Roles of Mitochondria in Oral Squamous Cell Carcinoma Therapy: Friend or Foe?
Abstract
Simple Summary
Abstract
1. Introduction
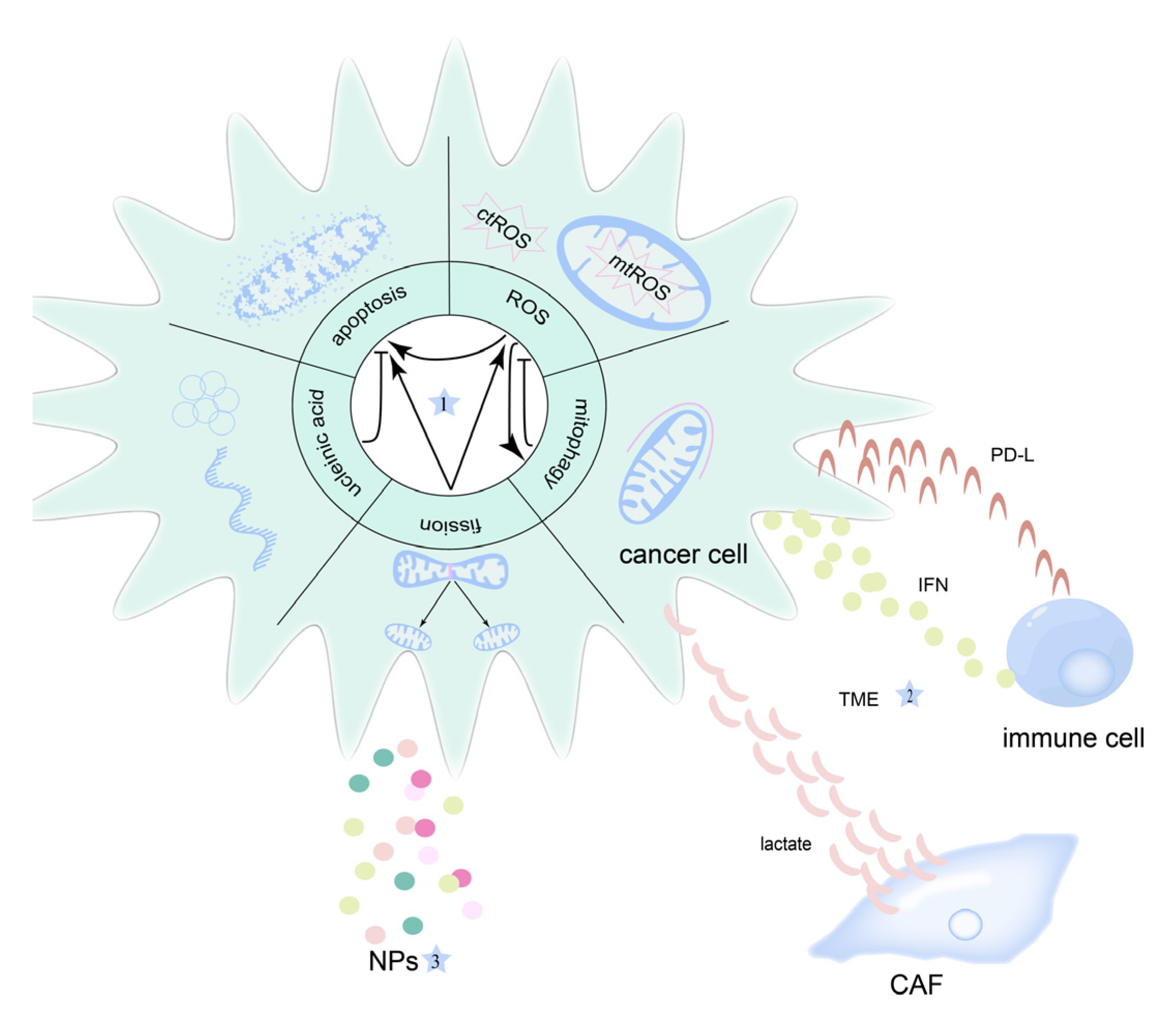
2. Mitochondria and OSCC
3. Mitochondrial-Targeted Therapeutic Strategies for OSCC
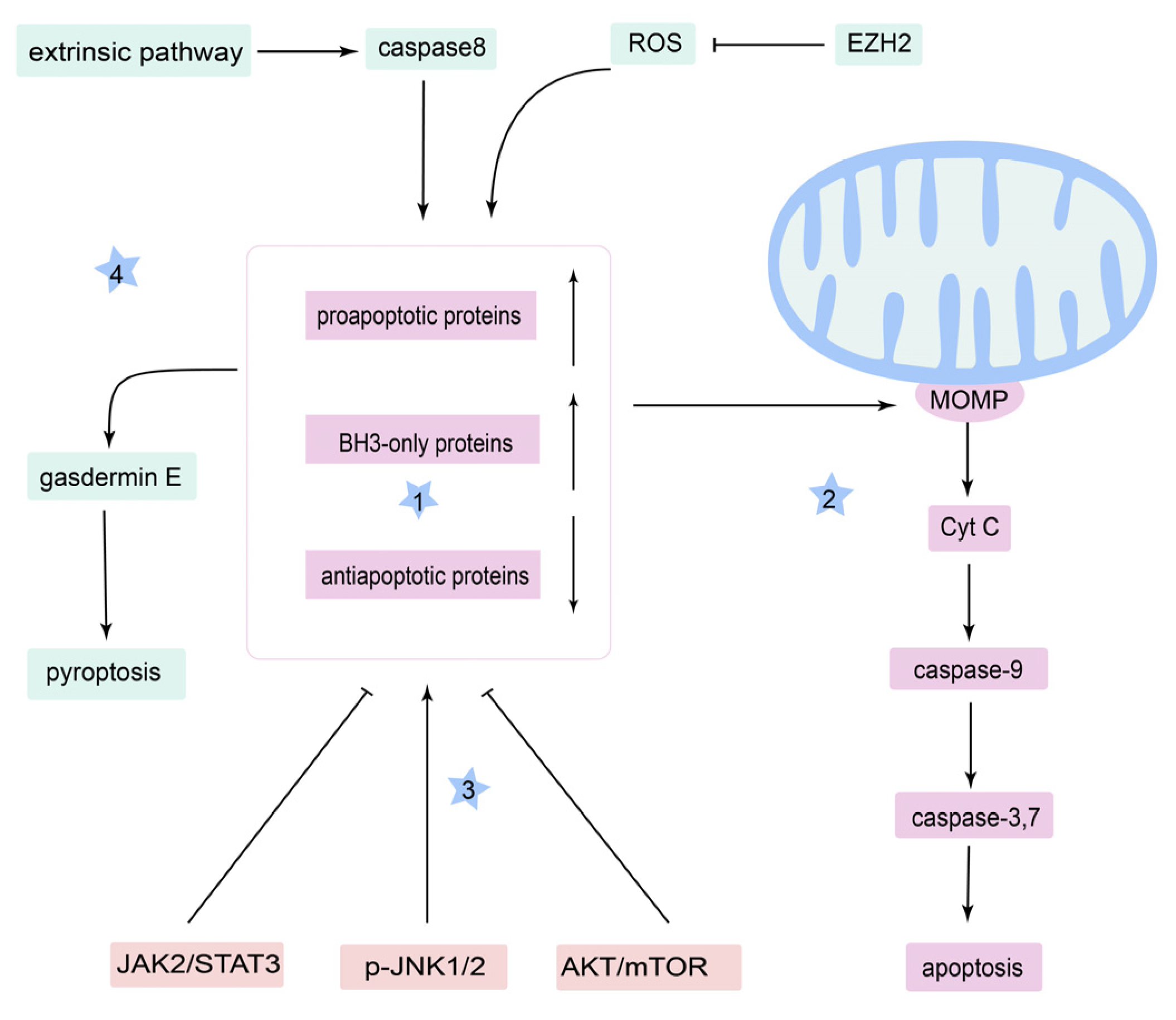
3.1. Apoptosis
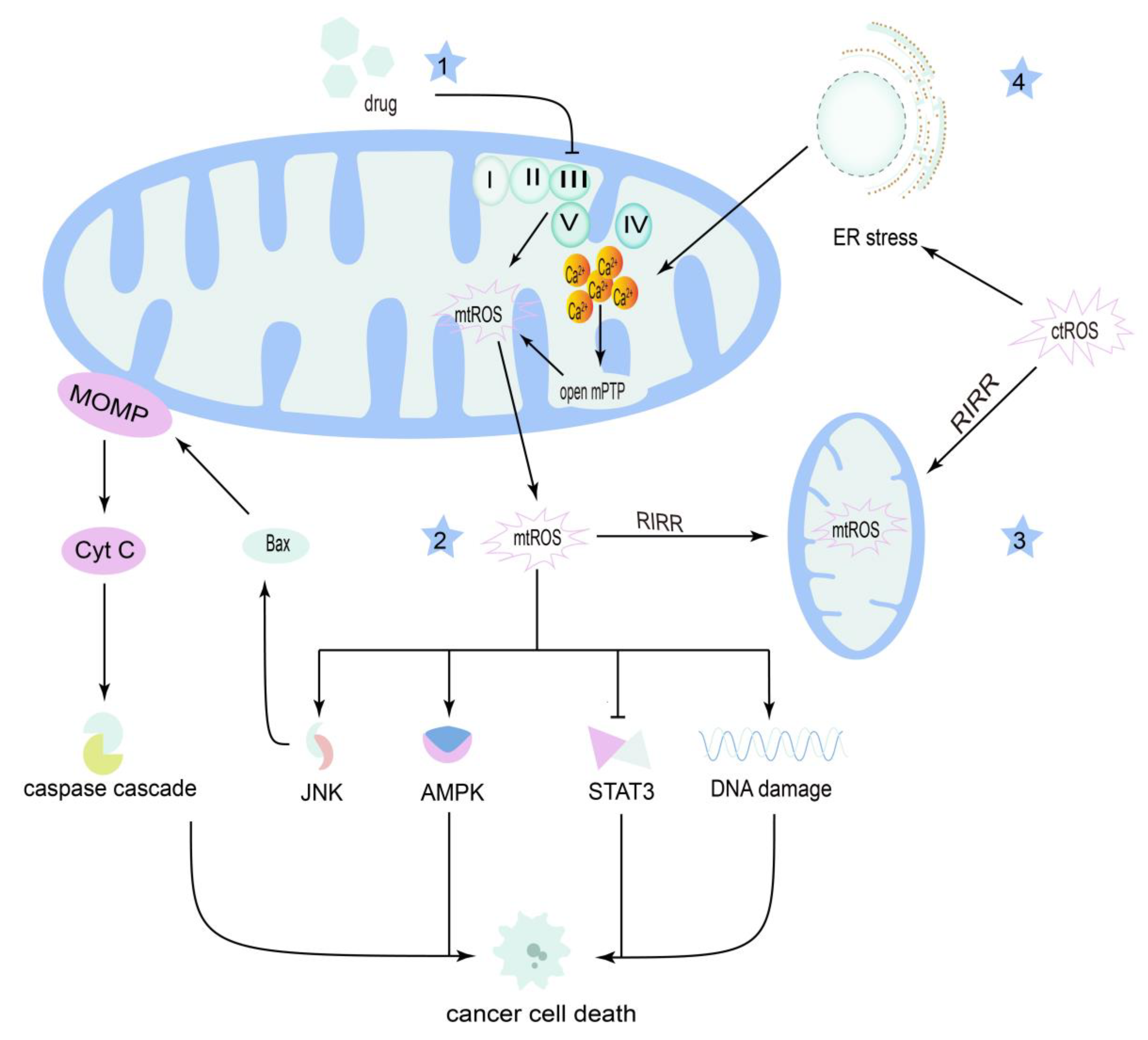
3.2. Reactive Oxygen Species
3.3. Mitochondrial Fission
4. Mitochondria in OSCC Therapy Resistance
4.1. Mitophagy
4.2. Mitochondrial MicroRNAs and Mitochondrial DNA
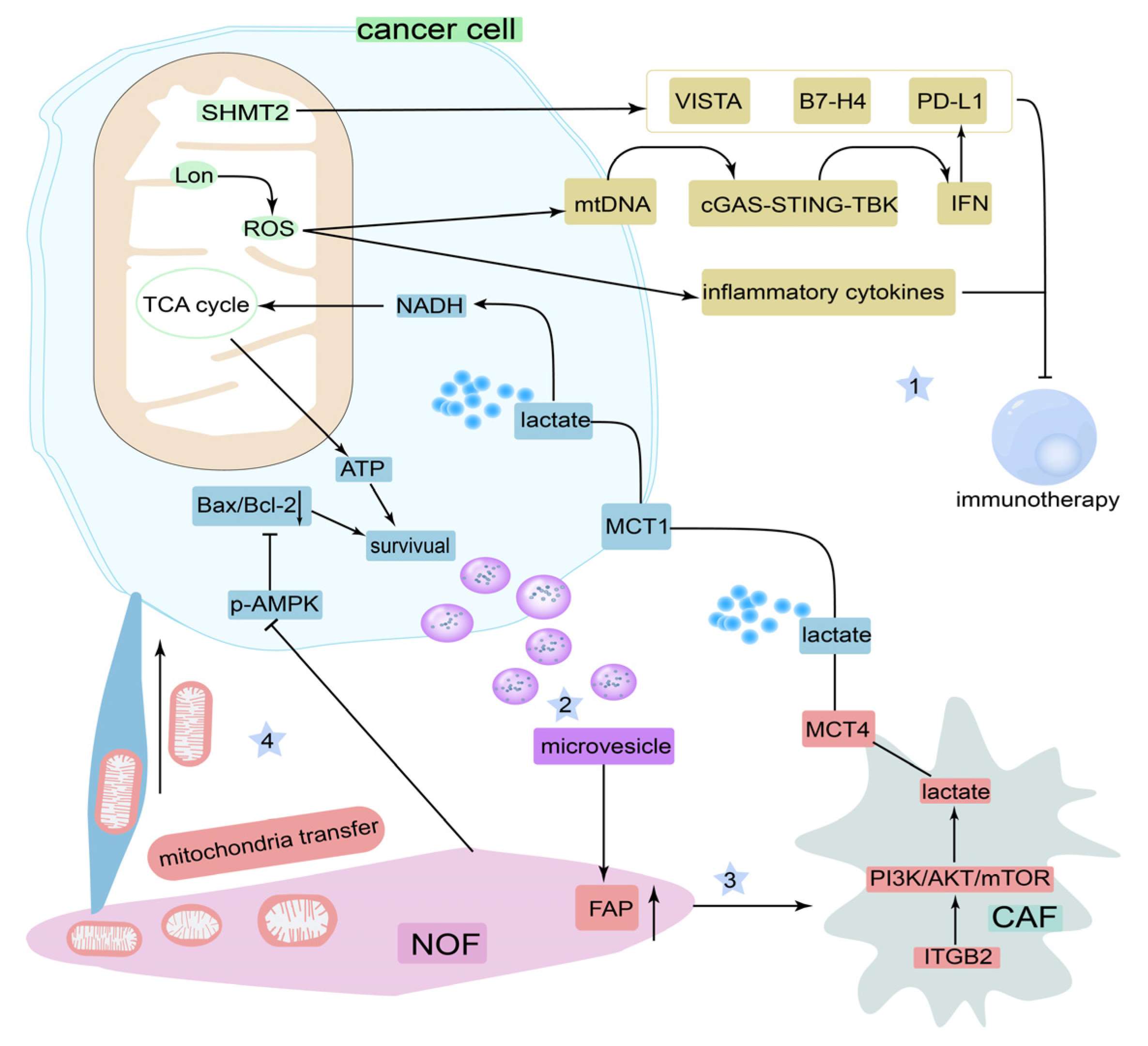
4.3. Tumor Microenvironment
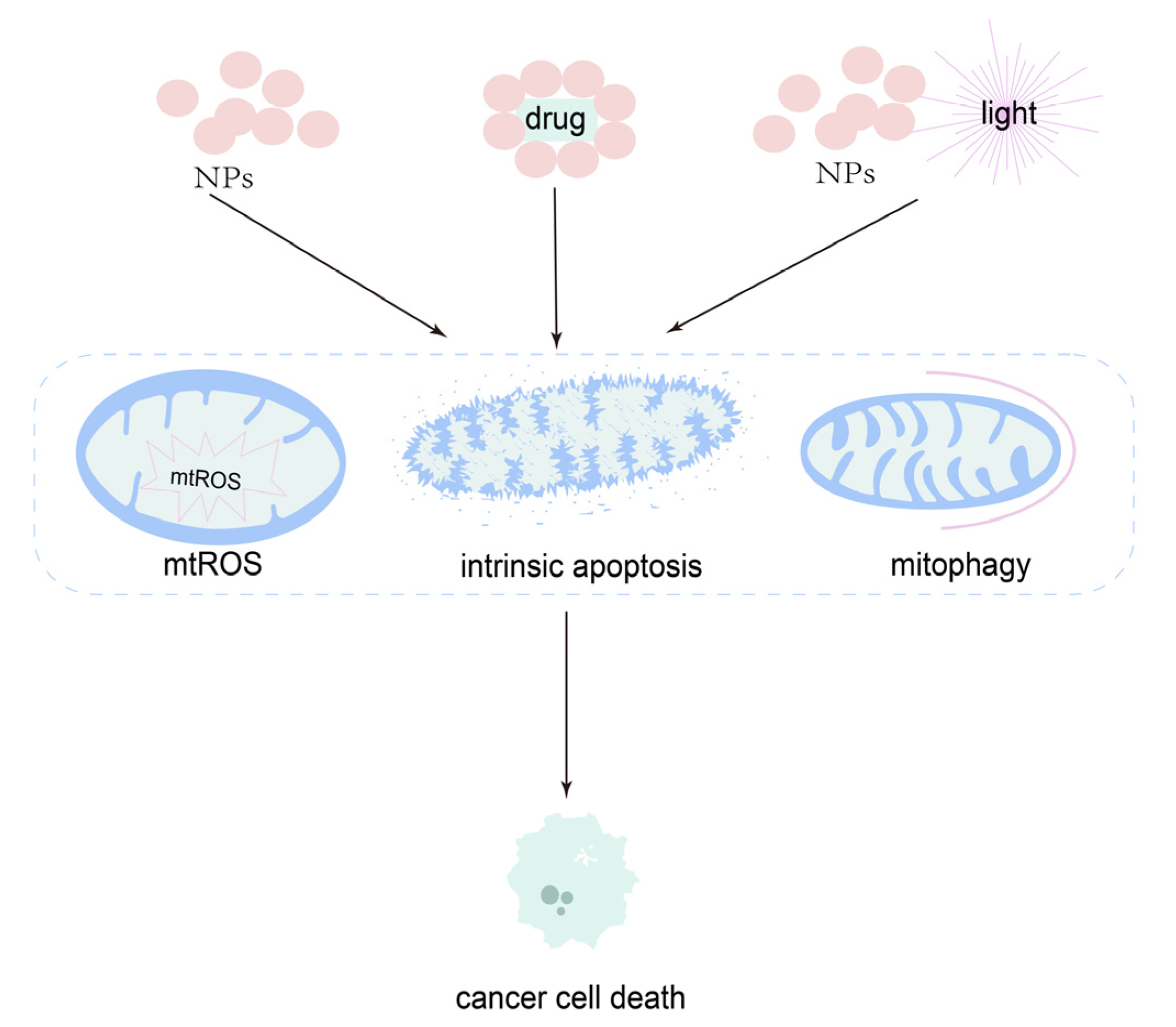
5. Nanoparticles in Mitochondria-Targeted Therapy in OSCC
6. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meng, X.; Lou, Q.; Yang, W.; Wang, Y.; Chen, R.; Wang, L.; Xu, T.; Zhang, L. The role of non-coding RNAs in drug resistance of oral squamous cell carcinoma and therapeutic potential. Cancer Commun. 2021, 41, 981–1006. [Google Scholar] [CrossRef] [PubMed]
- Chai, A.W.Y.; Lim, K.P.; Cheong, S.C. Translational genomics and recent advances in oral squamous cell carcinoma. Semin. Cancer Biol. 2020, 61, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Thomson, P.J. Perspectives on oral squamous cell carcinoma prevention—Proliferation, position, progression and prediction. J. Oral Pathol. Med. 2018, 47, 803–807. [Google Scholar] [CrossRef]
- Ng, J.H.; Iyer, N.G.; Tan, M.-H.; Edgren, G. Changing epidemiology of oral squamous cell carcinoma of the tongue: A global study. Head Neck 2016, 39, 297–304. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Zanoni, D.K.; Montero, P.H.; Migliacci, J.C.; Shah, J.P.; Wong, R.J.; Ganly, I.; Patel, S.G. Survival outcomes after treatment of cancer of the oral cavity (1985–2015). Oral Oncol. 2019, 90, 115–121. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, P. Resistance and recurrence of malignancies after CAR-T cell therapy. Exp. Cell Res. 2021, 410, 112971. [Google Scholar] [CrossRef]
- Mahato, R.; Tai, W.; Cheng, K. Prodrugs for improving tumor targetability and efficiency. Adv. Drug Deliv. Rev. 2011, 63, 659–670. [Google Scholar] [CrossRef]
- Kummer, E.; Ban, N. Mechanisms and regulation of protein synthesis in mitochondria. Nat. Rev. Mol. Cell Biol. 2021, 22, 307–325. [Google Scholar] [CrossRef]
- Barba-Aliaga, M.; Alepuz, P. Role of eIF5A in Mitochondrial Function. Int. J. Mol. Sci. 2022, 23, 1284. [Google Scholar] [CrossRef]
- Porporato, P.E.; Filigheddu, N.; Pedro, J.M.B.-S.; Kroemer, G.; Galluzzi, L. Mitochondrial metabolism and cancer. Cell Res. 2018, 28, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Chou, G.-L.; Peng, S.-F.; Liao, C.; Ho, H.-C.; Lu, K.-W.; Lien, J.-C.; Fan, M.-J.; La, K.-C.; Chung, J.-G. Casticin impairs cell growth and induces cell apoptosis via cell cycle arrest in human oral cancer SCC-4 cells. Environ. Toxicol. 2018, 33, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, L.; Lu, Y.; Shen, Y.; Zhang, M.; Ge, L.; Wang, M.; Yang, J.; Tian, Z.; Tang, X. Azoxystrobin Reduces Oral Carcinogenesis by Suppressing Mitochondrial Complex III Activity and Inducing Apoptosis. Cancer Manag. Res. 2020, 12, 11573–11583. [Google Scholar] [CrossRef]
- Lei, X.; Lin, H.; Wang, J.; Ou, Z.; Ruan, Y.; Sadagopan, A.; Chen, W.; Xie, S.; Chen, B.; Li, Q.; et al. Mitochondrial fission induces immunoescape in solid tumors through decreasing MHC-I surface expression. Nat. Commun. 2022, 13, 3882. [Google Scholar] [CrossRef] [PubMed]
- Kraus, F.; Roy, K.; Pucadyil, T.J.; Ryan, M.T. Function and regulation of the divisome for mitochondrial fission. Nature 2021, 590, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Genovese, I.; Carinci, M.; Modesti, L.; Aguiari, G.; Pinton, P.; Giorgi, C. Mitochondria: Insights into Crucial Features to Overcome Cancer Chemoresistance. Int. J. Mol. Sci. 2021, 22, 4770. [Google Scholar] [CrossRef]
- Guerra, F.; Arbini, A.A.; Moro, L. Mitochondria and cancer chemoresistance. Biochim. Biophys. Acta (BBA)—Bioenerg. 2017, 1858, 686–699. [Google Scholar] [CrossRef]
- Guerra-Librero, A.; Fernandez-Gil, B.; Florido, J.; Martinez-Ruiz, L.; Rodríguez-Santana, C.; Shen, Y.-Q.; García-Verdugo, J.; López-Rodríguez, A.; Rusanova, I.; Quiñones-Hinojosa, A.; et al. Melatonin Targets Metabolism in Head and Neck Cancer Cells by Regulating Mitochondrial Structure and Function. Antioxidants 2021, 10, 603. [Google Scholar] [CrossRef] [PubMed]
- Purohit, P.K.; Saini, N. Mitochondrial microRNA (MitomiRs) in cancer and complex mitochondrial diseases: Current status and future perspectives. Cell. Mol. Life Sci. 2020, 78, 1405–1421. [Google Scholar] [CrossRef]
- Hsieh, Y.-T.; Tu, H.-F.; Yang, M.-H.; Chen, Y.-F.; Lan, X.-Y.; Huang, C.-L.; Chen, H.-M.; Li, W.-C. Mitochondrial genome and its regulator TFAM modulates head and neck tumourigenesis through intracellular metabolic reprogramming and activation of oncogenic effectors. Cell Death Dis. 2021, 12, 96. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, K.X.; Fan, Y.; Gao, Z.; Bindoff, L.A.; Costea, D.E.; Li, L. Fibroblasts rescue oral squamous cancer cell from metformin-induced apoptosis via alleviating metabolic disbalance and inhibiting AMPK pathway. Cell Cycle 2019, 18, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Cho, U.; Kim, S.; Park, I.S.; Cho, J.H.; Dhanasekaran, D.N.; Song, Y.S. Tumour microenvironment on mitochondrial dynamics and chemoresistance in cancer. Free Radic. Res. 2018, 52, 1271–1287. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Swargiary, G.; Tyagi, S.; Singh, M.; Jha, N.K.; Singh, K.K. Nanotherapeutic approaches to target mitochondria in cancer. Life Sci. 2021, 281, 119773. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.-X.; Rabinowitz, J.D.; White, E. Mitochondria and Cancer. Mol. Cell 2016, 61, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Sainz, A.G.; Shadel, G.S. Mitochondrial DNA: Cellular genotoxic stress sentinel. Trends Biochem. Sci. 2021, 46, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Varela-López, A.; Vera-Ramírez, L.; Giampieri, F.; Navarro-Hortal, M.D.; Forbes-Hernández, T.Y.; Battino, M.; Quiles, J.L. The central role of mitochondria in the relationship between dietary lipids and cancer progression. Semin. Cancer Biol. 2021, 73, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. Mitochondria and cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Vidal, C.; Dey, S.; Zhang, L. Mitochondria Targeting as an Effective Strategy for Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 3363. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.D.; Shao, S.X.; Jiang, H.P.; Cao, Y.W.; Wang, Y.H.; Yang, X.C.; Wang, Y.L.; Wang, X.S.; Niu, H.T. Warburg Effect or Reverse Warburg Effect? A Review of Cancer Metabolism. Oncol. Res. Treat. 2015, 38, 117–122. [Google Scholar] [CrossRef]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg effect: Historical dogma versus current understanding. J. Physiol. 2020, 599, 1745–1757. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Missiroli, S.; Perrone, M.; Genovese, I.; Pinton, P.; Giorgi, C. Cancer metabolism and mitochondria: Finding novel mechanisms to fight tumours. eBioMedicine 2020, 59, 102943. [Google Scholar] [CrossRef] [PubMed]
- Badrinath, N.; Yoo, S.Y. Mitochondria in cancer: In the aspects of tumorigenesis and targeted therapy. Carcinogenesis 2018, 39, 1419–1430. [Google Scholar] [CrossRef] [PubMed]
- Sessions, D.T.; Kashatus, D.F. Mitochondrial dynamics in cancer stem cells. Cell. Mol. Life Sci. 2021, 78, 3803–3816. [Google Scholar] [CrossRef]
- Scheid, A.D.; Beadnell, T.C.; Welch, D.R. Roles of mitochondria in the hallmarks of metastasis. Br. J. Cancer 2020, 124, 124–135. [Google Scholar] [CrossRef]
- van der Merwe, M.; van Niekerk, G.; Fourie, C.; du Plessis, M.; Engelbrecht, A.-M. The impact of mitochondria on cancer treatment resistance. Cell Oncol. 2021, 44, 983–995. [Google Scholar] [CrossRef]
- Hou, X.-S.; Wang, H.-S.; Mugaka, B.P.; Yang, G.-J.; Ding, Y. Mitochondria: Promising organelle targets for cancer diagnosis and treatment. Biomater. Sci. 2018, 6, 2786–2797. [Google Scholar] [CrossRef]
- Hu, Q.; Peng, J.; Chen, X.; Li, H.; Song, M.; Cheng, B.; Wu, T. Obesity and genes related to lipid metabolism predict poor survival in oral squamous cell carcinoma. Oral Oncol. 2018, 89, 14–22. [Google Scholar] [CrossRef]
- de Mattos, S.; Diel, L.; Bittencourt, L.; Schnorr, C.; Gonçalves, F.; Bernardi, L.; Lamers, M. Glycolytic pathway candidate markers in the prognosis of oral squamous cell carcinoma: A systematic review with meta-analysis. Braz. J. Med. Biol. Res. 2021, 54, e10504. [Google Scholar] [CrossRef]
- Wu, R.; Zuo, W.; Xu, X.; Bi, L.; Zhang, C.; Chen, H.; Liu, H. MCU That Is Transcriptionally Regulated by Nrf2 Augments Malignant Biological Behaviors in Oral Squamous Cell Carcinoma Cells. BioMed. Res. Int. 2021, 2021, 6650791. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, Y.; Zhao, M.; Ding, L.; Yang, X.; Jing, Y.; Song, Y.; Chen, S.; Hu, Q.; Ni, Y. ITGB2-mediated metabolic switch in CAFs promotes OSCC proliferation by oxidation of NADH in mitochondrial oxidative phosphorylation system. Theranostics 2020, 10, 12044–12059. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhao, Y.; Li, T.; Gan, X.; Yu, H. The Role of PKM2 in the Regulation of Mitochondrial Function: Focus on Mitochondrial Metabolism, Oxidative Stress, Dynamic, and Apoptosis. PKM2 in Mitochondrial Function. Oxidative Med. Cell Longev. 2022, 2022, 770268. [Google Scholar] [CrossRef] [PubMed]
- Kurihara-Shimomura, M.; Sasahira, T.; Nakashima, C.; Kuniyasu, H.; Shimomura, H.; Kirita, T. The Multifarious Functions of Pyruvate Kinase M2 in Oral Cancer Cells. Int. J. Mol. Sci. 2018, 19, 2907. [Google Scholar] [CrossRef] [PubMed]
- Ashton, T.M.; McKenna, W.G.; Kunz-Schughart, L.A.; Higgins, G.S. Oxidative Phosphorylation as an Emerging Target in Cancer Therapy. Clin. Cancer Res. 2018, 24, 2482–2490. [Google Scholar] [CrossRef] [PubMed]
- Vasan, K.; Werner, M.; Chandel, N.S. Mitochondrial Metabolism as a Target for Cancer Therapy. Cell Metab. 2020, 32, 341–352. [Google Scholar] [CrossRef]
- Panigrahi, D.P.; Praharaj, P.P.; Bhol, C.S.; Mahapatra, K.K.; Patra, S.; Behera, B.P.; Mishra, S.R.; Bhutia, S.K. The emerging, multifaceted role of mitophagy in cancer and cancer therapeutics. Semin. Cancer Biol. 2019, 66, 45–58. [Google Scholar] [CrossRef]
- Burke, P.J. Mitochondria, Bioenergetics and Apoptosis in Cancer. Trends Cancer 2017, 3, 857–870. [Google Scholar] [CrossRef]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef]
- Gan, B. Mitochondrial regulation of ferroptosis. J. Cell Biol. 2021, 220. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Kroemer, G. Cuproptosis: A copper-triggered modality of mitochondrial cell death. Cell Res. 2022, 32, 417–418. [Google Scholar] [CrossRef]
- Faizan, I.; Ahmad, T. Altered mitochondrial calcium handling and cell death by necroptosis: An emerging paradigm. Mitochondrion 2021, 57, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Bernard, N.J. Mitochondria control pyroptosis. Nat. Immunol. 2021, 22, 1071. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Festa, A.; Falco, M.; Lombardi, A.; Luce, A.; Grimaldi, A.; Zappavigna, S.; Sperlongano, P.; Irace, C.; Caraglia, M.; et al. Mitochondria as playmakers of apoptosis, autophagy and senescence. Semin. Cell Dev. Biol. 2020, 98, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Diepstraten, S.T.; Anderson, M.A.; Czabotar, P.E.; Lessene, G.; Strasser, A.; Kelly, G.L. The manipulation of apoptosis for cancer therapy using BH3-mimetic drugs. Nat. Rev. Cancer 2021, 22, 45–64. [Google Scholar] [CrossRef]
- Messmer, M.N.; Snyder, A.G.; Oberst, A. Comparing the effects of different cell death programs in tumor progression and immunotherapy. Cell Death Differ. 2019, 26, 115–129. [Google Scholar] [CrossRef]
- Fontana, F.; Limonta, P. The multifaceted roles of mitochondria at the crossroads of cell life and death in cancer. Free Radic. Biol. Med. 2021, 176, 203–221. [Google Scholar] [CrossRef]
- Adams, J.; Cory, S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018, 25, 27–36. [Google Scholar] [CrossRef]
- Cowan, A.D.; Smith, N.A.; Sandow, J.J.; Kapp, E.A.; Rustam, Y.H.; Murphy, J.M.; Brouwer, J.M.; Bernardini, J.P.; Roy, M.J.; Wardak, A.Z.; et al. BAK core dimers bind lipids and can be bridged by them. Nat. Struct. Mol. Biol. 2020, 27, 1024–1031. [Google Scholar] [CrossRef]
- Cosentino, K.; García-Sáez, A.J. Bax and Bak Pores: Are We Closing the Circle? Trends Cell Biol. 2016, 27, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Ow, Y.-L.P.; Green, D.R.; Hao, Z.; Mak, T.W. Cytochrome c: Functions beyond respiration. Nat. Rev. Mol. Cell Biol. 2008, 9, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Kalkavan, H.; Green, D. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ. 2017, 25, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, M.B.; Ford, A.E.; Liu, Y.; Zhang, J.Y. The JNK Signaling Pathway in Inflammatory Skin Disorders and Cancer. Cells 2020, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-Y.; Lo, Y.-S.; Lin, C.-C.; Chuang, Y.-C.; Chen, M.-K.; Chou, M.-C. Modulating effect of Coronarin D in 5-fluorouracil resistance human oral cancer cell lines induced apoptosis and cell cycle arrest through JNK1/2 signaling pathway. Biomed. Pharmacother. 2020, 128, 110318. [Google Scholar] [CrossRef]
- Huang, C.-F.; Liu, S.-H.; Ho, T.-J.; Lee, K.-I.; Fang, K.-M.; Lo, W.-C.; Liu, J.-M.; Wu, C.-C.; Su, C.-C. Quercetin induces tongue squamous cell carcinoma cell apoptosis via the JNK activation-regulated ERK/GSK-3α/β-mediated mitochondria-dependent apoptotic signaling pathway. Oncol. Lett. 2022, 23, 78. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Hsieh, M.-J.; Lin, J.-T.; Chen, G.; Lin, C.-C.; Lo, Y.-S.; Chuang, Y.-C.; Hsi, Y.-T.; Chen, M.-K.; Chou, M.-C. Coronarin D induces human oral cancer cell apoptosis though upregulate JNK1/2 signaling pathway. Environ. Toxicol. 2019, 34, 513–520. [Google Scholar] [CrossRef]
- Chen, C.; Yang, J.; Chen, W.; Lu, C.; Chiang, J.; Chiu, H.; Tsai, S.; Juan, Y.; Huang, H.; Way, T. Ursolic acid elicits intrinsic apoptotic machinery by downregulating the phosphorylation of AKT/BAD signaling in human cisplatin-resistant oral cancer CAR cells. Oncol. Rep. 2018, 40, 1752–1760. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, R.; Liu, Z.; Yan, T.; Xia, Y.; Zhao, L.; Lin, F.; Zhang, X.; Li, C.; Wang, Y. Curcumin analog, WZ37, promotes G2/M arrest and apoptosis of HNSCC cells through Akt/mTOR inhibition. Toxicol. Vitr. 2019, 65, 104754. [Google Scholar] [CrossRef]
- Seo, J.-H.; Choi, H.W.; Oh, H.-N.; Lee, M.-H.; Kim, E.; Yoon, G.; Cho, S.-S.; Park, S.-M.; Cho, Y.S.; Chae, J.; et al. Licochalcone D directly targets JAK2 to induced apoptosis in human oral squamous cell carcinoma. J. Cell Physiol. 2018, 234, 1780–1793. [Google Scholar] [CrossRef]
- Zacks, D.N.; Zheng, Q.-D.; Bakhru, R.; Han, Y.; Miller, J.W. FAS-Mediated Apoptosis and Its Relation to Intrinsic Pathway Activation in an Experimental Model of Retinal Detachment. Investig. Opthalmology Vis. Sci. 2004, 45, 4563–4569. [Google Scholar] [CrossRef]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef]
- Cai, J.; Yi, M.; Tan, Y.; Li, X.; Li, G.; Zeng, Z.; Xiong, W.; Xiang, B. Natural product triptolide induces GSDME-mediated pyroptosis in head and neck cancer through suppressing mitochondrial hexokinase-ΙΙ. J. Exp. Clin. Cancer Res. 2021, 40, 190. [Google Scholar] [CrossRef]
- Dev, A.; Sardoiwala, M.N.; Kushwaha, A.C.; Karmakar, S.; Choudhury, S.R. Genistein nanoformulation promotes selective apoptosis in oral squamous cell carcinoma through repression of 3PK-EZH2 signalling pathway. Phytomedicine 2020, 80, 153386. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Villani, R.M.; Wang, H.; Simpson, M.J.; Roberts, M.S.; Tang, M.; Liang, X. The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer Res. 2018, 37, 266. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; O’Neill, L.A.J. The role of the electron transport chain in immunity. FASEB J. 2021, 35, e21974. [Google Scholar] [CrossRef]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Idelchik, M.D.P.S.; Begley, U.; Begley, T.J.; Melendez, J.A. Mitochondrial ROS control of cancer. Semin. Cancer Biol. 2017, 47, 57–66. [Google Scholar] [CrossRef]
- Rosini, E.; Pollegioni, L. Reactive oxygen species as a double-edged sword: The role of oxidative enzymes in antitumor therapy. BioFactors 2021, 48, 384–399. [Google Scholar] [CrossRef]
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef]
- Görlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef]
- Halestrap, A.P.; McStay, G.P.; Clarke, S.J. The permeability transition pore complex: Another view. Biochimie 2002, 84, 153–166. [Google Scholar] [CrossRef]
- Simon, H.-U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.M.; Murphy, E. Role of Mitochondrial Calcium and the Permeability Transition Pore in Regulating Cell Death. Circ. Res. 2020, 126, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Hsieh, C.; Tang, J.; Lin, L.; Huang, H.; Wang, H.; Yeh, Y.; Chuang, Y.; Ou-Yang, F.; Chang, H. Antimycin A shows selective antiproliferation to oral cancer cells by oxidative stress-mediated apoptosis andDNAdamage. Environ. Toxicol. 2020, 35, 1212–1224. [Google Scholar] [CrossRef]
- Li, D.; Ueta, E.; Kimura, T.; Yamamoto, T.; Osaki, T. Reactive oxygen species (ROS) control the expression of Bcl-2 family proteins by regulating their phosphorylation and ubiquitination. Cancer Sci. 2004, 95, 644–650. [Google Scholar] [CrossRef]
- Orrenius, S.; Gogvadze, V.; Zhivotovsky, B. Calcium and mitochondria in the regulation of cell death. Biochem. Biophys. Res. Commun. 2015, 460, 72–81. [Google Scholar] [CrossRef]
- Li, M.-H.; Liao, X.; Li, C.; Wang, T.-T.; Sun, Y.-S.; Yang, K.; Jiang, P.-W.; Shi, S.-T.; Zhang, W.-X.; Zhang, K.; et al. Lycorine hydrochloride induces reactive oxygen species-mediated apoptosis via the mitochondrial apoptotic pathway and the JNK signaling pathway in the oral squamous cell carcinoma HSC-3 cell line. Oncol. Lett. 2021, 21, 236. [Google Scholar] [CrossRef]
- Chong, S.J.F.; Low, I.C.C.; Pervaiz, S. Mitochondrial ROS and involvement of Bcl-2 as a mitochondrial ROS regulator. Mitochondrion 2014, 19, 39–48. [Google Scholar] [CrossRef]
- Han, D.; Antunes, F.; Canali, R.; Rettori, D.; Cadenas, E. Voltage-dependent Anion Channels Control the Release of the Superoxide Anion from Mitochondria to Cytosol. J. Biol. Chem. 2003, 278, 5557–5563. [Google Scholar] [CrossRef]
- Vo, T.T.T.; Liu, J.-F.; Wu, C.-Z.; Lin, W.-N.; Chen, Y.-L.; Lee, I.-T. Surfactin from Bacillus subtilis induces apoptosis in human oral squamous cell carcinoma through ROS-regulated mitochondrial pathway. J. Cancer 2020, 11, 7253–7263. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, G.; Pan, J.; Zielonka, J.; Xiong, D.; Myers, C.R.; Feng, L.; Shin, S.S.; Kim, Y.H.; Bui, D.; et al. Magnolia extract is effective for the chemoprevention of oral cancer through its ability to inhibit mitochondrial respiration at complex I. Cell Commun. Signal. 2020, 18, 58. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.-Y.; Wu, K.-H.; Wang, Y.-Y.; Farooqi, A.; Huang, H.-W.; Yuan, S.F.; Jian, R.-I.; Tsao, L.-Y.; Chen, P.-A.; Chang, F.-R.; et al. Methanol Extract of Usnea barbata Induces Cell Killing, Apoptosis, and DNA Damage against Oral Cancer Cells through Oxidative Stress. Antioxidants 2020, 9, 694. [Google Scholar] [CrossRef]
- Wang, H.-R.; Tang, J.-Y.; Wang, Y.-Y.; Farooqi, A.A.; Yen, C.-Y.; Yuan, S.-S.F.; Huang, H.-W.; Chang, H.-W. Manoalide Preferentially Provides Antiproliferation of Oral Cancer Cells by Oxidative Stress-Mediated Apoptosis and DNA Damage. Cancers 2019, 11, 1303. [Google Scholar] [CrossRef] [PubMed]
- Saluja, T.S.; Kumar, V.; Agrawal, M.; Tripathi, A.; Meher, R.K.; Srivastava, K.; Gupta, A.; Singh, A.; Chaturvedi, A.; Singh, S.K. Mitochondrial Stress–Mediated Targeting of Quiescent Cancer Stem Cells in Oral Squamous Cell Carcinoma. Cancer Manag. Res. 2020, 12, 4519–4530. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial ROS-induced ROS release: An update and review. Biochim. Biophys. Acta (BBA)—Bioenerg. 2006, 1757, 509–517. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Lai, R.; Lin, Q.; Huang, Y.; Wang, L. Arglabin is a plant sesquiterpene lactone that exerts potent anticancer effects on human oral squamous cancer cells via mitochondrial apoptosis and downregulation of the mTOR/PI3K/Akt signaling pathway to inhibit tumor growth in vivo. J. Buon Off. J. Balk. Union Oncol. 2019, 23, 1679–1685. [Google Scholar]
- Martucciello, S.; Masullo, M.; Cerulli, A.; Piacente, S. Natural Products Targeting ER Stress, and the Functional Link to Mitochondria. Int. J. Mol. Sci. 2020, 21, 1905. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Pizzo, P.; Filadi, R. Calcium, mitochondria and cell metabolism: A functional triangle in bioenergetics. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1068–1078. [Google Scholar] [CrossRef]
- Doghman, M.; Lalli, E. ER-mitochondria interactions: Both strength and weakness within cancer cells. Biochim. Biophys. Acta 2019, 1866, 650–662. [Google Scholar] [CrossRef]
- Madreiter-Sokolowski, C.T.; Thomas, C.; Ristow, M. Interrelation between ROS and Ca2+ in aging and age-related diseases. Redox Biol. 2020, 36, 101678. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, R.; Rehman, M.U.; Zhao, Q.-L.; Jawaid, P.; Mitsuhashi, Y.; Sakurai, K.; Heshiki, W.; Ogawa, R.; Tomihara, K.; Saitoh, J.-I.; et al. Combination of 5-aminosalicylic acid and hyperthermia synergistically enhances apoptotic cell death in HSC-3 cells due to intracellular nitric oxide/peroxynitrite generation. Cancer Lett. 2019, 451, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.S.; Sharma, A.K.; Soni, H.; Ali, D.M.; Tews, B.; König, R.; Eibl, H.; Berger, M.R. Induction of ER and mitochondrial stress by the alkylphosphocholine erufosine in oral squamous cell carcinoma cells. Cell Death Dis. 2018, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Trotta, A.P.; Chipuk, J.E. Mitochondrial dynamics as regulators of cancer biology. Cell. Mol. Life Sci. 2017, 74, 1999–2017. [Google Scholar] [CrossRef] [PubMed]
- Tilokani, L.; Nagashima, S.; Paupe, V.; Prudent, J. Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem. 2018, 62, 341–360. [Google Scholar] [CrossRef]
- Liu, R.; Chan, D.C. The mitochondrial fission receptor Mff selectively recruits oligomerized Drp1. Mol. Biol. Cell 2015, 26, 4466–4477. [Google Scholar] [CrossRef]
- Yu, R.; Jin, S.; Lendahl, U.; Nistér, M.; Zhao, J. Human Fis1 regulates mitochondrial dynamics through inhibition of the fusion machinery. EMBO J. 2019, 38, e99748. [Google Scholar] [CrossRef]
- Kamerkar, S.C.; Kraus, F.; Sharpe, A.J.; Pucadyil, T.J.; Ryan, M.T. Dynamin-related protein 1 has membrane constricting and severing abilities sufficient for mitochondrial and peroxisomal fission. Nat. Commun. 2018, 9, 5239. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Lin, S.-C.A.; Kuo, C.-H.; Li, C.-J. Molecular Machinery and Pathophysiology of Mitochondrial Dynamics. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Chiang, S.-F.; Chen, W.T.-L.; Ke, T.-W.; Chen, T.-W.; You, Y.-S.; Lin, C.-Y.; Chao, K.S.C. HMGB1 promotes ERK-mediated mitochondrial Drp1 phosphorylation for chemoresistance through RAGE in colorectal cancer. Cell Death Dis. 2018, 9, 743892. [Google Scholar] [CrossRef]
- Huang, L.; Luan, T.; Chen, Y.; Bao, X.; Huang, Y.; Fu, S.; Wang, H.; Wang, J. LASS2 regulates invasion and chemoresistance via ERK/Drp1 modulated mitochondrial dynamics in bladder cancer cells. J. Cancer 2018, 9, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Kim, B.; Cho, U.; Park, I.S.; Kim, S.I.; Dhanasekaran, D.N.; Tsang, B.K.; Song, Y.S. Mitochondrial fission causes cisplatin resistance under hypoxic conditions via ROS in ovarian cancer cells. Oncogene 2019, 38, 7089–7105. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Chen, K.-W.; Chen, L. Mitochondrial ROS1 Increases Mitochondrial Fission and Respiration in Oral Squamous Cancer Carcinoma. Cancers 2020, 12, 2845. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Chatterjee, K.; Chowdhury, A.R.; Barui, A. Clinico-pathological significance of Drp1 dysregulation and its correlation to apoptosis in oral cancer patients. Mitochondrion 2020, 52, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, Y.; Ding, Y.; Yu, M.; Ai, Z. CerS6 regulates cisplatin resistance in oral squamous cell carcinoma by altering mitochondrial fission and autophagy. J. Cell Physiol. 2018, 233, 9416–9425. [Google Scholar] [CrossRef] [PubMed]
- Tsao, Y.-C.; Chang, Y.-J.; Wang, C.-H.; Chen, L. Discovery of Isoplumbagin as a Novel NQO1 Substrate and Anti-Cancer Quinone. Int. J. Mol. Sci. 2020, 21, 4378. [Google Scholar] [CrossRef]
- Pearson, H.E.; Iida, M.; Orbuch, R.A.; McDaniel, N.K.; Nickel, K.P.; Kimple, R.J.; Arbiser, J.L.; Wheeler, D.L. Overcoming Resistance to Cetuximab with Honokiol, A Small-Molecule Polyphenol. Mol. Cancer Ther. 2018, 17, 204–214. [Google Scholar] [CrossRef]
- Wu, K.; Mao, Y.-Y.; Chen, Q.; Zhang, B.; Zhang, S.; Wu, H.-J.; Li, Y. Hypoxia-induced ROS promotes mitochondrial fission and cisplatin chemosensitivity via HIF-1α/Mff regulation in head and neck squamous cell carcinoma. Cell Oncol. 2021, 44, 1167–1181. [Google Scholar] [CrossRef]
- Ma, C.; Fan, L.; Wang, J.; Hao, L.; He, J. Hippo/Mst1 overexpression induces mitochondrial death in head and neck squamous cell carcinoma via activating β-catenin/Drp1 pathway. Cell Stress Chaperon- 2019, 24, 807–816. [Google Scholar] [CrossRef]
- Tian, T.; Lv, X.; Pan, G.; Lu, Y.; Chen, W.; He, W.; Lei, X.; Zhang, H.; Liu, M.; Sun, S.; et al. Long Noncoding RNA MPRL Promotes Mitochondrial Fission and Cisplatin Chemosensitivity via Disruption of Pre-miRNA Processing. Clin. Cancer Res. 2019, 25, 3673–3688. [Google Scholar] [CrossRef]
- Fan, S.; Tian, T.; Lv, X.; Lei, X.; Yang, Z.; Liu, M.; Liang, F.; Li, S.; Lin, X.; Lin, Z.; et al. lncRNA CISAL Inhibits BRCA1 Transcription by Forming a Tertiary Structure at Its Promoter. iScience 2020, 23, 100835. [Google Scholar] [CrossRef] [PubMed]
- Westermann, B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010, 11, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Lyakhovich, A.; Lleonart, M.E. Bypassing Mechanisms of Mitochondria-Mediated Cancer Stem Cells Resistance to Chemo- and Radiotherapy. Oxidative Med. Cell Longev. 2015, 2016, 1716341. [Google Scholar] [CrossRef]
- Fernandez, H.R.; Gadre, S.M.; Tan, M.; Graham, G.T.; Mosaoa, R.; Ongkeko, M.S.; Kim, K.A.; Riggins, R.B.; Parasido, E.; Petrini, I.; et al. The mitochondrial citrate carrier, SLC25A1, drives stemness and therapy resistance in non-small cell lung cancer. Cell Death Differ. 2018, 25, 1239–1258. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Z. Potential Mechanism Underlying the Role of Mitochondria in Breast Cancer Drug Resistance and Its Related Treatment Prospects. Front. Oncol. 2021, 11, 629614. [Google Scholar] [CrossRef]
- Shen, L.; Xia, M.; Zhang, Y.; Luo, H.; Dong, D.; Sun, L. Mitochondrial integration and ovarian cancer chemotherapy resistance. Exp. Cell Res. 2021, 401, 112549. [Google Scholar] [CrossRef]
- Fu, Y.; Ricciardiello, F.; Yang, G.; Qiu, J.; Huang, H.; Xiao, J.; Cao, Z.; Zhao, F.; Liu, Y.; Luo, W.; et al. The Role of Mitochondria in the Chemoresistance of Pancreatic Cancer Cells. Cells 2021, 10, 497. [Google Scholar] [CrossRef]
- Onishi, M.; Yamano, K.; Sato, M.; Matsuda, N.; Okamoto, K. Molecular mechanisms and physiological functions of mitophagy. EMBO J. 2021, 40, e104705. [Google Scholar] [CrossRef]
- Shen, Y.-Q.; Guerra-Librero, A.; Fernandez-Gil, B.I.; Florido, J.; García-López, S.; Ruiz, L.M.; Mendivil-Perez, M.; Soto-Mercado, V.; Acuña-Castroviejo, D.; Ortega-Arellano, H.; et al. Combination of melatonin and rapamycin for head and neck cancer therapy: Suppression of AKT/mTOR pathway activation, and activation of mitophagy and apoptosis via mitochondrial function regulation. J. Pineal Res. 2017, 64, e12461. [Google Scholar] [CrossRef]
- Naik, P.P.; Mukhopadhyay, S.; Praharaj, P.P.; Bhol, C.S.; Panigrahi, D.P.; Mahapatra, K.K.; Patra, S.; Saha, S.; Panda, A.K.; Panda, K.; et al. Secretory clusterin promotes oral cancer cell survival via inhibiting apoptosis by activation of autophagy in AMPK/mTOR/ULK1 dependent pathway. Life Sci. 2020, 264, 118722. [Google Scholar] [CrossRef]
- Shin, Y.Y.; Seo, Y.; Oh, S.; Ahn, J.; Song, M.; Kang, M.; Oh, J.; Lee, D.; Kim, Y.H.; Sung, E.; et al. Melatonin and verteporfin synergistically suppress the growth and stemness of head and neck squamous cell carcinoma through the regulation of mitochondrial dynamics. J. Pineal Res. 2021, 72, e12779. [Google Scholar] [CrossRef] [PubMed]
- Nazio, F.; Bordi, M.; Cianfanelli, V.; Locatelli, F.; Cecconi, F. Autophagy and cancer stem cells: Molecular mechanisms and therapeutic applications. Cell Death Differ. 2019, 26, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Praharaj, P.P.; Panigrahi, D.P.; Bhol, C.S.; Patra, S.; Mishra, S.R.; Mahapatra, K.K.; Behera, B.P.; Singh, A.; Patil, S.; Bhutia, S.K. Mitochondrial rewiring through mitophagy and mitochondrial biogenesis in cancer stem cells: A potential target for anti-CSC cancer therapy. Cancer Lett. 2020, 498, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Naik, P.P.; Mukhopadhyay, S.; Panda, P.K.; Sinha, N.; Das, C.K.; Mishra, R.; Patil, S.; Bhutia, S.K. Autophagy regulates cisplatin-induced stemness and chemoresistance via the upregulation of CD 44, ABCB 1 and ADAM 17 in oral squamous cell carcinoma. Cell Prolif. 2017, 51. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.J.; Boyer, T.L.; Venner, E.; Beck, P.J.; Slamowitz, T.; Caste, T.; Hickman, A.; Raymond, M.H.; Costa-Pinheiro, P.; Jameson, M.J.; et al. Inhibition of Lysosomal Function Mitigates Protective Mitophagy and Augments Ceramide Nanoliposome–Induced Cell Death in Head and Neck Squamous Cell Carcinoma. Mol. Cancer Ther. 2020, 19, 2621–2633. [Google Scholar] [CrossRef]
- Wang, J.; Gao, S.; Wang, S.; Xu, Z.; Wei, L. Zinc oxide nanoparticles induce toxicity in CAL 27 oral cancer cell lines by activating PINK1/Parkin-mediated mitophagy. Int. J. Nanomed. 2018, 13, 3441–3450. [Google Scholar] [CrossRef]
- Rencelj, A.; Gvozdenovic, N.; Cemazar, M. MitomiRs: Their roles in mitochondria and importance in cancer cell metabolism. Radiol. Oncol. 2021, 55, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, P.; Lu, Y.; Jin, T.; Lei, X.; Liu, M.; Zhuang, P.; Liao, J.; Lin, Z.; Li, B.; et al. Decreased expression of mitochondrial miR-5787 contributes to chemoresistance by reprogramming glucose metabolism and inhibiting MT-CO3 translation. Theranostics 2019, 9, 5739–5754. [Google Scholar] [CrossRef]
- Fan, S.; Tian, T.; Chen, W.; Lv, X.; Lei, X.; Zhang, H.; Sun, S.; Cai, L.; Pan, G.; He, L.; et al. Mitochondrial miRNA Determines Chemoresistance by Reprogramming Metabolism and Regulating Mitochondrial Transcription. Cancer Res. 2019, 79, 1069–1084. [Google Scholar] [CrossRef]
- Kao, Y.-Y.; Chou, C.-H.; Yeh, L.-Y.; Chen, Y.-F.; Chang, K.-W.; Liu, C.-J.; Chiang, C.-Y.F.; Lin, S.-C. MicroRNA miR-31 targets SIRT3 to disrupt mitochondrial activity and increase oxidative stress in oral carcinoma. Cancer Lett. 2019, 456, 40–48. [Google Scholar] [CrossRef]
- Lo, Y.-L.; Wang, C.-S.; Chen, Y.-C.; Wang, T.-Y.; Chang, Y.-H.; Chen, C.-J.; Yang, C.-P. Mitochondrion-Directed Nanoparticles Loaded with a Natural Compound and a microRNA for Promoting Cancer Cell Death via the Modulation of Tumor Metabolism and Mitochondrial Dynamics. Pharmaceutics 2020, 12, 756. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Bao, X.; Liu, Q.; Li, Q.; Huang, L.; Wang, H.; Jiao, K. MicroRNA-378-3p/5p represses proliferation and induces apoptosis of oral squamous carcinoma cells via targeting KLK4. Clin. Exp. Pharmacol. Physiol. 2019, 47, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Oeck, S.; West, A.P.; Mangalhara, K.C.; Sainz, A.G.; Newman, L.; Zhang, X.-O.; Wu, L.; Yan, Q.; Bosenberg, M.; et al. Mitochondrial DNA stress signalling protects the nuclear genome. Nat. Metab. 2019, 1, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Lim, S.-N.; Chen, C.-Y.; Chi, H.-C.; Yeh, C.-T.; Lin, W.-R. Functional Role of Mitochondrial DNA in Cancer Progression. Int. J. Mol. Sci. 2022, 23, 1659. [Google Scholar] [CrossRef] [PubMed]
- Grasso, D.; Medeiros, H.; Zampieri, L.X.; Bol, V.; Danhier, P.; Van Gisbergen, M.W.; Bouzin, C.; Brusa, D.; Grégoire, V.; Smeets, H.; et al. Fitter Mitochondria Are Associated With Radioresistance in Human Head and Neck SQD9 Cancer Cells. Front. Pharmacol. 2020, 11, 263. [Google Scholar] [CrossRef]
- Aminuddin, A.; Ng, P.Y.; Leong, C.-O.; Chua, E.W. Mitochondrial DNA alterations may influence the cisplatin responsiveness of oral squamous cell carcinoma. Sci. Rep. 2020, 10, 7885. [Google Scholar] [CrossRef]
- Wang, L.; Lv, H.; Ji, P.; Zhu, X.; Yuan, H.; Jin, G.; Dai, J.; Hu, Z.; Su, Y.; Ma, H. Mitochondrial DNA copy number is associated with risk of head and neck squamous cell carcinoma in Chinese population. Cancer Med. 2018, 7, 2776–2782. [Google Scholar] [CrossRef]
- Brandon, M.; Baldi, P.; Wallace, D.C. Mitochondrial mutations in cancer. Oncogene 2006, 25, 4647–4662. [Google Scholar] [CrossRef]
- Ju, Y.S.; Alexandrov, L.B.; Gerstung, M.; Martincorena, I.; Nik-Zainal, S.; Ramakrishna, M.; Davies, H.R.; Papaemmanuil, E.; Gundem, G.; Shlien, A.; et al. Origins and functional consequences of somatic mitochondrial DNA mutations in human cancer. eLife 2014, 3. [Google Scholar] [CrossRef]
- Fendt, L.; Fazzini, F.; Weissensteiner, H.; Bruckmoser, E.; Schönherr, S.; Schäfer, G.; Losso, J.L.; Streiter, G.A.; Lamina, C.; Rasse, M.; et al. Profiling of Mitochondrial DNA Heteroplasmy in a Prospective Oral Squamous Cell Carcinoma Study. Cancers 2020, 12, 1933. [Google Scholar] [CrossRef]
- Bonekamp, N.A.; Peter, B.; Hillen, H.S.; Felser, A.; Bergbrede, T.; Choidas, A.; Horn, M.; Unger, A.; Di Lucrezia, R.; Atanassov, I.; et al. Small-molecule inhibitors of human mitochondrial DNA transcription. Nature 2020, 588, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.-T.; Li, Y.-J.; Wu, S.-B.; Yang, C.-N.; Wu, T.-S.; Wei, Y.-H.; Deng, Y.-T. Connective tissue growth factor decreases mitochondrial metabolism through ubiquitin-mediated degradation of mitochondrial transcription factor A in oral squamous cell carcinoma. J. Formos. Med. Assoc. 2018, 117, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, L. Rewiring Mitochondrial Metabolism for CD8+ T Cell Memory Formation and Effective Cancer Immunotherapy. Front. Immunol. 2020, 11, 1834. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-Z.; Wang, S.; Yang, Q.-C.; Wang, X.-L.; Yang, L.-L.; Liu, B.; Sun, Z.-J. Increased Expression of SHMT2 Is Associated With Poor Prognosis and Advanced Pathological Grade in Oral Squamous Cell Carcinoma. Front. Oncol. 2020, 10, 588530. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, F.; Zhang, Y.; Cai, H.; Song, F.; Hou, J. Silencing SHMT2 inhibits the progression of tongue squamous cell carcinoma through cell cycle regulation. Cancer Cell Int. 2021, 21, 220. [Google Scholar] [CrossRef]
- Cheng, A.N.; Cheng, L.-C.; Kuo, C.-L.; Lo, Y.K.; Chou, H.-Y.; Chen, C.-H.; Wang, Y.-H.; Chuang, T.-H.; Cheng, S.-J.; Lee, A.Y.-L. Mitochondrial Lon-induced mtDNA leakage contributes to PD-L1–mediated immunoescape via STING-IFN signaling and extracellular vesicles. J. Immunother. Cancer 2020, 8, e001372. [Google Scholar] [CrossRef]
- Kuo, C.-L.; Chou, H.-Y.; Chiu, Y.-C.; Cheng, A.N.; Fan, C.-C.; Chang, Y.-N.; Chen, C.-H.; Jiang, S.S.; Chen, N.-J.; Lee, A.Y.-L. Mitochondrial oxidative stress by Lon-PYCR1 maintains an immunosuppressive tumor microenvironment that promotes cancer progression and metastasis. Cancer Lett. 2020, 474, 138–150. [Google Scholar] [CrossRef]
- Nocquet, L.; Juin, P.P.; Souazé, F. Mitochondria at Center of Exchanges between Cancer Cells and Cancer-Associated Fibroblasts during Tumor Progression. Cancers 2020, 12, 3017. [Google Scholar] [CrossRef]
- Hui, S.; Ghergurovich, J.M.; Morscher, R.J.; Jang, C.; Teng, X.; Lu, W.; Esparza, L.A.; Reya, T.; Zhan, L.; Guo, J.Y.; et al. Glucose feeds the TCA cycle via circulating lactate. Nature 2017, 551, 115–118. [Google Scholar] [CrossRef]
- Jiang, E.; Xu, Z.; Wang, M.; Yan, T.; Huang, C.; Zhou, X.; Liu, Q.; Wang, L.; Chen, Y.; Wang, H.; et al. Tumoral microvesicle–activated glycometabolic reprogramming in fibroblasts promotes the progression of oral squamous cell carcinoma. FASEB J. 2019, 33, 5690–5703. [Google Scholar] [CrossRef]
- Zhao, H.; Jiang, E.; Shang, Z. 3D Co-culture of Cancer-Associated Fibroblast with Oral Cancer Organoids. J. Dent. Res. 2021, 100, 201–208. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, Z.; Rajthala, S.; Sapkota, D.; Dongre, H.; Parajuli, H.; Suliman, S.; Das, R.; Li, L.; Bindoff, L.A.; et al. Metabolic reprogramming of normal oral fibroblasts correlated with increased glycolytic metabolism of oral squamous cell carcinoma and precedes their activation into carcinoma associated fibroblasts. Cell Mol. Life Sci. 2020, 77, 1115–1133. [Google Scholar] [CrossRef]
- Saha, T.; Dash, C.; Jayabalan, R.; Khiste, S.; Kulkarni, A.; Kurmi, K.; Mondal, J.; Majumder, P.K.; Bardia, A.; Jang, H.L.; et al. Intercellular nanotubes mediate mitochondrial trafficking between cancer and immune cells. Nat. Nanotechnol. 2021, 17, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Sigdel, K.; Yang, L.; Liu, Y.; Xuan, M.; Wang, X.; Gu, Z.; Wu, J.; Xie, H. Nanotechnology-based drug delivery systems for enhanced diagnosis and therapy of oral cancer. J. Mater. Chem. B 2020, 8, 8781–8793. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Das, U.; Dimmock, J.R. Recent advances in α,β-unsaturated carbonyl compounds as mitochondrial toxins. Eur. J. Med. Chem. 2019, 183, 111687. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Pandey, S. Exploiting Mitochondrial Vulnerabilities to Trigger Apoptosis Selectively in Cancer Cells. Cancers 2019, 11, 916. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Swargiary, G.; Singh, K.K. Natural Agents Targeting Mitochondria in Cancer. Int. J. Mol. Sci. 2020, 21, 6992. [Google Scholar] [CrossRef]
- Qian, Q.; Chen, W.; Cao, Y.; Cao, Q.; Cui, Y.; Li, Y.; Wu, J. Targeting Reactive Oxygen Species in Cancer via Chinese Herbal Medicine. Oxidative Med. Cell Longev. 2019, 2019, 9240426. [Google Scholar] [CrossRef]
- Shakeri, F.; Bianconi, V.; Pirro, M.; Sahebkar, A. Effects of Plant and Animal Natural Products on Mitophagy. Oxidative Med. Cell Longev. 2020, 2020, 6969402. [Google Scholar] [CrossRef]
- Zhu, Y.; Wen, L.-M.; Li, R.; Dong, W.; Jia, S.-Y.; Qi, M.-C. Recent advances of nano-drug delivery system in oral squamous cell carcinoma treatment. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9445–9453. [Google Scholar]
- Li, H.; Zhang, Y.; Xu, M.; Yang, D. Current trends of targeted therapy for oral squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2022, 148, 2169–2186. [Google Scholar] [CrossRef] [PubMed]
- Cryer, A.M.; Thorley, A.J. Nanotechnology in the diagnosis and treatment of lung cancer. Pharmacol. Ther. 2019, 198, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Gao, X.; Chen, Y.; Liu, T. Nanotechnology in cancer diagnosis: Progress, challenges and opportunities. J. Hematol. Oncol. 2019, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Şen, Ö.; Emanet, M.; Ciofani, G. Nanotechnology-Based Strategies to Evaluate and Counteract Cancer Metastasis and Neoangiogenesis. Adv. Health Mater. 2021, 10, e2002163. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Sun, P.; Cai, Y.; Xu, W.; Fan, Q.; Hu, Q.; Han, W. A Novel Multimodal NIR-II Nanoprobe for the Detection of Metastatic Lymph Nodes and Targeting Chemo-Photothermal Therapy in Oral Squamous Cell Carcinoma. Theranostics 2019, 9, 391–404. [Google Scholar] [CrossRef]
- Li, R.; Gao, R.; Wang, Y.; Liu, Z.; Xu, H.; Duan, A.; Zhang, F.; Ma, L. Gastrin releasing peptide receptor targeted nano-graphene oxide for near-infrared fluorescence imaging of oral squamous cell carcinoma. Sci. Rep. 2020, 10, 11434. [Google Scholar] [CrossRef]
- Allemailem, K.S.; Almatroudi, A.; Alsahli, M.A.; Aljaghwani, A.; El-Kady, A.M.; Rahmani, A.H.; Khan, A.A. Novel Strategies for Disrupting Cancer-Cell Functions with Mitochondria-Targeted Antitumor Drug–Loaded Nanoformulations. Int. J. Nanomed. 2021, 16, 3907–3936. [Google Scholar] [CrossRef]
- Afrasiabi, M.; Seydi, E.; Rahimi, S.; Tahmasebi, G.; Jahanbani, J.; Pourahmad, J. The selective toxicity of superparamagnetic iron oxide nanoparticles (SPIONs) on oral squamous cell carcinoma (OSCC) by targeting their mitochondria. J. Biochem. Mol. Toxicol. 2021, 35, e22769. [Google Scholar] [CrossRef]
- Jahanbani, J.; Ghotbi, M.; Shahsavari, F.; Seydi, E.; Rahimi, S.; Pourahmad, J. Selective anticancer activity of superparamagnetic iron oxide nanoparticles (SPIONs) against oral tongue cancer using in vitro methods: The key role of oxidative stress on cancerous mitochondria. J. Biochem. Mol. Toxicol. 2020, 34, e22557. [Google Scholar] [CrossRef]
- Wang, S.-W.; Lee, C.-H.; Lin, M.-S.; Chi, C.-W.; Chen, Y.-J.; Wang, G.-S.; Liao, K.-W.; Chiu, L.-P.; Wu, S.-H.; Huang, D.-M.; et al. ZnO Nanoparticles Induced Caspase-Dependent Apoptosis in Gingival Squamous Cell Carcinoma through Mitochondrial Dysfunction and p70S6K Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 1612. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, S.R.; Nayak, A.; Siddharth, S.; Das, S.; Nayak, D.; Kundu, C.N. Metallic gold and bioactive quinacrine hybrid nanoparticles inhibit oral cancer stem cell and angiogenesis by deregulating inflammatory cytokines in p53 dependent manner. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Qin, Z.; Shen, C.; Gong, H.-L.; He, Z.-Y. Multifunctional Mitochondria-Targeting Nanosystems for Enhanced Anticancer Efficacy. Front. Bioeng. Biotechnol. 2021, 9, 786621. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.-C.; Chueh, F.-S.; Hsiao, Y.-T.; Cheng, Z.-Y.; Lien, J.-C.; Liu, K.-C.; Peng, S.-F.; Chung, J.-G. Gefitinib and curcumin-loaded nanoparticles enhance cell apoptosis in human oral cancer SAS cells in vitro and inhibit SAS cell xenografted tumor in vivo. Toxicol. Appl. Pharmacol. 2019, 382, 114734. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Vinayagam, R.; Senthilkumar, V.; Paulpandi, M.; Murugan, K.; Xu, B.; Gothandam, K.M.; Kotakadi, V.S.; David, E. Phloretin loaded chitosan nanoparticles augments the pH-dependent mitochondrial-mediated intrinsic apoptosis in human oral cancer cells. Int. J. Biol. Macromol. 2019, 130, 997–1008. [Google Scholar] [CrossRef]
- Fan, H.-Y.; Zhu, Z.-L.; Zhang, W.-L.; Yin, Y.-J.; Tang, Y.-L.; Liang, X.-H.; Zhang, L. Light stimulus responsive nanomedicine in the treatment of oral squamous cell carcinoma. Eur. J. Med. Chem. 2020, 199, 112394. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Liu, H. Co-delivery of chitosan nanoparticles of 5-aminolevulinic acid and shGBAS for improving photodynamic therapy efficacy in oral squamous cell carcinomas. Photodiagnosis Photodyn. Ther. 2021, 34, 102218. [Google Scholar] [CrossRef]
- Dash, S.R.; Chatterjee, S.; Sinha, S.; Das, B.; Paul, S.; Pradhan, R.; Sethy, C.; Panda, R.; Tripathy, J.; Kundu, C.N. NIR irradiation enhances the apoptotic potentiality of quinacrine-gold hybrid nanoparticles by modulation of HSP-70 in oral cancer stem cells. Nanomed. Nanotechnol. Biol. Med. 2021, 40, 102502. [Google Scholar] [CrossRef]
- Mendes, B.B.; Sousa, D.P.; Conniot, J.; Conde, J. Nanomedicine-based strategies to target and modulate the tumor microenvironment. Trends Cancer 2021, 7, 847–862. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Gong, X.; Tang, H.; Yang, K. PER1 suppresses glycolysis and cell proliferation in oral squamous cell carcinoma via the PER1/RACK1/PI3K signaling complex. Cell Death Dis. 2021, 12, 276. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.; Yadav, V.K.; Kuo, K.-T.; Pikatan, N.W.; Lin, C.-S.; Chien, M.-H.; Lee, W.-H.; Hsiao, M.; Chiu, S.-C.; Yeh, C.-T.; et al. PDK1 Inhibitor BX795 Improves Cisplatin and Radio-Efficacy in Oral Squamous Cell Carcinoma by Downregulating the PDK1/CD47/Akt-Mediated Glycolysis Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 11492. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Pasala, M.S.; Prakash, A. Mitochondrial DNA: Epigenetics and environment. Environ. Mol. Mutagen. 2019, 60, 668–682. [Google Scholar] [CrossRef] [PubMed]
- Ghosh-Choudhary, S.K.; Liu, J.; Finkel, T. The role of mitochondria in cellular senescence. FASEB J. 2021, 35, e21991. [Google Scholar] [CrossRef]
- Andrieux, P.; Chevillard, C.; Cunha-Neto, E.; Nunes, J.P.S. Mitochondria as a Cellular Hub in Infection and Inflammation. Int. J. Mol. Sci. 2021, 22, 11338. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Sant’Ana, M.S.P.; de Cáceres, C.V.B.L.; Lima, L.A.; Soares, C.D.; Radhakrishnan, R.; Gomez, R.S.; Vargas, P.A.; Fonseca, F.P. Expression of mitochondrial dynamic markers in adenoid cystic carcinoma. J. Oral Pathol. Med. 2022, 51, 702–709. [Google Scholar] [CrossRef]
- Qiu, L.; Liu, Z.; Wu, C.; Chen, W.; Chen, Y.; Zhang, B.; Li, J.; Liu, H.; Huang, N.; Jiang, Z.; et al. C6-ceramide induces salivary adenoid cystic carcinoma cell apoptosis via IP3R-activated UPR and UPR-independent pathways. Biochem. Biophys. Res. Commun. 2020, 525, 997–1003. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, J.; Wu, L.; Wang, X.; Wang, Y.; Shang, Z.; Jiang, E.; Shao, Z. Roles of Mitochondria in Oral Squamous Cell Carcinoma Therapy: Friend or Foe? Cancers 2022, 14, 5723. https://doi.org/10.3390/cancers14235723
Bai J, Wu L, Wang X, Wang Y, Shang Z, Jiang E, Shao Z. Roles of Mitochondria in Oral Squamous Cell Carcinoma Therapy: Friend or Foe? Cancers. 2022; 14(23):5723. https://doi.org/10.3390/cancers14235723
Chicago/Turabian StyleBai, Junqiang, Luping Wu, Xinmiao Wang, Yifan Wang, Zhengjun Shang, Erhui Jiang, and Zhe Shao. 2022. "Roles of Mitochondria in Oral Squamous Cell Carcinoma Therapy: Friend or Foe?" Cancers 14, no. 23: 5723. https://doi.org/10.3390/cancers14235723
APA StyleBai, J., Wu, L., Wang, X., Wang, Y., Shang, Z., Jiang, E., & Shao, Z. (2022). Roles of Mitochondria in Oral Squamous Cell Carcinoma Therapy: Friend or Foe? Cancers, 14(23), 5723. https://doi.org/10.3390/cancers14235723





