Cancer Patients and the COVID-19 Vaccines: Considerations and Challenges
Abstract
Simple Summary
Abstract
1. Introduction
Cancer Patients and COVID-19
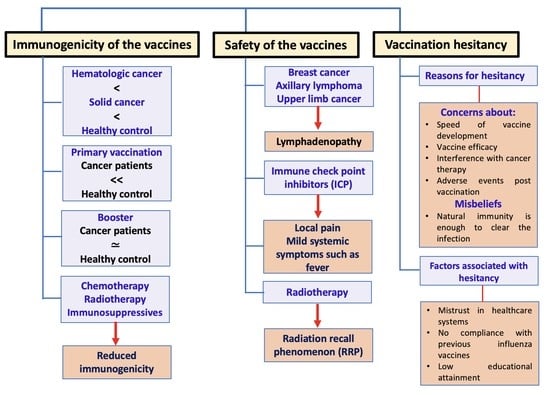
2. Immunogenicity of the COVID-19 Vaccines in Solid and Hematologic Cancer Patients
2.1. Lower Seropositivity in Patients Receiving Active Therapy Compared with Healthy Controls or Patients Not Receiving Active Therapy
2.2. Lower Seropositivity in Cancer Patients Receiving Chemotherapy Compared to Healthy Controls or Patients Receiving Other Treatments
2.3. Effect of Radiotherapy on the Immunogenicity of COVID-19 Vaccines in Cancer Patients
2.4. Effect of Checkpoint Inhibitors on the Immunogenicity of COVID-19 Vaccines in Cancer Patients
2.5. Lower Seropositivity in Cancer Patients Receiving Immunosuppressives Compared to Healthy Controls or Patients Receiving Other Treatments
2.6. Effect of Other Treatments on the Immunogenicity of COVID-19 Vaccines in Cancer Patients
2.6.1. Endocrine Therapy
2.6.2. Cyclin Dependent Kinase Inhibitors
2.6.3. Stem Cell Transplants
2.6.4. Combination Treatment with Chemotherapy and Immunotherapy
2.7. Effect of the Number of the COVID-19 Vaccine Doses on the Seropositivity of the Cancer Patients
3. Safety of the COVID-19 Vaccines on the Cancer Patients
3.1. Safety of the COVID-19 Vaccines in Cancer Patients with Different Treatments
3.2. Safety of the COVID-19 Vaccines in Cancer Patients Receiving Radiotherapy
3.3. Safety of the COVID-19 Vaccines in Cancer Patients Receiving Immune Checkpoint Inhibitors
3.4. Lymphadenopathy Post-COVID-19 Vaccination in Cancer Patients
4. Hesitancy/Acceptance of the COVID-19 Vaccination among Cancer Patients
4.1. Major Reasons for COVID-19 Vaccination Hesitancy
4.2. Significantly Associated Factors with Vaccine Hesitancy/Acceptance
4.3. How to Combat Vaccination Hesitancy among Cancer Patients?
5. Ongoing Clinical Trials and Future Challenges
6. Discussion
7. Conclusions and Recommendations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yasin, A.I.; Aydin, S.G.; Sümbül, B.; Koral, L.; Simşek, M.; Geredeli, Ç.; Öztürk, A.; Perkin, P.; Demirtaş, D.; Erdemoglu, E.; et al. Efficacy and Safety Profile of COVID-19 Vaccine in Cancer Patients: A Prospective, Multicenter Cohort Study. Futur. Oncol. 2022, 18, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, Y.; Okwan-Duodu, D.; Basho, R.; Cui, X. COVID-19 in Cancer Patients: Risk, Clinical Features, and Management. Cancer Biol. Med. 2020, 17, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Matovina Brko, G.; Popovic, M.; Jovic, M.; Radic, J.; Bodlovic Kladar, M.; Nikolic, I.; Vidovic, V.; Kolarov Bjelobrk, I.; Kukic, B.; Salma, S.; et al. COVID-19 Vaccines and Cancer Patients: Acceptance, Attitudes and Safety. JBUON 2021, 26, 2188–2195. [Google Scholar]
- Monin, L.; Laing, A.G.; Muñoz-Ruiz, M.; McKenzie, D.R.; del Molino del Barrio, I.; Alaguthurai, T.; Domingo-Vila, C.; Hayday, T.S.; Graham, C.; Seow, J.; et al. Safety and Immunogenicity of One versus Two Doses of the COVID-19 Vaccine BNT162b2 for Patients with Cancer: Interim Analysis of a Prospective Observational Study. Lancet Oncol. 2021, 22, 765–778. [Google Scholar] [CrossRef]
- Sinha, S.; Kundu, C.N. Cancer and COVID-19: Why Are Cancer Patients More Susceptible to COVID-19? Med. Oncol. 2021, 38, 101. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A New Coronavirus Associated with Human Respiratory Disease in China. Nature 2020, 579, 265. [Google Scholar] [CrossRef]
- Smith, J.C.; Sausville, E.L.; Girish, V.; Yuan, M.L.; Vasudevan, A.; John, K.M.; Sheltzer, J.M. Cigarette Smoke Exposure and Inflammatory Signaling Increase the Expression of the SARS-CoV-2 Receptor ACE2 in the Respiratory Tract. Dev. Cell 2020, 53, 514–529.e3. [Google Scholar] [CrossRef]
- Sica, A.; Colombo, M.P.; Trama, A.; Horn, L.; Garassino, M.C.; Torri, V. Immunometabolic Status of COVID-19 Cancer Patients. Physiol. Rev. 2020, 100, 1839. [Google Scholar] [CrossRef]
- Ligumsky, H.; Safadi, E.; Etan, T.; Vaknin, N.; Waller, M.; Croll, A.; Nikolaevski-Berlin, A.; Greenberg, I.; Halperin, T.; Wasserman, A.; et al. Immunogenicity and Safety of the BNT162b2 MRNA COVID-19 Vaccine Among Actively Treated Cancer Patients. JNCI J. Natl. Cancer Inst. 2022, 114, 203–209. [Google Scholar] [CrossRef]
- Palaia, I.; Caruso, G.; Di Donato, V.; Vestri, A.; Napoli, A.; Perniola, G.; Casinelli, M.; Alunni Fegatelli, D.; Campagna, R.; Tomao, F.; et al. Pfizer-BioNTech COVID-19 Vaccine in Gynecologic Oncology Patients: A Prospective Cohort Study. Vaccines 2021, 10, 12. [Google Scholar] [CrossRef]
- Margalit, O.; Shacham-Shmueli, E.; Itay, A.; Berger, R.; Halperin, S.; Jurkowicz, M.; Levin, E.G.; Olmer, L.; Regev-Yochay, G.; Lustig, Y.; et al. Seropositivity and Neutralising Antibodies at Six Months after BNT162b2 Vaccination in Patients with Solid Tumours. Eur. J. Cancer 2022, 168, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Massarweh, A.; Tschernichovsky, R.; Stemmer, A.; Benouaich-Amiel, A.; Siegal, T.; Eliakim-Raz, N.; Stemmer, S.M.; Yust-Katz, S. Immunogenicity of the BNT162b2 MRNA COVID-19 Vaccine in Patients with Primary Brain Tumors: A Prospective Cohort Study. J. Neurooncol. 2022, 156, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Fendler, A.; Shepherd, S.T.C.; Au, L.; Wilkinson, K.A.; Wu, M.; Byrne, F.; Cerrone, M.; Schmitt, A.M.; Joharatnam-Hogan, N.; Shum, B.; et al. Adaptive Immunity and Neutralizing Antibodies against SARS-CoV-2 Variants of Concern Following Vaccination in Patients with Cancer: The CAPTURE Study. Nat. cancer 2021, 2, 1305–1320. [Google Scholar] [CrossRef] [PubMed]
- Singer, J.; Le, N.-S.; Mattes, D.; Klamminger, V.; Hackner, K.; Kolinsky, N.; Scherb, M.; Errhalt, P.; Kreye, G.; Pecherstorfer, M.; et al. Evaluation of Antibody Responses to COVID-19 Vaccines among Solid Tumor and Hematologic Patients. Cancers 2021, 13, 4312. [Google Scholar] [CrossRef] [PubMed]
- Agha, M.; Blake, M.; Chilleo, C.; Wells, A.; Haidar, G. Suboptimal Response to COVID-19 MRNA Vaccines in Hematologic Malignancies Patients. medRxiv 2021. [Google Scholar] [CrossRef]
- Lim, S.H.; Stuart, B.; Joseph-Pietras, D.; Johnson, M.; Campbell, N.; Kelly, A.; Jeffrey, D.; Turaj, A.H.; Rolfvondenbaumen, K.; Galloway, C.; et al. Immune Responses against SARS-CoV-2 Variants after Two and Three Doses of Vaccine in B-Cell Malignancies: UK PROSECO Study. Nat. Cancer 2022, 3, 552–564. [Google Scholar] [CrossRef]
- Tran, S.; Truong, T.H.; Narendran, A. Evaluation of COVID-19 Vaccine Response in Patients with Cancer: An Interim Analysis. Eur. J. Cancer 2021, 159, 259. [Google Scholar] [CrossRef]
- Nelli, F.; Fabbri, A.; Onorato, A.; Giannarelli, D.; Silvestri, M.A.; Giron Berrios, J.R.; Virtuoso, A.; Marrucci, E.; Signorelli, C.; Chilelli, M.G.; et al. Effects of Active Cancer Treatment on Safety and Immunogenicity of COVID-19 MRNA-BNT162b2 Vaccine: Preliminary Results from the Prospective Observational Vax-On Study. Ann. Oncol. 2022, 33, 107–108. [Google Scholar] [CrossRef]
- Cavanna, L.; Citterio, C.; Biasini, C.; Madaro, S.; Bacchetta, N.; Lis, A.; Cremona, G.; Muroni, M.; Bernuzzi, P.; Lo Cascio, G.; et al. COVID-19 Vaccines in Adult Cancer Patients with Solid Tumours Undergoing Active Treatment: Seropositivity and Safety. A Prospective Observational Study in Italy. Eur. J. Cancer 2021, 157, 441–449. [Google Scholar] [CrossRef]
- Cavanna, L.; Proietto, M.; Citterio, C.; Anselmi, E.; Zaffignani, E.; Stroppa, E.M.; Borsotti, M.T.; Contini, A.; Di Girolamo, G.; Quitadamo, V.M.; et al. COVID-19 Vaccination in Cancer Patients Older than 70 Years Undergoing Active Treatment. Seroconversion Rate and Safety. Vaccines 2022, 10, 164. [Google Scholar] [CrossRef]
- Corrie, P.G. Cytotoxic Chemotherapy: Clinical Aspects. Medicine 2008, 36, 24–28. [Google Scholar] [CrossRef]
- Garcia, G.; Odaimi, M. Systemic Combination Chemotherapy in Elderly Pancreatic Cancer: A Review. J. Gastrointest. Cancer 2017, 48, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.R.; Suhag, V.; Semwal, M.; Sharma, N. Radiotherapy: Basic Concepts and Recent Advances. Med. J. Armed Forces India 2010, 66, 158. [Google Scholar] [CrossRef]
- Figueiredo, J.C.; Merin, N.M.; Hamid, O.; Choi, S.Y.; Lemos, T.; Cozen, W.; Nguyen, N.; Finster, L.J.; Foley, J.; Darrah, J.; et al. Longitudinal SARS-CoV-2 MRNA Vaccine-Induced Humoral Immune Responses in Patients with Cancer. Cancer Res. 2021, 81, 6273–6280. [Google Scholar] [CrossRef] [PubMed]
- Grinshpun, A.; Rottenberg, Y.; Ben-Dov, I.Z.; Djian, E.; Wolf, D.G.; Kadouri, L. Serologic Response to COVID-19 Infection and/or Vaccine in Cancer Patients on Active Treatment. ESMO Open 2021, 6, 100283. [Google Scholar] [CrossRef]
- Ariamanesh, M.; Porouhan, P.; PeyroShabany, B.; Fazilat-Panah, D.; Dehghani, M.; Nabavifard, M.; Hatami, F.; Fereidouni, M.; Welsh, J.S.; Javadinia, S.A. Immunogenicity and Safety of the Inactivated SARS-CoV-2 Vaccine (BBIBP-CorV) in Patients with Malignancy. Cancer Investig. 2022, 40, 26–34. [Google Scholar] [CrossRef]
- Agbarya, A.; Sarel, I.; Ziv-Baran, T.; Agranat, S.; Schwartz, O.; Shai, A.; Nordheimer, S.; Fenig, S.; Shechtman, Y.; Kozlener, E.; et al. Efficacy of the Mrna-Based Bnt162b2 COVID-19 Vaccine in Patients with Solid Malignancies Treated with Anti-Neoplastic Drugs. Cancers 2021, 13, 4191. [Google Scholar] [CrossRef]
- Addeo, A.; Shah, P.K.; Bordry, N.; Hudson, R.D.; Albracht, B.; Di Marco, M.; Kaklamani, V.; Dietrich, P.Y.; Taylor, B.S.; Simand, P.F.; et al. Immunogenicity of SARS-CoV-2 Messenger RNA Vaccines in Patients with Cancer. Cancer Cell 2021, 39, 1091–1098.e2. [Google Scholar] [CrossRef]
- Funakoshi, Y.; Yakushijin, K.; Ohji, G.; Hojo, W.; Sakai, H.; Takai, R.; Nose, T.; Ohata, S.; Nagatani, Y.; Koyama, T.; et al. Safety and Immunogenicity of the COVID-19 Vaccine BNT162b2 in Patients Undergoing Chemotherapy for Solid Cancer. J. Infect. Chemother. 2022, 28, 516–520. [Google Scholar] [CrossRef]
- Ruggeri, E.M.; Nelli, F.; Fabbri, A.; Onorato, A.; Giannarelli, D.; Giron Berrios, J.R.; Virtuoso, A.; Marrucci, E.; Mazzotta, M.; Schirripa, M.; et al. Antineoplastic Treatment Class Modulates COVID-19 MRNA-BNT162b2 Vaccine Immunogenicity in Cancer Patients: A Secondary Analysis of the Prospective Vax-On Study. ESMO Open 2022, 7, 100350. [Google Scholar] [CrossRef]
- Bowes, C.L.; Naranbhai, V.; St Denis, K.J.; Lam, E.C.; Bertaux, B.; Keane, F.K.; Khandekar, M.J.; Balazs, A.B.; Iafrate, J.A.; Gainor, J.F.; et al. Heterogeneous Immunogenicity of SARS-CoV-2 Vaccines in Cancer Patients Receiving Radiotherapy. Radiother. Oncol. 2022, 166, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252. [Google Scholar] [CrossRef] [PubMed]
- Butte, M.J.; Keir, M.E.; Phamduy, T.B.; Sharpe, A.H.; Freeman, G.J. PD-L1 Interacts Specifically with B7-1 to Inhibit T Cell Proliferation. Immunity 2007, 27, 111. [Google Scholar] [CrossRef] [PubMed]
- Karwacz, K.; Bricogne, C.; MacDonald, D.; Arce, F.; Bennett, C.L.; Collins, M.; Escors, D. PD-L1 Co-Stimulation Contributes to Ligand-Induced T Cell Receptor down-Modulation on CD8+ T Cells. EMBO Mol. Med. 2011, 3, 581. [Google Scholar] [CrossRef] [PubMed]
- Naranbhai, V.; Pernat, C.A.; Gavralidis, A.; St Denis, K.J.; Lam, E.C.; Spring, L.M.; Isakoff, S.J.; Farmer, J.R.; Zubiri, L.; Hobbs, G.S.; et al. Immunogenicity and Reactogenicity of SARS-CoV-2 Vaccines in Patients with Cancer: The CANVAX Cohort Study. J. Clin. Oncol. 2022, 40, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, N.; Wang, Y.; Zeng, J.; Hu, Y.Y.; Hao, W.; Shi, H.; Zhu, P.; Lv, J.; Fan, W.; et al. Immune Checkpoint Blocking Impact and Nomogram Prediction of COVID-19 Inactivated Vaccine Seroconversion in Patients with Cancer: A Propensity-Score Matched Analysis. J. Immunother. Cancer 2021, 9, e003712. [Google Scholar] [CrossRef]
- Lasagna, A.; Agustoni, F.; Percivalle, E.; Borgetto, S.; Paulet, A.; Comolli, G.; Sarasini, A.; Bergami, F.; Sammartino, J.C.; Ferrari, A.; et al. A Snapshot of the Immunogenicity, Efficacy and Safety of a Full Course of BNT162b2 Anti-SARS-CoV-2 Vaccine in Cancer Patients Treated with PD-1/PD-L1 Inhibitors: A Longitudinal Cohort Study. ESMO Open 2021, 6, 100272. [Google Scholar] [CrossRef]
- Lasagna, A.; Lilleri, D.; Agustoni, F.; Percivalle, E.; Borgetto, S.; Alessio, N.; Comolli, G.; Sarasini, A.; Bergami, F.; Sammartino, J.C.; et al. Analysis of the Humoral and Cellular Immune Response after a Full Course of BNT162b2 Anti-SARS-CoV-2 Vaccine in Cancer Patients Treated with PD-1/PD-L1 Inhibitors with or without Chemotherapy: An Update after 6 Months of Follow-Up. ESMO Open 2022, 7, 100359. [Google Scholar] [CrossRef]
- Wiseman, A.C. Immunosuppressive Medications. Clin. J. Am. Soc. Nephrol. 2016, 11, 332. [Google Scholar] [CrossRef]
- Zitvogel, L.; Apetoh, L.; Ghiringhelli, F.; Kroemer, G. Immunological Aspects of Cancer Chemotherapy. Nat. Rev. Immunol. 2008, 8, 59–73. [Google Scholar] [CrossRef]
- Ferrara, J.L.; Levine, J.E.; Reddy, P.; Holler, E. Graft-versus-Host Disease. Lancet 2009, 373, 1550. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T Cell Therapy: Current Limitations and Potential Strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Riddell, S.R. Engineering CAR-T Cells: Design Concepts. Trends Immunol. 2015, 36, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, A.; Gonzalez-Lugo, J.D.; Goradia, N.; Gali, R.; Shapiro, L.C.; Pradhan, K.; Rahman, S.; Kim, S.Y.; Ko, B.; Sica, R.A.; et al. Seroconversion Rates Following COVID-19 Vaccination among Patients with Cancer. Cancer Cell 2021, 39, 1081–1090.e2. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.C.; Thakkar, A.; Campbell, S.T.; Forest, S.K.; Pradhan, K.; Gonzalez-Lugo, J.D.; Quinn, R.; Bhagat, T.D.; Choudhary, G.S.; McCort, M.; et al. Efficacy of Booster Doses in Augmenting Waning Immune Responses to COVID-19 Vaccine in Patients with Cancer. Cancer Cell 2022, 40, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.; Verbruggen, L.; Teuwen, L.; Vanhoutte, G.; Vande Kerckhove, S.; Peeters, B.; Raats, S.; Van der Massen, I.; De Keersmaecker, S.; Debie, Y.; et al. Reduced Humoral Immune Response after BNT162b2 Coronavirus Disease 2019 Messenger RNA Vaccination in Cancer Patients under Antineoplastic Treatment. ESMO Open 2021, 6, 100274. [Google Scholar] [CrossRef] [PubMed]
- Nelli, F.; Fabbri, A.; Onorato, A.; Giannarelli, D.; Silvestri, M.A.; Pessina, G.; Giron Berrios, J.R.; Virtuoso, A.; Marrucci, E.; Schirripa, M.; et al. Six Month Immunogenicity of COVID-19 MRNA-BNT162b2 Vaccine in Actively Treated Cancer Patients: Updated Results of the Vax-On Study. Ann. Oncol. 2022, 33, 352–354. [Google Scholar] [CrossRef]
- Pimpinelli, F.; Marchesi, F.; Piaggio, G.; Giannarelli, D.; Papa, E.; Falcucci, P.; Pontone, M.; Di Martino, S.; Laquintana, V.; La Malfa, A.; et al. Fifth-Week Immunogenicity and Safety of Anti-SARS-CoV-2 BNT162b2 Vaccine in Patients with Multiple Myeloma and Myeloproliferative Malignancies on Active Treatment: Preliminary Data from a Single Institution. J. Hematol. Oncol. 2021, 14, 81. [Google Scholar] [CrossRef]
- Puhalla, S.; Bhattacharya, S.; Davidson, N.E. Hormonal Therapy in Breast Cancer: A Model Disease for the Personalization of Cancer Care. Mol. Oncol. 2012, 6, 222–236. [Google Scholar] [CrossRef]
- Cortés, A.; Casado, J.L.; Longo, F.; Serrano, J.J.; Saavedra, C.; Velasco, H.; Martin, A.; Chamorro, J.; Rosero, D.; Fernández, M.; et al. Limited T Cell Response to SARS-CoV-2 MRNA Vaccine among Patients with Cancer Receiving Different Cancer Treatments. Eur. J. Cancer 2022, 166, 229–239. [Google Scholar] [CrossRef]
- Khan, Q.J.; Bivona, C.R.; Martin, G.A.; Zhang, J.; Liu, B.; He, J.; Li, K.H.; Nelson, M.; Williamson, S.; Doolittle, G.C.; et al. Evaluation of the Durability of the Immune Humoral Response to COVID-19 Vaccines in Patients with Cancer Undergoing Treatment or Who Received a Stem Cell Transplant. JAMA Oncol. 2022, 66210, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Massarweh, A.; Eliakim-Raz, N.; Stemmer, A.; Levy-Barda, A.; Yust-Katz, S.; Zer, A.; Benouaich-Amiel, A.; Ben-Zvi, H.; Moskovits, N.; Brenner, B.; et al. Evaluation of Seropositivity Following BNT162b2 Messenger RNA Vaccination for SARS-CoV-2 in Patients Undergoing Treatment for Cancer. JAMA Oncol. 2021, 7, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Goshen-Lago, T.; Waldhorn, I.; Holland, R.; Szwarcwort-Cohen, M.; Reiner-Benaim, A.; Shachor-Meyouhas, Y.; Hussein, K.; Fahoum, L.; Baruch, M.; Peer, A.; et al. Serologic Status and Toxic Effects of the SARS-CoV-2 BNT162b2 Vaccine in Patients Undergoing Treatment for Cancer. JAMA Oncol. 2021, 7, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Joudi, M.; Moradi Binabaj, M.; Porouhan, P.; PeyroShabany, B.; Tabasi, M.; Fazilat-Panah, D.; Khajeh, M.; Mehrabian, A.; Dehghani, M.; Welsh, J.S.; et al. A Cohort Study on the Immunogenicity and Safety of the Inactivated SARS-CoV-2 Vaccine (BBIBP-CorV) in Patients with Breast Cancer; Does Trastuzumab Interfere with the Outcome? Front. Endocrinol. 2022, 13, 162. [Google Scholar] [CrossRef]
- Trontzas, I.P.; Vathiotis, I.; Economidou, C.; Petridou, I.; Gomatou, G.; Grammoustianou, M.; Tsamis, I.; Syrigos, N.; Anagnostakis, M.; Fyta, E.; et al. Assessment of Seroconversion after SARS-CoV-2 Vaccination in Patients with Lung Cancer. Vaccines 2022, 10, 618. [Google Scholar] [CrossRef]
- Di Noia, V.; Pimpinelli, F.; Renna, D.; Barberi, V.; Maccallini, M.T.; Gariazzo, L.; Pontone, M.; Monti, A.; Campo, F.; Taraborelli, E.; et al. Immunogenicity and Safety of COVID-19 Vaccine BNT162b2 for Patients with Solid Cancer: A Large Cohort Prospective Study from a Single Institution. Clin. Cancer Res. 2021, 27, 6815–6823. [Google Scholar] [CrossRef]
- Shulman, R.M.; Weinberg, D.S.; Ross, E.A.; Ruth, K.; Rall, G.F.; Olszanski, A.J.; Helstrom, J.; Hall, M.J.; Judd, J.; Chen, D.Y.T.; et al. Adverse Events Reported by Patients with Cancer after Administration of a 2-Dose MRNA COVID-19 Vaccine. J. Natl. Compr. Cancer Netw. 2022, 20, 160–166. [Google Scholar] [CrossRef]
- Kian, W.; Zemel, M.; Kestenbaum, E.H.; Rouvinov, K.; Alguayn, W.; Levitas, D.; Ievko, A.; Michlin, R.; Abod, M.A.; Massalha, I.; et al. Safety of the BNT162b2 MRNA COVID-19 Vaccine in Oncologic Patients Undergoing Numerous Cancer Treatment Options: A Retrospective Single-Center Study. Medicine 2022, 101, E28561. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Izumi, K.; Kadomoto, S.; Iwamoto, H.; Yaegashi, H.; Iijima, M.; Nohara, T.; Shigehara, K.; Kadono, Y.; Mizokami, A. Influence of the Coronavirus Disease 2019 Vaccine on Drug Therapy for Urological Cancer. Anticancer Res. 2022, 42, 2105–2111. [Google Scholar] [CrossRef]
- So, A.C.P.; McGrath, H.; Ting, J.; Srikandarajah, K.; Germanou, S.; Moss, C.; Russell, B.; Monroy-Iglesias, M.; Dolly, S.; Irshad, S.; et al. COVID-19 Vaccine Safety in Cancer Patients: A Single Centre Experience. Cancers 2021, 13, 3573. [Google Scholar] [CrossRef]
- Soyfer, V.; Gutfeld, O.; Shamai, S.; Schlocker, A.; Merimsky, O. COVID-19 Vaccine-Induced Radiation Recall Phenomenon. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 957. [Google Scholar] [CrossRef] [PubMed]
- Marples, R.; Douglas, C.; Xavier, J.; Collins, A.-J. Breast Radiation Recall Phenomenon after Astra-Zeneca COVID-19 Vaccine: A Case Series. Cureus 2022, 14, e21499. [Google Scholar] [CrossRef]
- Scoccianti, S.; Delli Paoli, C.; Grilli Leonulli, B.; Paoletti, L.; Alpi, P.; Caini, S.; Barca, R.; Fondelli, S.; Russo, S.; Perna, M.; et al. Acute Tolerance of Moderna MRNA-1273 Vaccine against COVID-19 in Patients with Cancer Treated with Radiotherapy. Lancet. Oncol. 2021, 22, 1212. [Google Scholar] [CrossRef]
- Waissengrin, B.; Agbarya, A.; Safadi, E.; Padova, H.; Wolf, I. Short-Term Safety of the BNT162b2 MRNA COVID-19 Vaccine in Patients with Cancer Treated with Immune Checkpoint Inhibitors. Lancet Oncol. 2021, 22, 581–583. [Google Scholar] [CrossRef]
- Strobel, S.B.; Machiraju, D.; Kälber, K.A.; Hassel, J.C. Immune-Related Adverse Events of COVID-19 Vaccination in Skin Cancer Patients Receiving Immune-Checkpoint Inhibitor Treatment. Cancer Immunol. Immunother. 2021, 71, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.; Hu, G.; Yang, Y.; Liu, B.; Yin, J.; Li, M.; Huang, Q.; Tang, X.; Böhner, A.; Bryant, A.; et al. Impact of COVID-19 Vaccination on the Use of PD-1 Inhibitor in Treating Patients with Cancer: A Real-World Study. J. Immunother. Cancer 2022, 10, e004157. [Google Scholar] [CrossRef]
- Au, L.; Fendler, A.; Shepherd, S.T.C.; Rzeniewicz, K.; Cerrone, M.; Byrne, F.; Carlyle, E.; Edmonds, K.; Del Rosario, L.; Shon, J.; et al. Cytokine Release Syndrome in a Patient with Colorectal Cancer after Vaccination with BNT162b2. Nat. Med. 2021, 27, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Bshesh, K.; Khan, W.; Vattoth, A.L.; Janjua, E.; Nauman, A.; Almasri, M.; Mohamed Ali, A.; Ramadorai, V.; Mushannen, B.; AlSubaie, M.; et al. Lymphadenopathy Post-COVID-19 Vaccination with Increased FDG Uptake May Be Falsely Attributed to Oncological Disorders: A Systematic Review. J. Med. Virol. 2022, 94, 1833. [Google Scholar] [CrossRef]
- Avner, M.; Orevi, M.; Caplan, N.; Popovtzer, A.; Lotem, M.; Cohen, J.E. COVID-19 Vaccine as a Cause for Unilateral Lymphadenopathy Detected by 18F-FDG PET/CT in a Patient Affected by Melanoma. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2659–2660. [Google Scholar] [CrossRef]
- Hiller, N.; Goldberg, S.N.; Cohen-Cymberknoh, M.; Vainstein, V.; Simanovsky, N. Lymphadenopathy Associated with the COVID-19 Vaccine. Cureus 2021, 13, e13524. [Google Scholar] [CrossRef]
- Bernstine, H.; Priss, M.; Anati, T.; Turko, O.; Gorenberg, M.; Steinmetz, A.P.; Groshar, D. Axillary Lymph Nodes Hypermetabolism after BNT162b2 MRNA COVID-19 Vaccination in Cancer Patients Undergoing 18F-FDG PET/CT: A Cohort Study. Clin. Nucl. Med. 2021, 46, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Krauthammer, S.H.; Wolf, I.; Even-Sapir, E. Hypermetabolic Lymphadenopathy Following Administration of BNT162b2 MRNA COVID-19 Vaccine: Incidence Assessed by [18F]FDG PET-CT and Relevance to Study Interpretation. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1854. [Google Scholar] [CrossRef] [PubMed]
- Eifer, M.; Tau, N.; Alhoubani, Y.; Kanana, N.; Domachevsky, L.; Shams, J.; Keret, N.; Gorfine, M.; Eshet, Y. COVID-19 MRNA Vaccination: Age and Immune Status and Its Association with Axillary Lymph Node PET/CT Uptake. J. Nucl. Med. 2022, 63, 134. [Google Scholar] [CrossRef] [PubMed]
- Placke, J.M.; Reis, H.; Hadaschik, E.; Roesch, A.; Schadendorf, D.; Stoffels, I.; Klode, J. Coronavirus Disease 2019 Vaccine Mimics Lymph Node Metastases in Patients Undergoing Skin Cancer Follow-up: A Monocentre Study. Eur. J. Cancer 2021, 154, 167. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Setola, S.V.; Galdiero, R.; Picone, C.; Izzo, F.; D’aniello, R.; Miele, V.; Grassi, R.; Grassi, R.; et al. Lymphadenopathy after BNT162b2 COVID-19 Vaccine: Preliminary Ultrasound Findings. Biology 2021, 10, 214. [Google Scholar] [CrossRef]
- Hagen, C.; Nowack, M.; Messerli, M.; Saro, F.; Mangold, F.; Bode, P.K. Fine Needle Aspiration in COVID-19 Vaccine-Associated Lymphadenopathy. Swiss Med. Wkly. 2021, 151, w20557. [Google Scholar] [CrossRef]
- Ko, G.; Hota, S.; Cil, T.D. COVID-19 Vaccination and Breast Cancer Surgery Timing. Breast Cancer Res. Treat. 2021, 188, 825–826. [Google Scholar] [CrossRef]
- Shirone, N.; Shinkai, T.; Yamane, T.; Uto, F.; Yoshimura, H.; Tamai, H.; Imai, T.; Inoue, M.; Kitano, S.; Kichikawa, K.; et al. Axillary Lymph Node Accumulation on FDG-PET/CT after Influenza Vaccination. Ann. Nucl. Med. 2012, 26, 248–252. [Google Scholar] [CrossRef]
- Thomassen, A.; Lerberg Nielsen, A.; Gerke, O.; Johansen, A.; Petersen, H. Duration of 18F-FDG Avidity in Lymph Nodes after Pandemic H1N1v and Seasonal Influenza Vaccination. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 894–898. [Google Scholar] [CrossRef]
- McIntosh, L.J.; Bankier, A.A.; Vijayaraghavan, G.R.; Licho, R.; Rosen, M.P. COVID-19 Vaccination-Related Uptake on FDG PET/CT: An Emerging Dilemma and Suggestions for Management. Am. J. Roentgenol. 2021, 217, 975–983. [Google Scholar] [CrossRef]
- Becker, A.S.; Perez-Johnston, R.; Chikarmane, S.A.; Chen, M.M.; El Homsi, M.; Feigin, K.N.; Gallagher, K.M.; Hanna, E.Y.; Hicks, M.; Ilica, A.T.; et al. Multidisciplinary Recommendations Regarding Post-Vaccine Adenopathy and Radiologic Imaging: Radiology Scientific Expert Panel. Radiology 2021, 300, E323–E327. [Google Scholar] [CrossRef] [PubMed]
- Lehman, C.D.; D’Alessandro, H.A.; Mendoza, D.P.; Succi, M.D.; Kambadakone, A.; Lamb, L.R. Unilateral Lymphadenopathy after COVID-19 Vaccination: A Practical Management Plan for Radiologists Across Specialties. J. Am. Coll. Radiol. 2021, 18, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.L.; Neelapu, S.S.; Xu, G.; Weaver, O. COVID-19 Vaccine-Related Axillary and Cervical Lymphadenopathy in Patients with Current or Prior Breast Cancer and Other Malignancies: Cross-Sectional Imaging Findings on MRI, CT, and PET-CT. Korean J. Radiol. 2021, 22, 1938–1945. [Google Scholar] [CrossRef] [PubMed]
- Locklin, J.N.; Woodard, G.A. Mammographic and Sonographic Findings in the Breast and Axillary Tail Following a COVID-19 Vaccine. Clin. Imaging 2021, 80, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Whitman, G.; Leung, J.; Sun, J.; Middleton, L.; Le Petross, H. Ultrasound Features to Differentiate COVID-19 Vaccine-Induced Benign Adenopathy from Breast Cancer Related Malignant Adenopathy. Acad. Radiol. 2022, 29, 1004–1012. [Google Scholar] [CrossRef]
- Adin, M.E.; Wu, J.; Isufi, E.; Tsui, E.; Pucar, D. Ipsilateral Malignant Axillary Lymphadenopathy and Contralateral Reactive Lymph Nodes in a COVID-19 Vaccine Recipient with Breast Cancer. J. Breast Cancer 2022, 25, 140. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Vallone, P.; Setola, S.V.; Picone, C.; Grassi, F.; Patrone, R.; Belli, A.; Izzo, F.; Petrillo, A. Not Only Lymphadenopathy: Case of Chest Lymphangitis Assessed with MRI after COVID 19 Vaccine. Infect. Agent. Cancer 2022, 17, 8. [Google Scholar] [CrossRef]
- Moujaess, E.; Zeid, N.B.; Samaha, R.; Sawan, J.; Kourie, H.; Labaki, C.; Chebel, R.; Chahine, G.; Karak, F.E.; Nasr, F.; et al. Perceptions of the COVID-19 Vaccine among Patients with Cancer: A Single-Institution Survey. Futur. Oncol. 2021, 17, 4071–4079. [Google Scholar] [CrossRef]
- Kufel-Grabowska, J.; Bartoszkiewicz, M.; Ramlau, R.; Litwiniuk, M. Cancer Patients and Internal Medicine Patients Attitude towards COVID-19 Vaccination in Poland. Adv. Clin. Exp. Med. 2021, 30, 805–811. [Google Scholar] [CrossRef]
- Villarreal-Garza, C.; Vaca-Cartagena, B.F.; Becerril-Gaitan, A.; Ferrigno, A.S.; Mesa-Chavez, F.; Platas, A.; Platas, A. Attitudes and Factors Associated with COVID-19 Vaccine Hesitancy among Patients with Breast Cancer. JAMA Oncol. 2021, 7, 1242. [Google Scholar] [CrossRef]
- Marijanović, I.; Kraljevic, M.; Buhovac, T.; Sokolovic, E. Acceptance of COVID-19 Vaccination and Its Associated Factors among Cancer Patients Attending the Oncology Clinic of University Clinical Hospital Mostar, Bosnia and Herzegovina: A Cross-Sectional Study. Med. Sci. Monit. 2021, 27, e932788-1. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.L.; Ho, Y.H.T.; Wong, C.K.H.; Choi, H.C.W.; Lam, K.O.; Yuen, K.K.; Kwong, D.; Hung, I. Acceptance of COVID-19 Vaccination in Cancer Patients in Hong Kong: Approaches to Improve the Vaccination Rate. Vaccines 2021, 9, 792. [Google Scholar] [CrossRef] [PubMed]
- Forster, M.; Wuerstlein, R.; Koenig, A.; Amann, N.; Beyer, S.; Kaltofen, T.; Degenhardt, T.; Burges, A.; Trillsch, F.; Mahner, S.; et al. COVID-19 Vaccination in Patients with Breast Cancer and Gynecological Malignancies: A German Perspective. Breast Off. J. Eur. Soc. Mastology 2021, 60, 214. [Google Scholar] [CrossRef] [PubMed]
- Tsai, R.; Hervey, J.; Hoffman, K.; Wood, J.; Johnson, J.; Deighton, D.; Clermont, D.; Loew, B.; Goldberg, S.L. COVID-19 Vaccine Hesitancy and Acceptance among Individuals with Cancer, Autoimmune Diseases, or Other Serious Comorbid Conditions: Cross-Sectional, Internet-Based Survey. JMIR Public Health Surveill. 2022, 8, e29872. [Google Scholar] [CrossRef]
- Khiari, H.; Cherif, I.; M’ghirbi, F.; Mezlini, A.; Hsairi, M. COVID-19 Vaccination Acceptance and Its Associated Factors among Cancer Patients in Tunisia. Asian Pac. J. Cancer Prev. 2021, 22, 3499. [Google Scholar] [CrossRef]
- Mejri, N.; Berrazega, Y.; Ouertani, E.; Rachdi, H.; Bohli, M.; Kochbati, L.; Boussen, H. Understanding COVID-19 Vaccine Hesitancy and Resistance: Another Challenge in Cancer Patients. Support. Care Cancer 2022, 30, 289. [Google Scholar] [CrossRef]
- Peng, X.; Gao, P.; Wang, Q.; Wu, H.G.; Yan, Y.L.; Xia, Y.; Wang, J.Y.; Lu, F.; Pan, H.; Yang, Y.; et al. Prevalence and Impact Factors of COVID-19 Vaccination Hesitancy among Breast Cancer Survivors: A Multicenter Cross-Sectional Study in China. Front. Med. 2021, 8, 741204. [Google Scholar] [CrossRef]
- Heyne, S.; Esser, P.; Werner, A.; Lehmann-Laue, A.; Mehnert-Theuerkauf, A. Attitudes toward a COVID-19 Vaccine and Vaccination Status in Cancer Patients: A Cross-Sectional Survey. J. Cancer Res. Clin. Oncol. 2022, 148, 1363. [Google Scholar] [CrossRef]
- Chun, J.Y.; Kim, S.I.; Park, E.Y.; Park, S.Y.; Koh, S.J.; Cha, Y.; Yoo, H.J.; Joung, J.Y.; Yoon, H.M.; Eom, B.W.; et al. Cancer Patients’ Willingness to Take COVID-19 Vaccination: A Nationwide Multicenter Survey in Korea. Cancers 2021, 13, 3883. [Google Scholar] [CrossRef]
- Erdem, D.; Karaman, I. Impact of Corona-Phobia on Attitudes and Acceptance towards COVID-19 Vaccine among Cancer Patients: A Single-Center Study. Futur. Oncol. 2021, 18, 457–469. [Google Scholar] [CrossRef]
- Home—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/home (accessed on 18 October 2022).
- Shroff, R.T.; Chalasani, P.; Wei, R.; Pennington, D.; Quirk, G.; Schoenle, M.V.; Peyton, K.L.; Uhrlaub, J.L.; Ripperger, T.J.; Jergović, M.; et al. Immune Responses to Two and Three Doses of the BNT162b2 MRNA Vaccine in Adults with Solid Tumors. Nat. Med. 2021, 27, 2002–2011. [Google Scholar] [CrossRef] [PubMed]
- Herishanu, Y.; Avivi, I.; Aharon, A.; Shefer, G.; Levi, S.; Bronstein, Y.; Morales, M.; Ziv, T.; Shorer Arbel, Y.; Scarfò, L.; et al. Efficacy of the BNT162b2 MRNA COVID-19 Vaccine in Patients with Chronic Lymphocytic Leukemia. Blood 2021, 137, 3165. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, S.L.; Yasawardene, P.; Wijerathne, W.; Somawardana, B. COVID-19 Vaccination in Cancer Patients: A Narrative Review. J. Int. Med. Res. 2022, 50, 3000605221086155. [Google Scholar] [CrossRef]
- He, Y.; Ding, Y.; Cao, B.; Huang, Y.; Wang, X. COVID-19 Vaccine Development from the Perspective of Cancer Patients. Hum. Vaccin. Immunother. 2021, 17, 3281–3287. [Google Scholar] [CrossRef]
- Hwang, J.K.; Zhang, T.; Wang, A.Z.; Li, Z. COVID-19 Vaccines for Patients with Cancer: Benefits Likely Outweigh Risks. J. Hematol. Oncol. 2021, 14, 38. [Google Scholar] [CrossRef]
- Yekedüz, E.; Ayasun, R.; Köksoy, E.B.; Utkan, G.; Ürün, Y.; Akbulut, H. MRNA-Based COVID-19 Vaccines Appear Not to Increase Immune Events in Cancer Patients Receiving Immune Checkpoint Inhibitors. Future Virol. 2021, 16, 583–585. [Google Scholar] [CrossRef]
- Malek, A.E.; Cornejo, P.P.; Daoud, N.; Alam, M. The MRNA COVID-19 Vaccine in Patients with Cancer Receiving Checkpoint Inhibitor Therapy: What We Know and What We Don’t. Immunotherapy 2021, 14, 91–94. [Google Scholar] [CrossRef]
- Esechie, A.; Fang, X.; Banerjee, P.; Rai, P.; Thottempudi, N. A Case Report of Longitudinal Extensive Transverse Myelitis: Immunotherapy Related Adverse Effect vs. COVID-19 Related Immunization Complications. Int. J. Neurosci. 2022; online ahead of print. [Google Scholar] [CrossRef]



| Study Title & ClinicalTrials.gov Identifier | Country | Study Phase | Primary Outcome Measures | Study Participants & Inclusion Criteria | Intervention & Model | Results |
|---|---|---|---|---|---|---|
| Evaluation of the Effect and Side Effect Profile of COVID-19 Vaccine in Cancer Patients Identifier: NCT04771559 [1,101] | Turkey | Complete | COVID-19 antibody titers. Time frame: 1 month | N = 1500 Patient group: Individuals aged 18+ diagnosed with cancer, who had received two doses of the COVID-19 vaccine Control group: Individuals aged 18+ with no history of cancer, who had received two doses of the COVID-19 vaccine | Intervention: COVID-19 antibody test. Non-randomized, parallel assignment | Seropositivity rates at one month: Patient group: 85.2% Control group: 97.5% (p < 0.001) Lower seropositivity in cancer patients associated with chemotherapy and age 60+ (p < 0.001) |
| Immune Response to the COVID-19 Vaccine Identifier: NCT04936997 [101,102] | USA | Early Phase 1 | Immune response to 2nd COVID-19 vaccination booster (3rd vaccine) in patients with solid malignancies on immunosuppressive therapy Time frame: 3 months | N = 20 Patient group: Individuals aged 18+ with active solid tumor malignancy on active chemotherapy, who had received two doses of the Pfizer COVID-19 vaccine | Intervention: SARS-COV2 Pfizer Vaccine Single group assignment | 80% (16/20) of participants demonstrated a median threefold increase in antibody response one week following a third dose of the Pfizer vaccine. No improvement was noted in T-cell responses. Adverse events were mild in nature. |
| Impact of the Immune System on Response to Anti-Coronavirus Disease 19 (COVID-19) Vaccine in Allogeneic Stem Cell Recipients (Covid Vaccin Allo) Identifier: NCT04951323 [101] | Belgium | Phase 3 | Quantification of anti-SARS-CoV-2 IgG antibodies after vaccination in allogenic stem cell recipients Time frame: 49 days following first injection | Estimated N = 50 Patient group: Individuals aged 18+ who had undergone allogeneic hematopoietic stem cell transplantation 3 months to 5 months prior. Patients were excluded if they had active malignant disease at the time of inclusion | Intervention: Anti-COVID19 mRNA-based vaccine (BNT162b2, Comirnaty®, commercialized by Pfizer) Single group assignment | N/A |
| Safety and Immunogenicity of COVID-19 Vaccination in Patients With Cancer Identifier: NCT05018078 [101] | China | N/A | Primary Outcome 1: Safety of the COVID-19 vaccine, monitoring the occurrence of adverse effects Time frame: Within 2 months following the first vaccine dose Primary Outcome 2: Immunogenicity of the COVID-19 vaccine, measuring antibody titers against SARS-CoV-2 Time frame: Within 2 months following the first vaccine dose | Estimated N = 300 Patient group: Individuals aged 18+, with a cancer diagnosis including hepatocellular carcinoma, breast cancer, lung cancer, esophageal cancer, gastric cancer or colorectal cancer. Individuals must have local or systemic anti-cancer therapies according to the treatment guidelines previously or currently, in stable condition with an Eastern Cooperative Oncology Group (ECOG) score below 2. Additionally, patients must have normal or basically normal multi-organ function, without contraindications to vaccination | Intervention: Coronavirus vaccine Single group assignment | N/A |
| A Trial of the Safety and Immunogenicity of the COVID-19 Vaccine (mRNA-1273) in Participants With Hematologic Malignancies and Various Regimens of Immunosuppression, and in Participants With Solid Tumors on PD1/PDL1 Inhibitor Therapy, Including Booster Doses of Vaccine Identifier: NCT04847050 [101] | USA | Phase 2 | Primary Outcome 1: Safety and reactogenicity of the mRNA-1273 vaccine, soliciting local and systemic adverse reactions 7 days after each injection, and unsolicited adverse events up to 28 days post-injection Time frame: 14 months Primary Outcome 2: Immunogenicity of the mRNA-1273 vaccine in patients with a hematological malignancy and are immunosuppressed due to their disease, and/or receiving PD-1/PDL-1 inhibitor for treatment of a solid tumor. Measured titers of specific binding antibody (bAb) on day 1, 29, 36, 57, 209, and 394 Time frame: 14 months | Estimated N = 220 Patient group: Individuals aged 18+ with either:
Individuals must demonstrate adequate organ and bone marrow function on laboratory assessment within 4 weeks of vaccine administration | Intervention: mRNA-1273 injection. Non-randomized, parallel assignment | N/A |
| The Immune Reaction Upon COVID-19 Vaccination in the Belgian Cancer Population. Identifier: NCT05033158 [101] | Belgium | N/A | Immune response measuring quantification of anti-SARS-CoV-2 IgG antibodies (against full Spike, S1, S2, RBD, and N proteins) 4 weeks after first vaccine administration Time frame: 4 months | Estimated N = 3000 Patient group: Individuals aged 18+ with oncological or hematological malignancy, or a history of it, with a life expectancy >3 months | Intervention: Blood sampling Single group assignment | N/A |
| SARS-CoV-2 Vaccine (COH04S1) Versus Emergency Use Authorization SARS-COV-2 Vaccine for the Treatment of COVID-19 in Patients With Blood Cancer Identifier: NCT04977024 [101] | USA | Phase 2 | Biological response, based on at least a 3-fold increase in anti-SARS-CoV-2 antibodies or interferon gamma levels Time frame: At 28 days post the second vaccine injection | Estimated N = 240 Patient group: Individuals aged 18+ with hematologic malignancy and an ECOG score of 2 or less. They must have received either allogenic or autologous hematopoietic cell transplant, or cellular therapy (chimeric antigen receptor [CAR] T-cell) therapy and be at least 3 months post treatment infusion | Interventions: COVID-19 Vaccine, Diagnostic Laboratory Biomarker Analysis, and Synthetic MVA-based SARS-CoV-2 Vaccine COH04S1 Randomized. parallel assignment | N/A |
| Safety and Immunogenicity of Prime-boost Vaccination of SARS-CoV-2 in Patients With Cancer Identifier: NCT05273541 [101] | China | Phase 1 Phase 2 | Primary Outcome 1: Determining the safety of the prime-boost vaccine, measuring the occurrence of adverse effects post-vaccination Time frame: Within 1 week after the prime-boost vaccination Primary Outcome 2: Determining immunogenicity by titers of anti-SARS-CoV-2 antibodies Time frame: Within 3 months after the prime-boost vaccination | Estimated N = 100 Patient group: Individuals aged 18+, with a cancer diagnosis including hepatocellular carcinoma, breast cancer, lung cancer, esophageal cancer, gastric cancer or colorectal cancer. Individuals must have local or systemic anti-cancer therapies according to the treatment guidelines previously or currently, in stable condition with an ECOG score below 2. Additionally, patients must have normal or basically normal multi-organ function, without contraindications to vaccination. | Intervention: Coronavirus vaccination Single group assignment | N/A |
| Study Evaluating SARS-CoV-2 (COVID-19) Humoral Response After BNT162b2 Vaccine in Immunocompromised Adults Compared to Healthy Adults Identifier: NCT04952766 [101] | France | Phase 4 | Protective humoral response post-vaccination, measuring the proportion of immunocompromised individuals with neutralizing activity against the “Wuhan” stain of SARS-CoV-2, as compared to healthy subjects Time frame: 2 months | N = 196 Adult volunteers belonging to one of the following groups: Immunocompromised group (~15 participants per subgroup):
Non-immunocompromised group: vaccinated with either Comirnaty TM or AstraZeneca’s Vaxzevria TM for the first dose | Intervention: Biological samples Single group assignment | N/A |
| COVID-19 VAX Booster Dosing in Patients With Hematologic Malignancies Identifier: NCT05028374 [101] | USA | Phase 2 | Seroconversion rates of anti-SARS-CoV-2 antibody following a booster dose of the Moderna mRNA COVID-19 vaccine Time frame: 28 (±3 days) following booster dose | N = 119 Patient group: Individuals aged 18+ who have been previously diagnosed with multiple myeloma (MM)/amyloid light-chain amyloidosis, or other hematologic malignancy. They must have previously received any one of the available COVID-19 vaccines between 4–36 months prior to study enrollment, with anti-SARS-CoV-2 IgG titers less than 1.0 unit, or between 1.0–1.99 units. If patients are currently receiving potentially immunosuppressive cancer therapy, a two-week interruption before and after the booster dose of the vaccine is encouraged, but not required (at physician discretion) | Intervention: A single “booster” dose of the Moderna mRNA COVID-19 vaccine Single group assignment | N/A |
| Booster Dose Trial Identifier: NCT05016622 [101] | USA | Phase 2 | Rates of seroconversion for SARS-CoV-2 anti-spike antibody Time frame: 4 weeks after booster dose | Estimated N = 100 Patient group: Individuals aged 18+ with a known diagnosis of any malignancy (either active or post completion of therapy), with negative SARS-CoV-2 spike IgG at least 14 days post-2nd dose of an mRNA-based COVID-19 vaccine, or 28 days after a single dose of the adenovirus-based Johnson & Johnson vaccine | Intervention: BNT162b2 vaccine Single group assignment | N/A |
| Passive Antibodies Against COVID-19 With EVUSHELD in Vaccine Non-responsive CLL Identifier: NCT05465876 [101] | Canada | Phase 2 | Conferring passive immunity to CLL patients, measuring the proportion of participants with anti-spike antibodies after EVUSHELD administration Time frame: 12 months | Estimated N = 200 Patient group: Individuals aged 18+ with a diagnosis of CLL, who are either treatment-naïve, post-treatment, or on-treatment for CLL, and an ECOG score between 0–2. They must have received at least two doses of the Pfizer, Moderna, or AstraZeneca COVID-19 vaccines between 28 days-18 months prior to enrollment, demonstrating absent or suboptimal response. Participants must weigh at least 40 kg, have adequate organ function laboratory values, and have a life expectancy >6 months | Intervention: EVUSHELD Single group assignment | N/A |
| Bringing Optimised COVID-19 Vaccine Schedules To ImmunoCompromised Populations (BOOST-IC): an Adaptive Randomised Controlled Clinical Trial Identifier: NCT05556720 [101] | Australia | Phase 3 | Measuring the geometric mean concentration (GMC) of anti-spike SARS-CoV-2 IgG antibody Time frame: 28 days after completion of vaccination trials | Estimated N = 960 Patient group: Individuals aged 16+ who have completed 3–5 doses of an Australian Therapeutic Goods Administration approved COVID-19 vaccine (Pfizer, Moderna, AstaZeneca, or Novavax). Patients must be in one of the following populations:
| Interventions: BNT162b2, mRNA-1273, or NVX-COV2373 Randomized, parallel assignment | N/A |
| Anti-COVID-19 Vaccine in Children With Acute Leukemia and Their Siblings Identifier: NCT04969601 [101] | France | Phase 1 Phase 2 | Primary Objective 1: Dose limiting toxicity, determined by the presence of grade ≥3 adverse events within 7 days following vaccine injection, that are deemed to be related to the vaccine Time frame: Within 7 days from first dose Primary Objective 2: Four-times or higher increase in the anti-spike IgG titer, AND positive anti-spike neutralizing test, indicating significant seroconversion Time frame: At 2 months from first dose | Estimated N = 150 Patient group: Individuals aged 1–15 years, with either:
Control group: Healthy siblings aged 1–15 years, living in the same household as the child with ALL/AML more than 50% of the time | Intervention: Vaccine COMIRNATY® (BNT162b2) Single group assignment | N/A |
| Safety, Efficacy of BNT162b2 mRNA Vaccine in CLL Identifier: NCT04862806 [101,103] | Israel | Complete | Primary Objective 1: Change in the number of participants with adverse events related to the BNT162b2 mRNA vaccine, assessed by a questionnaire with answers reported on a scale of 0–5 Time frame: 2–6 weeks after 2nd vaccination, 3 months after 2nd vaccination, 6 months after 2nd vaccination Primary Objective 2: Antibody persistence following the 3rd dose of the BNT162b2 mRNA vaccine in seronegative patients with chronic lymphocytic leukemia Time frame: 6 months | Estimated N = 1000 Patient group: Individuals aged 18+ with a diagnosis of CLL, who have received two 30-μg doses of BNT162b2 3 weeks apart | Intervention: COVID-19 serology Single group assignment | Of patients with CLL who failed to demonstrate a seropositive response following two doses of the BNT162b2 vaccine, nearly one fourth responded to a third dose of the vaccine. However, antibody responses were lower in patients undergoing active treatment, and patients with recent exposure (<12 months prior) to anti-CD20 therapy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almasri, M.; Bshesh, K.; Khan, W.; Mushannen, M.; Salameh, M.A.; Shafiq, A.; Vattoth, A.L.; Elkassas, N.; Zakaria, D. Cancer Patients and the COVID-19 Vaccines: Considerations and Challenges. Cancers 2022, 14, 5630. https://doi.org/10.3390/cancers14225630
Almasri M, Bshesh K, Khan W, Mushannen M, Salameh MA, Shafiq A, Vattoth AL, Elkassas N, Zakaria D. Cancer Patients and the COVID-19 Vaccines: Considerations and Challenges. Cancers. 2022; 14(22):5630. https://doi.org/10.3390/cancers14225630
Chicago/Turabian StyleAlmasri, Muna, Khalifa Bshesh, Wafa Khan, Malik Mushannen, Mohammad A. Salameh, Ameena Shafiq, Ahamed Lazim Vattoth, Nadine Elkassas, and Dalia Zakaria. 2022. "Cancer Patients and the COVID-19 Vaccines: Considerations and Challenges" Cancers 14, no. 22: 5630. https://doi.org/10.3390/cancers14225630
APA StyleAlmasri, M., Bshesh, K., Khan, W., Mushannen, M., Salameh, M. A., Shafiq, A., Vattoth, A. L., Elkassas, N., & Zakaria, D. (2022). Cancer Patients and the COVID-19 Vaccines: Considerations and Challenges. Cancers, 14(22), 5630. https://doi.org/10.3390/cancers14225630