The Monocyte, a Maestro in the Tumor Microenvironment (TME) of Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Introduction to BC
1.2. Introduction to the Tumor Microenvironment (TME)
2. Methods
3. Results
3.1. Monocytes’ Classification in the TME of BC
3.2. Monocytes and Diagnosis
3.3. Monocytes’ Expansion in the TME
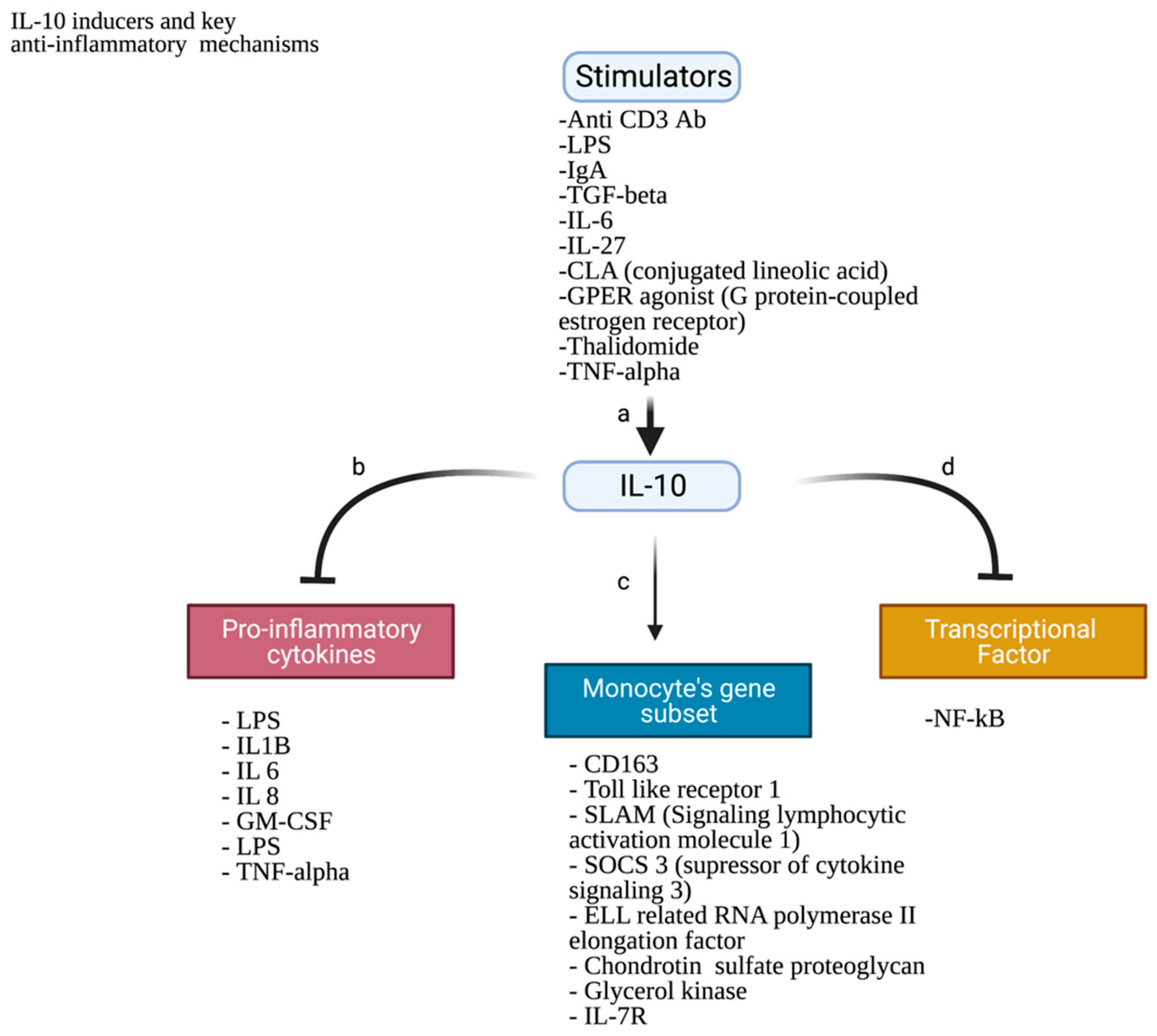
3.3.1. (A) Cytokine-Related Factors
Macrophage Colony-Stimulating Factor (M-CSF)
CXCL16
YKL-39 as a Monocyte Attracting Factor Produced by TAMs
3.3.2. (B) Non-Cytokine-Related Factors
Stage of Cancer
BC Subtype
3.4. Monocytes’ Differentiation
3.4.1. Monocytes’ Differentiation to Dendritic Cells
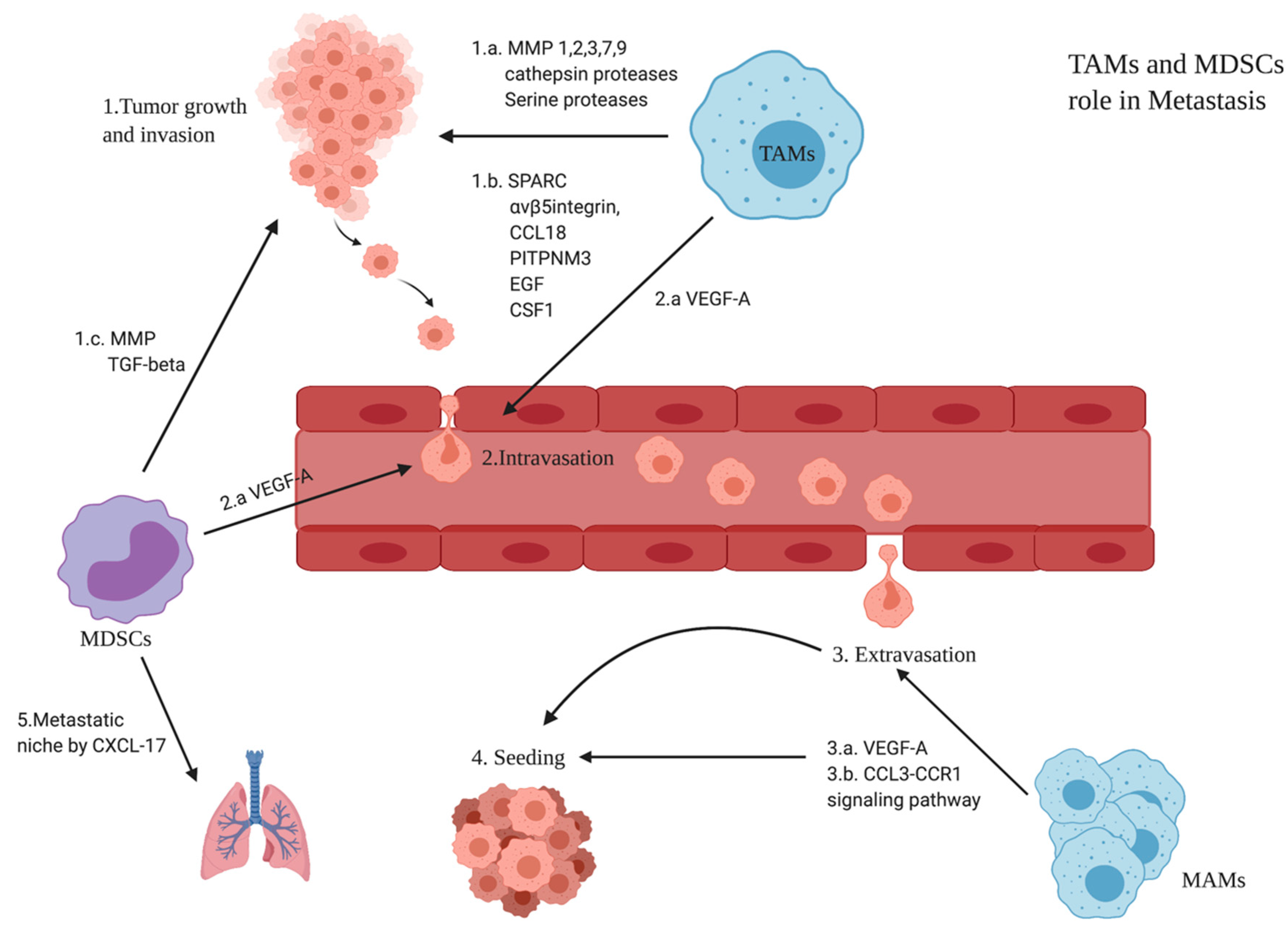
3.4.2. Monocytes’ Differentiation to MDSCs
3.4.3. Monocytes’ Differentiation to Macrophages
Monocytes’ Differentiation to M1 Population (Pro-Inflammatory)
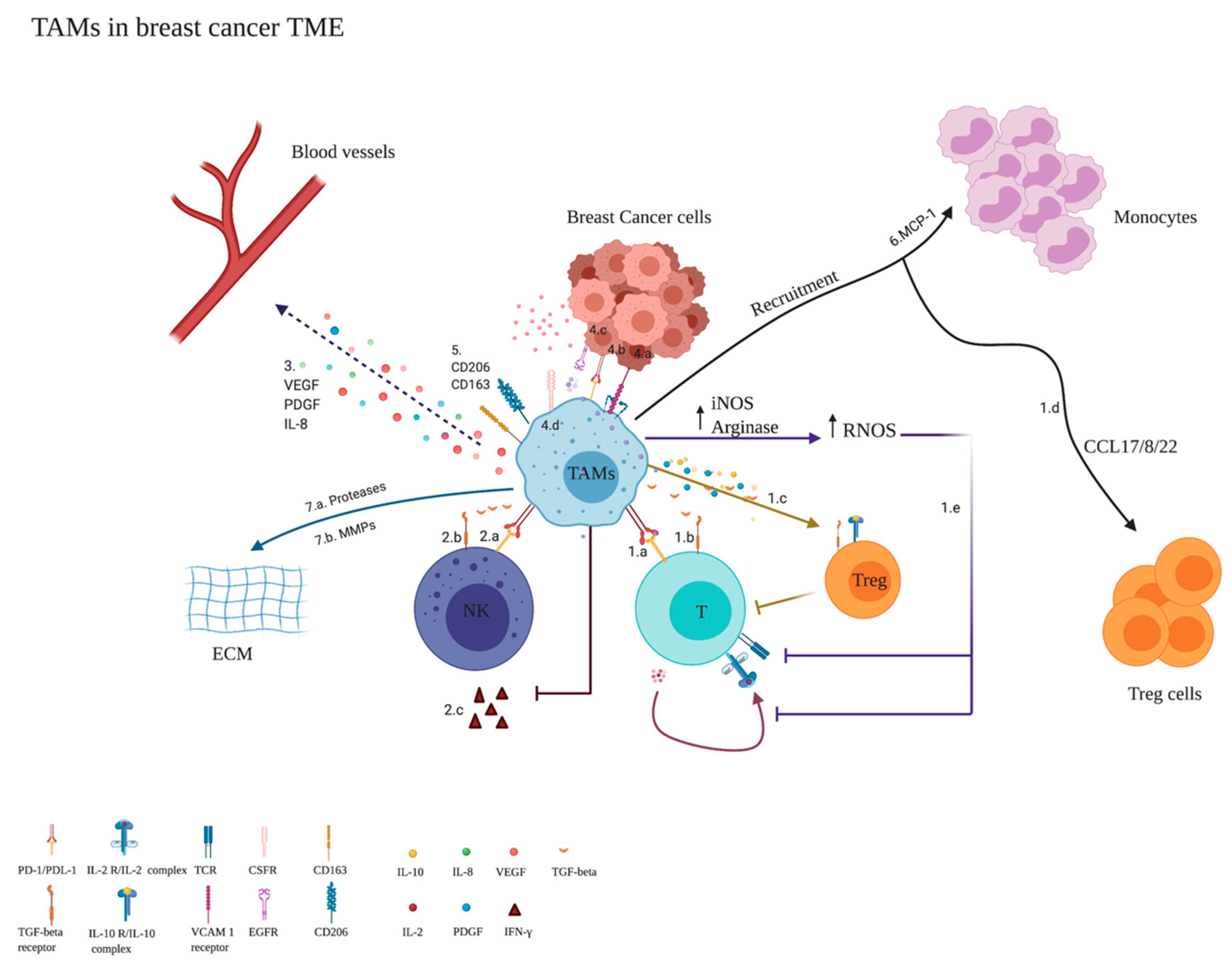
Monocytes’ Differentiation to M2 Population/TAMs (Anti-Inflammatory)
Key Players Affecting the Differentiation of Monocytes to TAMs/M2
- A
- Cytokine-Related Factors
- B
- Non-Cytokine-Related Factors
3.5. TAM Gene Signature
3.6. Role of TAMs and MDSCs in BC Metastasis
3.6.1. Monocytes’ Differentiation to M0 Macrophages
3.6.2. Monocytes’ Differentiation to Mregs
3.6.3. Monocytes’ Differentiation to M3
3.6.4. Monocytes’ Differentiation to M4
3.6.5. Monocytes’ Differentiation to Mox
3.6.6. Monocytes’ Differentiation to Hemorrhage-Associated Macrophages (Mhas)
3.6.7. Monocytes’ Differentiation to M17
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ding, J.; Guo, C.; Hu, P.; Chen, J.; Liu, Q.; Wu, X.; Cao, Y.; Wu, J. CSF1 Is Involved in Breast Cancer Progression through Inducing Monocyte Differentiation and Homing. Int. J. Oncol. 2016, 49, 2064–2074. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Tian, Y.; Guo, F.; Yu, B.; Li, J.; Xu, H.; Su, Z. LincRNA-P21 Knockdown Reversed Tumor-Associated Macrophages Function by Promoting MDM2 to Antagonize* P53 Activation and Alleviate Breast Cancer Development. Cancer Immunol. Immunother. 2020, 69, 835–846. [Google Scholar] [CrossRef]
- Blows, F.M.; Driver, K.E.; Schmidt, M.K.; Broeks, A.; van Leeuwen, F.E.; Wesseling, J.; Cheang, M.C.; Gelmon, K.; Nielsen, T.O.; Blomqvist, C.; et al. Subtyping of Breast Cancer by Immunohistochemistry to Investigate a Relationship between Subtype and Short and Long Term Survival: A Collaborative Analysis of Data for 10,159 Cases from 12 Studies. PLoS Med. 2010, 7, e1000279. [Google Scholar] [CrossRef] [PubMed]
- Kondov, B.; Milenkovikj, Z.; Kondov, G.; Petrushevska, G.; Basheska, N. Presentation of the Molecular Subtypes of Breast Cancer Detected by Immunohistochemistry in Surgically Treated Patients. Open Access Maced. J. Med. Sci. 2018, 6, 961–967. [Google Scholar] [CrossRef]
- Kennecke, H.; Yerushalmi, R.; Woods, R.; Cheang, M.C.U.; Voduc, D.; Speers, C.H.; Nielsen, T.O.; Gelmon, K. Metastatic Behavior of Breast Cancer Subtypes. J. Clin. Oncol. 2010, 28, 3271–3277. [Google Scholar] [CrossRef]
- Perou, C.M.; Sùrlie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular Portraits of Human Breast Tumours. Letters to Nature 748. Nature 2000, 533, 747–752. [Google Scholar] [CrossRef]
- Cheang, M.C.U.; Chia, S.K.; Voduc, D.; Gao, D.; Leung, S.; Snider, J.; Watson, M.; Davies, S.; Bernard, P.S.; Parker, J.S.; et al. Ki67 Index, HER2 Status, and Prognosis of Patients with Luminal B Breast Cancer. J. Natl. Cancer Inst. 2009, 101, 736–750. [Google Scholar] [CrossRef]
- Staaf, J.; Ringnér, M.; Vallon-Christersson, J.; Jönsson, G.; Bendahl, P.O.; Holm, K.; Arason, A.; Gunnarsson, H.; Hegardt, C.; Agnarsson, B.A.; et al. Identification of Subtypes in Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer Reveals a Gene Signature Prognostic of Outcome. J. Clin. Oncol. 2010, 28, 1813–1820. [Google Scholar] [CrossRef]
- Bosch, A.; Eroles, P.; Zaragoza, R.; Viña, J.R.; Lluch, A. Triple-Negative Breast Cancer: Molecular Features, Pathogenesis, Treatment and Current Lines of Research. Cancer Treat. Rev. 2010, 36, 206–215. [Google Scholar] [CrossRef]
- Sørlie, T.; Tibshirani, R.; Parker, J.; Hastie, T.; Marron, J.S.; Nobel, A.; Deng, S.; Johnsen, H.; Pesich, R.; Geisler, S.; et al. Repeated Observation of Breast Tumor Subtypes in Independent Gene Expression Data Sets. Proc. Natl. Acad. Sci. USA 2003, 100, 8418–8423. [Google Scholar] [CrossRef] [PubMed]
- Eroles, P.; Bosch, A.; Pérez-fidalgo, J.A.; Lluch, A. Molecular Biology in Breast Cancer: Intrinsic Subtypes and Signaling Pathways. Cancer Treat. Rev. 2012, 38, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Mozdarani, H.; Ezzatizadeh, V.; Parvaneh, R.R. The Emerging Role of the Long Non-Coding RNA HOTAIR in Breast Cancer Development and Treatment. J. Transl. Med. 2020, 18, 152. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Guo, H.; Ganesan, P.; Parker, J.S.; Gao, M.; Moulder, S.; Karginova, O.; Lu, Y.; Lee, J.J.; Fan, C.; et al. Phenotypic and Molecular Characterization of the Claudin-Low Intrinsic Subtype of Breast Cancer. Breast Cancer Res. 2010, 12, 68–86. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Perou, C.M. Deconstructing the Molecular Portraits of Breast Cancer. Mol. Oncol. 2011, 5, 5–23. [Google Scholar] [CrossRef]
- Netanely, D.; Avraham, A.; Ben-Baruch, A.; Evron, E.; Shamir, R. Expression and Methylation Patterns Partition Luminal-A Breast Tumors into Distinct Prognostic Subgroups. Breast Cancer Res. 2016, 18, 74. [Google Scholar] [CrossRef]
- Jézéquel, P.; Kerdraon, O.; Hondermarck, H.; Guérin-Charbonnel, C.; Lasla, H.; Gouraud, W.; Canon, J.L.; Gombos, A.; Dalenc, F.; Delaloge, S.; et al. Identification of Three Subtypes of Triple-Negative Breast Cancer with Potential Therapeutic Implications. Breast Cancer Res. 2019, 21, 65. [Google Scholar] [CrossRef]
- MacGregor, H.L.; Ohashi, P.S. Molecular Pathways: Evaluating the Potential for B7-H4 as an Immunoregulatory Target. Clin. Cancer Res. 2017, 23, 2934–2941. [Google Scholar] [CrossRef]
- Kryczek, I.; Zou, L.; Rodriguez, P.; Zhu, G.; Wei, S.; Mottram, P.; Brumlik, M.; Cheng, P.; Curiel, T.; Myers, L.; et al. B7-H4 Expression Identifies a Novel Suppressive Macrophage Population in Human Ovarian Carcinoma. J. Exp. Med. 2006, 203, 871–881. [Google Scholar] [CrossRef]
- de Palma, M.; Lewis, C.E. Macrophage Regulation of Tumor Responses to Anticancer Therapies. Cancer Cell 2013, 23, 277–286. [Google Scholar] [CrossRef]
- Pollard, J.W. Tumour-Educated Macrophages Promote Tumour Progression and Metastasis. Nat. Rev. Cancer 2004, 4, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. The Tumor Microenvironment and Its Role in Promoting Tumor Growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef] [PubMed]
- Barriga, V.; Kuol, N.; Nurgali, K.; Apostolopoulos, V. The Complex Interaction between the Tumor Micro-Environment and Immune Checkpoints in Breast Cancer. Cancers 2019, 11, 1205. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, G.S.; Poutahidis, T.; Erdman, S.E.; Kirsch, R.; Riddell, R.H.; Diamandis, E.P. Cancer-Associated Fibroblasts Drive the Progression of Metastasis through Both Paracrine and Mechanical Pressure on Cancer Tissue. Mol. Cancer Res. 2015, 10, 1403–1418. [Google Scholar] [CrossRef]
- Treffers, L.W.; Hiemstra, I.H.; Kuijpers, T.W.; van den Berg, T.K.; Matlung, H.L. Neutrophils in Cancer. Immunol. Rev. 2016, 273, 312–328. [Google Scholar] [CrossRef]
- Queen, M.M.; Ryan, R.E.; Holzer, R.G.; Keller-Peck, C.R.; Jorcyk, C.L. Breast Cancer Cells Stimulate Neutrophils to Produce Oncostatin M: Potential Implications for Tumor Progression. Cancer Res. 2005, 65, 8896–8904. [Google Scholar] [CrossRef]
- Perret, J.; McDonald, C.; Apostolopoulos, V. Elevated Serum Interleukin-5 Levels in Severe Chronic Obstructive Pulmonary Disease. Acta Biochim. Biophys. Sin. 2017, 49, 560–563. [Google Scholar] [CrossRef]
- Szalayova, G.; Ogrodnik, A.; Spencer, B.; Wade, J.; Bunn, J.; Ambaye, A.; James, T.; Rincon, M. Human Breast Cancer Biopsies Induce Eosinophil Recruitment and Enhance Adjacent Cancer Cell Proliferation. Breast Cancer Res. Treat. 2016, 157, 461–474. [Google Scholar] [CrossRef]
- Sakkal, S.; Miller, S.; Apostolopoulos, V.; Nurgali, K. Eosinophils in Cancer: Favourable or Unfavourable? Curr. Med. Chem. 2016, 23, 650–666. [Google Scholar] [CrossRef]
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Lucarini, V.; Marone, G.; Mattei, F.; Marone, G.; Schiavoni, G. Eosinophils: The Unsung Heroes in Cancer? Oncoimmunology 2018, 7, e1393134. [Google Scholar] [CrossRef]
- Allaoui, R.; Bergenfelz, C.; Mohlin, S.; Hagerling, C.; Salari, K.; Werb, Z.; Anderson, R.L.; Ethier, S.P.; Jirström, K.; Påhlman, S.; et al. Cancer-Associated Fibroblast-Secreted CXCL16 Attracts Monocytes to Promote Stroma Activation in Triple-Negative Breast Cancers. Nat. Commun. 2016, 7, 13050. [Google Scholar] [CrossRef] [PubMed]
- Coillard, A.; Segura, E. In Vivo Differentiation of Human Monocytes. Front. Immunol. 2019, 10, 1907. [Google Scholar] [CrossRef] [PubMed]
- Feng, A.L.; Zhu, J.K.; Sun, J.T.; Yang, M.X.; Neckenig, M.R.; Wang, X.W.; Shao, Q.Q.; Song, B.F.; Yang, Q.F.; Kong, B.H.; et al. CD16+ Monocytes in Breast Cancer Patients: Expanded by Monocyte Chemoattractant Protein-1 and May Be Useful for Early Diagnosis. Clin. Exp. Immunol. 2011, 164, 57–65. [Google Scholar] [CrossRef]
- Yamamoto, K.; Murphy, G.; Troeberg, L. Extracellular Regulation of Metalloproteinases. Matrix Biol. 2015, 44–46, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, J.; Wang, X.; Deng, S.; Ye, H.; Guan, W.; Wu, M.; Zhu, S.; Yu, Y.; Han, W. CXCL4 Mediates Tumor Regrowth after Chemotherapy by Suppression of Antitumor Immunity. Cancer Biol. Ther. 2015, 16, 1775–1783. [Google Scholar] [CrossRef]
- Liu, T.; Larionova, I.; Litviakov, N.; Riabov, V.; Zavyalova, M.; Tsyganov, M.; Buldakov, M.; Song, B.; Moganti, K.; Kazantseva, P.; et al. Tumor-Associated Macrophages in Human Breast Cancer Produce New Monocyte Attracting and pro-Angiogenic Factor YKL-39 Indicative for Increased Metastasis after Neoadjuvant Chemotherapy. Oncoimmunology 2018, 7, e1436922. [Google Scholar] [CrossRef]
- Soria, G.; Ben-Baruch, A. The Inflammatory Chemokines CCL2 and CCL5 in Breast Cancer. Cancer Lett. 2008, 267, 271–285. [Google Scholar] [CrossRef]
- Cao, H.; Huang, Y.; Wang, L.; Wang, H.; Pang, X.; Li, K.; Dang, W.; Tang, H.; Wei, L.; Su, M.; et al. Leptin Promotes Migration and Invasion of Breast Cancer Cells by Stimulating IL-8 Production in M2 Macrophages. Oncotarget 2016, 7, 65441–65453. [Google Scholar] [CrossRef]
- Tsai, C.S.; Chen, F.H.; Wang, C.C.; Huang, H.L.; Jung, S.M.; Wu, C.J.; Lee, C.C.; McBride, W.H.; Chiang, C.S.; Hong, J.H. Macrophages from Irradiated Tumors Express Higher Levels of INOS, Arginase-I and COX-2, and Promote Tumor Growth. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 499–507. [Google Scholar] [CrossRef]
- Rey-Giraud, F.; Hafner, M.; Ries, C.H. In Vitro Generation of Monocyte-Derived Macrophages under Serum-Free Conditions Improves Their Tumor Promoting Functions. PLoS ONE 2012, 7, e42656. [Google Scholar] [CrossRef]
- Sousa, S.; Brion, R.; Lintunen, M.; Kronqvist, P.; Sandholm, J.; Mönkkönen, J.; Kellokumpu-Lehtinen, P.L.; Lauttia, S.; Tynninen, O.; Joensuu, H.; et al. Human Breast Cancer Cells Educate Macrophages toward the M2 Activation Status. Breast Cancer Res. 2015, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Benner, B.; Scarberry, L.; Suarez-Kelly, L.P.; Duggan, M.C.; Campbell, A.R.; Smith, E.; Lapurga, G.; Jiang, K.; Butchar, J.P.; Tridandapani, S.; et al. Generation of Monocyte-Derived Tumor-Associated Macrophages Using Tumor-Conditioned Media Provides a Novel Method to Study Tumor-Associated Macrophages in Vitro. J. Immunother. Cancer 2019, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.N.; Rodriguez, C.; Hubert, M.; Ardin, M.; Treilleux, I.; Ries, C.H.; Lavergne, E.; Chabaud, S.; Colombe, A.; Trédan, O.; et al. CD163+ Tumor-Associated Macrophage Accumulation in Breast Cancer Patients Reflects Both Local Differentiation Signals and Systemic Skewing of Monocytes. Clin. Transl. Immunol. 2020, 9, e1108. [Google Scholar] [CrossRef] [PubMed]
- Toor, S.M.; Syed Khaja, A.S.; El Salhat, H.; Faour, I.; Kanbar, J.; Quadri, A.A.; Albashir, M.; Elkord, E. Myeloid Cells in Circulation and Tumor Microenvironment of Breast Cancer Patients. Cancer Immunol. Immunother. 2017, 66, 753–764. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, X.; Wu, Y.; Wang, X. Interaction between Treg Cells and Tumor-Associated Macrophages in the Tumor Microenvironment of Epithelial Ovarian Cancer. Oncol. Rep. 2016, 36, 3472–3478. [Google Scholar] [CrossRef]
- Bonapace, L.; Coissieux, M.M.; Wyckoff, J.; Mertz, K.D.; Varga, Z.; Junt, T.; Bentires-Alj, M. Cessation of CCL2 Inhibition Accelerates Breast Cancer Metastasis by Promoting Angiogenesis. Nature 2014, 515, 130–133. [Google Scholar] [CrossRef]
- Fang, M.; Yuan, J.; Peng, C.; Li, Y. Collagen as a Double-Edged Sword in Tumor Progression. Tumor Biol. 2014, 35, 2871–2882. [Google Scholar] [CrossRef]
- Manuscript, A. Cellular and Molecular Origin of Tam. Changes 2012, 29, 997–1003. [Google Scholar] [CrossRef]
- Xu, H.; Lai, W.; Zhang, Y.; Liu, L.; Luo, X.; Zeng, Y.; Wu, H.; Lan, Q.; Chu, Z. Tumor-Associated Macrophage-Derived IL-6 and IL-8 Enhance Invasive Activity of LoVo Cells Induced by PRL-3 in a KCNN4 Channel-Dependent Manner. BMC Cancer 2014, 14, 330. [Google Scholar] [CrossRef]
- Van Den Brandt, P.A.; Spiegelman, D.; Yaun, S.S.; Adami, H.O.; Beeson, L.; Folsom, A.R.; Fraser, G.; Goldbohm, R.A.; Graham, S.; Kushi, L.; et al. Pooled Analysis of Prospective Cohort Studies on Height, Weight, and Breast Cancer Risk. Am. J. Epidemiol. 2000, 152, 514–527. [Google Scholar] [CrossRef]
- Guo, S.; Liu, M.; Wang, G.; Torroella-Kouri, M.; Gonzalez-Perez, R.R. Oncogenic Role and Therapeutic Target of Leptin Signaling in Breast Cancer and Cancer Stem Cells. Biochim. Biophys. Acta Rev. Cancer 2012, 1825, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Kunz, S.; Wolk, K.; Witte, E.; Witte, K.; Doecke, W.D.; Volk, H.D.; Sterry, W.; Asadullah, K.; Sabat, R. Interleukin (IL)-19, IL-20 and IL-24 Are Produced by and Act on Keratinocytes and Are Distinct from Classical ILs. Exp. Dermatol. 2006, 15, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Jose, P.; Avdiushko, M.G.; Akira, S.; Kaplan, A.M.; Cohen, D.A. Inhibition of Interleukin-10 Signaling in Lung Dendritic Cells by Toll-like Receptor 4 Ligands. Exp. Lung Res. 2009, 35, 1–28. [Google Scholar] [CrossRef]
- Fitzgerald, D.C.; Zhang, G.X.; El-Behi, M.; Fonseca-Kelly, Z.; Li, H.; Yu, S.; Saris, C.J.; Gran, B.; Ciric, B.; Rostami, A. Suppression of Autoimmune Inflammation of the Central Nervous System by Interleukin 10 Secreted by Interleukin 27-Stimulated T Cells. Nat. Immunol. 2007, 8, 1372–1379. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Bak-Jensen, K.S.; Chen, Y.; Tato, C.M.; Blumenschein, W.; McClanahan, T.; Cua, D.J. TGF-β and IL-6 Drive the Production of IL-17 and IL-10 by T Cells and Restrain TH-17 Cell-Mediated Pathology. Nat. Immunol. 2007, 8, 1390–1397. [Google Scholar] [CrossRef]
- Williams, L.; Jarai, G.; Smith, A.; Finan, P. L-10 expression profiling in human monocytes. J. Leukoc. Biol. 2002, 72, 800–809. [Google Scholar] [CrossRef]
- Wang, P.; Wu, P.; Siegel, M.I.; Egan, R.W.; Billah, M.M. Interleukin (IL)-10 Inhibits Nuclear Factor ΚB (NFκB) Activation in Human Monocytes. IL-10 and IL-4 Suppress Cytokine Synthesis by Different Mechanisms. J. Biol. Chem. 1995, 270, 9558–9563. [Google Scholar] [CrossRef]
- Carninci, P.; Kasukawa, T.; Katayama, S.; Gough, J.; Frith, M.C.; Maeda, N.; Oyama, R.; Ravasi, T.; Lenhard, B.; Wells, C.; et al. Molecular Biology: The Transcriptional Landscape of the Mammalian Genome. Science 2005, 309, 1559–1563. [Google Scholar] [CrossRef]
- Liu, M.; Xing, L.Q.; Liu, Y.J. A Three-Long Noncoding RNA Signature as a Diagnostic Biomarker for Differentiating between Triple-Negative and Non-Triple-Negative Breast Cancers. Medicine 2017, 96, e6222. [Google Scholar] [CrossRef] [PubMed]
- Pulikkan, J.A.; Dengler, V.; Peramangalam, P.S.; Peer Zada, A.A.; Müller-Tidow, C.; Bohlander, S.K.; Tenen, D.G.; Behre, G. Cell-Cycle Regulator E2F1 and MicroRNA-223 Comprise an Autoregulatory Negative Feedback Loop in Acute Myeloid Leukemia. Blood 2010, 115, 1768–1778. [Google Scholar] [CrossRef]
- Shen, C.; Chen, M.-T.; Zhang, X.-H.; Yin, X.-L.; Ning, H.-M.; Su, R.; Lin, H.-S.; Song, L.; Wang, F.; Ma, Y.-N.; et al. The PU.1-Modulated MicroRNA-22 Is a Regulator of Monocyte/Macrophage Differentiation and Acute Myeloid Leukemia. PLoS Genet. 2016, 12, e1006259. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, B.; Saha, P.; Bose, S.; Shukla, D.; Chatterjee, N.; Kumar, S.; Tripathi, P.P.; Srivastava, A.K. MicroRNAs: As Critical Regulators of Tumor- Associated Macrophages. Int. J. Mol. Sci. 2020, 21, 7117. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.-C.; Ebersberger, S.; Fink, A.F.; Lampe, S.; Weigert, A.; Schmid, T.; Ebersberger, I.; Syed, S.N.; Brüne, B. Apoptotic Tumor Cell-Derived MicroRNA-375 Uses CD36 to Alter the Tumor-Associated Macrophage Phenotype. Nat. Commun. 2019, 10, 1135. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jiang, Z.; Chen, C.; Hu, Q.; Fu, Z.; Chen, J.; Wang, Z.; Wang, Q.; Li, A.; Marks, J.R.; et al. CircIRAK3 Sponges MiR-3607 to Facilitate Breast Cancer Metastasis. Cancer Lett. 2018, 430, 179–192. [Google Scholar] [CrossRef]
- Ray, T.; Ryusaki, T.; Ray, P.S. Therapeutically Targeting Cancers That Overexpress FOXC1: A Transcriptional Driver of Cell Plasticity, Partial EMT, and Cancer Metastasis. Front. Oncol. 2021, 11, 721959. [Google Scholar] [CrossRef]
- Michea, P.; Noël, F.; Zakine, E.; Czerwinska, U.; Sirven, P.; Abouzid, O.; Goudot, C.; Scholer-Dahirel, A.; Vincent-Salomon, A.; Reyal, F.; et al. Adjustment of Dendritic Cells to the Breast-Cancer Microenvironment Is Subset Specific. Nat. Immunol. 2018, 19, 885–897. [Google Scholar] [CrossRef]
- Cassetta, L.; Fragkogianni, S.; Sims, A.H.; Swierczak, A.; Forrester, L.M.; Zhang, H.; Soong, D.Y.H.; Cotechini, T.; Anur, P.; Lin, E.Y.; et al. Human Tumor-Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specific Reprogramming, Biomarkers, and Therapeutic Targets. Cancer Cell 2019, 35, 588–602.e10. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, D.; Wang, X.; Celkan, T.; Nordenskjld, M.; Henter, J.-I.; Fadeel, B.; Zheng, C. Syntaxin-11 Is Expressed in Primary Human Monocytesmacrophages and Acts as a Negative Regulator of Macrophage Engulfment of Apoptotic Cells and IgG-Opsonized Target Cells. Br. J. Haematol. 2008, 142, 469–479. [Google Scholar] [CrossRef]
- Roychaudhuri, R.; Hergrueter, A.H.; Polverino, F.; Laucho-Contreras, M.E.; Gupta, K.; Borregaard, N.; Owen, C.A. ADAM9 Is a Novel Product of Polymorphonuclear Neutrophils: Regulation of Expression and Contributions to Extracellular Matrix Protein Degradation during Acute Lung Injury. J. Immunol. 2014, 193, 2469–2482. [Google Scholar] [CrossRef]
- Liu, B.; Sun, L.; Liu, Q.; Gong, C.; Yao, Y.; Lv, X.; Lin, L.; Yao, H.; Su, F.; Li, D.; et al. A Cytoplasmic NF-ΚB Interacting Long Noncoding RNA Blocks IκB Phosphorylation and Suppresses Breast Cancer Metastasis. Cancer Cell 2015, 27, 370–381. [Google Scholar] [CrossRef]
- Mouton, A.J.; Li, X.; Hall, M.E.; Hall, J.E. Obesity, Hypertension, and Cardiac Dysfunction. Circ. Res. 2020, 126, 789–806. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.Q.; Waaijer, S.J.H.; Zwager, M.C.; de Vries, E.G.E.; van der Vegt, B.; Schröder, C.P. Tumor-Associated Macrophages in Breast Cancer: Innocent Bystander or Important Player? Cancer Treat. Rev. 2018, 70, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Trovato, R.; Canè, S.; Petrova, V.; Sartoris, S.; Ugel, S.; De Sanctis, F. The Engagement between MDSCs and Metastases: Partners in Crime. Front. Oncol. 2020, 10, 165. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Yen, M.C.; Chang, W.A.; Tsai, P.H.; Pan, Y.C.; Liao, S.H.; Kuo, P.L. CXCL17-Derived CD11b+Gr-1+ Myeloid-Derived Suppressor Cells Contribute to Lung Metastasis of Breast Cancer through Platelet-Derived Growth Factor-BB. Breast Cancer Res. 2019, 21, 23. [Google Scholar] [CrossRef] [PubMed]
- Gabrusiewicz, K.; Rodriguez, B.; Wei, J.; Hashimoto, Y.; Healy, L.M.; Maiti, S.N.; Thomas, G.; Zhou, S.; Wang, Q.; Elakkad, A.; et al. Glioblastoma-Infiltrated Innate Immune Cells Resemble M0 Macrophage Phenotype. JCI Insight 2016, 1, e85841. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J.A.; Riquelme, P.; Sawitzki, B.; Tomiuk, S.; Miqueu, P.; Zuhayra, M.; Oberg, H.H.; Pascher, A.; Lützen, U.; Janßen, U.; et al. Cutting Edge: Immunological Consequences and Trafficking of Human Regulatory Macrophages Administered to Renal Transplant Recipients. J. Immunol. 2011, 187, 2072–2078. [Google Scholar] [CrossRef]
- Riquelme, P.; Amodio, G.; Macedo, C.; Moreau, A.; Obermajer, N.; Brochhausen, C.; Ahrens, N.; Kekarainen, T.; Fändrich, F.; Cuturi, C.; et al. DHRS9 Is a Stable Marker of Human Regulatory Macrophages. Transplantation 2017, 101, 2731–2738. [Google Scholar] [CrossRef]
- Krijgsman, D.; de Vries, N.L.; Andersen, M.N.; Skovbo, A.; Tollenaar, R.A.E.M.; Møller, H.J.; Hokland, M.; Kuppen, P.J.K. CD163 as a Biomarker in Colorectal Cancer: The Expression on Circulating Monocytes and Tumor-Associated Macrophages, and the Soluble Form in the Blood. Int. J. Mol. Sci. 2020, 21, 5925. [Google Scholar] [CrossRef]
- Kalish, S.; Lyamina, S.; Manukhina, E.; Malyshev, Y.; Raetskaya, A.; Malyshev, I. M3 Macrophages Stop Division of Tumor Cells In Vitro and Extend Survival of Mice with Ehrlich Ascites Carcinoma. Med. Sci. Monit. Basic Res. 2017, 23, 8–19. [Google Scholar] [CrossRef][Green Version]
- Chen, F.-H.; Chiang, C.-S.; Wang, C.-C.; Tsai, C.-S.; Jung, S.-M.; Lee, C.-C.; McBride, W.H.; Hong, J.-H. Radiotherapy Decreases Vascular Density and Causes Hypoxia with Macrophage Aggregation in TRAMP-C1 Prostate Tumors. Clin. Cancer Res. 2009, 15, 1721–1729. [Google Scholar] [CrossRef]
- Gleissner, C.A.; Shaked, I.; Little, K.M.; Ley, K. CXC Chemokine Ligand 4 Induces a Unique Transcriptome in Monocyte-Derived Macrophages. J. Immunol. 2010, 184, 4810–4818. [Google Scholar] [CrossRef] [PubMed]
- Butcher, M.J.; Galkina, E.V. Phenotypic and Functional Heterogeneity of Macrophages and Dendritic Cell Subsets in the Healthy and Atherosclerosis-Prone Aorta. Front. Physiol. 2012, 3, 44. [Google Scholar] [CrossRef] [PubMed]
- Boyle, J.J.; Harrington, H.A.; Piper, E.; Elderfield, K.; Stark, J.; Landis, R.C.; Haskard, D.O. Coronary Intraplaque Hemorrhage Evokes a Novel Atheroprotective Macrophage Phenotype. Am. J. Pathol. 2009, 174, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Hristodorov, D.; Mladenov, R.; Huhn, M.; Barth, S.; Thepen, T. Macrophage-Targeted Therapy: CD64-Based Immunotoxins for Treatment of Chronic Inflammatory Diseases. Toxins 2012, 4, 676–694. [Google Scholar] [CrossRef] [PubMed]
- Zizzo, G.; Cohen, P.L. IL-17 Stimulates Differentiation of Human Anti-Inflammatory Macrophages and Phagocytosis of Apoptotic Neutrophils in Response to IL-10 and Glucocorticoids. J. Immunol. 2013, 190, 5237–5246. [Google Scholar] [CrossRef]
- Duluc, D.E.; Delneste, Y.; Tan, F.; Moles, M.-P.; Grimaud, L.; Lenoir, J.; Preisser, L.; Anegon, I.; Catala, L.; Ifrah, N.; et al. Tumor-Associated Leukemia Inhibitory Factor and IL-6 Skew Monocyte Differentiation into Tumor-Associated Macrophage-like Cells. Blood J. Am. Soc. Hematol. 2007, 110, 4319–4330. [Google Scholar] [CrossRef]
| M0-MΦ | M1-MΦ | M2-MΦ | SnDil-MΦ | mo-DCs | |
|---|---|---|---|---|---|
| CD14 | + | + | + | + | −/lo |
| CD64 | + | + | + | + | − |
| BDCA | − | − | − | − | + |
| CD163 | −/lo | − | + | +/− | N/A |
| CD86 | N/A | + | − | +/− | N/A |
| Macrophage Phenotype | Stimulation | Function | References |
|---|---|---|---|
| M0 | Resting-state macrophages | Commonly, they are considered only as precursors to M1 or M2 phenotypes. However, they might have tumorigenic activity in glioma | [75] |
| M1 | LPS and IFN-γ | IL-23, IL-12, and promotes Th1 responses. It can also secrete IL-6, ROS, and TNF-α | [40,41,42] |
| M2a | IL-13 and IL-4 | Produce matrix remodeling cytokines. They are considered anti-inflammatory immune cells with an elevation in the expression of both CD200R and CD86 | [35] |
| M2b | IL-1β or LPS | Immunosuppressive | [35] |
| M2c | IL-10 and TGF-β | Immunosuppressive activity is achieved by production of IL-10 and MMPs with a significant elevation in CD163 expression | [35] |
| M2d | Leukemia inhibitory factor, IL-6 | Not clear | [86] |
| Mreg | IFN-Y | Suppress mitogen-stimulated T-cell proliferation in vitro through interferon-gamma (IFN-γ)-induced indoleamine 2,3-dioxygenase (IDO) activity. Activates Tregs that in turn suppress effector T cells | [76,77] |
| M3 | Unknown | Known as TAMs with an M1/M2 or M2/M1 switch phenotype. It was reported to have anti-tumor activity in Ehrlich ascites and a prostate cancer mouse model | [78,79,80] |
| M4 | CXCL4 | Pro-inflammatory in the context of atherosclerosis | [81] |
| Mox | ox-PL 1-palmitoyl 2arachidonoyl-sn-glycero-3-phosphorylcholine (might be a strong stimulator in vitro) | Produces IL-10 and VEGF; thus, it might have anti-inflammatory/angiogenic activity | [82,83] |
| Mha | hapto-hemoglobin complexes or oxidized red blood cells in vitro | CD163 and IL-10 upregulation; thus, it might have anti-inflammatory activity | [84] |
| M17 | IL-17 | Anti-inflammatory activity through the polarization to M2c | [85] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amer, H.T.; Stein, U.; El Tayebi, H.M. The Monocyte, a Maestro in the Tumor Microenvironment (TME) of Breast Cancer. Cancers 2022, 14, 5460. https://doi.org/10.3390/cancers14215460
Amer HT, Stein U, El Tayebi HM. The Monocyte, a Maestro in the Tumor Microenvironment (TME) of Breast Cancer. Cancers. 2022; 14(21):5460. https://doi.org/10.3390/cancers14215460
Chicago/Turabian StyleAmer, Hoda T., Ulrike Stein, and Hend M. El Tayebi. 2022. "The Monocyte, a Maestro in the Tumor Microenvironment (TME) of Breast Cancer" Cancers 14, no. 21: 5460. https://doi.org/10.3390/cancers14215460
APA StyleAmer, H. T., Stein, U., & El Tayebi, H. M. (2022). The Monocyte, a Maestro in the Tumor Microenvironment (TME) of Breast Cancer. Cancers, 14(21), 5460. https://doi.org/10.3390/cancers14215460







