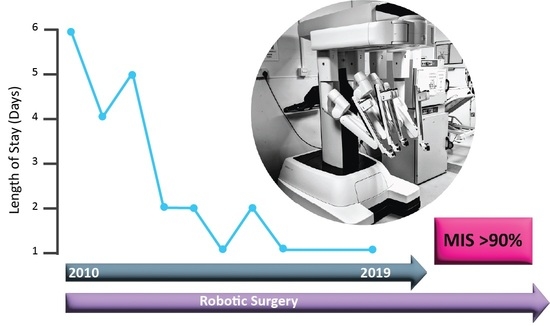
Enhanced Recovery after Uterine Corpus Cancer Surgery: A 10 Year Retrospective Cohort Study of Robotic Surgery in an NHS Cancer Centre
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Body Mass Index (BMI)
3.2. Age
3.3. Length of Surgery
3.4. Survival
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.A.; Higham-Kessler, J.; Yokoe, D.S.; Butler, A.M.; Vostok, J.; Stevenson, K.B.; Khan, Y.; Fraser, V.J.; Prevention Epicenter Program; Centers for Disease Control and Prevention. Developing a risk stratification model for surgical site infection after abdominal hysterectomy. Infect. Control. Hosp. Epidemiol. 2009, 30, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Nugent, E.K.; Hoff, J.T.; Gao, F.; Massad, L.S.; Case, A.; Zighelboim, I.; Mutch, D.G.; Thaker, P.H. Wound complications after gynecologic cancer surgery. Gynecol. Oncol. 2011, 121, 347–352. [Google Scholar] [CrossRef]
- Parkin, L.; Sweetland, S.; Balkwill, A.; Green, J.; Reeves, G.; Beral, V.; Million Women Study, C. Body mass index, surgery, and risk of venous thromboembolism in middle-aged women: A cohort study. Circulation 2012, 125, 1897–1904. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Sundar, S.B.J.; Crosbie, E.; Drake, A.; Edmondson, R.; Fotopoulou, C.; Gallos, I.; Ganesan, R.; Gupta, J.; Johnson, N.; Kitson, S.; et al. BGCS Uterine Cancer Guidelines: Recommendations for Practice. Available online: https://www.bgcs.org.uk/wp-content/uploads/2019/05/BGCS-Endometrial-Guidelines-2017.pdf (accessed on 25 January 2021).
- Nelson, G.; Bakkum-Gamez, J.; Kalogera, E.; Glaser, G.; Altman, A.; Meyer, L.A.; Taylor, J.S.; Iniesta, M.; Lasala, J.; Mena, G.; et al. Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery After Surgery (ERAS) Society recommendations-2019 update. Int. J. Gynecol. Cancer 2019, 29, 651–668. [Google Scholar] [CrossRef]
- Simpson, A.N.; Sutradhar, R.; Ferguson, S.E.; Robertson, D.; Cheng, S.Y.; Li, Q.; Baxter, N.N. Perioperative outcomes of women with and without class III obesity undergoing hysterectomy for endometrioid endometrial cancer: A population-based study. Gynecol. Oncol. 2020, 158, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Stephan, J.M.; Goodheart, M.J.; McDonald, M.; Hansen, J.; Reyes, H.D.; Button, A.; Bender, D. Robotic surgery in supermorbidly obese patients with endometrial cancer. Am. J. Obstet. Gynecol. 2015, 213, 49.e1–49.e8. [Google Scholar] [CrossRef]
- Janda, M.; Gebski, V.; Davies, L.C.; Forder, P.; Brand, A.; Hogg, R.; Jobling, T.W.; Land, R.; Manolitsas, T.; Nascimento, M.; et al. Effect of Total Laparoscopic Hysterectomy vs Total Abdominal Hysterectomy on Disease-Free Survival Among Women With Stage I Endometrial Cancer: A Randomized Clinical Trial. JAMA 2017, 317, 1224–1233. [Google Scholar] [CrossRef]
- Walker, J.L.; Piedmonte, M.R.; Spirtos, N.M.; Eisenkop, S.M.; Schlaerth, J.B.; Mannel, R.S.; Spiegel, G.; Barakat, R.; Pearl, M.L.; Sharma, S.K. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J. Clin. Oncol. 2009, 27, 5331–5336. [Google Scholar] [CrossRef]
- Walker, J.L.; Piedmonte, M.R.; Spirtos, N.M.; Eisenkop, S.M.; Schlaerth, J.B.; Mannel, R.S.; Barakat, R.; Pearl, M.L.; Sharma, S.K. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J. Clin. Oncol. 2012, 30, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Cusimano, M.C.; Simpson, A.N.; Dossa, F.; Liani, V.; Kaur, Y.; Acuna, S.A.; Robertson, D.; Satkunaratnam, A.; Bernardini, M.Q.; Ferguson, S.E.; et al. Laparoscopic and robotic hysterectomy in endometrial cancer patients with obesity: A systematic review and meta-analysis of conversions and complications. Am. J. Obstet. Gynecol. 2019, 221, 410–428.e419. [Google Scholar] [CrossRef] [PubMed]
- Fornalik, H.; Zore, T.; Fornalik, N.; Foster, T.; Katschke, A.; Wright, G. Can Teamwork and High-Volume Experience Overcome Challenges of Lymphadenectomy in Morbidly Obese Patients (Body Mass Index of 40 kg/m2 or Greater) with Endometrial Cancer?: A Cohort Study of Robotics and Laparotomy and Review of Literature. Int. J. Gynecol. Cancer 2018, 28, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Uwins, C.; Tailor, A.; Chatterjee, J.; Ellis, P.; Madhuri, T.; Michael, A.; Butler-Manuel, S. Robotic corpus cancer surgery: Ten year mortality data from the UK epicentre in guildford. Int. J. Gynecol. Cancer 2020, 30, A31–A32. [Google Scholar]
- Park, H.K.; Helenowski, I.B.; Berry, E.; Lurain, J.R.; Neubauer, N.L. A Comparison of Survival and Recurrence Outcomes in Patients With Endometrial Cancer Undergoing Robotic Versus Open Surgery. J. Minim. Invasive Gynecol. 2015, 22, 961–967. [Google Scholar] [CrossRef]
- Kilgore, J.E.; Jackson, A.L.; Ko, E.M.; Soper, J.T.; Van Le, L.; Gehrig, P.A.; Boggess, J.F. Recurrence-free and 5-year survival following robotic-assisted surgical staging for endometrial carcinoma. Gynecol. Oncol. 2013, 129, 49–53. [Google Scholar] [CrossRef]
- Mäenpää, M.M.; Nieminen, K.; Tomás, E.I.; Laurila, M.; Luukkaala, T.; Mäenpää, J.U. Robotic-assisted vs traditional laparoscopic surgery for endometrial cancer: A randomized controlled trial. Am. J. Obstet. Gynecol. 2016, 215, 588.e1–588.e7. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Wu, H.; Zhang, Y.; Wang, F. A Meta-Analysis of Robotic Surgery in Endometrial Cancer: Comparison with Laparoscopy and Laparotomy. Dis. Markers 2020, 2020, 2503753. [Google Scholar] [CrossRef]
- Muaddi, H.; Hafid, M.E.; Choi, W.J.; Lillie, E.; de Mestral, C.; Nathens, A.; Stukel, T.A.; Karanicolas, P.J. Clinical Outcomes of Robotic Surgery Compared to Conventional Surgical Approaches (Laparoscopic or Open): A Systematic Overview of Reviews. Ann. Surg. 2021, 273, 467–473. [Google Scholar] [CrossRef]
- Office for National Statistics. Cancer Survival by Stage at Diagnosis for England. Available online: www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/cancersurvivalratescancersurvivalinenglandadultsdiagnosed (accessed on 21 February 2021).
- Bourgin, C.; Lambaudie, E.; Houvenaeghel, G.; Foucher, F.; Leveque, J.; Lavoue, V. Impact of age on surgical staging and approaches (laparotomy, laparoscopy and robotic surgery) in endometrial cancer management. Eur. J. Surg. Oncol. 2017, 43, 703–709. [Google Scholar] [CrossRef]
- Bishop, E.A.; Java, J.J.; Moore, K.N.; Walker, J.L. Pathologic and Treatment Outcomes Among a Geriatric Population of Endometrial Cancer Patients: An NRG Oncology/Gynecologic Oncology Group Ancillary Data Analysis of LAP2. Int. J. Gynecol. Cancer 2017, 27, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Lindfors, A.; Akesson, A.; Staf, C.; Sjoli, P.; Sundfeldt, K.; Dahm-Kahler, P. Robotic vs Open Surgery for Endometrial Cancer in Elderly Patients: Surgical Outcome, Survival, and Cost Analysis. Int. J. Gynecol. Cancer 2018, 28, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Bishop, E.A.; Java, J.J.; Moore, K.N.; Spirtos, N.M.; Pearl, M.L.; Zivanovic, O.; Kushner, D.M.; Backes, F.; Hamilton, C.A.; Geller, M.A.; et al. Surgical outcomes among elderly women with endometrial cancer treated by laparoscopic hysterectomy: A NRG/Gynecologic Oncology Group study. Am. J. Obstet. Gynecol. 2018, 218, 109.e1–109.e11. [Google Scholar] [CrossRef] [PubMed]
- Uccella, S.; Bonzini, M.; Palomba, S.; Fanfani, F.; Malzoni, M.; Ceccaroni, M.; Seracchioli, R.; Ferrero, A.; Berretta, R.; Vizza, E.; et al. Laparoscopic vs. open treatment of endometrial cancer in the elderly and very elderly: An age-stratified multicenter study on 1606 women. Gynecol. Oncol. 2016, 141, 211–217. [Google Scholar] [CrossRef]
- Lavoue, V.; Zeng, X.; Lau, S.; Press, J.Z.; Abitbol, J.; Gotlieb, R.; How, J.; Wang, Y.; Gotlieb, W.H. Impact of robotics on the outcome of elderly patients with endometrial cancer. Gynecol. Oncol. 2014, 133, 556–562. [Google Scholar] [CrossRef]
- Bogani, G.; Cromi, A.; Uccella, S.; Serati, M.; Casarin, J.; Pinelli, C.; Ghezzi, F. Perioperative and long-term outcomes of laparoscopic, open abdominal, and vaginal surgery for endometrial cancer in patients aged 80 years or older. Int. J. Gynecol. Cancer 2014, 24, 894–900. [Google Scholar] [CrossRef]
- Frey, M.K.; Ihnow, S.B.; Worley, M.J., Jr.; Heyman, K.P.; Kessler, R.; Slomovitz, B.M.; Holcomb, K.M. Minimally invasive staging of endometrial cancer is feasible and safe in elderly women. J. Minim. Invasive Gynecol. 2011, 18, 200–204. [Google Scholar] [CrossRef]
- NCIN. Outline of Uterine Cancer in the United Kingdom: Incidence, Mortality and Survival. Available online: http://www.ncin.org.uk/cancer_type_and_topic_specific_work/cancer_type_specific_work/gynaecological_cancer/gynaecological_cancer_hub/resources/uterine_cancer (accessed on 13 July 2021).
- Jorgensen, S.L.; Mogensen, O.; Wu, C.S.; Korsholm, M.; Lund, K.; Jensen, P.T. Survival after a nationwide introduction of robotic surgery in women with early-stage endometrial cancer: A population-based prospective cohort study. Eur. J. Cancer 2019, 109, 1–11. [Google Scholar] [CrossRef]
- Levy, B.F.; Scott, M.J.; Fawcett, W.J.; Rockall, T.A. 23-hour-stay laparoscopic colectomy. Dis. Colon Rectum 2009, 52, 1239–1243. [Google Scholar] [CrossRef]
- Fawcett, W.J.; Mythen, M.G.; Scott, M.J. Enhanced recovery: More than just reducing length of stay? Br. J. Anaesth. 2012, 109, 671–674. [Google Scholar] [CrossRef]
- Jones, C.; Smith, R.L.; Fawcett, W.J. Optimal analgesia for laparoscopic colorectal surgery in an enhanced recovery programme. Br. J. Hosp. Med. 2012, 73, 178. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Kelliher, L.; Dickinson, M.; Riga, A.; Worthington, T.; Scott, M.J.; Vandrevala, T.; Fry, C.H.; Karanjia, N.; Quiney, N. Randomized clinical trial on enhanced recovery versus standard care following open liver resection. Br. J. Surg. 2013, 100, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- National Health Service (NHS). National Schedule of Reference Costs 2017/18; NHS: Redditch, UK, 2018.
- National Health Service (NHS). NHS Reference Costs 2012/13; NHS: Redditch, UK, 2013.
- NICE. NICE National Costing Statement: Blood Transfusion (November 2015); NICE: London, UK, 2015. [Google Scholar]
- Rouanet, P.; Mermoud, A.; Jarlier, M.; Bouazza, N.; Laine, A.; Mathieu Daude, H. Combined robotic approach and enhanced recovery after surgery pathway for optimization of costs in patients undergoing proctectomy. BJS Open 2020, 4, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Straatman, J.; Cuesta, M.A.; de Lange-de Klerk, E.S.; van der Peet, D.L. Hospital cost-analysis of complications after major abdominal surgery. Dig. Surg. 2015, 32, 150–156. [Google Scholar] [CrossRef] [PubMed]
- NICE. NICE National Costing Statement: Implementing the NICE Guideline on Transition between Inpatient Hospitalsettings and Community or Care Homesettings for Adults with Social Care Needs (December 2015); NICE: London, UK, 2015. [Google Scholar]
- Volpin, A.; Khan, O.; Haddad, F.S. Theater Cost Is pound16/Minute So What Are You Doing Just Standing There? J. Arthroplast. 2016, 31, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Kitchener, H.; Swart, A.M.; Qian, Q.; Amos, C.; Parmar, M.K. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): A randomised study. Lancet 2009, 373, 125–136. [Google Scholar] [CrossRef]
- de Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; Colombo, A.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): Final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 295–309. [Google Scholar] [CrossRef]
- Nout, R.A.; Smit, V.T.; Putter, H.; Jurgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Mens, J.W.; Slot, A.; Kroese, M.C.; et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): An open-label, non-inferiority, randomised trial. Lancet 2010, 375, 816–823. [Google Scholar] [CrossRef]
- Creutzberg, C.L.; van Putten, W.L.; Koper, P.C.; Lybeert, M.L.; Jobsen, J.J.; Warlam-Rodenhuis, C.C.; De Winter, K.A.; Lutgens, L.C.; van den Bergh, A.C.; van de Steen-Banasik, E.; et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: Multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet 2000, 355, 1404–1411. [Google Scholar] [CrossRef]
- Moss, E.L.; Morgan, G.; Martin, A.P.; Sarhanis, P.; Ind, T. Surgical trends, outcomes and disparities in minimal invasive surgery for patients with endometrial cancer in England: A retrospective cohort study. BMJ Open 2020, 10, e036222. [Google Scholar] [CrossRef]




| Corpus Cancer Primary Urgery | 2010–2019 | ||||||
|---|---|---|---|---|---|---|---|
| 2008/2009 | Overall | Robotic | Other MIS | Open | Comparative Data | ||
| Number (n) | 62 | 952 | 734 (77.1%) | 54 (5.7%) | 164 (17.2%) | Laparotomy | Laparoscopy |
| Median Age | 67.5 | 68.0 | 67.0 | 70.5 | 69.0 | 63.1 yrs $ | 63.3 yrs $ |
| Age range | 33–89 | 31–91 | 31–90 | 35–91 | 37–9 | ||
| Median ASA | 2 | 2 | 2 | 2 | 2 | ||
| ASA range | 1–4 | 1–4 | 1–4 | 1–3 | 1–4 | ||
| ASA1 | 35.5% (22) | 9.7% (92) | 10.8% (79) | 5.6% (3) | 6.1% (10) | ||
| ASA2 | 45.2% (28) | 65.0% (619) | 63.8% (468) | 66.7% (36) | 70.1% (115) | ||
| ASA ≥ 3 | 19.4% (12) | 22.5% (214) | 23.4% (172) | 22.2% (12) | 18.3% (30) | ||
| ASA Data unavailable (n) | 0 | 2.8% (27) | 2.0% (15) | 5.6% (3) | 5.5% (9) | ||
| BMI Median | 29.9 | 30.5 | 31.1 | 29.0 | 27.7 | ||
| BMI Range | 17.7–54.4 | 16.1–75.2 | 16.4–75.2 | 18.6–55.0 | 16.1–51.4 | ||
| BMI Data unavailable (n) | 12.9% (8) | 3.9% (37) | 1.6% (12) | 13.0% (7) | 11.0% (18) | ||
| Stage I | 66.1% (41) | 72.7% (692) | 79.8% (586) | 75.9% (41) | 39.6% (65) | ||
| Stage II | 14.5% (9) | 7.7% (73) | 7.4% (54) | 7.4% (4) | 9.2% (15) | ||
| Stage III | 12.9% (8) | 13.6% (129) | 9.8% (72) | 13.0% (7) | 30.5% (50) | ||
| Stage IV | 6.5% (4) | 6.0% (57) | 3.0% (22) | 3.7% (2) | 20.1% (33) | ||
| Unknown | 0 | 0.1% (1) | 0 | 0 | 0.6% (1) | ||
| Grade 1 | 32.3% (20) | 31.1% (296) | 35.3% (259) | 33.3% (18) | 11.6% (19) | 63.2% $ | 63.6% $ |
| Grade 2 | 29.0% (18) | 29.4% (280) | 32.4% (238) | 25.9% (14) | 17.1% (28) | 30.3% $ | 29.5% $ |
| Grade 3 | 38.7% (24) | 39.5% (376) | 32.3% (237) | 40.7% (22) | 71.3% (117) | 6.5 $ | 6.9% $ |
| Endometrioid | 74.2% (46) | 70.8% (674) | 77.4% (568) | 66.7% (36) | 42.7% (70) | 76.9% * | |
| Serous | 9.7% (6) | 14.5% (138) | 12.1% (89) | 14.8% (8) | 25.0% (41) | 7.4% * Combined | |
| Clear Cell | 4.8% (3) | 2.2% (21) | 1.9% (14) | 1.9% (1) | 3.7% (6) | ||
| Adenosarcoma/Leiomyosarcoma | 0 | 2.7% (26) | 1.5% (11) | 1.9% (1) | 8.5% (14) | 3.4% * | |
| MMMT | 9.7% (6) | 7.5% (71) | 5.2% (38) | 11.1% (6) | 16.5% (27) | 6.2% * | |
| Other | 1.6% (1) | 2.3% (22) | 1.9% (14) | 3.7% (2) | 3.7% (6) | ||
| Any Lymph node sampling/dissection | 64.5% (40) | 64.3% (612) | 64.2% (471) | 55.6% (30) | 67.7% (111) | ||
| Pelvic nodes | 64.5% (40) | 60.0% (571) | 59.4% (436) | 55.6% (30) | 64.0% (105) | ||
| Para-aortic | 46.8% (29) | 20.6% (196) | 15.8% (116) | 9.3% (5) | 45.7% (75) | ||
| Omentectomy/biopsy | 17.7% (11) | 23.0% (219) | 14.4% (106) | 24.1% (13) | 61.0% (100) | ||
| Median EBL (ml) | 300 | 70 | 50 | 100 | 500 | ||
| Range (ml) | 50–3800 | 0–8000 | 0–2500 | 10–8000 | 50–8000 | ||
| EBL Data unavailable (n) | 2 | 2.6% (25) | 3.0% (22) | 3.7% (2) | 0.6% (1) | ||
| Median LOS | 6 | 1 | 1 | 2 | 6 | ||
| Range LOS | 1–59 | 0–84 | 0–84 | 0–17 | 1–42 | ||
| Return to Theatre <30 Days | 1.6% (1) | 0.7% (7) | 0.5% (4) | 1.9% (1) | 1.2% (2) | ||
| 30 Day Mortality | 0 | 0.6% (6) | 0.1% (1) | 1.9% (1) | 2.4% (4) | ||
| Required any blood Transfusion | 5.4% (51) | 1.8% (13) | 13.0% (7) | 18.9% (31) | |||
| Required any ITU admission | 15.5% (148) | 7.2% (53) | 18.5% (10) | 51.8% (85) | |||
| Conversion to open | 21.4% (3/14) | 0.5% (4) | 24.1% (13) | ||||
| Post Operative 30 day Morbidity | |||||||
| Clavien-Dindo Grade II | 9.6% (91) | 5.3% (39) | 33.3% (18) | 20.7% (34) | |||
| Clavien-Dindo Grade III | 1.5% (14) | 1.0% (7) | 5.6% (3) | 2.4% (4) | |||
| Clavien-Dindo Grade IV | 0.4% (4) | 0.4% (3) | 0 | 0.6% (1) | |||
| Clavien-Dindo Grade V | 0.6% (6) | 0.1% (1) | 1.9% (1) | 2.4% (4) | |||
| BMI | (n) | Median Age | Median EBL (Range) | Blood Transfusion | ITU Use | Median LOS Days (Range) | Conversion (n) | Reason for Conversion | ||
|---|---|---|---|---|---|---|---|---|---|---|
| <25 | 138 | 66 | 50 mL | (0–300) | 0.72% | 2.17% | 1 | (1–31) | ||
| 25–29.9 | 179 | 72 | 50 mL | (8–400) | 1.12% | 5.03% | 1 | (0–84) | 1 | Adhesions |
| 30–34.9 | 151 | 69 | 50 mL | (10–800) | 1.99% | 1.32% | 1 | (0–11) | 1 | Adhesions |
| 35–39.9 | 116 | 67 | 75 mL | (5–2500) | 1.72% | 6.90% | 1 | (0–17) | 1 | Vascular injury |
| 40–44.9 | 67 | 64 | 75 mL | (10–940) | 1.49% | 11.94% | 1 | (0–5) | ||
| 45–49.9 | 44 | 65 | 80 mL | (15–1500) | 4.55% | 22.73% | 1 | (0–11) | ||
| 50+ | 27 | 60 | 150 mL | (20–1900) | 7.41% | 48.15% | 1 | (0–8) | 1 | Vascular injury |
| Age | (n) | Median BMI | Median EBL (Range) | Blood Transfusion | ITU Use | Median LOS Days (Range) | ||
|---|---|---|---|---|---|---|---|---|
| <55 | 88 | 33.41 | 50 | (10–1900) | 3.41% | 5.68% | 1 | (0–8) |
| 55–59 | 102 | 30.84 | 50 | (0–400) | 0% | 8.82% | 1 | (0–9) |
| 60–64 | 114 | 32.58 | 50 | (10–1700) | 0% | 6.14% | 1 | (0–8) |
| 65–69 | 117 | 33.43 | 50 | (8–1300) | 3.42% | 6.84% | 1 | (0–31) |
| 70–74 | 149 | 30.67 | 50 | (10–2500) | 1.34% | 6.04% | 1 | (0–17) |
| 75–79 | 84 | 28.28 | 50 | (10–1500) | 3.57% | 10.71% | 1 | (0–11) |
| 80+ | 80 | 29.03 | 50 | (5–300) | 1.25% | 7.50% | 1 | (0–84) |
| 2010–2019 | Overall | Robotic 2010–2019 | Robotic 2015–2019 | Other MIS | Open |
|---|---|---|---|---|---|
| Corpus Cancer primary surgery (n) | 952 | 734 (77.10%) | 487 | 54 (5.67%) | 164 (17.23%) |
| Median Length of surgery (HH:MM) | 02:53 | 02:54 | 02:37 | 02:54 | 02:50 |
| Range | 00:32–07:03 | 00:32–07:03 | 00:32–05:45 | 00:53–05:53 | 01:05–06:44 |
| Data Missing (n) | 66 | 22 | 19 | 13 | 31 |
| Median Length of surgery no nodes (n) | 02:37 (340) | 02:41 (263) | 02:27 (169) | 02:15 (24) | 02:31 (53) |
| Median length of surgery any nodes (n) | 03:00 (612) | 03:00 (471) | 02:43 (318) | 03:23 (30) | 02:53 (111) |
| Median length of surgery with pelvic nodes not para-aortic nodes (n) a | 03:00 (387) | 03:00 (326) | 02:40 (205) | 03:15 (25) | 02:30 (36) |
| Median length of surgery with para-aortic nodes (n) a | 03:07 (196) | 03:10 (116) | 03:00 (85) | 04:07 (5) | 02:57 (75) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uwins, C.; Hablase, R.; Assalaarachchi, H.; Tailor, A.; Stewart, A.; Chatterjee, J.; Ellis, P.; Skene, S.S.; Michael, A.; Butler-Manuel, S. Enhanced Recovery after Uterine Corpus Cancer Surgery: A 10 Year Retrospective Cohort Study of Robotic Surgery in an NHS Cancer Centre. Cancers 2022, 14, 5463. https://doi.org/10.3390/cancers14215463
Uwins C, Hablase R, Assalaarachchi H, Tailor A, Stewart A, Chatterjee J, Ellis P, Skene SS, Michael A, Butler-Manuel S. Enhanced Recovery after Uterine Corpus Cancer Surgery: A 10 Year Retrospective Cohort Study of Robotic Surgery in an NHS Cancer Centre. Cancers. 2022; 14(21):5463. https://doi.org/10.3390/cancers14215463
Chicago/Turabian StyleUwins, Christina, Radwa Hablase, Hasanthi Assalaarachchi, Anil Tailor, Alexandra Stewart, Jayanta Chatterjee, Patricia Ellis, Simon S. Skene, Agnieszka Michael, and Simon Butler-Manuel. 2022. "Enhanced Recovery after Uterine Corpus Cancer Surgery: A 10 Year Retrospective Cohort Study of Robotic Surgery in an NHS Cancer Centre" Cancers 14, no. 21: 5463. https://doi.org/10.3390/cancers14215463
APA StyleUwins, C., Hablase, R., Assalaarachchi, H., Tailor, A., Stewart, A., Chatterjee, J., Ellis, P., Skene, S. S., Michael, A., & Butler-Manuel, S. (2022). Enhanced Recovery after Uterine Corpus Cancer Surgery: A 10 Year Retrospective Cohort Study of Robotic Surgery in an NHS Cancer Centre. Cancers, 14(21), 5463. https://doi.org/10.3390/cancers14215463