Allogeneic Hematopoietic Stem Cell Transplantation in Transformed Follicular Lymphoma (tFL): Results of a Retrospective Multicenter Study from GELTAMO/GETH-TC Spanish Groups
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Objectives
2.2. Patient Enrollment
2.3. Study Endpoints and Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Donors
3.3. Engraftmet and Chimerism
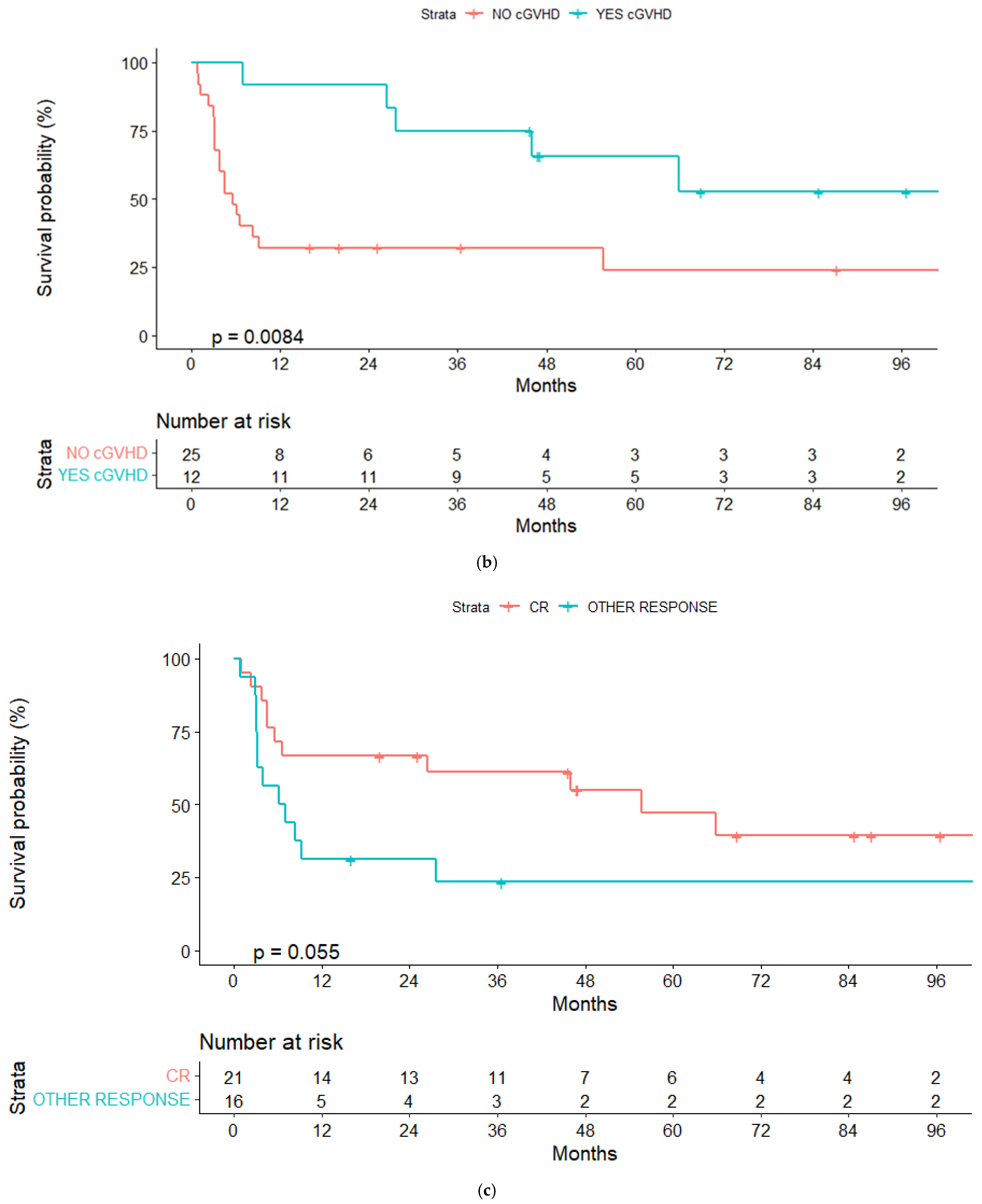
3.4. Graft-Versus-Host Disease
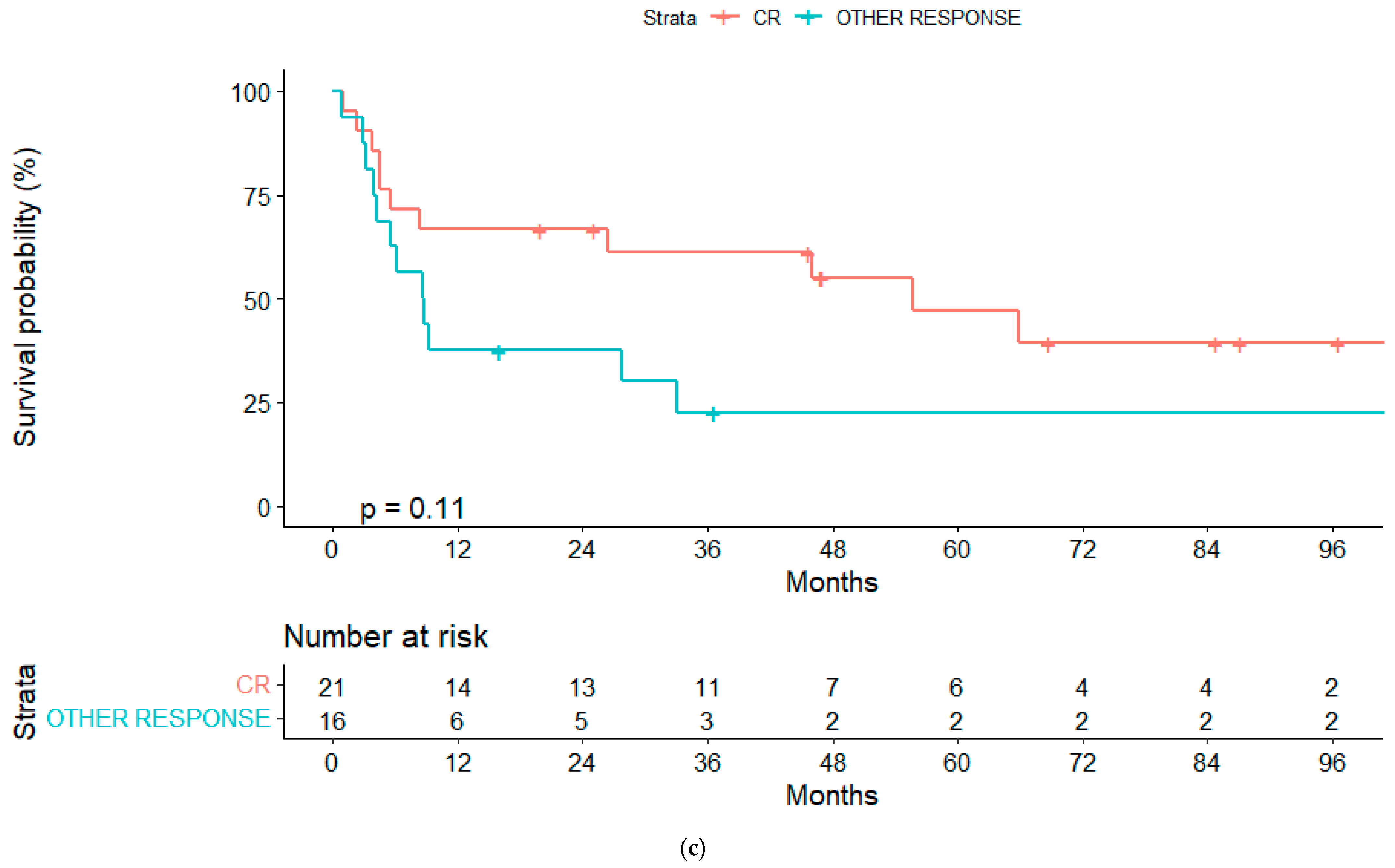
3.5. Response and Relapse Rate
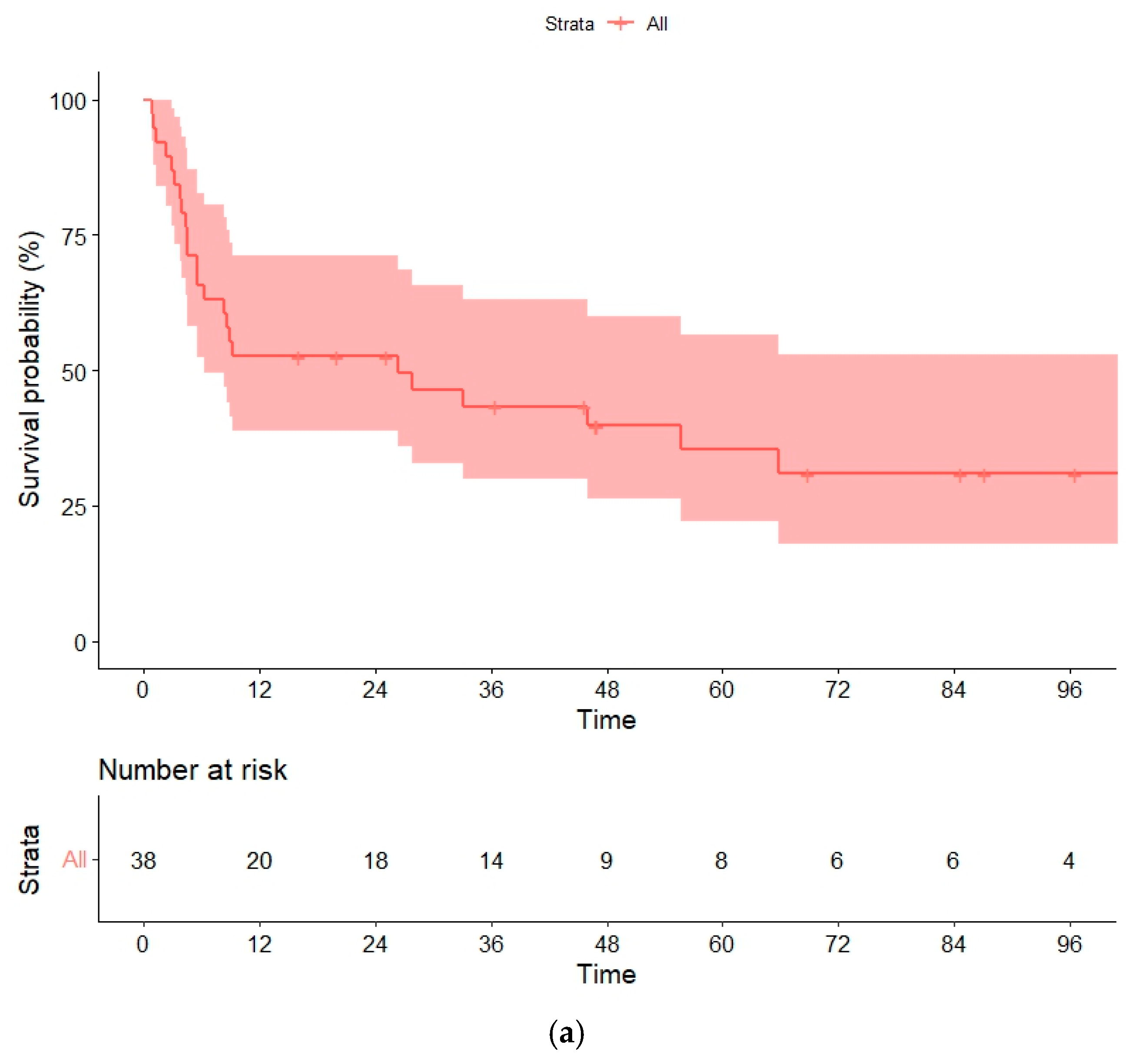
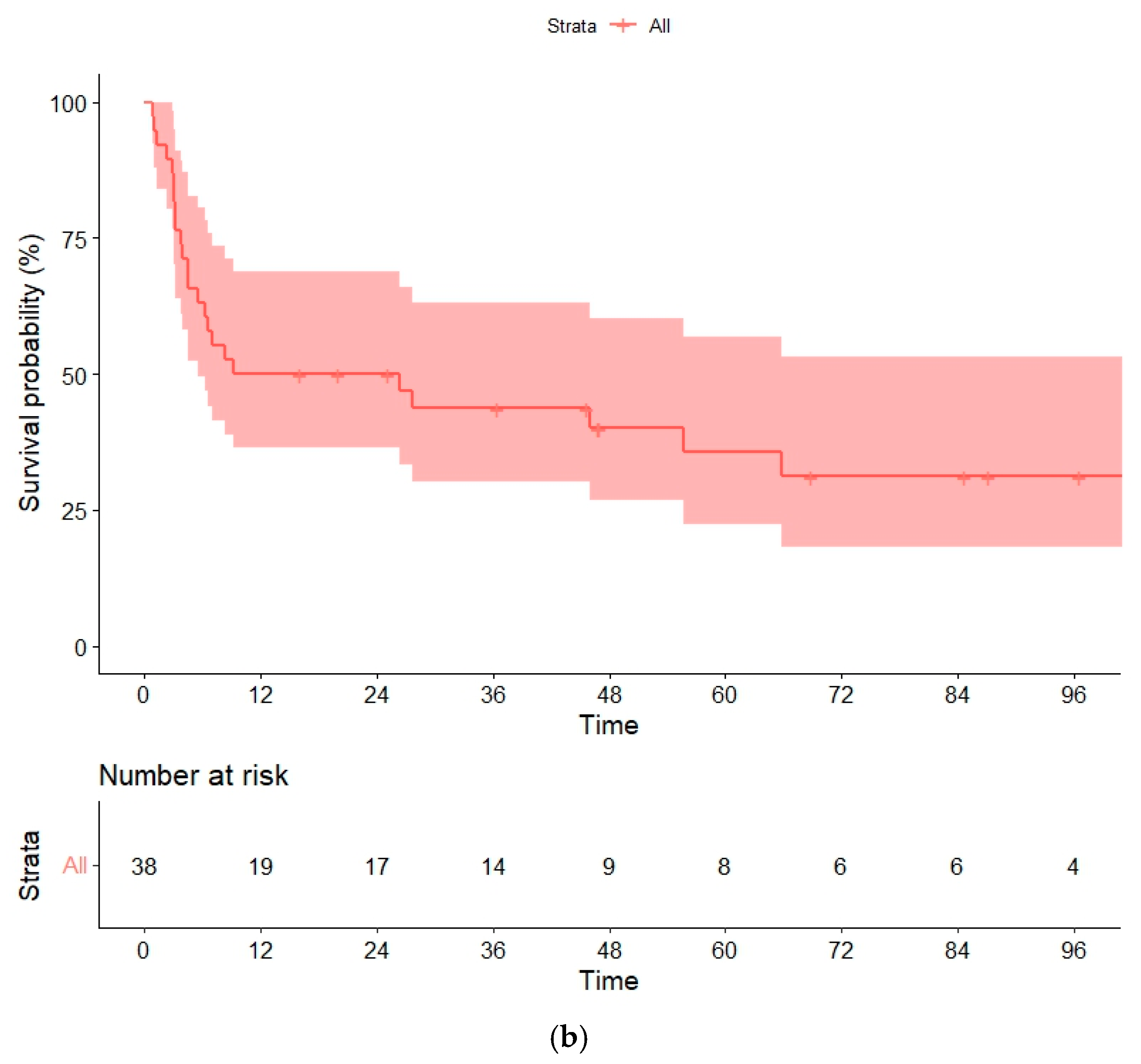
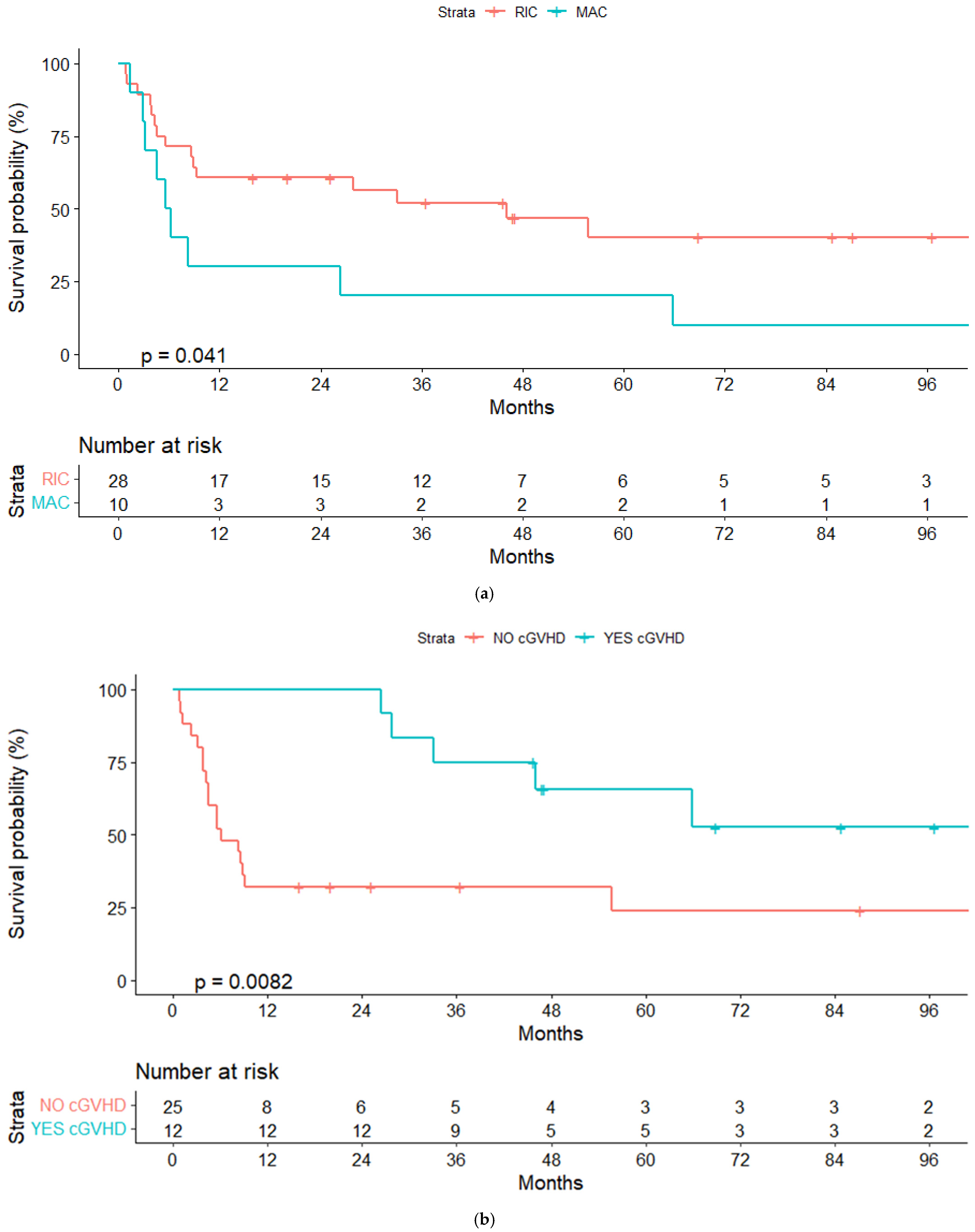
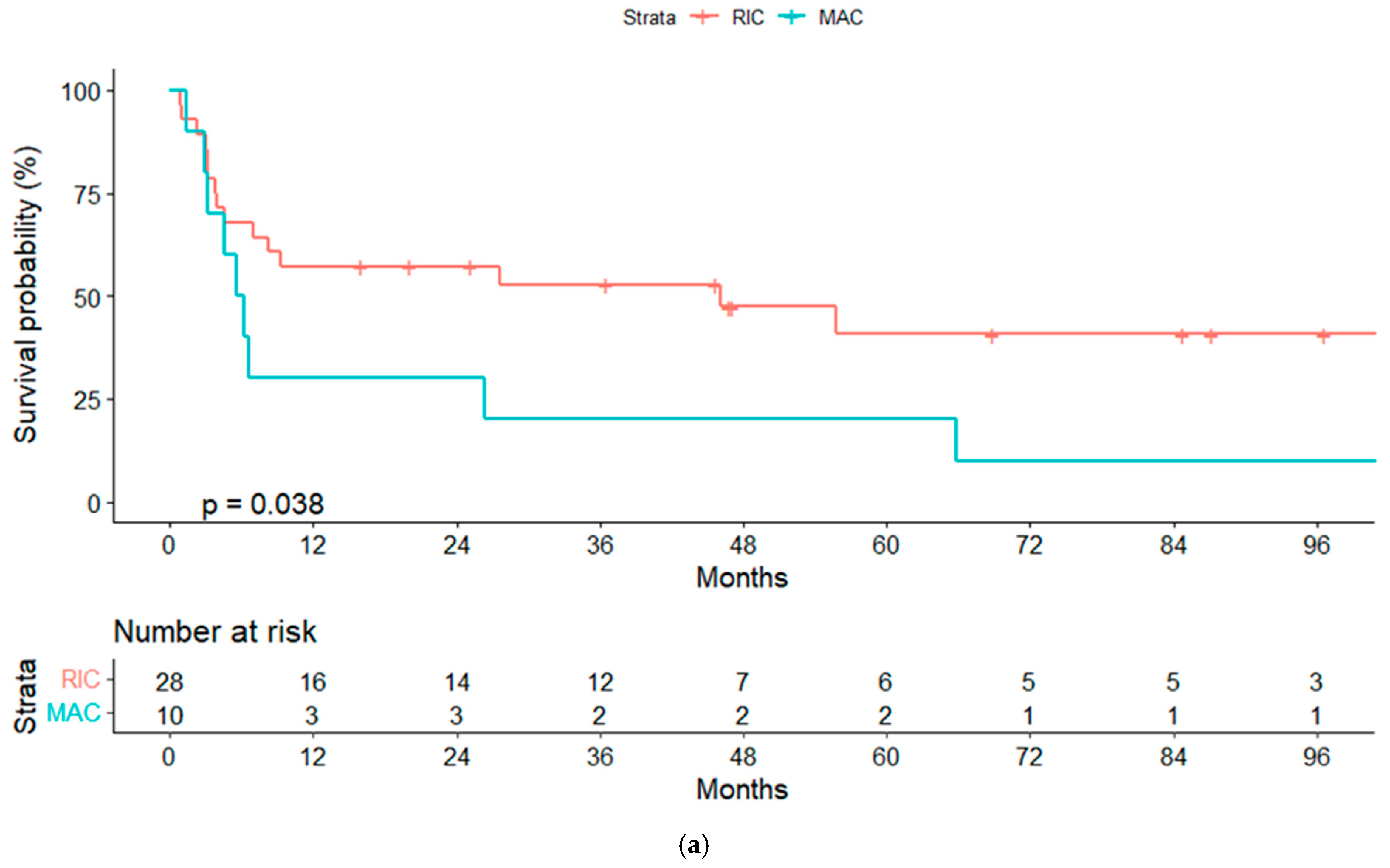
3.6. Overall and Progression-Free Survival
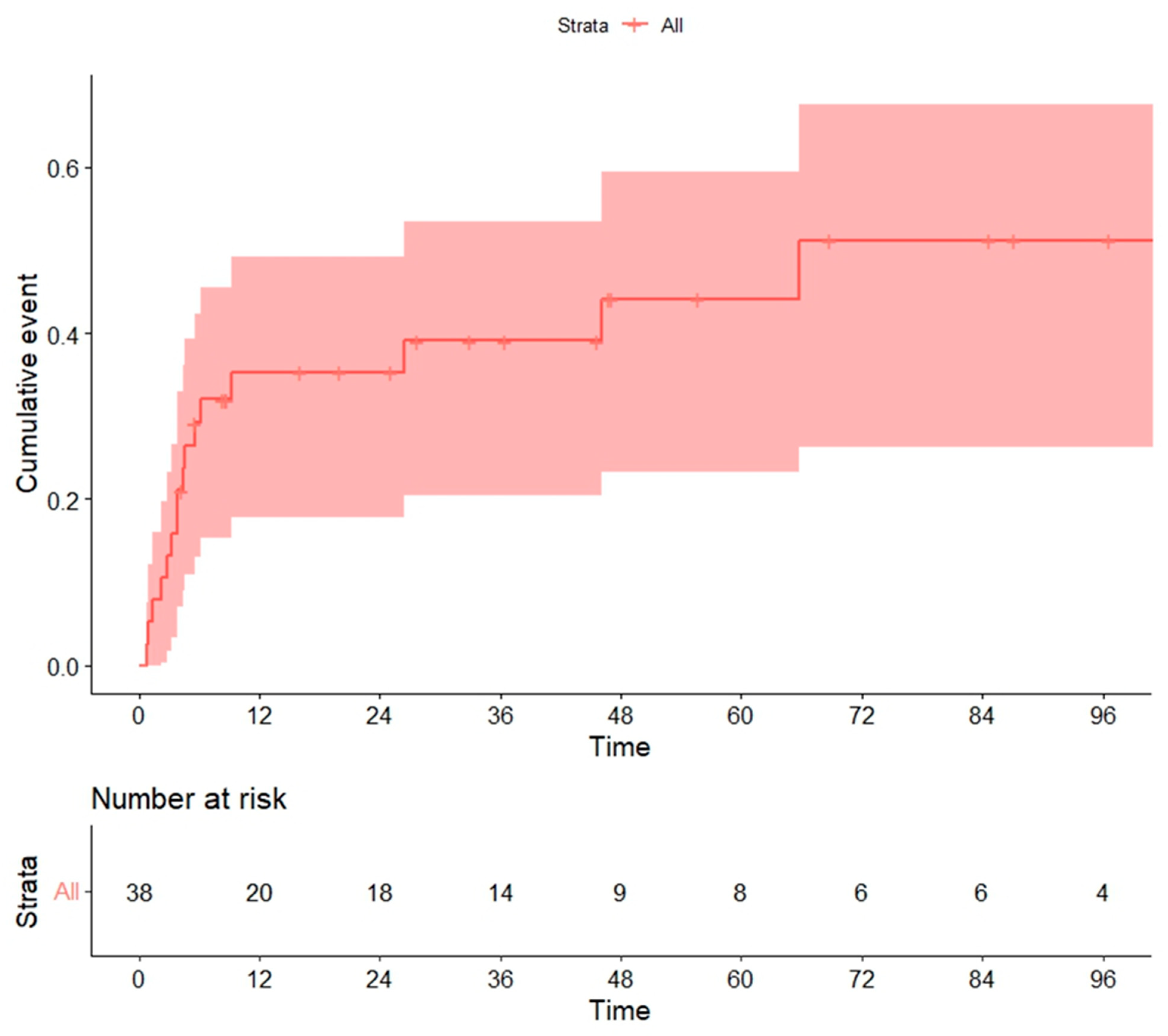
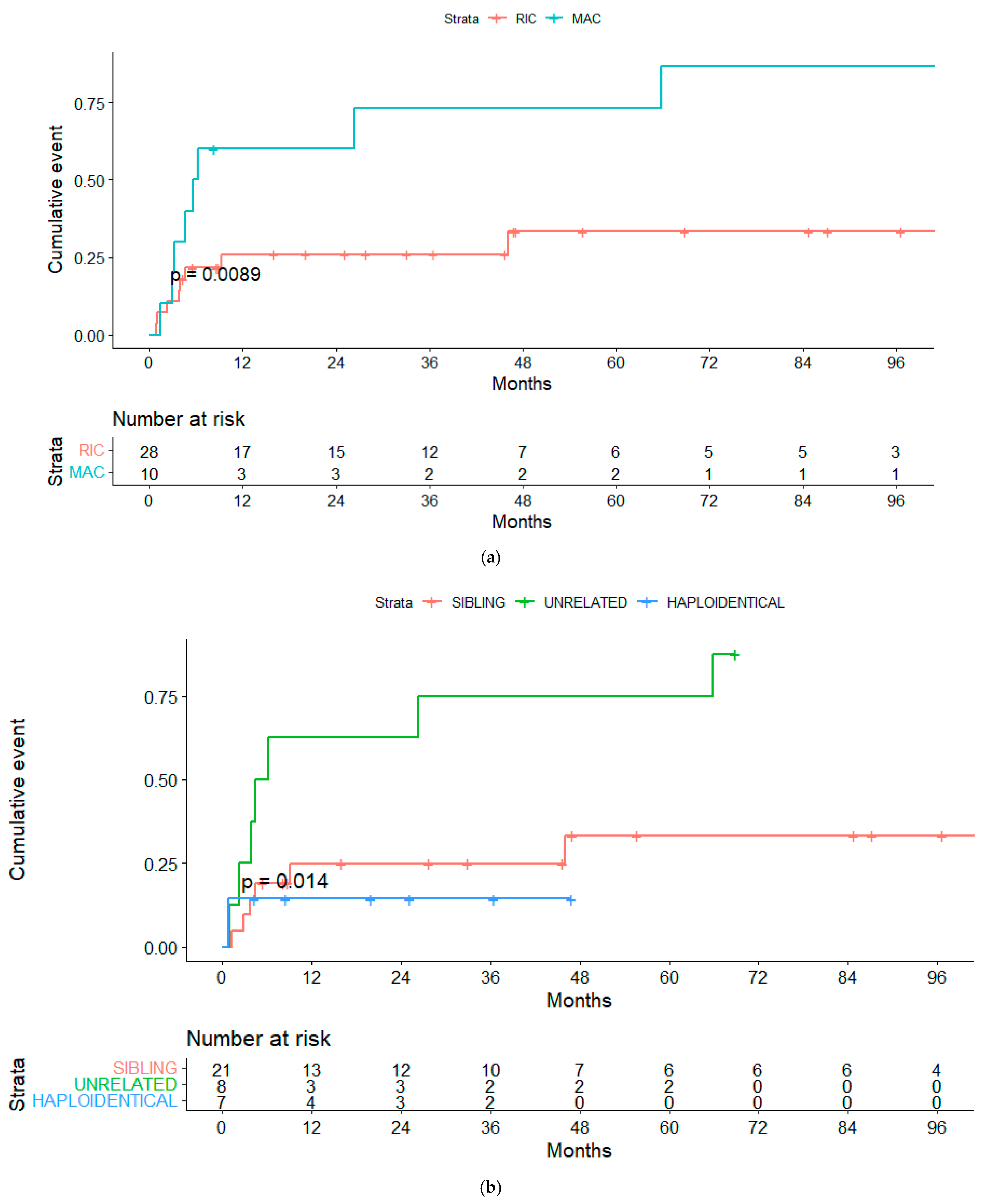
3.7. Global Mortality and Non-Relapse Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campo, E.; Swerdlow, S.H.; Harris, N.L.; Pileri, S.; Stein, H.; Jaffe, E.S. The 2008 WHO Classification of Lymphoid Neoplasms and beyond: Evolving Concepts and Practical Applications. Blood 2011, 117, 5019–5032. [Google Scholar] [CrossRef] [PubMed]
- Herold, M.; Haas, A.; Srock, S.; Neser, S.; Al-Ali, H.; Neubauer, A.; Dölken, G.; Naumann, R.; Knauf, W.; Freund, M.; et al. Rituximab Added to First-Line Mitoxantrone, Chlorambucil, and Prednisolone Chemotherapy Followed by Interferon Maintenance Prolongs Survival in Patients With Advanced Follicular Lymphoma: An East German Study Group Hematology and Oncology Study. J. Clin. Oncol. 2007, 25, 1986–1992. [Google Scholar] [CrossRef] [PubMed]
- Marcus, R.; Imrie, K.; Solal-Celigny, P.; Catalano, J.; Dmoszynska, A.; Raposo, J.; Offner, F.; Gomez-Codina, J.; Belch, A.; Cunningham, D.; et al. Phase III Study of R-CVP Compared With Cyclophosphamide, Vincristine, and Prednisone Alone in Patients With Previously Untreated Advanced Follicular Lymphoma. J. Clin. Oncol. 2008, 26, 4579–4586. [Google Scholar] [CrossRef] [PubMed]
- Salles, G.; Seymour, J.F.; Offner, F.; López-Guillermo, A.; Belada, D.; Xerri, L.; Feugier, P.; Bouabdallah, R.; Catalano, J.V.; Brice, P.; et al. Rituximab Maintenance for 2 Years in Patients with High Tumour Burden Follicular Lymphoma Responding to Rituximab plus Chemotherapy (PRIMA): A Phase 3, Randomised Controlled Trial. Lancet 2011, 377, 42–51. [Google Scholar] [CrossRef]
- Magnano, L.; Alonso-Alvarez, S.; Alcoceba, M.; Rivas-Delgado, A.; Muntañola, A.; Nadeu, F.; Setoain, X.; Rodríguez, S.; Andrade-Campos, M.; Espinosa-Lara, N.; et al. Life Expectancy of Follicular Lymphoma Patients in Complete Response at 30 Months Is Similar to That of the Spanish General Population. Br. J. Haematol. 2019, 185, 480–491. [Google Scholar] [CrossRef]
- Sarkozy, C.; Maurer, M.J.; Link, B.K.; Ghesquieres, H.; Nicolas, E.; Thompson, C.A.; Traverse-Glehen, A.; Feldman, A.L.; Allmer, C.; Slager, S.L.; et al. Cause of Death in Follicular Lymphoma in the First Decade of the Rituximab Era: A Pooled Analysis of French and US Cohorts. J. Clin. Oncol. 2019, 37, 144–152. [Google Scholar] [CrossRef]
- Montoto, S.; Davies, A.J.; Matthews, J.; Calaminici, M.; Norton, A.J.; Amess, J.; Vinnicombe, S.; Waters, R.; Rohatiner, A.Z.S.; Lister, T.A. Risk and Clinical Implications of Transformation of Follicular Lymphoma to Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2007, 25, 2426–2433. [Google Scholar] [CrossRef]
- Alonso-Álvarez, S.; Magnano, L.; Alcoceba, M.; Andrade-Campos, M.; Espinosa-Lara, N.; Rodríguez, G.; Mercadal, S.; Carro, I.; Sancho, J.M.; Moreno, M.; et al. Risk of, and Survival Following, Histological Transformation in Follicular Lymphoma in the Rituximab Era. A Retrospective Multicentre Study by the Spanish GELTAMO Group. Br. J. Haematol. 2017, 178, 699–708. [Google Scholar] [CrossRef]
- Bastion, Y.; Brice, P.; Haioun, C.; Sonet, A.; Salles, G.; Marolleau, J.P.; Espinouse, D.; Reyes, F.; Gisselbrecht, C.; Coiffier, B. Intensive Therapy with Peripheral Blood Progenitor Cell Transplantation in 60 Patients with Poor-Prognosis Follicular Lymphoma. Blood 1995, 86, 3257–3262. [Google Scholar] [CrossRef]
- Al-Tourah, A.J.; Gill, K.K.; Chhanabhai, M.; Hoskins, P.J.; Klasa, R.J.; Savage, K.J.; Sehn, L.H.; Shenkier, T.N.; Gascoyne, R.D.; Connors, J.M. Population-Based Analysis of Incidence and Outcome of Transformed Non-Hodgkin’s Lymphoma. J. Clin. Oncol. 2008, 26, 5165–5169. [Google Scholar] [CrossRef]
- Link, B.K.; Maurer, M.J.; Nowakowski, G.S.; Ansell, S.M.; Macon, W.R.; Syrbu, S.I.; Slager, S.L.; Thompson, C.A.; Inwards, D.J.; Johnston, P.B.; et al. Rates and Outcomes of Follicular Lymphoma Transformation in the Immunochemotherapy Era: A Report from the University of Iowa/MayoClinic Specialized Program of Research Excellence Molecular Epidemiology Resource. J. Clin. Oncol. 2013, 31, 3272–3278. [Google Scholar] [CrossRef] [PubMed]
- Federico, M.; Caballero Barrigón, M.D.; Marcheselli, L.; Tarantino, V.; Manni, M.; Sarkozy, C.; Alonso-Álvarez, S.; Wondergem, M.; Cartron, G.; Lopez-Guillermo, A.; et al. Rituximab and the Risk of Transformation of Follicular Lymphoma: A Retrospective Pooled Analysis. Lancet Haematol. 2018, 5, e359–e367. [Google Scholar] [CrossRef]
- Villa, D.; Crump, M.; Panzarella, T.; Savage, K.J.; Toze, C.L.; Stewart, D.A.; MacDonald, D.A.; Buckstein, R.; Lee, C.; Alzahrani, M.; et al. Autologous and Allogeneic Stem-Cell Transplantation for Transformed Follicular Lymphoma: A Report of the Canadian Blood and Marrow Transplant Group. J. Clin. Oncol. 2013, 31, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Hamadani, M.; Benson, D.M.; Lin, T.S.; Porcu, P.; Blum, K.A.; Devine, S.M. High-Dose Therapy and Autologous Stem Cell Transplantation for Follicular Lymphoma Undergoing Transformation to Diffuse Large B-Cell Lymphoma. Eur. J. Haematol. 2008, 81, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.; Pedersen, M.B.; Vase, M.Ø.; Bendix, K.; Møller, M.B.; Johansen, P.; Jensen, B.A.; Jensen, P.; Munksgaard, L.; Brown, P.D.; et al. Outcome Determinants for Transformed Indolent Lymphomas Treated with or without Autologous Stem-Cell Transplantation. Ann. Oncol. 2015, 26, 393–399. [Google Scholar] [CrossRef]
- Chen, C.I.; Crump, M.; Tsang, R.; Stewart, A.K.; Keating, A. Autotransplants for Histologically Transformed Follicular Non-Hodgkin’s Lymphoma. Br. J. Haematol. 2001, 113, 202–208. [Google Scholar] [CrossRef]
- Schouten, H.; Bierman, P.; Vaughan, W.; Kessinger, A.; Vose, J.; Weisenburger, D.; Armitage, J. Autologous Bone Marrow Transplantation in Follicular Non-Hodgkin’s Lymphoma before and after Histologic Transformation. Blood 1989, 74, 2579–2584. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef]
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-Term Safety and Activity of Axicabtagene Ciloleucel in Refractory Large B-Cell Lymphoma (ZUMA-1): A Single-Arm, Multicentre, Phase 1-2 Trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene Maraleucel for Patients with Relapsed or Refractory Large B-Cell Lymphomas (TRANSCEND NHL 001): A Multicentre Seamless Design Study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef]
- Corradini, P.; Dodero, A.; Farina, L.; Fanin, R.; Patriarca, F.; Miceli, R.; Matteucci, P.; Bregni, M.; Scimè, R.; Narni, F.; et al. Allogeneic Stem Cell Transplantation Following Reduced-Intensity Conditioning Can Induce Durable Clinical and Molecular Remissions in Relapsed Lymphomas: Pre-Transplant Disease Status and Histotype Heavily Influence Outcome. Leukemia 2007, 21, 2316–2323. [Google Scholar] [CrossRef] [PubMed]
- Khouri, I.F.; McLaughlin, P.; Saliba, R.M.; Hosing, C.; Korbling, M.; Lee, M.S.; Medeiros, L.J.; Fayad, L.; Samaniego, F.; Alousi, A.; et al. Eight-Year Experience with Allogeneic Stem Cell Transplantation for Relapsed Follicular Lymphoma after Nonmyeloablative Conditioning with Fludarabine, Cyclophosphamide, and Rituximab. Blood 2008, 111, 5530–5536. [Google Scholar] [CrossRef] [PubMed]
- Heinzelmann, F.; Bethge, W.; Beelen, D.W.; Stelljes, M.; Dreger, P.; Engelhard, M.; Finke, J.; Kröger, N.; Holler, E.; Bornhäuser, M.; et al. Allogeneic Haematopoietic Cell Transplantation Offers the Chance of Cure for Patients with Transformed Follicular Lymphoma. J. Cancer Res. Clin. Oncol. 2018, 144, 1173–1183. [Google Scholar] [CrossRef]
- Glucksberg, H.; Storb, R.; Fefer, A.; Buckner, C.D.; Neiman, P.E.; Clift, R.A.; Lerner, K.G.; Thomas, E.D. Clinical Manifestations of Graft-versus-Host Disease in Human Recipients of Marrow from HL-A-Matched Sibling Donors. Transplantation 1974, 18, 295–304. [Google Scholar] [CrossRef]
- Filipovich, A.H.; Weisdorf, D.; Pavletic, S.; Socie, G.; Wingard, J.R.; Lee, S.J.; Martin, P.; Chien, J.; Przepiorka, D.; Couriel, D.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. Diagnosis and Staging Working Group Report. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2005, 11, 945–956. [Google Scholar] [CrossRef]
- Sorror, M.L.; Maris, M.B.; Storb, R.; Baron, F.; Sandmaier, B.M.; Maloney, D.G.; Storer, B. Hematopoietic Cell Transplantation (HCT)-Specific Comorbidity Index: A New Tool for Risk Assessment before Allogeneic HCT. Blood 2005, 106, 2912–2919. [Google Scholar] [CrossRef]
- Cabrero, M.; Martin, A.; Briones, J.; Gayoso, J.; Jarque, I.; López, J.; Grande, C.; Heras, I.; Arranz, R.; Bernal, T.; et al. Phase II Study of Yttrium-90-Ibritumomab Tiuxetan as Part of Reduced-Intensity Conditioning (with Melphalan, Fludarabine ± Thiotepa) for Allogeneic Transplantation in Relapsed or Refractory Aggressive B Cell Lymphoma: A GELTAMO Trial. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2017, 23, 53–59. [Google Scholar] [CrossRef]
- Rezvani, A.R.; Storer, B.; Maris, M.; Sorror, M.L.; Agura, E.; Maziarz, R.T.; Wade, J.C.; Chauncey, T.; Forman, S.J.; Lange, T.; et al. Nonmyeloablative Allogeneic Hematopoietic Cell Transplantation in Relapsed, Refractory, and Transformed Indolent Non-Hodgkin’s Lymphoma. JCO 2008, 26, 211–217. [Google Scholar] [CrossRef]
- Wirk, B.; Fenske, T.S.; Hamadani, M.; Zhang, M.-J.; Hu, Z.-H.; Akpek, G.; Aljurf, M.D.; Armand, P.; Ayala, E.; Bachanova, V.; et al. Outcomes of Hematopoietic Cell Transplantation for Diffuse Large B Cell Lymphoma Transformed from Follicular Lymphoma. Biol. Blood Marrow Transplant. 2014, 20, 951–959. [Google Scholar] [CrossRef]
- On behalf of GETH and GELTAMO Group; Montoro, J.; Chorão, P.; Bento, L.; Cabrero, M.; Martín, C.; Novelli, S.; Cadenas, I.G.; Gutiérrez, G.; López-Godino, O.; et al. Risk Factors and Outcomes of Follicular Lymphoma after Allogeneic Hematopoietic Stem Cell Transplantation Using HLA-Matched Sibling, Unrelated, and Haploidentical-Related Donors. Bone Marrow Transplant. 2021, 56, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Bento, L.; Gutiérrez, A.; Novelli, S.; Montoro, J.; Piñana, J.L.; López-Corral, L.; Cabrero, M.; Martín-Sancho, A.; Gutiérrez-García, G.; Ortiz-Moscovich, M.; et al. Allogeneic Stem Cell Transplantation as a Curative Option in Relapse/Refractory Diffuse Large B Cell Lymphoma: Spanish Multicenter GETH/GELTAMO Study. Bone Marrow Transplant. 2021, 56, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Maloney, D.G. Graft-vs.-Lymphoma Effect in Various Histologies of Non-Hodgkin’s Lymphoma. Leuk. Lymphoma 2003, 44, S99–S105. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Zhang, M.; Dreger, P.; Carreras, J.; Fenske, T.; Finel, H.; Schouten, H.; Montoto, S.; Robinson, S.; Smith, S.M.; et al. Allogeneic Hematopoietic Stem Cell Transplantation for Relapsed Follicular Lymphoma: A Combined Analysis on Behalf of the Lymphoma Working Party of the EBMT and the Lymphoma Committee of the CIBMTR. Cancer 2018, 124, 1733–1742. [Google Scholar] [CrossRef]
- Heinzelmann, F.; Bethge, W.; Beelen, D.W.; Engelhard, M.; Kröger, N.; Dreger, P.; Niederwieser, D.; Finke, J.; Bunjes, D.; Tischer, J.; et al. Allogeneic Hematopoietic Cell Transplantation as Curative Therapy for Non-Transformed Follicular Lymphomas. Bone Marrow Transplant. 2016, 51, 654–662. [Google Scholar] [CrossRef]
- van Kampen, R.J.W.; Canals, C.; Schouten, H.C.; Nagler, A.; Thomson, K.J.; Vernant, J.-P.; Buzyn, A.; Boogaerts, M.A.; Luan, J.-J.; Maury, S.; et al. Allogeneic Stem-Cell Transplantation As Salvage Therapy for Patients With Diffuse Large B-Cell Non-Hodgkin’s Lymphoma Relapsing After an Autologous Stem-Cell Transplantation: An Analysis of the European Group for Blood and Marrow Transplantation Registry. J. Clin. Oncol. 2011, 29, 1342–1348. [Google Scholar] [CrossRef]
- Dreger, P.; Fenske, T.S.; Montoto, S.; Pasquini, M.C.; Sureda, A.; Hamadani, M. Cellular Immunotherapy for Refractory Diffuse Large B Cell Lymphoma in the Chimeric Antigen Receptor-Engineered T Cell Era: Still a Role for Allogeneic Transplantation? Biol. Blood Marrow Transplant. 2020, 26, e77–e85. [Google Scholar] [CrossRef]
| Characteristic | Frequency (Range or Percentage) | |
|---|---|---|
| Age, median (range) | 52 (31–66) | |
| Sex, n (%) | Male | 24 (63%) |
| Female | 14 (37%) | |
| Previous lines of treatment, median (range) | 4 (2–7) | |
| Previous lines of treatment, n (%) | 2 | 5 (13%) |
| 3 | 14 (37%) | |
| 4 or more | 19 (50%) | |
| Previous ASCT, n (%) | Yes | 24 (63%) |
| No | 14 (37%) | |
| Lymphoma status pre-AlloSCT, n (%) | CR | 21 (55%) |
| PR | 12 (32%) | |
| SD/PD | 4 (11%) | |
| Data not available | 1 (2%) | |
| HCT-CI, n (%) | 0 | 17 (44.7%) |
| 1 | 10 (26.3%) | |
| 2 | 3 (7.9%) | |
| ≥3 | 6 (15.8%) | |
| Data not available | 2 (5.3) | |
| HLA identical and donor, n (%) | Identical donor | 26 (68%) |
| Related | 21 (56%) | |
| Unrelated | 5 (12%) | |
| Haploidentical | 7 (18%) | |
| Mismatched donor unrelated | 3 (8%) | |
| Data not available | 2 (5%) | |
| Source of stem cells, n (%) | PB | 36 (95%) |
| BM | 1 (2.5%) | |
| CBU | 1 (2.5%) | |
| Intensity conditioning regimen, n (%) | Myeloablative (MAC) | 10 (23.3%) |
| Reduced intensity (RIC) | 26 (68.4%) | |
| Data not available | 2 (5.3%) | |
| Conditioning regimen, n (%) | Flu-Mel | 21 (55.3%) |
| Flu-Bu | 4 (23.7%) | |
| Flu-Bu-Thio | 4 (10.5%) | |
| CyTBI | 1 (2.6%) | |
| Flu-TBI | 2 (5.2%) | |
| Bu-Cy | 1 (2.6%) | |
| Monoclonal antibodies as part of conditioning regimen, n (%) | Rituximab | 2 (5.3%) |
| Ofatumumab | 7 (18.4%) | |
| Ibritumomab tiuxetan | 2 (5.3%) | |
| GVHD prophylaxis, n (%) | MTX + CI | 13 (34.2%) |
| Tacro + Rapa | 11 (28.9%) | |
| MMF + CI | 4 (10.6%) | |
| Cy post-alloSCT | 7 (18.4%) | |
| Alemtuzumab/ATG | 2 (5.3%) | |
| Status at alloSCT | Response at Day +100 | Status at Last Follow-Up | Cause of Death |
|---|---|---|---|
| CR, n = 21 | CR, n = 19 | Alive in CR, n = 10 | |
| Death while in CR, n = 8 | Infection, n = 5 aGVHD n = 2 Secondary neoplasm, n = 1 | ||
| Death after progression, n = 1 | Lymphoma, n = 1 | ||
| Relapse, n = 1 | Death, n = 1 | Lymphoma, n = 1 | |
| Death without evaluation, n = 1 | Death, n = 1 | SOS, n = 1 | |
| PR, n = 12 | CR, n = 5 | Alive in CR, n = 1 | |
| Death in CR, n = 2 | Infection, n = 2 | ||
| Death after progression, n = 2 | Lymphoma, n = 2 | ||
| PR, n = 3 | Alive in CR, n = 2 * | ||
| Death in CR, n = 1 | NRM, n = 1 | ||
| SD/PD, n = 3 | Death, n = 3 | Lymphoma, n = 3 | |
| Not evaluable, n = 1 | Death after progression, n = 1 | Lymphoma, n = 1 | |
| SD/PD, n = 4 | CR, n = 2 | Alive in CR, n = 1 | |
| Death in CR, n = 1 | Infection, n = 1 | ||
| Death without evaluation, n = 2 | Death, n = 2 | Infection, n = 2 | |
| No data, n = 1 | Death without evaluation, n = 1 | Death, n = 1 | MDS, n = 1 |
| Univariate Analysis | ||||
|---|---|---|---|---|
| Characteristics | 3y-PFS (95% CI) | p | 3y-OS (95% CI) | p |
| Age at alloSCT: | ||||
| <59 | 45% (28–62) | 44.6% (27.4–61.8) | ||
| >60 | 30% (16.9–76.9) | 0.86 | 30% (16.8–76.8) | 0.75 |
| Previous lines of therapy: | ||||
| 1–3 | 39.5% (16.4–62.6) | 39.5% (16.4–62.7) | ||
| >3 | 47.4% (24.9–69.9) | 0.95 | 46.80% | 0.95 |
| Previous ASCT: | ||||
| Yes | 56.4% (35.8–77) | 56.4% (36–77) | ||
| No | 21.4% (0.2–43) | 0.06 | 21.4% (0.1–43) | 0.13 |
| Conditioning regimen: | ||||
| MAC | 20% (4.7–44.7) | 20% (5–45) | ||
| RIC | 52.7% (33.9–71.5) | 0.04 | 52% (33–71) | 0.04 |
| Donor: | ||||
| Sibling | 52% (30.3–73.5) | 51.6% (29.8–73.4) | ||
| Haploidentical | 57% (20.4–93.8) | 57% (20.4–93.8) | ||
| Unrelated | 25% (5–55) | 0.3 | 25% (5–55) | 0.22 |
| Response pre alloSCT: | ||||
| CR | 61% (39.9–82.3) | 66.7% (46.5–86.9) | ||
| Other | 23.4% (1.8–45) | 0.06 | 22.5% (1–44) | 0.11 |
| aGVHD: | ||||
| Yes | 41.5% (22.3–60.7) | 41% (21.5–60.3) | ||
| No | 53% (22.6–83.4) | 0.66 | 53% (22.6–83.4) | 0.67 |
| aGVHD: | ||||
| 0–2 | 47% (46.8–47.2) | 46.5% (27.4–63.8) | ||
| >2 | 33.3% (31.9–33.7) | 0.9 | 33.3% (4.3–70.9) | 0.65 |
| cGVHD: | ||||
| Yes | 75% (50.5–99.5) | 75% (50.5–99.5) | ||
| No | 32% (13.8–50.2) | 0.008 | 32% (13.8–50.2) | 0.008 |
| Reference | Patients (n) | CR (%) | OS/PFS (%) | NRM (%) |
|---|---|---|---|---|
| Heinzelman et al., J. Cancer Res. Clin. Oncol. 2018 [24] | 33 | 62% | 1-y: 49/33 2-y: 39/30 5-y: 33/24 | 30-d: 9 100-d: 25 1-y: 38 2-y: 43 |
| Rezvani et al., Clin. Oncol. 2008 [29] | 16 | - | 3-y OS: 43 3-y PFS: 38 | 3-y: 42 |
| Villa et al., Clin. Oncol. 2013 [13] | 22 | 59% | 5-y OS: 45 | 5-y: 23 |
| Wirk et al., BBMT 2014 PMID: 24641828 [30] | 33 | - | 5-y: 22/18 | 1-y: 41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rey-Búa, B.; Cabrero, M.; Bento, L.; Montoro, J.; Bastos-Oreiro, M.; Parody, R.; Yañez, L.; Lopez-Godino, O.; Zanabili, J.; Herrera, P.; et al. Allogeneic Hematopoietic Stem Cell Transplantation in Transformed Follicular Lymphoma (tFL): Results of a Retrospective Multicenter Study from GELTAMO/GETH-TC Spanish Groups. Cancers 2022, 14, 5670. https://doi.org/10.3390/cancers14225670
Rey-Búa B, Cabrero M, Bento L, Montoro J, Bastos-Oreiro M, Parody R, Yañez L, Lopez-Godino O, Zanabili J, Herrera P, et al. Allogeneic Hematopoietic Stem Cell Transplantation in Transformed Follicular Lymphoma (tFL): Results of a Retrospective Multicenter Study from GELTAMO/GETH-TC Spanish Groups. Cancers. 2022; 14(22):5670. https://doi.org/10.3390/cancers14225670
Chicago/Turabian StyleRey-Búa, Beatriz, Mónica Cabrero, Leyre Bento, Juan Montoro, Mariana Bastos-Oreiro, Rocío Parody, Lucrecia Yañez, Oriana Lopez-Godino, Joud Zanabili, Pilar Herrera, and et al. 2022. "Allogeneic Hematopoietic Stem Cell Transplantation in Transformed Follicular Lymphoma (tFL): Results of a Retrospective Multicenter Study from GELTAMO/GETH-TC Spanish Groups" Cancers 14, no. 22: 5670. https://doi.org/10.3390/cancers14225670
APA StyleRey-Búa, B., Cabrero, M., Bento, L., Montoro, J., Bastos-Oreiro, M., Parody, R., Yañez, L., Lopez-Godino, O., Zanabili, J., Herrera, P., Gutierrez, G., Perez, A., Piñana, J. L., Novelli, S., Cortés, M., Sureda, A. M., Caballero, D., & García-Sancho, A. M. (2022). Allogeneic Hematopoietic Stem Cell Transplantation in Transformed Follicular Lymphoma (tFL): Results of a Retrospective Multicenter Study from GELTAMO/GETH-TC Spanish Groups. Cancers, 14(22), 5670. https://doi.org/10.3390/cancers14225670





