Evaluating Different Quantitative Shear Wave Parameters of Ultrasound Elastography in the Diagnosis of Lymph Node Malignancies: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Data Collection
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Quality Assessment
2.6. Endpoints
2.7. Meta-Analysis
3. Results
3.1. Study Characteristics
3.2. Risk of Bias
3.3. Meta-Analysis
3.4. Sub-Group Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosen, R.D.; Sapra, A. TNM Classification. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Mohseni, S.; Shojaiefard, A.; Khorgami, Z.; Alinejad, S.; Ghorbani, A.; Ghafouri, A. Peripheral lymphadenopathy: Approach and diagnostic tools. Iran. J. Med. Sci. 2014, 39, 158–170. [Google Scholar] [PubMed]
- Kawano, S.; Kojima, M.; Higuchi, Y.; Sugimoto, M.; Ikeda, K.; Sakuyama, N.; Takahashi, S.; Hayashi, R.; Ochiai, A.; Saito, N. Assessment of elasticity of colorectal cancer tissue, clinical utility, pathological and phenotypical relevance. Cancer Sci. 2015, 106, 1232–1239. [Google Scholar] [CrossRef]
- Ophir, J.; Céspedes, I.; Ponnekanti, H.; Yazdi, Y.; Li, X. Elastography: A quantitative method for imaging the elasticity of biological tissues. Ultrason. Imaging 1991, 13, 111–134. [Google Scholar] [CrossRef]
- Bazemore, A.W.; Smucker, D.R. Lymphadenopathy and malignancy. Am. Fam. Physician 2002, 66, 2103–2110. [Google Scholar]
- Na, D.G.; Lim, H.K.; Byun, H.S.; Kim, H.D.; Ko, Y.H.; Baek, J.H. Differential diagnosis of cervical lymphadenopathy: Usefulness of color Doppler sonography. AJR Am. J. Roentgenol. 1997, 168, 1311–1316. [Google Scholar] [CrossRef]
- Ahuja, A.T.; Ying, M.; Ho, S.Y.; Antonio, G.; Lee, Y.P.; King, A.D.; Wong, K.T. Ultrasound of malignant cervical lymph nodes. Cancer Imaging 2008, 8, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Lee, J.H.; Baek, J.H. Ultrasound elastography for evaluation of cervical lymph nodes. Ultrasonography 2015, 34, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Leenhardt, L.; Erdogan, M.F.; Hegedus, L.; Mandel, S.J.; Paschke, R.; Rago, T.; Russ, G. European thyroid association guidelines for cervical ultrasound scan and ultrasound-guided techniques in the postoperative management of patients with thyroid cancer. Eur. Thyroid J. 2013, 2, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, P.; Edel, G.; Roos, N.; Naguib, A.; Peters, P.E. In-vitro high-resolution ultrasonography of benign and malignant lymph nodes. A sonographic-pathologic correlation. Investig. Radiol. 1993, 28, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Bamber, J.; Cosgrove, D.; Dietrich, C.F.; Fromageau, J.; Bojunga, J.; Calliada, F.; Cantisani, V.; Correas, J.M.; D’Onofrio, M.; Drakonaki, E.E.; et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013, 34, 169–184. [Google Scholar] [CrossRef]
- Bhatia, K.S.S.; Cho, C.C.M.; Tong, C.S.L.; Yuen, E.H.Y.; Ahuja, A.T. Shear Wave Elasticity Imaging of Cervical Lymph Nodes. Ultrasound Med. Biol. 2012, 38, 195–201. [Google Scholar] [CrossRef]
- Melodelima, D.; Bamber, J.C.; Duck, F.A.; Shipley, J.A. Transient elastography using impulsive ultrasound radiation force: A preliminary comparison with surface palpation elastography. Ultrasound Med. Biol. 2007, 33, 959–969. [Google Scholar] [CrossRef]
- Hill, C.R.; Bamber, J.C. Methodology for Clinical Investigation. In Physical Principles of Medical Ultrasonics; John Wiley & Sons Ltd.: Chichester, UK, 2004; pp. 255–302. [Google Scholar]
- Ophir, J.; Alam, S.K.; Garra, B.S.; Kallel, F.; Konofagou, E.E.; Krouskop, T.; Merritt, C.R.B.; Righetti, R.; Souchon, R.; Srinivasan, S.; et al. Elastography: Imaging the elastic properties of soft tissues with ultrasound. J. Med. Ultrason. 2002, 29, 155. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.L.; Omar, N.; Ab Mumin, N.; Ramli Hamid, M.T.; Vijayananthan, A.; Rahmat, K. Diagnostic Accuracy of Shear Wave Elastography as an Adjunct Tool in Detecting Axillary Lymph Nodes Metastasis. Acad. Radiol. 2022, 29, S69–S78. [Google Scholar] [CrossRef]
- Wei, C.; Li, C.; Szewczyk-Bieda, M.; Upreti, D.; Lang, S.; Huang, Z.; Nabi, G. Performance Characteristics of Transrectal Shear Wave Elastography Imaging in the Evaluation of Clinically Localized Prostate Cancer: A Prospective Study. J. Urol. 2018, 200, 549–558. [Google Scholar] [CrossRef]
- Li, D.-D.; Xu, H.-X.; Guo, L.-H.; Bo, X.-W.; Li, X.-L.; Wu, R.; Xu, J.-M.; Zhang, Y.-F.; Zhang, K. Combination of two-dimensional shear wave elastography with ultrasound breast imaging reporting and data system in the diagnosis of breast lesions: A new method to increase the diagnostic performance. Eur. Radiol. 2016, 26, 3290–3300. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.S.; Kim, J.-A.; Son, E.J.; Youk, J.H.; Park, C.S. Shear Wave Elastography in Evaluation of Cervical Lymph Node Metastasis of Papillary Thyroid Carcinoma: Elasticity Index as a Prognostic Implication. Ann. Surg. Oncol. 2015, 22, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef]
- Samir, A.E.; Dhyani, M.; Anvari, A.; Prescott, J.; Halpern, E.F.; Faquin, W.C.; Stephen, A. Shear-Wave Elastography for the Preoperative Risk Stratification of Follicular-patterned Lesions of the Thyroid: Diagnostic Accuracy and Optimal Measurement Plane. Radiology 2015, 277, 565–573. [Google Scholar] [CrossRef]
- Youk, J.H.; Son, E.J.; Kim, J.-A.; Gweon, H.M. Pre-Operative Evaluation of Axillary Lymph Node Status in Patients with Suspected Breast Cancer Using Shear Wave Elastography. Ultrasound Med. Biol. 2017, 43, 1581–1586. [Google Scholar] [CrossRef]
- Choi, J.J.; Kang, B.J.; Kim, S.H.; Lee, J.H.; Jeong, S.H.; Yim, H.W.; Song, B.J.; Jung, S.S. Role of sonographic elastography in the differential diagnosis of axillary lymph nodes in breast cancer. J. Ultrasound Med. 2011, 30, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Bamber, J.; Berzigotti, A.; Bota, S.; Cantisani, V.; Castera, L.; Cosgrove, D.; Ferraioli, G.; Friedrich-Rust, M.; Gilja, O.H.; et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. 2017, 38, e16–e47. [Google Scholar] [CrossRef]
- Davis, L.C.; Baumer, T.G.; Bey, M.J.; Holsbeeck, M.V. Clinical utilization of shear wave elastography in the musculoskeletal system. Ultrasonography 2019, 38, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Sandrin, L.; Tanter, M.; Gennisson, J.; Catheline, S.; Fink, M. Shear elasticity probe for soft tissues with 1-D transient elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2002, 49, 436–446. [Google Scholar] [CrossRef]
- Bercoff, J.; Tanter, M.; Fink, M. Supersonic shear imaging: A new technique for soft tissue elasticity mapping. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 396–409. [Google Scholar] [CrossRef]
- Zhou, J.; Zhan, W.; Chang, C.; Zhang, X.; Jia, Y.; Dong, Y.; Zhou, C.; Sun, J.; Grant, E.G. Breast Lesions: Evaluation with Shear Wave Elastography, with Special Emphasis on the “Stiff Rim” Sign. Radiology 2014, 272, 63–72. [Google Scholar] [CrossRef]
- Seo, M.; Sohn, Y.-M. Differentiation of benign and metastatic axillary lymph nodes in breast cancer: Additive value of shear wave elastography to B-mode ultrasound. Clin. Imaging 2018, 50, 258–263. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, I.H.; Jin, S.-Y.; Park, H.K.; Byun, D.W.; Suh, K.; Yoo, M.H. Efficacy of Shear-Wave Elastography for Detecting Postoperative Cervical Lymph Node Metastasis in Papillary Thyroid Carcinoma. Int. J. Endocrinol. 2018, 2018, 9382649. [Google Scholar] [CrossRef]
- Luo, S.; Yao, G.; Hong, Z.; Zhang, S.; Wang, W.; Zhang, J.; Zhang, Y.; Wu, J.; Zhang, L.; Cheng, H.; et al. Qualitative Classification of Shear Wave Elastography for Differential Diagnosis Between Benign and Metastatic Axillary Lymph Nodes in Breast Cancer. Front. Oncol. 2019, 9, 533. [Google Scholar] [CrossRef]
- Yang, J.-R.; Song, Y.; Jia, Y.-L.; Ruan, L.-T. Application of multimodal ultrasonography for differentiating benign and malignant cervical lymphadenopathy. Jpn. J. Radiol. 2021, 39, 938–945. [Google Scholar] [CrossRef]
- Tourasse, C.; Dénier, J.F.; Awada, A.; Gratadour, A.-C.; Nessah-Bousquet, K.; Gay, J. Elastography in the assessment of sentinel lymph nodes prior to dissection. Eur. J. Radiol. 2012, 81, 3154–3159. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-B.; Li, J.; Guan, Y.; Xiao, W.-W.; Zhao, C.; Lu, T.-X.; Han, F. The value of shear wave elastography in predicting for undiagnosed small cervical lymph node metastasis in nasopharyngeal carcinoma: A preliminary study. Eur. J. Radiol. 2018, 103, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Lee, J.H.; Lim, H.K.; Kim, S.Y.; Han, M.W.; Cho, K.J.; Baek, J.H. Quantitative shear wave elastography in the evaluation of metastatic cervical lymph nodes. Ultrasound Med. Biol. 2013, 39, 935–940. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Chami, L.; Giron, A.; Ezziane, M.; Leblond, V.; Charlotte, F.; Pellot-Barakat, C.; Lucidarme, O. Quantitative and Qualitative Approach for Shear Wave Elastography in Superficial Lymph Nodes. Ultrasound Med. Biol. 2021, 47, 2117–2127. [Google Scholar] [CrossRef]
- Lo, W.C.; Hsu, W.L.; Wang, C.T.; Cheng, P.W.; Liao, L.J. Incorporation of shear wave elastography into a prediction model in the assessment of cervical lymph nodes. PLoS ONE 2019, 14, e0221062. [Google Scholar] [CrossRef]
- You, J.; Chen, J.; Xiang, F.; Song, Y.; Khamis, S.; Lu, C.; Lv, Q.; Zhang, Y.; Xie, M. The value of quantitative shear wave elastography in differentiating the cervical lymph nodes in patients with thyroid nodules. J. Med. Ultrason. 2018, 45, 251–259. [Google Scholar] [CrossRef]
- Tan, S.; Miao, L.Y.; Cui, L.G.; Sun, P.F.; Qian, L.X. Value of Shear Wave Elastography Versus Contrast-Enhanced Sonography for Differentiating Benign and Malignant Superficial Lymphadenopathy Unexplained by Conventional Sonography. J Ultrasound Med. 2017, 36, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Desmots, F.; Fakhry, N.; Mancini, J.; Reyre, A.; Vidal, V.; Jacquier, A.; Santini, L.; Moulin, G.; Varoquaux, A. Shear Wave Elastography in Head and Neck Lymph Node Assessment: Image Quality and Diagnostic Impact Compared with B-Mode and Doppler Ultrasonography. Ultrasound Med. Biol. 2016, 42, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Suh, C.H.; Choi, Y.J.; Baek, J.H.; Lee, J.H. The diagnostic performance of shear wave elastography for malignant cervical lymph nodes: A systematic review and meta-analysis. Eur. Radiol. 2017, 27, 222–230. [Google Scholar] [CrossRef]
- Wang, R.Y.; Zhang, Y.W.; Gao, Z.M.; Wang, X.M. Role of sonoelastography in assessment of axillary lymph nodes in breast cancer: A systematic review and meta-analysis. Clin. Radiol. 2020, 75, 320.e1–320.e7. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-W.; Huang, Q.-X.; Huang, H.; Cheng, M.-Q.; Tong, W.-J.; Xian, M.-F.; Liang, J.-Y.; Wang, W. Diagnostic Performance of Quantitative and Qualitative Elastography for Axillary Lymph Node Metastasis in Breast Cancer: A Systematic Review and Meta-Analysis. Front. Oncol 2020, 10, 552177. [Google Scholar] [CrossRef]
- Bae, S.J.; Park, J.T.; Park, A.Y.; Youk, J.H.; Lim, J.W.; Lee, H.W.; Lee, H.M.; Ahn, S.G.; Son, E.J.; Jeong, J. Ex Vivo Shear-Wave Elastography of Axillary Lymph Nodes to Predict Nodal Metastasis in Patients with Primary Breast Cancer. J. Breast Cancer 2018, 21, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Sun, J.-W.; Zhou, H.; Du, L.-Y.; Wang, X.-L.; Tao, L.; Jiang, Z.-P.; Zhou, X.-L. Pre-operative Conventional Ultrasound and Sonoelastography Evaluation for Predicting Axillary Lymph Node Metastasis in Patients with Malignant Breast Lesions. Ultrasound Med. Biol. 2018, 44, 2587–2595. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, K.; Tamaki, N.; Kamada, Y.; Uehara, K.; Miyashita, M.; Sm Chan, M.; Ishida, T.; Ohuchi, N.; Sasano, H. Non-invasive evaluation of axillary lymph node status in breast cancer patients using shear wave elastography. Tohoku J. Exp. Med. 2013, 231, 211–216. [Google Scholar] [CrossRef][Green Version]
- Sun, Y.; Wang, W.; Mi, C.; Zhang, Q.; Zhang, K. Differential Diagnosis Value of Shear-Wave Elastography for Superficial Enlarged Lymph Nodes. Front. Oncol. 2022, 12, 908085. [Google Scholar] [CrossRef]
- Cosgrove, D.O.; Berg, W.A.; Doré, C.J.; Skyba, D.M.; Henry, J.-P.; Gay, J.; Cohen-Bacrie, C.; The BE1 Study Group. Shear wave elastography for breast masses is highly reproducible. Eur. Radiol. 2012, 22, 1023–1032. [Google Scholar] [CrossRef]
- Bhatia, K.; Tong, C.S.; Cho, C.C.; Yuen, E.H.; Lee, J.; Ahuja, A.T. Reliability of shear wave ultrasound elastography for neck lesions identified in routine clinical practice. Ultraschall Med. 2012, 33, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Cronin, C.G.; Scott, J.; Kambadakone, A.; Catalano, O.A.; Sahani, D.; Blake, M.A.; McDermott, S. Utility of positron emission tomography/CT in the evaluation of small bowel pathology. Br. J. Radiol. 2012, 85, 1211–1221. [Google Scholar] [CrossRef]
- Metser, U.; Even-Sapir, E. Increased (18)F-fluorodeoxyglucose uptake in benign, nonphysiologic lesions found on whole-body positron emission tomography/computed tomography (PET/CT): Accumulated data from four years of experience with PET/CT. Semin. Nucl. Med. 2007, 37, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, H.B.; Sahani, D.V.; Fischman, A.J.; Mueller, P.R.; Blake, M.A. Bowel Hot Spots at PET-CT. Radiographics 2007, 27, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; He, X.; Chang, Y.; Sun, G.; Thabane, L. A sensitivity and specificity comparison of fine needle aspiration cytology and core needle biopsy in evaluation of suspicious breast lesions: A systematic review and meta-analysis. Breast 2017, 31, 157–166. [Google Scholar] [CrossRef]
- Mahmoud, O.M.; Khedrawy, M.E.-M.; Megally, H.I.; Mohamed, M.F.; Allam, M.T. Fine-needle aspiration cytology versus core needle lymph node biopsy in axillary staging of breast cancer. Egypt. J. Radiol. Nucl. Med. 2022, 53, 219. [Google Scholar] [CrossRef]
- Amador-Ortiz, C.; Chen, L.; Hassan, A.; Frater, J.L.; Burack, R.; Nguyen, T.T.; Kreisel, F. Combined core needle biopsy and fine-needle aspiration with ancillary studies correlate highly with traditional techniques in the diagnosis of nodal-based lymphoma. Am. J. Clin. Pathol. 2011, 135, 516–524. [Google Scholar] [CrossRef]
- Wilkinson, A.R.; Mahore, S.D.; Maimoon, S.A. FNAC in the diagnosis of lymph node malignancies: A simple and sensitive tool. Indian J. Med. Paediatr. Oncol. 2012, 33, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Höft, S.; Muhle, C.; Brenner, W.; Sprenger, E.; Maune, S. Fine-needle aspiration cytology of the sentinel lymph node in head and neck cancer. J. Nucl. Med. 2002, 43, 1585–1590. [Google Scholar]
- Garg, S.; Rohilla, M.; Srinivasan, R.; Bal, A.; Das, A.; Dey, P.; Gupta, N.; Gupta, P.; Rajwanshi, A. Fine-Needle Aspiration Diagnosis of Lymphoma Based on Cytomorphology Alone: How Accurate is it?—A Cyto-Histopathology Correlative Study. J. Cytol. 2021, 38, 164–170. [Google Scholar] [CrossRef]
- Kiliçarslan, A.; Doğan, M.; Süngü, N.; Karakök, E.; Karabekmez, L.; Akyol, M.; Doğan, H.T. Can Cutting-Needle Biopsy Be an Alternative to Excisional Biopsy in Lymph Node Pathologies? Türk Patoloji Dergisi 2017, 1, 235–239. [Google Scholar] [CrossRef]
- Wang, B.; Guo, Q.; Wang, J.-Y.; Yu, Y.; Yi, A.-J.; Cui, X.-W.; Dietrich, C.F. Ultrasound Elastography for the Evaluation of Lymph Nodes. Front. Oncol. 2021, 11, 3133. [Google Scholar] [CrossRef]
- Chae, S.Y.; Jung, H.N.; Ryoo, I.; Suh, S. Differentiating cervical metastatic lymphadenopathy and lymphoma by shear wave elastography. Sci. Rep. 2019, 9, 12396. [Google Scholar] [CrossRef] [PubMed]
- Săftoiu, A.; Gilja, O.H.; Sidhu, P.S.; Dietrich, C.F.; Cantisani, V.; Amy, D.; Bachmann-Nielsen, M.; Bob, F.; Bojunga, J.; Brock, M.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Elastography in Non-Hepatic Applications: Update 2018. Ultraschall Med. 2019, 40, 425–453. [Google Scholar] [CrossRef] [PubMed]




| Authors | Year of Publication | Study Design | No. of Patients | Mean Age (Range) (Years Old) | No. of Lymph Node Lesions | US Imaging System | SWE Imaging Strength (MHz) | SWE Parameters | Elastic Modulus Values in Malignant Lymph Nodes Mean ± SD or Median (IQR) (kPa) | LN Type | Reference Standard | Clinically Significant Definition |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ng et al. [16] | 2022 | Prospective | 107 | 58 (32–82) | 107 | Aixplorer (SuperSonic Imagine) | 15-4 | Emax | 40.0 ± 46.4 | Axillary | ALND or SLNB | Bloom and Richardson Grading ALN pathological staging: cut-off point of >3 mm |
| Emean | 28.9 ± 36.4 | |||||||||||
| Emin | 22.5 ± 33.5 | |||||||||||
| Esd | 8.7 ± 27.1 | |||||||||||
| Chami et al. [37] | 2021 | Prospective | 222 | N/A | 222 | Aixplorer (SuperSonic Imagine Ltd, Aix-en- Provence, France) | SL10-2 (central frequency at 10 MHz) | Emax | 36.1 ± 33.7 (lymphoma) 62 ± 58.2 (carcinoma) | Axillary, Head & Neck, Inguinal | CNB | Doppler criteria |
| Emean | 16.7 ± 12.3 (lymphoma) 29.5 ± 32.3 (carcinoma) | |||||||||||
| Esd | 6.4 ± 5.7 (lymphoma) 11.1 ± 10.6 (carcinoma) | |||||||||||
| Yang et al. [32] | 2021 | Retrospective | 103 | 43.9 (18–66) | 109 | Aixplorer (SuperSonic Imagine, Aixen-Provence, France) | 15-4 | Emax | 34.2 ± 7.0 | Cervical | US-guided biopsy | US criterion; transverse diameter of >7 mm (level II–VI), peripheral/mixed blood flow present |
| Lo et al. [38] | 2019 | Prospective | 109 | 46 (21–86) | 109 | Toshiba Aplio 500 US system (Otawara, Japan) | 15-4 | Emax | 66.3 ± 24.3 | Cervical | US-FNA or US-CNB | - |
| Luo et al. [31] | 2019 | Prospective | 118 | 46.7 (27–69) | 121 | Aixplorer ultrasound system (Supersonic Imagine, Aix-en-Provence, France) | 15-4 | Emax | 54.79 ± 37.42 | Axillary | ALNB or SLNB | Tumour deposit > 0.2 mm in diameter in at least one lymph node |
| Emean | 49.93 ± 35.68 | |||||||||||
| Emin | 41.88 ± 32.67 | |||||||||||
| Esd | 3.74 ± 3.16 | |||||||||||
| Chen et al. [34] | 2018 | Prospective | 62 | 43.5 (19–66) | 114 | Aixplorer (SuperSonic Imagine, Aixen-Provence, France) | 15-4 | Emax | 31.6 (IQR: 25.2; 55.9) | Cervical | US-Guided CNB | AJCC staging system |
| Emean | 22.4 (IQR: 18.8; 36.6) | |||||||||||
| Emin | 15.8 (IQR: 9.6; 22.4) | |||||||||||
| Kim et al. [30] | 2018 | Retrospective | 43 | 49 (29–81) | 43 | Aixplorer (SuperSonic Imagine, Aix-en-Provence, France) | 15-4 | Emax | 50.5 (IQR: 39.9; 88.0) | Cervical | FNAB | - |
| Emean | 37.1 (IQR: 20.0; 46.3) | |||||||||||
| Emin | 11.3 (IQR: 4.2; 34.7) | |||||||||||
| Esd | 7.8 (IQR: 4.6; 11.2) | |||||||||||
| Seo et al. [29] | 2018 | Retrospective | 53 | 54.7 (33–80) * | 54 | Aixplorer (Supersonic Imagine, Aix en Provence, France) | 15-4 | Emax | 79.80 ± 65.95 | Axillary | US-guided FNAB or SLNB | US criterion |
| Emean | 55.99 ± 49.19 | |||||||||||
| Emin | 29.29 ± 31.44 | |||||||||||
| Esd | 13.92 ± 11.46 | |||||||||||
| You et al. [39] | 2018 | Prospective | 39 | 45.6 (15–67) | 141 | Aixplorer US system (SuperSonic Imagine, Aix en Provence, France) | 15-4 | Emax | 58.7 ± 25.7 | Cervical | FNAB | US criterion 18 months follow-up |
| Emean | 30.6 ± 14.9 | |||||||||||
| Emin | 11.9 ± 9.1 | |||||||||||
| Esd | 10.2 ± 5.0 | |||||||||||
| Tan et al. [40] | 2017 | Prospective | 42 | 44 (23–61) | 42 | Aixplorer US system (SuperSonic Imagine, Aix-en-Provence, France) | SL10-2 | Emax | 52.0 (IQR: 38.1; 65.1) | Neck, Supraclavicular fossze, axilla | CNB | NA |
| Emean | 16.8 (IQR: 10.6; 26.1) | |||||||||||
| Emin | 0.1 (IQR: 0.1; 0.4) | |||||||||||
| Esd | 9.1 (IQR: 6.9; 11.7) | |||||||||||
| Youk et al. [22] | 2017 | Retrospective | 130 | 49.4 (18–84) | 130 | Aixplorer (SuperSonic Imagine, Aix-en-Provence, France) | 15-4 | Emax | 64.6 ± 41.9 | Axillary | ALND and SLNB | - |
| Emean | 50.2 ± 31.8 | |||||||||||
| Emin | 31.4 ± 24.8 | |||||||||||
| Esd | 9.0 ± 9.7 | |||||||||||
| Desmots et al. [41] | 2016 | Prospective | 56 | 49 (25–84) | 63 (62 involved in further analysis) | Aixplorer, SuperSonic Imagine, Aix-en-Provence, France) with a conventional 15- to 4-MHz transducer linear probe (SuperLinear SL15-4) | SL15–4 | Emax | 72 ± 59 | Head & Neck | Surgical resection, FNAC and US-follow up | AJCC staging system |
| Jung et al. [19] | 2015 | Retrospective | 66 | 45.2 | 84 | Aixplorer (SuperSonic Imagine, Les Jardins de la Duranne, Aix en Provence, France) | 15-4 | Emax | 79.61 ± 71.23 | Cervical | US-Guided FNAB | US criterion |
| Emean | 67.93 ± 62.52 | |||||||||||
| Emin | 48.49 ± 47.21 | |||||||||||
| Choi et al. [35] | 2013 | Prospective | 15 | 54.2 (38–73) | 67 | Aixplorer (SuperSonic Imagine, Aix en Provence, France) | 15-4 | Emax | 41.06 ± 36.34 | Cervical | Surgical resection | US criterion |
| Bhatia et al. [12] | 2012 | Prospective | 46 | 52.8 (7–74) | 55 | Aixplorer; (SuperSonic Imagine, Les Jardins de la Duranne, Aix en Provence, France) | 15-4 | Emax | 42.2 (IQR: 28.5; 126.4) | Cervical | US-Guided FNAB | Doppler criteria |
| Emean | 25.0 kPa (IQR: 19.3; 86.2) | |||||||||||
| Tourasse et al. [33] | 2012 | Prospective | 65 | - | 81 | SuperSonic Imagine device (Aix en Provence, France) | N/A | Emax | 6.71–44.18 (mean = 23.27) | Axillary | SLNB | - |
| Emean | 6.24–29.72 (mean = 17.47) | |||||||||||
| Esd | 0.3–9.7 (mean = 2.95) |
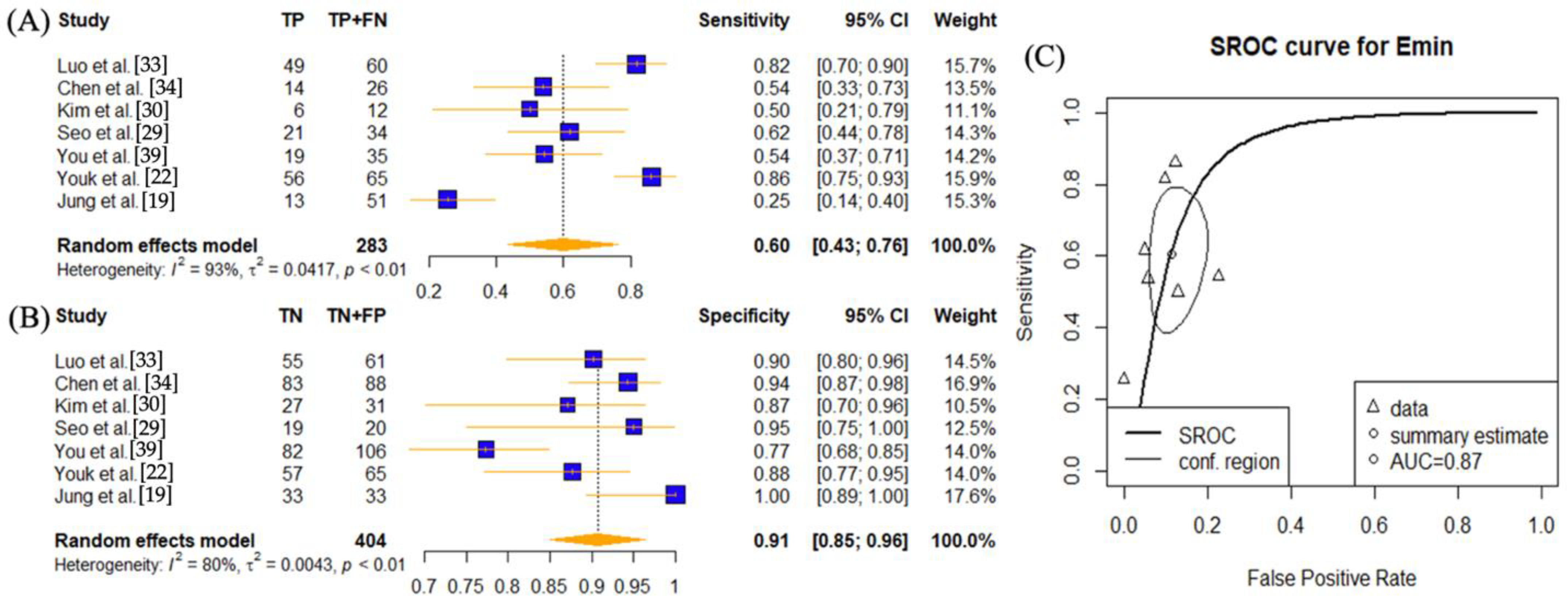
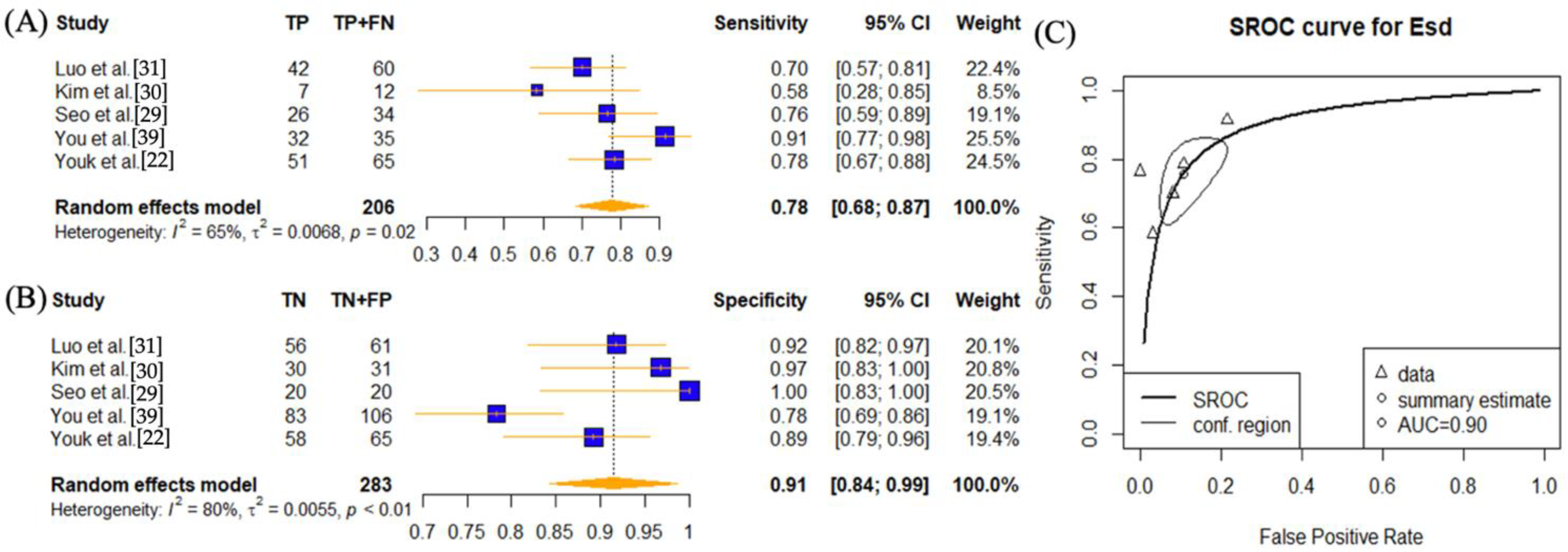
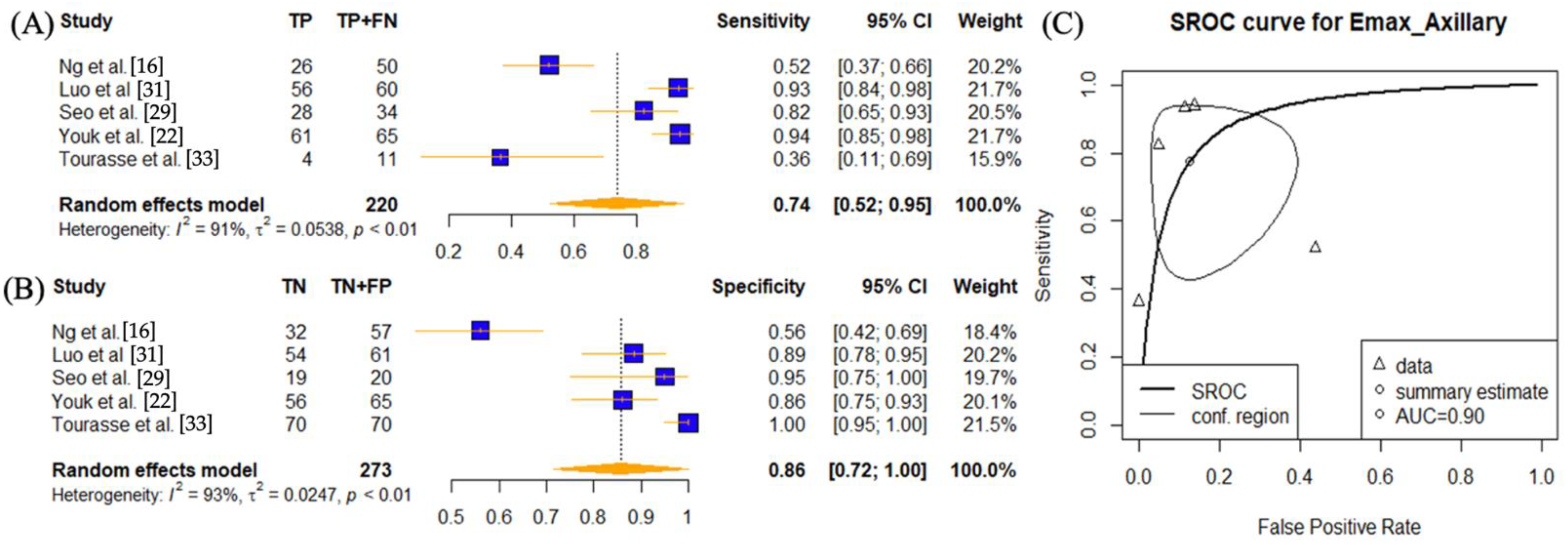
| SWE Parameter | Cutoff Values | Number of Lymph Node Lesions | Number of Disease Positive Lymph Nodes | TP | FP | TN | FN | Sensitivity | Spec | PPV | NPV | Accuracy | AUC | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ng et al. [16] | 2022 | Emax | 15.2 | 107 | 50 | 26 | 25 | 32 | 24 | 0.52 | 0.56 | 0.51 | 0.57 | 0.54 | 0.61 |
| Chami et al. [37] | 2021 | Emean | 15.2 | 222 | 151 | 66 | 12 | 59 | 85 | 0.44 | 0.83 | 0.85 | 0.41 | 0.56 | 0.66 (95% CI: 0.59–0.73) |
| Yang et al. [32] | 2021 | Emax | 31.6 | 109 | 66 | 47 | 1 | 24 | 29 | 0.56 | 0.96 | 0.97 | 0.45 | 0.65 | 0.825 (95% CI 0.741–0.891) |
| Lo et al. [38] | 2019 | Emax | 42 | 109 | 24 | 20 | 30 | 55 | 4 | 0.83 | 0.65 | 0.40 | 0.93 | 0.69 | 0.688 (0.601–0.775) |
| Luo et al. [31] | 2019 | Emax | 26.05 | 121 | 60 | 56 | 7 | 54 | 4 | 0.93 | 0.89 | 0.89 | 0.93 | 0.91 | 0.94 |
| Emean | 26.9 | 121 | 60 | 52 | 2 | 59 | 8 | 0.87 | 0.97 | 0.96 | 0.88 | 0.92 | 0.95 | ||
| Emin | 22.75 | 121 | 60 | 49 | 6 | 55 | 11 | 0.82 | 0.90 | 0.89 | 0.83 | 0.86 | 0.91 | ||
| Esd | 2.05 | 121 | 60 | 42 | 5 | 56 | 18 | 0.70 | 0.92 | 0.89 | 0.76 | 0.81 | 0.83 | ||
| Chen et al. [34] | 2018 | Emax | 20.6 | 114 | 26 | 26 | 44 | 44 | 0 | 1.00 | 0.50 | 0.37 | 1.00 | 0.61 | 0.82 |
| Emean | 18.4 | 114 | 26 | 22 | 15 | 73 | 4 | 0.85 | 0.83 | 0.59 | 0.95 | 0.83 | 0.88 | ||
| Emin | 15.5 | 114 | 26 | 14 | 5 | 83 | 12 | 0.54 | 0.94 | 0.74 | 0.87 | 0.85 | 0.80 | ||
| Kim et al. [30] | 2018 | Emax | 37.5 | 43 | 12 | 10 | 1 | 30 | 2 | 0.83 | 0.97 | 0.91 | 0.94 | 0.93 | 0.93 |
| Emean | 23 | 43 | 12 | 8 | 1 | 30 | 4 | 0.67 | 0.97 | 0.89 | 0.88 | 0.88 | 0.94 | ||
| Emin | 11.7 | 43 | 12 | 6 | 4 | 27 | 6 | 0.50 | 0.87 | 0.60 | 0.82 | 0.77 | 0.70 | ||
| Esd | 6.9 | 43 | 12 | 7 | 1 | 30 | 5 | 0.58 | 0.97 | 0.88 | 0.86 | 0.86 | 0.77 | ||
| Seo et al. [29] | 2018 | Emax | 20.9 | 54 | 34 | 28 | 1 | 19 | 6 | 0.82 | 0.95 | 0.97 | 0.76 | 0.87 | 0.89 |
| Emean | 23.8 | 54 | 34 | 26 | 0 | 20 | 8 | 0.76 | 1.00 | 1.00 | 0.71 | 0.85 | 0.88 | ||
| Emin | 11.4 | 54 | 34 | 21 | 1 | 19 | 13 | 0.62 | 0.95 | 0.95 | 0.59 | 0.74 | 0.78 | ||
| Esd | 4.05 | 54 | 34 | 26 | 0 | 20 | 8 | 0.76 | 1.00 | 1.00 | 0.71 | 0.85 | 0.88 | ||
| You et al. [39] | 2018 | Emax | 40.2 | 141 | 35 | 28 | 7 | 99 | 7 | 0.80 | 0.93 | 0.80 | 0.93 | 0.90 | 0.92 |
| Emean | 22.1 | 141 | 35 | 26 | 12 | 94 | 9 | 0.74 | 0.89 | 0.68 | 0.91 | 0.85 | 0.87 | ||
| Emin | 12.4 | 141 | 35 | 19 | 24 | 82 | 16 | 0.54 | 0.77 | 0.44 | 0.84 | 0.72 | 0.61 | ||
| Esd | 4.1 | 141 | 35 | 32 | 23 | 83 | 3 | 0.91 | 0.78 | 0.58 | 0.97 | 0.82 | 0.92 | ||
| Tan et al. [40] | 2017 | Emax | 37.9 | 42 | 34 | 18 | 4 | 16 | 4 | 0.82 | 0.80 | 0.82 | 0.80 | 0.81 | 0.845 (0.701–0.938) |
| Emean | 15.5 | 42 | 34 | 14 | 0 | 20 | 8 | 0.64 | 1.00 | 1.00 | 0.71 | 0.81 | 0.732 (0.573–0.857) | ||
| Esd | 6.3 | 42 | 34 | 18 | 6 | 14 | 4 | 0.82 | 0.70 | 0.75 | 0.78 | 0.76 | 0.777 (0.622–0.891) | ||
| Youk et al. [22] | 2017 | Emax | 25.8 | 130 | 65 | 61 | 9 | 56 | 4 | 0.94 | 0.86 | 0.87 | 0.93 | 0.90 | 0.941 (0.885, 0.974) |
| Emean | 18.7 | 130 | 65 | 61 | 8 | 57 | 4 | 0.94 | 0.88 | 0.88 | 0.93 | 0.91 | 0.946 (0.892, 0.978) | ||
| Emin | 12.3 | 130 | 65 | 56 | 8 | 57 | 9 | 0.86 | 0.88 | 0.88 | 0.86 | 0.87 | 0.915 (0.853, 0.956) | ||
| Esd | 4 | 130 | 65 | 51 | 7 | 58 | 14 | 0.78 | 0.89 | 0.88 | 0.81 | 0.84 | 0.900 (0.835, 0.945) | ||
| Desmots et al. [41] | 2016 | Emax | 31 | 62 | 30 | 26 | 4 | 28 | 4 | 0.87 | 0.88 | 0.87 | 0.88 | 0.87 | 0.903 ± 0.042 |
| Jung et al. [19] | 2015 | Emax | 57 | 84 | 51 | 43 | 23 | 10 | 8 | 0.84 | 0.30 | 0.65 | 0.56 | 0.63 | 0.738 (0.633–0.843) |
| Emean | 29 | 84 | 51 | 39 | 11 | 22 | 12 | 0.76 | 0.67 | 0.78 | 0.65 | 0.73 | 0.748 (0.644–0.852) | ||
| Emin | 24 | 84 | 51 | 13 | 0 | 33 | 38 | 0.25 | 1.00 | 1.00 | 0.46 | 0.55 | 0.737 (0.632–0.842) | ||
| Choi et al. [35] | 2013 | Emax | 19.44 | 67 | 34 | 31 | 1 | 32 | 3 | 0.91 | 0.97 | 0.97 | 0.91 | 0.94 | 0.96 (95% CI: 0.885, 0.993) |
| Bhatia et al. [12] | 2012 | Emax | 45 | 55 | 31 | 15 | 2 | 22 | 16 | 0.48 | 0.92 | 0.88 | 0.58 | 0.67 | 0.77 (95% CI 5 0.57–0.83) |
| Emean | 30.2 | 55 | 31 | 13 | 0 | 24 | 18 | 0.42 | 1.00 | 1.00 | 0.57 | 0.62 | 0.77 (95% CI 5 0.57–0.83) | ||
| Tourasse et al. [33] | 2012 | Emax | 26.4704 | 81 | 11 | 4 | 0 | 70 | 7 | 0.36 | 1.00 | 1.00 | 0.91 | 0.91 | 0.75 (95% CI: 0.55–0.95) |
| Emean | 23.5947 | 81 | 11 | 2 | 0 | 70 | 9 | 0.18 | 1.00 | 1.00 | 0.89 | 0.89 | 0.76 (95% CI: 0.58–0.94) |
| QUADAS | ||||||||
|---|---|---|---|---|---|---|---|---|
| Risk of Bias | Applicability Concerns | |||||||
| Study | Overall Diagnostic Quality | Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard |
| Ng et al. [16] | Fair | Unclear | Low | Low | Low | Low | Low | Low |
| Chami et al. [37] | Good | Low | Low | Low | Low | Low | Low | Low |
| Yang et al. [32] | Good | Low | Low | Low | Low | Low | Low | Low |
| Lo et al. [38] | Good | Low | Low | Low | Low | Low | Low | Low |
| Luo et al. [31] | Good | Low | Low | Low | Low | Low | Low | Low |
| Chen et al. [34] | Good | Low | Low | Low | Low | Low | Low | Low |
| Kim et al. [30] | Good | Low | Low | Low | Low | Low | Low | Low |
| Seo et al. [29] | Good | Low | Low | Low | Low | Low | Low | Low |
| You et al. [39] | Fair | Low | Low | Low | Unclear | Low | Low | Low |
| Tan et al. [40] | Good | Low | Low | Low | Low | Low | Low | Low |
| Youk et al. [22] | Fair | Low | Low | Low | Unclear | Low | Low | Low |
| Desmots et al. [41] | Good | Low | Low | Low | Low | Low | Low | Low |
| Jung et al. [19] | Good | Low | Low | Low | Low | Low | Low | Low |
| Choi et al. [35] | Good | Low | Low | Low | Low | Low | Low | Low |
| Bhatia et al. [12] | Good | Low | Low | Low | Low | Low | Low | Low |
| Tourasse et al. [33] | Fair | Low | Low | Low | Unclear | Low | Low | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Zhao, Y.; Choi, S.; Chaurasia, A.; Ding, H.; Haroon, A.; Wan, S.; Adeleke, S. Evaluating Different Quantitative Shear Wave Parameters of Ultrasound Elastography in the Diagnosis of Lymph Node Malignancies: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 5568. https://doi.org/10.3390/cancers14225568
Gao Y, Zhao Y, Choi S, Chaurasia A, Ding H, Haroon A, Wan S, Adeleke S. Evaluating Different Quantitative Shear Wave Parameters of Ultrasound Elastography in the Diagnosis of Lymph Node Malignancies: A Systematic Review and Meta-Analysis. Cancers. 2022; 14(22):5568. https://doi.org/10.3390/cancers14225568
Chicago/Turabian StyleGao, Yujia, Yi Zhao, Sunyoung Choi, Anjalee Chaurasia, Hao Ding, Athar Haroon, Simon Wan, and Sola Adeleke. 2022. "Evaluating Different Quantitative Shear Wave Parameters of Ultrasound Elastography in the Diagnosis of Lymph Node Malignancies: A Systematic Review and Meta-Analysis" Cancers 14, no. 22: 5568. https://doi.org/10.3390/cancers14225568
APA StyleGao, Y., Zhao, Y., Choi, S., Chaurasia, A., Ding, H., Haroon, A., Wan, S., & Adeleke, S. (2022). Evaluating Different Quantitative Shear Wave Parameters of Ultrasound Elastography in the Diagnosis of Lymph Node Malignancies: A Systematic Review and Meta-Analysis. Cancers, 14(22), 5568. https://doi.org/10.3390/cancers14225568








