the patient is removed from the CT-scan mobile couch. |
| 18. | The robot is removed, and the procedure is completed. |
| (3)—Patient follow-up | |
| 19. | The patient evolution is monitored and based on his/her evolution subsequent treatments are scheduled. |
| 20. | All the information is stored in a database recording the robot parameters, procedural times as well as all the medically relevant data such as needle accuracy and treatment efficiency. |
| Scenario | Maximum Normal Force | |
|---|---|---|
| Between Needle and Centering Device [N] | Between Stylet and Canula [N] | |
| 1 (250 mm/s) | 3.57 | 1.36 |
| 2 (125 mm/s) | 5.73 | 2.28 |
| 3 (25 mm/s) | 5.02 | 1.99 |
| 4 (12.5 mm/s) | 5.99 | 2.392 |
| 5 (250 mm/s + rotation) | 8.74 | 3.45 |
| 6 (125 mm/s + rotation) | 7.97 | 3.16 |
| 7 (12.5 mm/s + rotation) | 5.24 | 2.08 |
| Scenario | Wear [mm3] | Wear Rate [mm3/s] | ||
|---|---|---|---|---|
| ABS–Steel | Steel–Steel | ABS–Steel | Steel–Steel | |
| 1 (250 mm/s) | 0.003482927 | 0.008102128 | 0.004353659 | 0.01012766 |
| 2 (125 mm/s) | 0.005590244 | 0.013582979 | 0.003493902 | 0.008489362 |
| 3 (25 mm/s) | 0.004897561 | 0.011855319 | 0.000612195 | 0.001481915 |
| 4 (12.5 mm/s) | 0.005843902 | 0.014250213 | 0.000365244 | 0.000890638 |
| 5 (250 mm/s + rotation) | 0.008526829 | 0.020553191 | 0.010658537 | 0.025691489 |
| 6 (125 mm/s + rotation) | 0.00777561 | 0.018825532 | 0.004859756 | 0.011765957 |
| 7 (12.5 mm/s + rotation) | 0.005112195 | 0.012391489 | 0.000319512 | 0.000774468 |
| Min value | 0.003482927 | 0.008102128 | 0.000319512 | 0.000774468 |
| Max value | 0.008526829 | 0.020553191 | 0.010658537 | 0.025691489 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tucan, P.; Vaida, C.; Horvath, D.; Caprariu, A.; Burz, A.; Gherman, B.; Iakab, S.; Pisla, D. Design and Experimental Setup of a Robotic Medical Instrument for Brachytherapy in Non-Resectable Liver Tumors. Cancers 2022, 14, 5841. https://doi.org/10.3390/cancers14235841
Tucan P, Vaida C, Horvath D, Caprariu A, Burz A, Gherman B, Iakab S, Pisla D. Design and Experimental Setup of a Robotic Medical Instrument for Brachytherapy in Non-Resectable Liver Tumors. Cancers. 2022; 14(23):5841. https://doi.org/10.3390/cancers14235841
Chicago/Turabian StyleTucan, Paul, Calin Vaida, Daniel Horvath, Andrei Caprariu, Alin Burz, Bogdan Gherman, Stefan Iakab, and Doina Pisla. 2022. "Design and Experimental Setup of a Robotic Medical Instrument for Brachytherapy in Non-Resectable Liver Tumors" Cancers 14, no. 23: 5841. https://doi.org/10.3390/cancers14235841
APA StyleTucan, P., Vaida, C., Horvath, D., Caprariu, A., Burz, A., Gherman, B., Iakab, S., & Pisla, D. (2022). Design and Experimental Setup of a Robotic Medical Instrument for Brachytherapy in Non-Resectable Liver Tumors. Cancers, 14(23), 5841. https://doi.org/10.3390/cancers14235841










