Reproductive and Endocrine Outcomes in a Cohort of Danish Women following Auto-Transplantation of Frozen/Thawed Ovarian Tissue from a Single Center
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Ethics
3. Results
3.1. Pregnancy and Live Birth Rates following OTT
3.2. Fertility Treatment
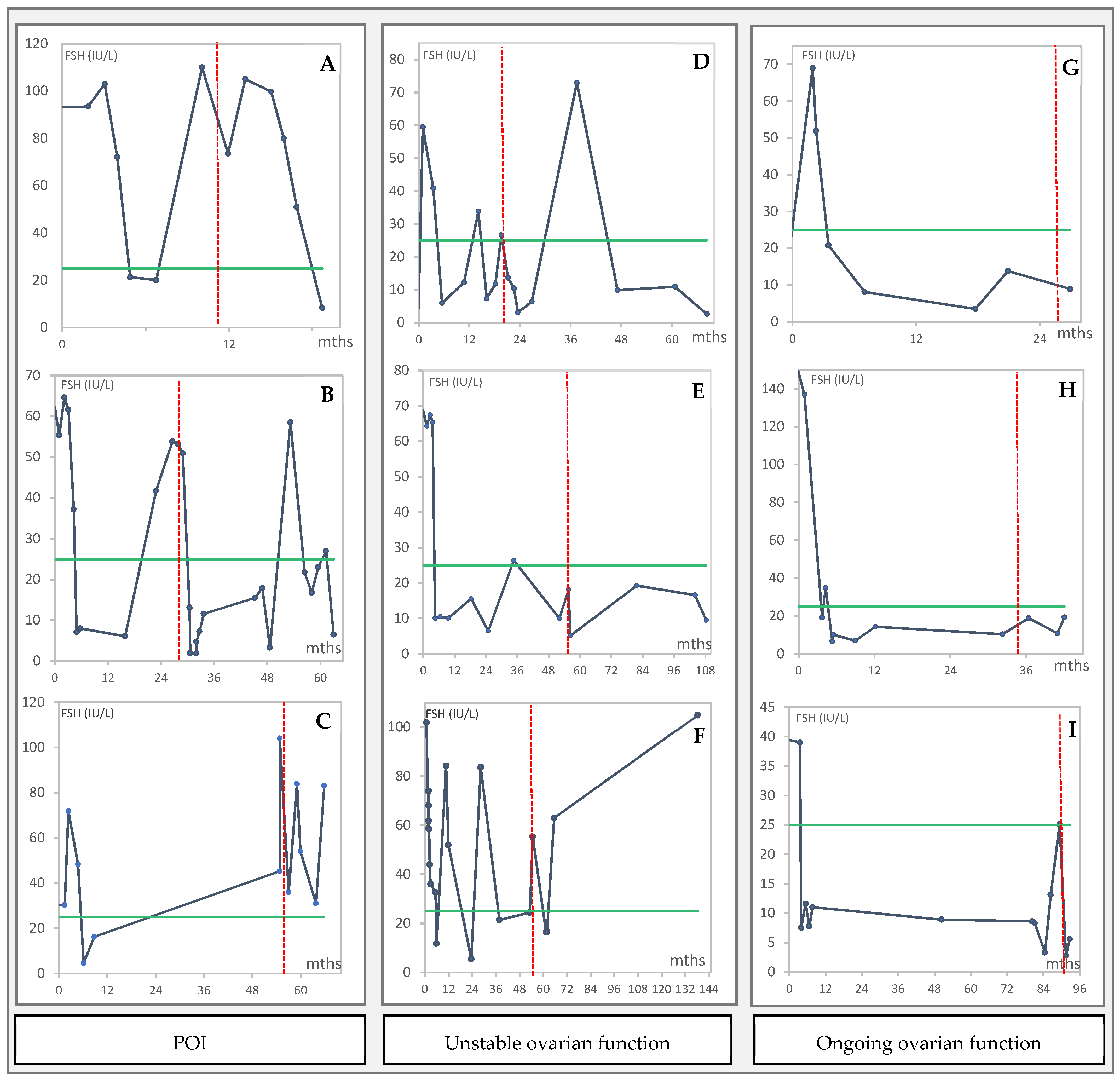
3.3. Endocrine Function of the Grafted Tissue in Women with POI
3.4. Repeated Transplantations
3.5. Relapse of Malignancy after OTT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Practice Committee of the American Society for Reproductive Medicine. Fertility Preservation in Patients Undergoing Gonadotoxic Therapy or Gonadectomy: A Committee Opinion. Fertil. Steril. 2019, 112, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.M.; von Wolff, M.; Poirot, C.; Diaz-Garcia, C.; Cacciottola, L.; Boissel, N.; Liebenthron, J.; Pellicer, A.; Donnez, J.; Andersen, C.Y. Transplantation of Cryopreserved Ovarian Tissue in a Series of 285 Women: A Review of Five Leading European Centers. Fertil. Steril. 2021, 115, 1102–1115. [Google Scholar] [CrossRef] [PubMed]
- Jadoul, P.; Guilmain, A.; Squifflet, J.; Luyckx, M.; Votino, R.; Wyns, C.; Dolmans, M.M. Efficacy of Ovarian Tissue Cryopreservation for Fertility Preservation: Lessons Learned from 545 Cases. Hum. Reprod. 2017, 32, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Lotz, L.; Maktabi, A.; Hoffmann, I.; Findeklee, S.; Beckmann, M.W.; Dittrich, R. Ovarian Tissue Cryopreservation and Retransplantation-What Do Patients Think about It? Reprod. Biomed. Online 2016, 32, 394–400. [Google Scholar] [CrossRef][Green Version]
- Gellert, S.E.; Pors, S.E.; Kristensen, S.G.; Bay-Bjørn, A.M.; Ernst, E.; Yding Andersen, C. Transplantation of Frozen-Thawed Ovarian Tissue: An Update on Worldwide Activity Published in Peer-Reviewed Papers and on the Danish Cohort. J. Assist. Reprod. Genet. 2018, 35, 561–570. [Google Scholar] [CrossRef]
- Pacheco, F.; Oktay, K. Current Success and Efficiency of Autologous Ovarian Transplantation: A Meta-Analysis. Reprod. Sci. 2017, 24, 1111–1120. [Google Scholar] [CrossRef]
- Khattak, H.; Malhas, R.; Craciunas, L.; Afifi, Y.; Amorim, C.A.; Fishel, S.; Silber, S.; Gook, D.; Demeestere, I.; Bystrova, O.; et al. Fresh and Cryopreserved Ovarian Tissue Transplantation for Preserving Reproductive and Endocrine Function: A Systematic Review and Individual Patient Data Meta-Analysis. Hum. Reprod. Update 2022, 28, 400–416. [Google Scholar] [CrossRef]
- Greve, T.; Schmidt, K.T.; Kristensen, S.G.; Ernst, E.; Andersen, C.Y. Evaluation of the Ovarian Reserve in Women Transplanted with Frozen and Thawed Ovarian Cortical Tissue. Fertil. Steril. 2012, 97, 1394–1398. [Google Scholar] [CrossRef]
- Jensen, A.K.; Kristensen, S.G.; MacKlon, K.T.; Jeppesen, J.V.; Fedder, J.; Ernst, E.; Andersen, C.Y. Outcomes of Transplantations of Cryopreserved Ovarian Tissue to 41 Women in Denmark. Hum. Reprod. 2015, 30, 2838–2845. [Google Scholar] [CrossRef]
- Andersen, S.T.; Pors, S.E.; la Cour Poulsen, L.; Colmorn, L.B.; Macklon, K.T.; Ernst, E.; Humaidan, P.; Andersen, C.Y.; Kristensen, S.G. Ovarian Stimulation and Assisted Reproductive Technology Outcomes in Women Transplanted with Cryopreserved Ovarian Tissue: A Systematic Review. Fertil. Steril. 2019, 112, 908–921. [Google Scholar] [CrossRef] [PubMed]
- Dueholm Hjorth, I.M.; Kristensen, S.G.; Dueholm, M.; Humaidan, P. Reproductive Outcomes after in Vitro Fertilization Treatment in a Cohort of Danish Women Transplanted with Cryopreserved Ovarian Tissue. Fertil. Steril. 2020, 114, 379–387. [Google Scholar] [CrossRef]
- Kristensen, S.G.; Wakimoto, Y.; Colmorn, L.B.; Dueholm, M.; Pors, S.E.; Macklon, K.T.; Mamsen, L.S.; Nikiforov, D.; Cadenas, J.; Greve, V.H.; et al. Use of Cryopreserved Ovarian Tissue in the Danish Fertility Preservation Cohort. Fertil. Steril. 2021, 116, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Eshre Guideline Group on POI; Webber, L.; Davies, M.; Anderson, R.; Bartlett, J.; Braat, D.; Cartwright, B.; Cifkova, R.; de Muinck Keizer-Schrama, S.; Hogervorst, E.; et al. ESHRE Guideline: Management of Women with Premature Ovarian Insufficiency. Hum. Reprod. 2016, 31, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Shapira, M.; Dolmans, M.M.; Silber, S.; Meirow, D. Evaluation of Ovarian Tissue Transplantation: Results from Three Clinical Centers. Fertil. Steril. 2020, 114, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Van der Ven, H.; Liebenthron, J.; Beckmann, M.; Toth, B.; Korell, M.; Krüssel, J.; Frambach, T.; Kupka, M.; Hohl, M.K.; Winkler-Crepaz, K.; et al. Ninety-Five Orthotopic Transplantations in 74 Women of Ovarian Tissue after Cytotoxic Treatment in a Fertility Preservation Network: Tissue Activity, Pregnancy and Delivery Rates. Hum. Reprod. 2016, 31, 2031–2041. [Google Scholar] [CrossRef]
- von Wolff, M.; Germeyer, A.; Liebenthron, J.; Korell, M.; Nawroth, F. Practical Recommendations for Fertility Preservation in Women by the FertiPROTEKT Network. Part II: Fertility Preservation Techniques. Arch. Gynecol. Obstet. 2018, 297, 257–267. [Google Scholar] [CrossRef]
- Diaz-Garcia, C.; Domingo, J.; Garcia-Velasco, J.A.; Herraiz, S.; Mirabet, V.; Iniesta, I.; Cobo, A.; Remohí, J.; Pellicer, A. Oocyte Vitrification versus Ovarian Cortex Transplantation in Fertility Preservation for Adult Women Undergoing Gonadotoxic Treatments: A Prospective Cohort Study. Fertil. Steril. 2018, 109, 478–485. [Google Scholar] [CrossRef]
- Lotz, L.; Bender-Liebenthron, J.; Dittrich, R.; Häberle, L.; Beckmann, M.W.; Germeyer, A.; Korell, M.; Sänger, N.; Kruessel, J.S.; von Wolff, M.; et al. Determinants of Transplantation Success with Cryopreserved Ovarian Tissue: Data from 196 Women of the FertiPROTEKT Network. Hum. Reprod. 2022; deac225, Epub ahead of print. [Google Scholar] [CrossRef]
- Wallace, W.H.B.; Kelsey, T.W. Human Ovarian Reserve from Conception to the Menopause. PLoS ONE 2010, 5, e8772. [Google Scholar] [CrossRef]
- Janse, F.; Donnez, J.; Anckaert, E.; De Jong, F.H.; Fauser, B.C.J.M.; Dolmans, M.M. Limited Value of Ovarian Function Markers Following Orthotopic Transplantation of Ovarian Tissue after Gonadotoxic Treatment. J. Clin. Endocrinol. Metab. 2011, 96, 1136–1144. [Google Scholar] [CrossRef]
- Schmidt, K.T.; Rosendahl, M.; Ernst, E.; Loft, A.; Andersen, A.N.; Dueholm, M.; Ottosen, C.; Andersen, C.Y. Autotransplantation of Cryopreserved Ovarian Tissue in 12 Women with Chemotherapy-Induced Premature Ovarian Failure: The Danish Experience. Fertil. Steril. 2011, 95, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Signorello, L.B.; Mulvihill, J.J.; Green, D.M.; Munro, H.M.; Stovall, M.; Weathers, R.E.; Mertens, A.C.; Whitton, J.A.; Robison, L.L.; Boice, J.D. Stillbirth and Neonatal Death in Relation to Radiation Exposure before Conception: A Retrospective Cohort Study. Lancet 2010, 376, 624–630. [Google Scholar] [CrossRef]
- Chiarelli, A.M.; Marrett, L.D.; Darlington, G.A. Pregnancy Outcomes in Females after Treatment for Childhood Cancer. Epidemiology 2000, 11, 161–166. [Google Scholar] [CrossRef]
- Rozen, G.; Rogers, P.; Chander, S.; Anderson, R.; McNally, O.; Umstad, M.; Winship, A.; Hutt, K.; Teh, W.T.; Dobrotwir, A.; et al. Clinical Summary Guide: Reproduction in Women with Previous Abdominopelvic Radiotherapy or Total Body Irradiation. Hum. Reprod. Open 2020, 2020, hoaa045. [Google Scholar] [CrossRef]
- Teh, W.T.; Stern, C.; Chander, S.; Hickey, M. The Impact of Uterine Radiation on Subsequent Fertility and Pregnancy Outcomes. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Wo, J.Y.; Viswanathan, A.N. Impact of Radiotherapy on Fertility, Pregnancy, and Neonatal Outcomes in Female Cancer Patients. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1304–1312. [Google Scholar] [CrossRef]
- Greve, V.H.; Dueholm, M.; Mamsen, L.S.; Kristensen, S.G.; Ernst, E.; Andersen, C.Y. Hormonal Characteristics of Women Receiving Ovarian Tissue Transplantation with or without Endogenous Ovarian Activity. J. Clin. Med. 2021, 10, 5217. [Google Scholar] [CrossRef]
- Vatel, M.; Torre, A.; Paillusson, B.; Scheffler, F.; Bergere, M.; Benkhalifa, M.; Le Martelot, M.T.; Leperlier, F.; Mirallié, S.; Selleret, L.; et al. Efficacy of Assisted Reproductive Technology after Ovarian Tissue Transplantation in a Cohort of 11 Patients with or without Associated Infertility Factors. J. Assist. Reprod. Genet. 2021, 38, 503–511. [Google Scholar] [CrossRef]
- Kyono, K.; Doshida, M.; Toya, M.; Sato, Y.; Akahira, J.; Sasano, H. Potential Indications for Ovarian Autotransplantation Based on the Analysis of 5,571 Autopsy Findings of Females under the Age of 40 in Japan. Fertil. Steril. 2010, 93, 2429–2430. [Google Scholar] [CrossRef]
- Dolmans, M.M.; Masciangelo, R. Risk of Transplanting Malignant Cells in Cryopreserved Ovarian Tissue. Minerva Ginecol. 2018, 70, 436–443. [Google Scholar] [CrossRef]
- Rodriguez-Wallberg, K.A.; Marklund, A.; Lundberg, F.; Wikander, I.; Milenkovic, M.; Anastacio, A.; Sergouniotis, F.; Wånggren, K.; Ekengren, J.; Lind, T.; et al. A Prospective Study of Women and Girls Undergoing Fertility Preservation Due to Oncologic and Non-Oncologic Indications in Sweden–Trends in Patients’ Choices and Benefit of the Chosen Methods after Long-Term Follow Up. Acta Obstet. Gynecol. Scand. 2019, 98, 604–615. [Google Scholar] [CrossRef]
- Imbert, R.; Moffa, F.; Tsepelidis, S.; Simon, P.; Delbaere, A.; Devreker, F.; Dechene, J.; Ferster, A.; Veys, I.; Fastrez, M.; et al. Safety and Usefulness of Cryopreservation of Ovarian Tissue to Preserve Fertility: A 12-Year Retrospective Analysis. Hum. Reprod. 2014, 29, 1931–1940. [Google Scholar] [CrossRef]
- Fauser, B.C.J.M.; Levy-Toledano, R. Public Perception of In-Vitro Fertilization (IVF) and Fertility Preservation: Assessed by the Listening IVF and Fertility in Europe (LIFE) Survey. Hum. Reprod. 2017, 32, 93. [Google Scholar]
- Macklon, K.T.; Pedersen, A.T.; Larsen, E.C.; Colmorn, L.B. Fertility Counselling of Younger Women after Cancer Treatment. Ugeskr. Laeger 2020, 182, 68–80. [Google Scholar]

| Women, n = 40 (%) | Pregnant, n = 22 (%) | Non-Pregnant, n = 17 (%) | p-Value | |
|---|---|---|---|---|
| Age at OTC a, mean (SD) | 28.9 (5.5) | 28.9 (5.0) | 28.7 (6.2) | 0.94 |
| 15–19 | 3 (7.5) | 2 (9) | 1 (6) | |
| 20–24 | 6 (15.0) | 2 (9) | 4 (24) | |
| 25–29 | 9 (22.5) | 5 (23) | 4 (24) | |
| 30–34 | 15 (37.5) | 11 (50) | 4 (24) | |
| 35–39 | 7 (17.5) | 2 (9) | 4 (24) | |
| AMH at OTC a, mean (SD) (Median, IQR) | 19.7 (19.9) (15.0, 14.8) | 25.8 (24.8) (19.0, 10.0) | 10.3 (6.4) (7.2, 7.1) | 0.14 |
| AFC at OTC a, mean (SD) (Median, IQR) | 15.8 (14.2) (12.0, 10.0) | 20.0 (16.7) (16.0, 18.0) | 8.7 (4.7) (10.0, 8.0) | 0.04 |
| Diagnosis at OTC a | ||||
| Breast cancer | 19 (48) | 11 (50) | 8 (47) | |
| Leukemia (CML) | 1 (3) | 0 (0) | 1 (6) | |
| Hodgkins disease | 7 (18) | 4 (18) | 3 (18) | |
| Non-Hodgkins lymphoma | 4 (10) | 2 (9) | 2 (12) | |
| Other malignancy | 3 (8) | 1 (5) | 2 (12) | |
| Benign disease | 6 (15) | 4 (18) | 1 (6) | |
| Previous deliveries | 6 (15) | 4 (18) | 2 (12) | |
| Pelvic radiation | 1.0 | |||
| Yes | 4 (10) | 2 (9) | 2 (12) | |
| No | 36 (90) | 20 (91) | 15 (88) | |
| Age at first OTT | 33.7 (5.5) | 28.9 (5.0) | 28.4 (6.2) | 0.76 |
| Time to first OTT (years) | 4.2 (2.9) | 3.6 (1.2) | 5.3 (4.0) | |
| Ovarian function before first OTT | 0.12 | |||
| POI | 19 (48) | 9 (41) | 9 (53) | |
| Unstable ovarian function | 10 (25) | 4 (18) | 6 (35) | |
| Ongoing ovarian function | 11 (28) | 9 (41) | 2 (12) | |
| Number of OTTs | ||||
| 1 | 40 (100) | 16 (73) | 11 (65) | |
| 2 | 12 (30) | 5 (23) | 6 (35) | |
| 3 | 1 (3) | 1 (5) | 0 (0) | |
| ART after OTT (from 39 women) | ||||
| Yes | 30 (77) | 11 (50) | 17 (100) | |
| No b | 9 (23) | 11 (50) | 0 (0) |
| Women with Pregnancy Wish | Pregnancies (n = 29) | Deliveries (n = 20) | |||
|---|---|---|---|---|---|
| n | Women, n | Rate % (95% CI) | Women, n | Rate % (95% CI) | |
| Overall | 39 | 22 | 56% (39.6, 72.2) | 16 | 41% (25.6, 57.9) |
| Method of Conception | Women with a Pregnancy Wish n | Women who Conceived n (%) | Women who Gave Birth n (%) | Pregnancies n (%) | Deliveries n (%) | Pregnancy Loss n (%) |
|---|---|---|---|---|---|---|
| ART | 30 | 11 (50) | 6 (38) | 14 (48) | 8 (40) | 6 (43) |
| Natural | 11 | 11 (50) | 10 (63) | 15 (52) | 12 (60) | 3 (20) |
| Cycles, n | Pregnancy Outcome, n | Pr Transfer (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ALL | Cancelled | Transfer | Ectopic | Early Miscarriage | Bio-Chemical | Deliveries | TOTAL | Clinical PR | LBR | |
| IVF/ICSI | 121 | 77 | 44 | 1 | 1 | 3 | 6 | 11 | 18.2% | 13.6% |
| FET | 6 | 1 | 5 | 0 | 0 | 1 | 2 | 3 | 40.0% | 40.0% |
| TOTAL | 127 | 78 | 49 | 1 | 1 | 4 | 8 | 14 | 20.4% | 16.4% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colmorn, L.B.; Pedersen, A.T.; Larsen, E.C.; Hansen, A.S.; Rosendahl, M.; Andersen, C.Y.; Kristensen, S.G.; Macklon, K.T. Reproductive and Endocrine Outcomes in a Cohort of Danish Women following Auto-Transplantation of Frozen/Thawed Ovarian Tissue from a Single Center. Cancers 2022, 14, 5873. https://doi.org/10.3390/cancers14235873
Colmorn LB, Pedersen AT, Larsen EC, Hansen AS, Rosendahl M, Andersen CY, Kristensen SG, Macklon KT. Reproductive and Endocrine Outcomes in a Cohort of Danish Women following Auto-Transplantation of Frozen/Thawed Ovarian Tissue from a Single Center. Cancers. 2022; 14(23):5873. https://doi.org/10.3390/cancers14235873
Chicago/Turabian StyleColmorn, Lotte B., Anette T. Pedersen, Elisabeth C. Larsen, Alexandra S. Hansen, Mikkel Rosendahl, Claus Yding Andersen, Stine G. Kristensen, and Kirsten T. Macklon. 2022. "Reproductive and Endocrine Outcomes in a Cohort of Danish Women following Auto-Transplantation of Frozen/Thawed Ovarian Tissue from a Single Center" Cancers 14, no. 23: 5873. https://doi.org/10.3390/cancers14235873
APA StyleColmorn, L. B., Pedersen, A. T., Larsen, E. C., Hansen, A. S., Rosendahl, M., Andersen, C. Y., Kristensen, S. G., & Macklon, K. T. (2022). Reproductive and Endocrine Outcomes in a Cohort of Danish Women following Auto-Transplantation of Frozen/Thawed Ovarian Tissue from a Single Center. Cancers, 14(23), 5873. https://doi.org/10.3390/cancers14235873






