Antitumor Activity of Simvastatin in Preclinical Models of Mantle Cell Lymphoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Simvastatin Activation
2.2. Cell Culture
2.3. Cytotoxicity Assay
2.4. Proliferation Rate Assays
2.5. Apoptosis Rate and Mitochondrial Transmembrane Potential (ΔΨm)
2.6. Reactive Oxygen Species Quantification
2.7. Cell Migration and Invasion Assays
2.8. Western Blot Analysis
2.9. Chick Chorioallantoic Membrane (CAM) Assay
2.10. Quantitative Real-Time PCR
2.11. Statistical Analysis
3. Results and Discussion
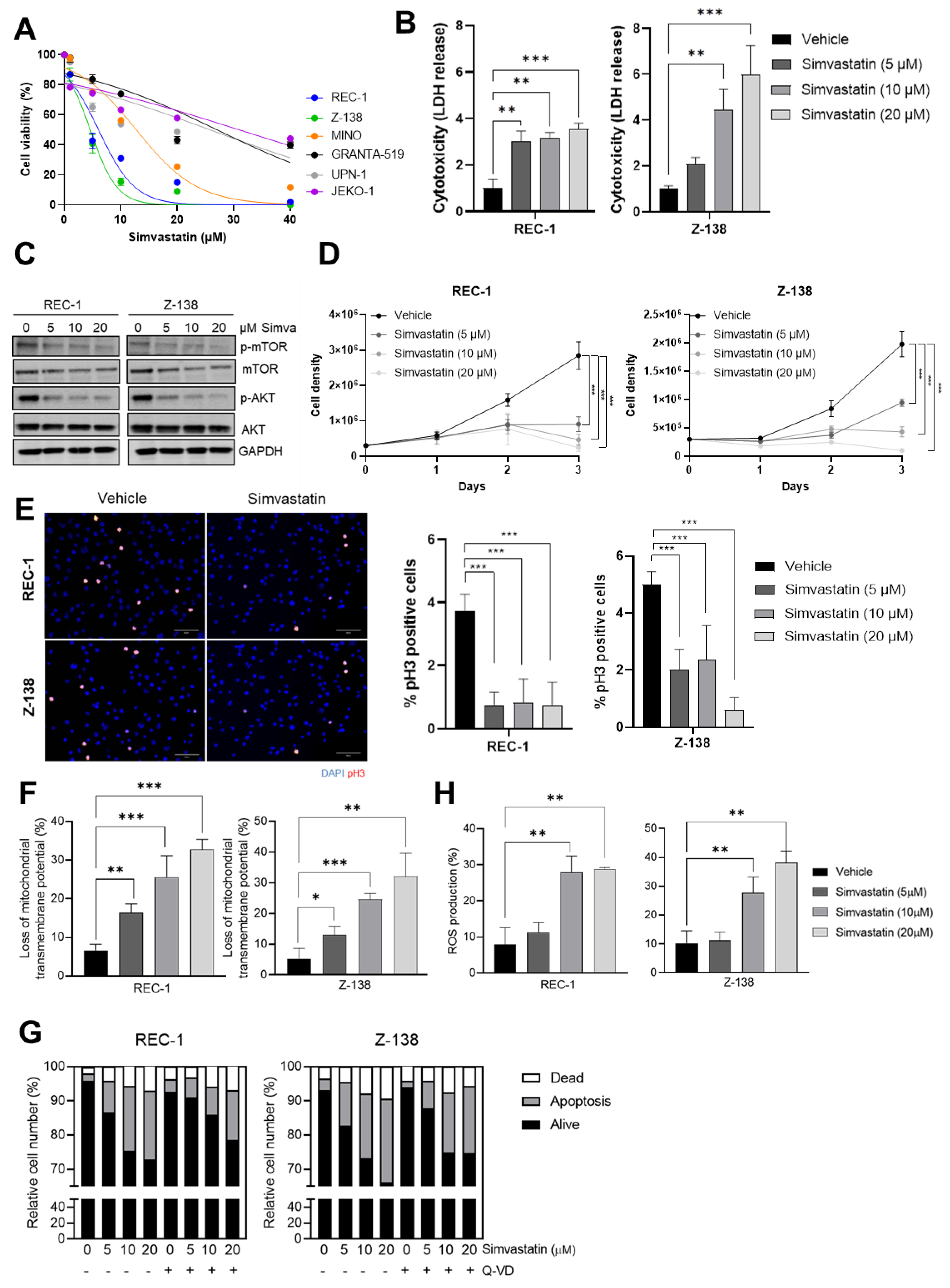
3.1. Simvastatin Impairs the Proliferation and Triggers a Caspase-Independent ROS-Mediated Cell Death of MCL Cells
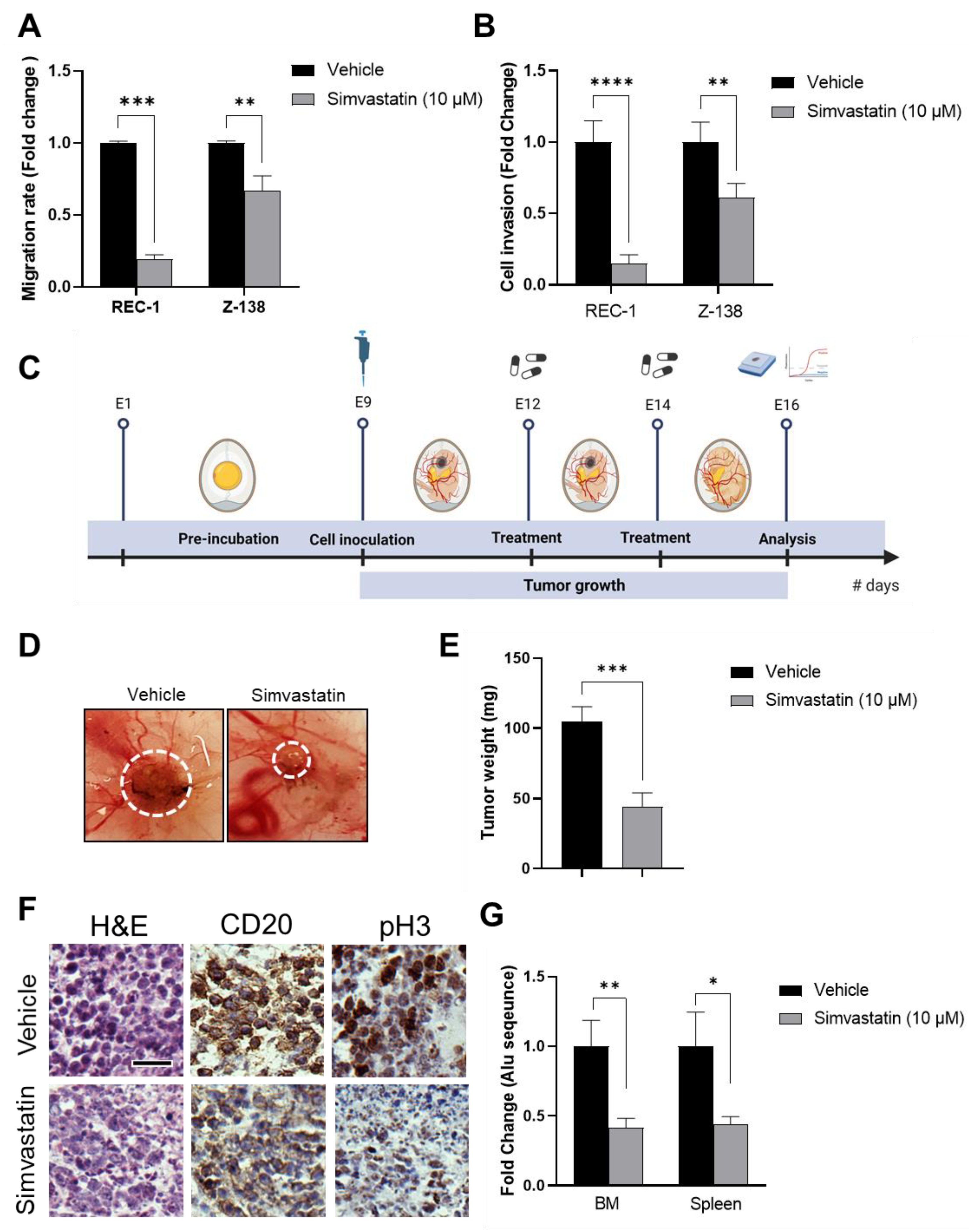
3.2. Simvastatin Inhibits MCL Migration, Invasion and In Vivo Tumorigenic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Lee Harris, N.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Rule, S. Mantle cell lymphoma: Evolving management strategies. Blood 2015, 125, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Rosenwald, A.; Wright, G.; Wiestner, A.; Chan, W.C.; Connors, J.M.; Campo, E.; Gascoyne, R.D.; Grogan, T.M.; Muller-Hermelink, H.K.; Smeland, E.B.; et al. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer Cell 2003, 3, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Kuroda, M.; Tanzawa, K. Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme a reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett. 1976, 72, 323–326. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. A century of cholesterol and coronaries: From plaques to genes to statins. Cell 2015, 161, 161–172. [Google Scholar] [CrossRef]
- Dimitroulakos, J.; Nohynek, D.; Backway, K.L.; Medley, D.W.; Yeger, H.; Freedman, M.H.; Minden, M.D.; Penn, L.Z. Increased sensitivity of acute myeloid leukemias to lovastatin-induced apoptosis: A potential therapeutic approach. Blood 1999, 93, 1308–1318. [Google Scholar] [CrossRef]
- Von Tresckow, B.; Von Strandmann, E.P.; Sasse, S.; Tawadros, S.; Engert, A.; Hansen, H.P. Simvastatin-dependent apoptosis in Hodgkin’s lymphoma cells and growth impairment of human Hodgkin’s tumors in vivo. Haematologica 2007, 92, 682–685. [Google Scholar] [CrossRef][Green Version]
- Qi, X.F.; Zheng, L.; Lee, K.J.; Kim, D.H.; Kim, C.S.; Cai, D.Q.; Wu, Z.; Qin, J.W.; Yu, Y.H.; Kim, S.K. HMG-CoA reductase inhibitors induce apoptosis of lymphoma cells by promoting ROS generation and regulating Akt, Erk and p38 signals via suppression of mevalonate pathway. Cell Death Dis. 2013, 4, e518. [Google Scholar] [CrossRef]
- Ukomadu, C.; Dutta, A. p21-dependent Inhibition of Colon Cancer Cell Growth by Mevastatin Is Independent of Inhibition of G1 Cyclin-dependent Kinases. J. Biol. Chem. 2003, 278, 43586–43594. [Google Scholar] [CrossRef]
- Jiang, Z.; Zheng, X.; Lytle, R.A.; Higashikubo, R.; Rich, K.M. Lovastatin-induced up-regulation of the BH3-only protein, Bim, and cell death in glioblastoma cells. J. Neurochem. 2004, 89, 168–178. [Google Scholar] [CrossRef]
- Campbell, M.J.; Esserman, L.J.; Zhou, Y.; Shoemaker, M.; Lobo, M.; Borman, E.; Baehner, F.; Kumar, A.S.; Adduci, K.; Marx, C.; et al. Breast cancer growth prevention by statins. Cancer Res. 2006, 66, 8707–8714. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.E.; Na, K.S.; Park, D.S.; Choi, K.H.; Kim, B.R.; Shim, H.; Jeong, E.T.; Kim, H.R. Apoptotic induction by simvastatin in human lung cancer A549 cells via Akt signaling dependent down-regulation of survivin. Investig. New Drugs 2011, 29, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Roberts, A.; Juarez, D.; Vo, T.T.T.; Bhatt, S.; Herzog, L.O.; Mallya, S.; Bellin, R.J.; Agarwal, S.K.; Salem, A.H.; et al. Statins enhance efficacy of venetoclax in blood cancers. Sci. Transl. Med. 2018, 10, eaaq1240. [Google Scholar] [CrossRef] [PubMed]
- Hoque, A.; Chen, H.; Xu, X.C. Statin induces apoptosis and cell growth arrest in prostate cancer cells. Cancer Epidemiol. Biomark. Prev. 2008, 17, 88–94. [Google Scholar] [CrossRef]
- Yi, X.; Jia, W.; Jin, Y.; Zhen, S. Statin use is associated with reduced risk of haematological malignancies: Evidence from a meta-analysis. PLoS ONE 2014, 9, e87019. [Google Scholar] [CrossRef]
- Sanfilippo, K.M.; Keller, J.; Gage, B.F.; Luo, S.; Wang, T.F.; Moskowitz, G.; Gumbel, J.; Blue, B.; O’Brian, K.; Carson, K.R. Statins are associated with reduced mortality in multiple myeloma. J. Clin. Oncol. 2016, 34, 4008. [Google Scholar] [CrossRef]
- Demierre, M.F.; Higgins, P.D.R.; Gruber, S.B.; Hawk, E.; Lippman, S.M. Statins and cancer prevention. Nat. Rev. Cancer 2005, 5. [Google Scholar] [CrossRef]
- Ye, X.; Mneina, A.; Johnston, J.B.; Mahmud, S.M. Associations between statin use and non-Hodgkin lymphoma (NHL) risk and survival: A meta-analysis. Hematol. Oncol. 2017, 35, 206–214. [Google Scholar] [CrossRef]
- Van De Donk, N.W.C.J.; Schotte, D.; Kamphuis, M.M.J.; Van Marion, A.M.W.; Van Kessel, B.; Bloem, A.C.; Lokhorst, H.M. Protein Geranylgeranylation Is Critical for the Regulation of Survival and Proliferation of Lymphoma Tumor Cells. Clin. Cancer Res. 2003, 9, 5735–5748. [Google Scholar]
- Osaki, M.; Oshimura, M.; Ito, H. PI3K-Akt pathway: Its functions and alterations in human cancer. Apoptosis 2004, 9, 667–676. [Google Scholar] [CrossRef]
- Zhuang, L.; Kim, J.; Adam, R.M.; Solomon, K.R.; Freeman, M.R. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J. Clin. Investig. 2005, 115, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Hassanabad, A.F. Current perspectives on statins as potential anti-cancer therapeutics: Clinical outcomes and underlying molecular mechanisms. Transl. Lung Cancer Res. 2019, 8, 692. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, Y.; Sekine, Y.; Kato, H.; Furuya, Y.; Koike, H.; Suzuki, K. Simvastatin Up-Regulates Annexin A10 That Can Inhibit the Proliferation, Migration, and Invasion in Androgen-Independent Human Prostate Cancer Cells. Prostate 2017, 77, 337–349. [Google Scholar] [CrossRef]
- Sánchez, C.A.; Rodríguez, E.; Varela, E.; Zapata, E.; Páez, A.; Massó, F.A.; Montaño, L.F.; López-Marure, R. Statin-induced inhibition of MCF-7 breast cancer cell proliferation is related to cell cycle arrest and apoptotic and necrotic cell death mediated by an enhanced oxidative stress. Cancer Investig. 2008, 26, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Gajski, G.; Garaj-Vrhovac, V.; Oreščanin, V. Cytogenetic status and oxidative DNA-damage induced by atorvastatin in human peripheral blood lymphocytes: Standard and Fpg-modified comet assay. Toxicol. Appl. Pharmacol. 2008, 231, 85–93. [Google Scholar] [CrossRef]
- Farina, H.G.; Bublik, D.R.; Alonso, D.F.; Gomez, D.E. Lovastatin alters cytoskeleton organization and inhibits experimental metastasis of mammary carcinoma cells. Clin. Exp. Metastasis 2002, 19, 551–560. [Google Scholar] [CrossRef]
- Kusama, T.; Mukai, M.; Iwasaki, T.; Tatsuta, M.; Matsumoto, Y.; Akedo, H.; Nakamura, H. Inhibition of epidermal growth factor-induced RhoA translocation and invasion of human pancreatic cancer cells by 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors. Cancer Res. 2001, 61, 4885–4891. [Google Scholar]
- Gliemroth, J.; Zulewski, H.; Arnold, H.; Terzis, A.J.A. Migration, proliferation, and invasion of human glioma cells following treatment with simvastatin. Neurosurg. Rev. 2003, 26, 117–124. [Google Scholar] [CrossRef]
- Collisson, E.A.; Kleer, C.; Wu, M.; De, A.; Gambhir, S.S.; Merajver, S.D.; Kolodney, M.S. Atorvastatin prevents RhoC isoprenylation, invasion, and metastasis in human melanoma cells. Mol. Cancer Ther. 2003, 2, 941–948. [Google Scholar]
- Horiguchi, A.; Sumitomo, M.; Asakuma, J.; Asano, T.; Asano, T.; Hayakawa, M. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor, fluvastatin, as a novel agent for prophylaxis of renal cancer metastasis. Clin. Cancer Res. 2004, 10, 8648–8655. [Google Scholar] [CrossRef]
- Kochuparambil, S.T.; Al-Husein, B.; Goc, A.; Soliman, S.; Somanath, P.R. Anticancer efficacy of simvastatin on prostate cancer cells and tumor xenografts is associated with inhibition of Akt and reduced prostate-specific antigen expression. J. Pharmacol. Exp. Ther. 2011, 336, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, W.; Ning, J.; Wang, J.; Lang, Y.; Jin, X.; Zhu, K.; Wang, X.; Li, X.; Yang, F.; et al. Simvastatin suppresses proliferation and migration in non-small cell lung cancer via pyroptosis. Int. J. Biol. Sci. 2018, 14, 406. [Google Scholar] [CrossRef] [PubMed]
- Gimenez, N.; Tripathi, R.; Giró, A.; Rosich, L.; López-Guerra, M.; López-Oreja, I.; Playa-Albinyana, H.; Arenas, F.; Mas, J.M.; Pérez-Galán, P.; et al. Systems biology drug screening identifies statins as enhancers of current therapies in chronic lymphocytic leukemia. Sci. Rep. 2020, 10, 22153. [Google Scholar] [CrossRef]
- Wu, H.; Jiang, H.; Lu, D.; Xiong, Y.; Qu, C.; Zhou, D.; Mahmood, A.; Chopp, M. Effect of simvastatin on glioma cell proliferation, migration, and apoptosis. Neurosurgery 2009, 65, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.H.; Kim, S.K.; Kim, H.J.; Chang, J.; Ahn, C.M.; Chang, Y.S. Lipid raft modulation inhibits NSCLC cell migration through delocalization of the focal adhesion complex. Lung Cancer 2010, 69, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Rous, P. Tumor implantations in the developing embryo. J. Am. Med. Assoc. 1911, 56, 741–742. [Google Scholar] [CrossRef]
- Achkar, I.W.; Kader, S.; Dib, S.S.; Junejo, K.; Al-Bader, S.B.; Hayat, S.; Bhagwat, A.M.; Rousset, X.; Wang, Y.; Viallet, J.; et al. Metabolic signatures of tumor responses to doxorubicin elucidated by metabolic profiling in ovo. Metabolites 2020, 10, 268. [Google Scholar] [CrossRef]
- El Hasasna, H.; Saleh, A.; Samri, H.A.; Athamneh, K.; Attoub, S.; Arafat, K.; Benhalilou, N.; Alyan, S.; Viallet, J.; Dhaheri, Y.A.; et al. Rhus coriaria suppresses angiogenesis, metastasis and tumor growth of breast cancer through inhibition of STAT3, NFκB and nitric oxide pathways. Sci. Rep. 2016, 6, 21144. [Google Scholar] [CrossRef]
- Garcia, P.; Wang, Y.; Viallet, J.; Macek Jilkova, Z. The Chicken Embryo Model: A Novel and Relevant Model for Immune-Based Studies. Front. Immunol. 2021, 12, 791081. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.C.; Profitós-Pelejà, N.; Ribeiro, M.L.; Roué, G. Antitumor Activity of Simvastatin in Preclinical Models of Mantle Cell Lymphoma. Cancers 2022, 14, 5601. https://doi.org/10.3390/cancers14225601
Santos JC, Profitós-Pelejà N, Ribeiro ML, Roué G. Antitumor Activity of Simvastatin in Preclinical Models of Mantle Cell Lymphoma. Cancers. 2022; 14(22):5601. https://doi.org/10.3390/cancers14225601
Chicago/Turabian StyleSantos, Juliana Carvalho, Núria Profitós-Pelejà, Marcelo Lima Ribeiro, and Gaël Roué. 2022. "Antitumor Activity of Simvastatin in Preclinical Models of Mantle Cell Lymphoma" Cancers 14, no. 22: 5601. https://doi.org/10.3390/cancers14225601
APA StyleSantos, J. C., Profitós-Pelejà, N., Ribeiro, M. L., & Roué, G. (2022). Antitumor Activity of Simvastatin in Preclinical Models of Mantle Cell Lymphoma. Cancers, 14(22), 5601. https://doi.org/10.3390/cancers14225601






