Protein Kinase C (PKC) Isozymes as Diagnostic and Prognostic Biomarkers and Therapeutic Targets for Cancer
Simple Summary
Abstract
1. Introduction
2. PKC Isozymes as Prognostic Biomarkers or Therapeutic Targets for Cancer
2.1. Bladder Cancer
2.2. Blood and Bone Marrow Cancers
2.2.1. MM
2.2.2. Leukemia
ALL and CLL
AML and CML
Myelodysplastic Syndromes (MDSs)
2.2.3. Lymphoma
2.3. Brain Cancer (Glioblastoma)
2.4. Breast Cancer
2.5. Colorectal (Colon) Cancer (CRC)
2.6. Gastric (Stomach) Cancer
2.7. Head and Neck Squamous Cell Carcinoma (HNSCC)
2.8. Liver Cancer (Hepatocellular Carcinoma)
2.9. Lung Cancer
2.10. Ovarian Cancer
2.11. Pancreatic, Bile Duct, and Gallbladder Cancer
2.11.1. Pancreatic Cancer
2.11.2. Bile Duct and Gallbladder Cancer
2.12. Prostate Cancer
2.13. Renal Cell Carcinoma (RCC)
2.14. Skin Cancer
2.14.1. Melanoma
2.14.2. NMSC
2.15. Thyroid Carcinoma
3. PKC Isozymes as Diagnostic Biomarkers for Cancer
3.1. PKC Isozymes as Diagnostic Immunohistochemical Biomarkers
3.2. PKC Isozymes as Diagnostic Biomarkers in Body Fluids
4. Summary and Overall Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Steinberg, S.F. Structural basis of protein kinase C isoform function. Physiol. Rev. 2008, 88, 1341–1378. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.C. Protein kinase C: Perfectly balanced. Crit. Rev. Biochem. Mol. Biol. 2018, 5, 208–230. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Toita, R.; Kim, C.W.; Katayama, Y. Protein kinase C (PKC) isozyme-specific substrates and their design. Biotechnol. Adv. 2012, 30, 1662–1672. [Google Scholar] [CrossRef] [PubMed]
- Jaken, S. Protein kinase C isozymes and substrates. Curr. Opin. Cell Biol. 1996, 8, 168–173. [Google Scholar] [CrossRef]
- Hofmann, J. The potential for isoenzyme-selective modulation of protein kinase C. FASEB J. 1997, 11, 649–669. [Google Scholar]
- Gonelli, A.; Mischiati, C.; Guerrini, R.; Voltan, R.; Salvadori, S.; Zauli, G. Perspectives of protein kinase C (PKC) inhibitors as anti-cancer agents. Mini Rev. Med. Chem. 2009, 9, 498–509. [Google Scholar] [CrossRef]
- Kawano, T.; Inokuchi, J.; Eto, M.; Murata, M.; Kang, J.H. Activators and inhibitors of protein kinase C (PKC): Their applications in clinical trials. Pharmaceutics 2021, 13, 1748. [Google Scholar]
- Langzam, L.; Koren, R.; Gal, R.; Kugel, V.; Paz, A.; Farkas, A.; Sampson, S.R. Patterns of protein kinase C isoenzyme expression in transitional cell carcinoma of bladder. Relation to degree of malignancy. Am. J. Clin. Pathol. 2001, 116, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Varga, A.; Czifra, G.; Tállai, B.; Németh, T.; Kovács, I.; Kovács, L.; Bíró, T. Tumor grade-dependent alterations in the protein kinase C isoform pattern in urinary bladder carcinomas. Eur. Urol. 2004, 46, 462–465. [Google Scholar] [CrossRef]
- Kang, J.H.; Inokuchi, J.; Kawano, T.; Murata, M. Protein kinase Cα as a therapeutic target in cancer. In Protein Kinase C: Emerging Roles and Therapeutic Potential; Pierce, D.N., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2018; pp. 25–47. [Google Scholar]
- Xu, W.; Anwaier, A.; Ma, C.; Liu, W.; Tian, X.; Palihati, M.; Hu, X.; Qu, Y.; Zhang, H.; Ye, D. Multi-omics reveals novel prognostic implication of SRC protein expression in bladder cancer and its correlation with immunotherapy response. Ann. Med. 2021, 53, 596–610. [Google Scholar] [CrossRef]
- Du, H.F.; Ou, L.P.; Yang, X.; Song, X.D.; Fan, Y.R.; Tan, B.; Luo, C.L.; Wu, X.H. A new PKCα/β/TBX3/E-cadherin pathway is involved in PLCε-regulated invasion and migration in human bladder cancer cells. Cell Signal. 2014, 26, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Chunli, L.; Xiaohou, W.; Qiaoling, Z. Involvement of the PLCε/PKCα pathway in human BIU-87 bladder cancer cell proliferation. Cell Biol. Int. 2011, 35, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Kong, C.; Yang, X.; Cui, X.; Lin, X.; Zhang, Z. Protein kinase C-α (PKCα) modulates cell apoptosis by stimulating nuclear translocation of NF-kappa-B p65 in urothelial cell carcinoma of the bladder. BMC Cancer 2017, 17, 432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, J.; Zhang, H.; Liu, Y.; Yin, L.; Liu, X.; Li, X.; Yu, X.; Yao, J.; Zhang, Z.; et al. Exploring the five different genes associated with PKCα in bladder cancer based on gene expression microarray. J. Cell Mol. Med. 2021, 25, 1759–1770. [Google Scholar] [PubMed]
- Liu, J.; Li, J. PKCα and Netrin-1/UNC5B positive feedback control in relation with chemical therapy in bladder cancer. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1712–1717. [Google Scholar] [PubMed]
- Tan, S.T.; Liu, S.Y.; Wu, B. TRIM29 overexpression promotes proliferation and survival of bladder cancer cells through NF-κB signaling. Cancer Res. Treat. 2016, 48, 1302–1312. [Google Scholar] [CrossRef]
- Kong, C.; Zhu, Y.; Liu, D.; Yu, M.; Li, S.; Li, Z.; Sun, Z.; Liu, G. Role of protein kinase C-α in superficial bladder carcinoma recurrence. Urology 2005, 65, 1228–1232. [Google Scholar] [CrossRef]
- Jiang, Z.; Kong, C.; Zhang, Z.; Zhu, Y.; Zhang, Y.; Chen, X. Reduction of protein kinase C α (PKC-α) promote apoptosis via down-regulation of Dicer in bladder cancer. J. Cell. Mol. Med. 2015, 19, 1085–1093. [Google Scholar]
- Namdarian, B.; Wong, E.; Galea, R.; Pedersen, J.; Chin, X.; Speirs, R.; Humbert, P.O.; Costello, A.J.; Corcoran, N.M.; Hovens, C.M. Loss of APKC expression independently predicts tumor recurrence in superficial bladder cancers. Urol. Oncol. 2013, 31, 649–655. [Google Scholar]
- Patel, R.; Islam, S.A.; Bommareddy, R.R.; Smalley, T.; Acevedo-Duncan, M. Simultaneous inhibition of atypical protein kinase-C and mTOR impedes bladder cancer cell progression. Int. J. Oncol. 2020, 56, 1373–1386. [Google Scholar] [CrossRef]
- Neri, A.; Marmiroli, S.; Tassone, P.; Lombardi, L.; Nobili, L.; Verdelli, D.; Civallero, M.; Cosenza, M.; Bertacchini, J.; Federico, M.; et al. The oral protein-kinase Cβ inhibitor enzastaurin (LY317615) suppresses signalling through the AKT pathway, inhibits proliferation and induces apoptosis in multiple myeloma cell lines. Leuk. Lymphoma 2008, 49, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Podar, K.; Raab, M.S.; Zhang, J.; McMillin, D.; Breitkreutz, I.; Tai, Y.T.; Lin, B.K.; Munshi, N.; Hideshima, T.; Chauhan, D.; et al. Targeting PKC in multiple myeloma: In vitro and in vivo effects of the novel, orally available small-molecule inhibitor enzastaurin (LY317615.HCl). Blood 2007, 109, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, E.; Leblond, V.; Maisonneuve, H.; Benhadji, K.A.; Hossain, A.M.; Nguyen, T.S.; Wooldridge, J.E.; Moreau, P. A multicenter phase II study of single-agent enzastaurin in previously treated multiple myeloma. Leuk. Lymphoma. 2014, 55, 2013–2017. [Google Scholar] [CrossRef]
- Alkan, S.; Huang, Q.; Ergin, M.; Denning, M.F.; Nand, S.; Maududi, T.; Paner, G.P.; Ozpuyan, F.; Izban, K.F. Survival role of protein kinase C (PKC) in chronic lymphocytic leukemia and determination of isoform expression pattern and genes altered by PKC inhibition. Am. J. Hematol. 2005, 79, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Frezzato, F.; Accordi, B.; Trimarco, V.; Gattazzo, C.; Martini, V.; Milani, G.; Bresolin, S.; Severin, F.; Visentin, A.; Basso, G.; et al. Profiling B cell chronic lymphocytic leukemia by reverse phase protein array: Focus on apoptotic proteins. J. Leukoc. Biol. 2016, 100, 1061–1070. [Google Scholar] [CrossRef]
- Lei, J.; Li, Q.; Gao, Y.; Zhao, L.; Liu, Y. Increased PKCα activity by Rack1 overexpression is responsible for chemotherapy resistance in T-cell acute lymphoblastic leukemia-derived cell line. Sci. Rep. 2016, 6, 33717. [Google Scholar] [CrossRef]
- Jiffar, T.; Kurinna, S.; Suck, G.; Carlson-Bremer, D.; Ricciardi, M.R.; Konopleva, M.; Andreeff, M.; Ruvolo, P.P. PKCα mediates chemoresistance in acute lymphoblastic leukemia through effects on Bcl2 phosphorylation. Leukemia 2004, 18, 505–512. [Google Scholar] [CrossRef]
- Lutzny, G.; Kocher, T.; Schmidt-Supprian, M.; Rudelius, M.; Klein-Hitpass, L.; Finch, A.J.; Dürig, J.; Wagner, M.; Haferlach, C.; Kohlmann, A.; et al. Protein kinase C-β-dependent activation of NF-κB in stromal cells is indispensable for the survival of chronic lymphocytic leukemia B cells in vivo. Cancer Cell 2013, 23, 77–92. [Google Scholar] [CrossRef]
- El-Gamal, D.; Williams, K.; LaFollette, T.D.; Cannon, M.; Blachly, J.S.; Zhong, Y.; Woyach, J.A.; Williams, E.; Awan, F.T.; Jones, J.; et al. PKC-β as a therapeutic target in CLL: PKC inhibitor AEB071 demonstrates preclinical activity in CLL. Blood 2014, 124, 1481–1491. [Google Scholar] [CrossRef]
- Handl, S.; von Heydebrand, F.; Voelkl, S.; Oostendorp, R.A.J.; Wilke, J.; Kremer, A.N.; Mackensen, A.; Lutzny-Geier, G. Immune modulatory effects of Idelalisib in stromal cells of chronic lymphocytic leukemia. Leuk. Lymphoma 2021, 62, 2679–2689. [Google Scholar] [CrossRef]
- Zum Büschenfelde, C.M.; Wagner, M.; Lutzny, G.; Oelsner, M.; Feuerstacke, Y.; Decker, T.; Bogner, C.; Peschel, C.; Ringshausen, I. Recruitment of PKC-βII to lipid rafts mediates apoptosis-resistance in chronic lymphocytic leukemia expressing ZAP-70. Leukemia 2010, 24, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Amigo-Jiménez, I.; Bailón, E.; Aguilera-Montilla, N.; Terol, M.J.; García-Marco, J.A.; García-Pardo, A. Bone marrow stroma-induced resistance of chronic lymphocytic leukemia cells to arsenic trioxide involves Mcl-1 upregulation and is overcome by inhibiting the PI3Kδ or PKCβ signaling pathways. Oncotarget 2015, 6, 44832–44848. [Google Scholar] [CrossRef] [PubMed]
- Loi, T.H.; Dai, P.; Carlin, S.; Melo, J.V.; Ma, D.D.F. Pro-survival role of protein kinase Cε in Philadelphia chromosome positive acute leukemia. Leuk. Lymphoma 2016, 57, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Ringshausen, I.; Schneller, F.; Bogner, C.; Hipp, S.; Duyster, J.; Peschel, C.; Decker, T. Constitutively activated phosphatidylinositol-3 kinase (PI-3K) is involved in the defect of apoptosis in B-CLL: Association with protein kinase Cδ. Blood 2002, 100, 3741–3748. [Google Scholar] [CrossRef] [PubMed]
- Hartsink-Segers, S.A.; Beaudoin, J.J.; Luijendijk, M.W.; Exalto, C.; Pieters, R.; Den Boer, M.L. PKCζ and PKMζ are overexpressed in TCF3-rearranged paediatric acute lymphoblastic leukaemia and are associated with increased thiopurine sensitivity. Leukemia 2015, 29, 304–311. [Google Scholar] [CrossRef][Green Version]
- Hubmann, R.; Düchler, M.; Schnabl, S.; Hilgarth, M.; Demirtas, D.; Mitteregger, D.; Hölbl, A.; Vanura, K.; Le, T.; Look, T.; et al. NOTCH2 links protein kinase Cδ to the expression of CD23 in chronic lymphocytic leukaemia (CLL) cells. Br. J. Haematol. 2010, 148, 868–878. [Google Scholar] [CrossRef]
- Giambra, V.; Jenkins, C.R.; Wang, H.; Lam, S.H.; Shevchuk, O.O.; Nemirovsky, O.; Wai, C.; Gusscott, S.; Chiang, M.Y.; Aster, J.C.; et al. NOTCH1 promotes T cell leukemia-initiating activity by RUNX-mediated regulation of PKC-θ and reactive oxygen species. Nat. Med. 2012, 18, 1693–1698. [Google Scholar] [CrossRef]
- Nayak, R.C.; Hegde, S.; Althoff, M.J.; Wellendorf, A.M.; Mohmoud, F.; Perentesis, J.; Reina-Campos, M.; Reynaud, D.; Zheng, Y.; Diaz-Meco, M.T.; et al. The signaling axis atypical protein kinase C λ/ι-Satb2 mediates leukemic transformation of B-cell progenitors. Nat. Commun. 2019, 10, 46. [Google Scholar] [CrossRef]
- Kurinna, S.; Konopleva, M.; Palla, S.L.; Chen, W.; Kornblau, S.; Contractor, R.; Deng, X.; May, W.S.; Andreeff, M.; Ruvolo, P.P. Bcl2 phosphorylation and active PKCα are associated with poor survival in AML. Leukemia 2006, 20, 1316–1319. [Google Scholar] [CrossRef]
- Ruvolo, P.P.; Deng, X.; Carr, B.K.; May, W.S. A functional role for mitochondrial protein kinase Cα in Bcl2 phosphorylation and suppression of apoptosis. J. Biol. Chem. 1998, 273, 25436–25442. [Google Scholar] [CrossRef]
- Li, Z.S.; Shi, K.J.; Guan, L.Y.; Jiang, Q.; Yang, Y.; Xu, C.M. Downregulation of protein kinase Cα was involved in selenite-induced apoptosis of NB4 cells. Oncol. Res. 2010, 19, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, R.; Menezes, A.C.; Azevedo, A.; Leckenby, A.; Davies, S.; Seedhouse, C.; Gilkes, A.; Knapper, S.; Tonks, A.; Darley, R.L. Protein kinase Cε overexpression is associated with poor patient outcomes in AML and promotes daunorubicin resistance through p-glycoprotein-mediated drug efflux. Front. Oncol. 2022, 12, 840046. [Google Scholar] [CrossRef] [PubMed]
- Di Marcantonio, D.; Martinez, E.; Sidoli, S.; Vadaketh, J.; Nieborowska-Skorska, M.; Gupta, A.; Meadows, J.M.; Ferraro, F.; Masselli, E.; Challen, G.A.; et al. Protein kinase Cε is a key regulator of mitochondrial redox homeostasis in acute myeloid leukemia. Clin. Cancer Res. 2018, 24, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Ozpolat, B.; Akar, U.; Tekedereli, I.; Alpay, S.N.; Barria, M.; Gezgen, B.; Zhang, N.; Coombes, K.; Kornblau, S.; Lopez-Berestein, G. PKCδ regulates translation initiation through PKR and eIF2α in response to retinoic acid in acute myeloid leukemia cells. Leuk. Res. Treat. 2012, 2012, 482905. [Google Scholar] [CrossRef] [PubMed]
- Song, M.G.; Gao, S.M.; Du, K.M.; Xu, M.; Yu, Y.; Zhou, Y.H.; Wang, Q.; Chen, Z.; Zhu, Y.S.; Chen, G.Q. Nanomolar concentration of NSC606985, a camptothecin analog, induces leukemic-cell apoptosis through protein kinase Cδ-dependent mechanisms. Blood 2005, 105, 3714–3721. [Google Scholar] [CrossRef]
- Yan, H.; Wang, Y.C.; Li, D.; Wang, Y.; Liu, W.; Wu, Y.L.; Chen, G.Q. Arsenic trioxide and proteasome inhibitor bortezomib synergistically induce apoptosis in leukemic cells: The role of protein kinase Cδ. Leukemia 2007, 21, 1488–1495. [Google Scholar] [CrossRef]
- Gao, F.H.; Wu, Y.L.; Zhao, M.; Liu, C.X.; Wang, L.S.; Chen, G.Q. Protein kinase C-δ mediates down-regulation of heterogeneous nuclear ribonucleoprotein K protein: Involvement in apoptosis induction. Exp. Cell Res. 2009, 315, 3250–3258. [Google Scholar] [CrossRef]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef]
- Weisberg, E.; Boulton, C.; Kelly, L.M.; Manley, P.; Fabbro, D.; Meyer, T.; Gilliland, D.G.; Griffin, J.D. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell 2002, 1, 433–443. [Google Scholar] [CrossRef]
- Ma, D.; Wang, P.; Fang, Q.; Yu, Z.; Zhou, Z.; He, Z.; Wei, D.; Yu, K.; Lu, T.; Zhang, Y.; et al. Low-dose staurosporine selectively reverses BCR-ABL-independent IM resistance through PKC-α-mediated G2/M phase arrest in chronic myeloid leukaemia. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 3), S208–S216. [Google Scholar] [CrossRef]
- Ma, D.; Liu, P.; Wang, P.; Zhou, Z.; Fang, Q.; Wang, J. PKC-β/Alox5 axis activation promotes Bcr-Abl-independent TKI-resistance in chronic myeloid leukemia. J. Cell. Physiol. 2021, 236, 6312–6327. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Shan, Y.; Bai, R.; Xue, L.; Eide, C.A.; Ou, J.; Zhu, L.J.; Hutchinson, L.; Cerny, J.; Khoury, H.J.; et al. A therapeutically targetable mechanism of BCR-ABL-independent imatinib resistance in chronic myeloid leukemia. Sci. Transl. Med. 2014, 6, 252ra121. [Google Scholar] [CrossRef] [PubMed]
- Gangat, N.; Patnaik, M.M.; Tefferi, A. Myelodysplastic syndromes: Contemporary review and how we treat. Am. J. Hematol. 2016, 91, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M. Myelodysplastic syndromes. N. Engl. J. Med. 2020, 383, 1358–1374. [Google Scholar] [CrossRef]
- Poli, A.; Ratti, S.; Finelli, C.; Mongiorgi, S.; Clissa, C.; Lonetti, A.; Cappellini, A.; Catozzi, A.; Barraco, M.; Suh, P.G.; et al. Nuclear translocation of PKC-α is associated with cell cycle arrest and erythroid differentiation in myelodysplastic syndromes (MDSs). FASEB J. 2018, 32, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, A.; Mongiorgi, S.; Finelli, C.; Fazio, A.; Ratti, S.; Marvi, M.V.; Curti, A.; Salvestrini, V.; Pellagatti, A.; Billi, A.M.; et al. Phospholipase C beta1 (PI-PLCbeta1)/Cyclin D3/protein kinase C (PKC) alpha signaling modulation during iron-induced oxidative stress in myelodysplastic syndromes (MDS). FASEB J. 2020, 34, 15400–15416. [Google Scholar] [CrossRef]
- Kamimura, K.; Hojo, H.; Abe, M. Characterization of expression of protein kinase C isozymes in human B-cell lymphoma: Relationship between its expression and prognosis. Pathol. Int. 2004, 54, 224–230. [Google Scholar] [CrossRef]
- Bakalova, R.; Ohba, H.; Zhelev, Z.; Kubo, T.; Fujii, M.; Ishikawa, M.; Shinohara, Y.; Baba, Y. Atypical protein-kinase Cζ, but neither conventional Ca2+-dependent protein-kinase C isoenzymes nor Ca2+-calmodulin, participates in regulation of telomerase activity in Burkitt’s lymphoma cells. Cancer Chemother. Pharmacol. 2004, 54, 161–172. [Google Scholar] [CrossRef]
- Decouvelaere, A.V.; Morschhauser, F.; Buob, D.; Copin, M.C.; Dumontet, C. Heterogeneity of protein kinase C β2 expression in lymphoid malignancies. Histopathology 2007, 50, 561–566. [Google Scholar] [CrossRef]
- Chao, C.; Silverberg, M.J.; Xu, L.; Chen, L.H.; Castor, B.; Martínez-Maza, O.; Abrams, D.I.; Zha, H.D.; Haque, R.; Said, J. A comparative study of molecular characteristics of diffuse large B-cell lymphoma from patients with and without human immunodeficiency virus infection. Clin. Cancer Res. 2015, 21, 1429–1437. [Google Scholar] [CrossRef]
- Li, S.; Phong, M.; Lahn, M.; Brail, L.; Sutton, S.; Lin, B.K.; Thornton, D.; Liao, B. Retrospective analysis of protein kinase C-β (PKC-β) expression in lymphoid malignancies and its association with survival in diffuse large B-cell lymphomas. Biol. Direct. 2007, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Chaiwatanatorn, K.; Stamaratis, G.; Opeskin, K.; Firkin, F.; Nandurkar, H. Protein kinase C-βII expression in diffuse large B-cell lymphoma predicts for inferior outcome of anthracycline-based chemotherapy with and without rituximab. Leuk. Lymphoma 2009, 50, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, I.; Briones, J.; Bordes, R.; Brunet, S.; Martino, R.; Sureda, A.; Prat, J.; Sierra, J. Membrane PKC-β2 protein expression predicts for poor response to chemotherapy and survival in patients with diffuse large B-cell lymphoma. Ann. Hematol. 2006, 85, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Hans, C.P.; Weisenburger, D.D.; Greiner, T.C.; Chan, W.C.; Aoun, P.; Cochran, G.T.; Pan, Z.; Smith, L.M.; Lynch, J.C.; Bociek, R.G.; et al. Expression of PKC-β or cyclin D2 predicts for inferior survival in diffuse large B-cell lymphoma. Mod. Pathol. 2005, 18, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Schaffel, R.; Morais, J.C.; Biasoli, I.; Lima, J.; Scheliga, A.; Romano, S.; Milito, C.; Spector, N. PKC-βII expression has prognostic impact in nodal diffuse large B-cell lymphoma. Mod. Pathol. 2007, 20, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Vinayak, M. Role of ellagic acid in regulation of apoptosis by modulating novel and atypical PKC in lymphoma bearing mice. BMC Complement Altern. Med. 2015, 15, 281. [Google Scholar] [CrossRef]
- Sumarni, U.; Reidel, U.; Eberle, J. Targeting cutaneous T-cell lymphoma cells by ingenol mebutate (PEP005) correlates with PKCδ activation, ROS induction as well as downregulation of XIAP and c-FLIP. Cells 2021, 10, 987. [Google Scholar] [CrossRef]
- Yanase, N.; Hayashida, M.; Kanetaka-Naka, Y.; Hoshika, A.; Mizuguchi, J. PKC-δ mediates interferon-α-induced apoptosis through c-Jun NH₂-terminal kinase activation. BMC Cell Biol. 2012, 13, 7. [Google Scholar] [CrossRef]
- Parent, N.; Scherer, M.; Liebisch, G.; Schmitz, G.; Bertrand, R. Protein kinase C-δ isoform mediates lysosome labilization in DNA damage-induced apoptosis. Int. J. Oncol. 2011, 38, 313–324. [Google Scholar]
- Leseux, L.; Laurent, G.; Laurent, C.; Rigo, M.; Blanc, A.; Olive, D.; Bezombes, C. PKCζ-mTOR pathway: A new target for rituximab therapy in follicular lymphoma. Blood 2008, 111, 285–291. [Google Scholar] [CrossRef]
- Arcos-Montoy, A.D.; Wegman-Ostrosky, T.; Mejía-Pérez, S.; De la Fuente-Granada, M.; Camacho-Arroyo, I.; García-Carrancá, A.; Velasco-Velázquez, M.A.; Manjarrez-Marmolejo, J.; González-Arenas, A. Progesterone receptor together with PKCα expression as prognostic factors for astrocytomas malignancy. Onco Targets Ther. 2021, 14, 3757–3768. [Google Scholar] [CrossRef] [PubMed]
- Chandrika, G.; Natesh, K.; Ranade, D.; Chugh, A.; Shastry, P. Suppression of the invasive potential of glioblastoma cells by mTOR inhibitors involves modulation of NFκB and PKC-α signaling. Sci. Rep. 2016, 6, 22455. [Google Scholar] [CrossRef] [PubMed]
- Amos, S.; Mut, M.; diPierro, C.G.; Carpenter, J.E.; Xiao, A.; Kohutek, Z.A.; Redpath, G.T.; Zhao, Y.; Wang, J.; Shaffrey, M.E.; et al. Protein kinase C-α-mediated regulation of low-density lipoprotein receptor related protein and urokinase increases astrocytoma invasion. Cancer Res. 2007, 67, 10241–10251. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Wang, Y.; Zhang, L.; Wang, J.; Wang, W.; Han, X.; Mu, C.; Gao, D. Identification of novel LncRNA targeting Smad2/PKCα signal pathway to negatively regulate malignant progression of glioblastoma. J. Cell. Physiol. 2020, 235, 3835–3848. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Rives, S.A.; Arcos-Montoya, D.; de la Fuente-Granada, M.; Zamora-Sánchez, C.J.; Arias-Romero, L.E.; Villamar-Cruz, O.; Camacho-Arroyo, I.; Pérez-Tapia, S.M.; González-Arenas, A. LPA1 receptor promotes progesterone receptor phosphorylation through PKCα in human glioblastoma cells. Cells 2021, 10, 807. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Rives, S.A.; de la Fuente-Granada, M.; Velasco-Velázquez, M.A.; González-Flores, O.; González-Arenas, A. LPA1 receptor activation induces PKCα nuclear translocation in glioblastoma cells. Int. J. Biochem. Cell Biol. 2019, 110, 91–102. [Google Scholar] [CrossRef]
- Grossman, S.A.; Alavi, J.B.; Supko, J.G.; Carson, K.A.; Priet, R.; Dorr, F.A.; Grundy, J.S.; Holmlund, J.T. Efficacy and toxicity of the antisense oligonucleotide aprinocarsen directed against protein kinase C-α delivered as a 21-day continuous intravenous infusion in patients with recurrent high-grade astrocytomas. Neuro. Oncol. 2005, 7, 32–40. [Google Scholar] [CrossRef]
- Wong, R.A.; Luo, X.; Lu, M.; An, Z.; Haas-Kogan, D.A.; Phillips, J.J.; Shokat, K.M.; Weiss, W.A.; Fan, Q.W. Cooperative blockade of PKCα and JAK2 drives apoptosis in glioblastoma. Cancer Res. 2020, 80, 709–718. [Google Scholar] [CrossRef]
- Cameron, A.J.; Procyk, K.J.; Leitges, M.; Parker, P.J. PKC alpha protein but not kinase activity is critical for glioma cell proliferation and survival. Int. J. Cancer 2008, 123, 769–779. [Google Scholar] [CrossRef]
- Baldwin, R.M.; Barrett, G.M.; Parolin, D.A.; Gillies, J.K.; Paget, J.A.; Lavictoire, S.J.; Gray, D.A.; Lorimer, I.A. Coordination of glioblastoma cell motility by PKCι. Mol. Cancer 2010, 9, 233. [Google Scholar] [CrossRef]
- Desai, S.R.; Pillai, P.P.; Patel, R.S.; McCray, A.N.; Win-Piazza, H.Y.; Acevedo-Duncan, M.E. Regulation of Cdk7 activity through a phosphatidylinositol (3)-kinase/PKC-ι-mediated signaling cascade in glioblastoma. Carcinogenesis 2012, 33, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Islam, S.M.A.; Patel, R.; Acevedo-Duncan, M. The interruption of atypical PKC signaling and temozolomide combination therapy against glioblastoma. Cell. Signal. 2021, 77, 109819. [Google Scholar] [CrossRef] [PubMed]
- Kenchappa, R.S.; Liu, Y.; Argenziano, M.G.; Banu, M.A.; Mladek, A.C.; West, R.; Luu, A.; Quiñones-Hinojosa, A.; Hambardzumyan, D.; Justilien, V.; et al. Protein kinase Cι and SRC signaling define reciprocally related subgroups of glioblastoma with distinct therapeutic vulnerabilities. Cell Rep. 2021, 37, 110054. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, R.M.; Garratt-Lalonde, M.; Parolin, D.A.; Krzyzanowski, P.M.; Andrade, M.A.; Lorimer, I.A. Protection of glioblastoma cells from cisplatin cytotoxicity via protein kinase Cι-mediated attenuation of p38 MAP kinase signaling. Oncogene 2006, 25, 2909–2919. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.; Lang, V.; Bohlen, J.; Bethke, F.; Puccio, L.; Tichy, D.; Herold-Mende, C.; Hielscher, T.; Lichter, P.; Goidts, V. Targeting atypical protein kinase C iota reduces viability in glioblastoma stem-like cells via a notch signaling mechanism. Int. J. Cancer 2016, 139, 1776–1787. [Google Scholar] [CrossRef]
- Desai, S.; Pillai, P.; Win-Piazza, H.; Acevedo-Duncan, M. PKC-ι promotes glioblastoma cell survival by phosphorylating and inhibiting BAD through a phosphatidylinositol 3-kinase pathway. Biochim. Biophys. Acta 2011, 1813, 1190–1197. [Google Scholar] [CrossRef]
- McCray, A.N.; Desai, S.; Acevedo-Duncan, M. The interruption of PKC-ι signaling and TRAIL combination therapy against glioblastoma cells. Neurochem. Res. 2014, 39, 1691–1701. [Google Scholar] [CrossRef]
- Mandil, R.; Ashkenazi, E.; Blass, M.; Kronfeld, I.; Kazimirsky, G.; Rosenthal, G.; Umansky, F.; Lorenzo, P.S.; Blumberg, P.M.; Brodie, C. Protein kinase Cα and protein kinase Cδ play opposite roles in the proliferation and apoptosis of glioma cells. Cancer Res. 2001, 61, 4612–4619. [Google Scholar]
- Assad Kahn, S.; Costa, S.L.; Gholamin, S.; Nitta, R.T.; Dubois, L.G.; Fève, M.; Zeniou, M.; Coelho, P.L.; El-Habr, E.; Cadusseau, J.; et al. The anti-hypertensive drug prazosin inhibits glioblastoma growth via the PKCδ-dependent inhibition of the AKT pathway. EMBO Mol. Med. 2016, 8, 511–526. [Google Scholar] [CrossRef]
- Misuth, M.; Joniova, J.; Horvath, D.; Dzurova, L.; Nichtova, Z.; Novotova, M.; Miskovsky, P.; Stroffekova, K.; Huntosova, V. The flashlights on a distinct role of protein kinase C δ: Phosphorylation of regulatory and catalytic domain upon oxidative stress in glioma cells. Cell. Signal. 2017, 34, 11–22. [Google Scholar] [CrossRef]
- Sharif, T.R.; Sharif, M. Overexpression of protein kinase C epsilon in astroglial brain tumor derived cell lines and primary tumor samples. Int. J. Oncol. 1999, 15, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Toton, E.; Romaniuk, A.; Konieczna, N.; Hofmann, J.; Barciszewski, J.; Rybczynska, M. Impact of PKCε downregulation on autophagy in glioblastoma cells. BMC Cancer 2018, 18, 85. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Gu, F.; Li, W.; Zhang, B.; Niu, R.; Fu, L.; Zhang, N.; Ma, Y. Reduction of protein kinase Cζ inhibits migration and invasion of human glioblastoma cells. J. Neurochem. 2009, 109, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Donson, A.M.; Banerjee, A.; Gamboni-Robertson, F.; Fleitz, J.M.; Foreman, N.K. Protein kinase C ζ isoform is critical for proliferation in human glioblastoma cell lines. J. Neurooncol. 2000, 47, 109–115. [Google Scholar] [CrossRef]
- Uht, R.M.; Amos, S.; Martin, P.M.; Riggan, A.E.; Hussaini, I.M. The protein kinase C-η isoform induces proliferation in glioblastoma cell lines through an ERK/Elk-1 pathway. Oncogene 2007, 26, 2885–2893. [Google Scholar] [CrossRef]
- Ali, S.; Al-Sukhun, S.; El-Rayes, B.F.; Sarkar, F.H.; Heilbrun, L.K.; Philip, P.A. Protein kinases C isozymes are differentially expressed in human breast carcinomas. Life Sci. 2009, 84, 766–771. [Google Scholar] [CrossRef]
- Pan, Q.; Bao, L.W.; Kleer, C.G.; Sabel, M.S.; Griffith, K.A.; Teknos, T.N.; Merajver, S.D. Protein kinase Cε is a predictive biomarker of aggressive breast cancer and a validated target for RNA interference anticancer therapy. Cancer Res. 2005, 65, 8366–8371. [Google Scholar] [CrossRef]
- Khan, K.; Safi, S.; Abbas, A.; Badshah, Y.; Dilshad, E.; Rafiq, M.; Zahra, K.; Shabbir, M. Unravelling structure, localization, and genetic crosstalk of KLF3 in human breast cancer. Biomed. Res. Int. 2020, 2020, 1354381. [Google Scholar] [CrossRef]
- Lønne, G.K.; Cornmark, L.; Zahirovic, I.O.; Landberg, G.; Jirström, K.; Larsson, C. PKCα expression is a marker for breast cancer aggressiveness. Mol. Cancer 2010, 9, 76. [Google Scholar] [CrossRef]
- Pham, T.N.D.; Perez White, B.E.; Zhao, H.; Mortazavi, F.; Tonetti, D.A. Protein kinase Cα enhances migration of breast cancer cells through FOXC2-mediated repression of p120-catenin. BMC Cancer 2017, 17, 832. [Google Scholar] [CrossRef]
- Frankel, L.B.; Lykkesfeldt, A.E.; Hansen, J.B.; Stenvang, J. Protein Kinase C α is a marker for antiestrogen resistance and is involved in the growth of tamoxifen resistant human breast cancer cells. Breast Cancer Res. Treat. 2007, 104, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Li, Z.; Tian, F.; Feng, Y.; Huang, J.; Li, C.; Xie, F. PKCα inhibited apoptosis by decreasing the activity of JNK in MCF-7/ADR cells. Exp. Toxicol. Pathol. 2012, 64, 459–464. [Google Scholar] [CrossRef]
- Tonetti, D.A.; Gao, W.; Escarzaga, D.; Walters, K.; Szafran, A.; Coon, J.S. PKCα and ERβ are associated with triple-negative breast cancers in African American and Caucasian patients. Int. J. Breast Cancer 2012, 2012, 740353. [Google Scholar] [CrossRef] [PubMed]
- Tam, W.L.; Lu, H.; Buikhuisen, J.; Soh, B.S.; Lim, E.; Reinhardt, F.; Wu, Z.J.; Krall, J.A.; Bierie, B.; Guo, W.; et al. Protein kinase C α is a central signaling node and therapeutic target for breast cancer stem cells. Cancer Cell 2013, 24, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Fournier, D.B.; Chisamore, M.; Lurain, J.R.; Rademaker, A.W.; Jordan, V.C.; Tonetti, D.A. Protein kinase C α expression is inversely related to ER status in endometrial carcinoma: Possible role in AP-1-mediated proliferation of ER-negative endometrial cancer. Gynecol. Oncol. 2001, 81, 366–372. [Google Scholar] [CrossRef]
- Kim, C.W.; Asai, D.; Kang, J.H.; Kishimura, A.; Mori, T.; Katayama, Y. Reversal of efflux of an anticancer drug in human drug-resistant breast cancer cells by inhibition of protein kinase Cα (PKCα) activity. Tumor Biol. 2016, 37, 1901–1908. [Google Scholar] [CrossRef] [PubMed]
- Reedijk, M.; Odorcic, S.; Chang, L.; Zhang, H.; Miller, N.; McCready, D.R.; Lockwood, G.; Egan, S.E. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res. 2005, 65, 8530–8537. [Google Scholar] [CrossRef]
- Dickson, B.C.; Mulligan, A.M.; Zhang, H.; Lockwood, G.; O’Malley, F.P.; Egan, S.E.; Reedijk, M. High level JAG1 mRNA and protein predict poor outcome in breast cancer. Mod. Pathol. 2007, 20, 85–693. [Google Scholar] [CrossRef]
- BeLow, M.; Osipo, C. Notch signaling in breast cancer: A role in drug resistance. Cells 2020, 9, 2204. [Google Scholar] [CrossRef]
- Pandya, K.; Wyatt, D.; Gallagher, B.; Shah, D.; Baker, A.; Bloodworth, J.; Zlobin, A.; Pannuti, A.; Green, A.; Ellis, I.O.; et al. PKCα attenuates Jagged-1-mediated notch signaling in ErbB-2-positive breast cancer to reverse trastuzumab resistance. Clin. Cancer Res. 2016, 22, 175–186. [Google Scholar] [CrossRef]
- Berardi, D.E.; Ariza Bareño, L.; Amigo, N.; Cañonero, L.; Pelagatti, M.L.N.; Motter, A.N.; Taruselli, M.A.; Díaz Bessone, M.I.; Cirigliano, S.M.; Edelstein, A.; et al. All-trans retinoic acid and protein kinase C α/β1 inhibitor combined treatment targets cancer stem cells and impairs breast tumor progression. Sci. Rep. 2021, 11, 6044. [Google Scholar] [CrossRef]
- Bessone, M.I.D.; Berardi, D.E.; Cirigliano, S.M.; Delbart, D.I.; Peters, M.G.; Todaro, L.B.; Urtreger, A.J. Protein Kinase C Alpha (PKCα) overexpression leads to a better response to retinoid acid therapy through Retinoic Acid Receptor Beta (RARβ) activation in mammary cancer cells. J. Cancer Res. Clin. Oncol. 2020, 146, 3241–3253. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.A.; Luan, H.; Tom, E.; Bielecki, T.A.; Mohapatra, B.; Ahmad, G.; George, M.; Kelly, D.L.; Natarajan, A.; Raja, S.M.; et al. A kinase inhibitor screen reveals protein kinase C-dependent endocytic recycling of ErbB2 in breast cancer cells. J. Biol. Chem. 2014, 89, 30443–30458. [Google Scholar] [CrossRef] [PubMed]
- Allen-Petersen, B.L.; Carter, C.J.; Ohm, A.M.; Reyland, M.E. Protein kinase Cδ is required for ErbB2-driven mammary gland tumorigenesis and negatively correlates with prognosis in human breast cancer. Oncogene 2014, 33, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Forman, L.W.; Williams, R.M.; Faller, D.V. Protein kinase C-δ inactivation inhibits the proliferation and survival of cancer stem cells in culture and in vivo. BMC Cancer 2014, 14, 90. [Google Scholar] [CrossRef]
- Assender, J.W.; Gee, J.M.; Lewis, I.; Ellis, I.O.; Robertson, J.F.; Nicholson, R.I. Protein kinase C isoform expression as a predictor of disease outcome on endocrine therapy in breast cancer. J. Clin. Pathol. 2007, 60, 1216–1221. [Google Scholar] [CrossRef]
- He, Y.; Liu, J.; Durrant, D.; Yang, H.S.; Sweatman, T.; Lothstein, L.; Lee, R.M. N-benzyladriamycin-14-valerate (AD198) induces apoptosis through protein kinase C-delta-induced phosphorylation of phospholipid scramblase 3. Cancer Res. 2005, 65, 10016–10023. [Google Scholar] [CrossRef]
- Díaz Bessone, M.I.; Berardi, D.E.; Campodónico, P.B.; Todaro, L.B.; Lothstein, L.; Bal de Kier Joffé, E.D.; Urtreger, A.J. Involvement of PKC delta (PKCδ) in the resistance against different doxorubicin analogs. Breast Cancer Res. Treat. 2011, 126, 577–587. [Google Scholar] [CrossRef]
- Yin, J.; Liu, Z.; Li, H.; Sun, J.; Chang, X.; Liu, J.; He, S.; Li, B. Association of PKCζ expression with clinicopathological characteristics of breast cancer. PLoS ONE 2014, 9, e90811. [Google Scholar] [CrossRef]
- Smalley, T.; Islam, S.M.A.; Apostolatos, C.; Apostolatos, A.; Acevedo-Duncan, M. Analysis of PKC-ζ protein levels in normal and malignant breast tissue subtypes. Oncol. Lett. 2019, 17, 1537–1546. [Google Scholar]
- Belguise, K.; Cherradi, S.; Sarr, A.; Boissière, F.; Boulle, N.; Simony-Lafontaine, J.; Choesmel-Cadamuro, V.; Wang, X.; Chalbos, D. PKCθ-induced phosphorylations control the ability of Fra-1 to stimulate gene expression and cancer cell migration. Cancer Lett. 2017, 385, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.; McCuaig, R.D.; Tan, A.H.Y.; Tu, W.J.; Wu, F.; Wagstaff, K.M.; Zafar, A.; Ali, S.; Diwakar, H.; Dahlstrom, J.E.; et al. Selective targeting of protein kinase C (PKC)-θ nuclear translocation reduces mesenchymal gene signatures and reinvigorates dysfunctional CD8+ T cells in immunotherapy-resistant and metastatic cancers. Cancers 2022, 14, 1596. [Google Scholar] [CrossRef] [PubMed]
- Byerly, J.; Halstead-Nussloch, G.; Ito, K.; Katsyv, I.; Irie, H.Y. PRKCQ promotes oncogenic growth and anoikis resistance of a subset of triple-negative breast cancer cells. Breast Cancer Res. 2016, 18, 95. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Outram, S.P.; Basu, A. Upregulation of PKCη by PKCε and PDK1 involves two distinct mechanisms and promotes breast cancer cell survival. Biochim. Biophys. Acta. 2013, 1830, 4040–4045. [Google Scholar] [CrossRef]
- Karp, G.; Abu-Ghanem, S.; Novack, V.; Mermershtain, W.; Ariad, S.; Sion-Vardy, N.; Livneh, E. Localization of PKCη in cell membranes as a predictor for breast cancer response to treatment. Onkologie 2012, 35, 260–266. [Google Scholar] [CrossRef]
- Zurgil, U.; Ben-Ari, A.; Rotem-Dai, N.; Karp, G.; Krasnitsky, E.; Frost, S.A.; Livneh, E. PKCη is an anti-apoptotic kinase that predicts poor prognosis in breast and lung cancer. Biochem. Soc. Trans. 2014, 42, 1519–1523. [Google Scholar] [CrossRef]
- Motomura, H.; Nozaki, Y.; Onaga, C.; Ozaki, A.; Tamori, S.; Shiina, T.A.; Kanai, S.; Ohira, C.; Hara, Y.; Harada, Y.; et al. High expression of c-Met, PKCλ and ALDH1A3 predicts a poor prognosis in late-stage breast cancer. Anticancer Res. 2020, 40, 35–52. [Google Scholar] [CrossRef]
- Nozaki, Y.; Motomura, H.; Tamori, S.; Kimura, Y.; Onaga, C.; Kanai, S.; Ishihara, Y.; Ozaki, A.; Hara, Y.; Harada, Y.; et al. High PKCλ expression is required for ALDH1-positive cancer stem cell function and indicates a poor clinical outcome in late-stage breast cancer patients. PLoS ONE 2020, 15, e0235747. [Google Scholar] [CrossRef]
- Motomura, H.; Tamori, S.; Yatani, M.A.; Namiki, A.; Onaga, C.; Ozaki, A.; Takasawa, R.; Mano, Y.; Sato, T.; Hara, Y.; et al. GLO 1 and PKCλ regulate ALDH1-positive breast cancer stem cell survival. Anticancer Res. 2021, 41, 5959–5971. [Google Scholar] [CrossRef]
- Blanchard, A.A.; Ma, X.; Wang, N.; Hombach-Klonisch, S.; Penner, C.; Ozturk, A.; Klonisch, T.; Pitz, M.; Murphy, L.; Leygue, E.; et al. Claudin 1 is highly upregulated by PKC in MCF7 human breast cancer cells and correlates positively with PKCε in patient biopsies. Transl. Oncol. 2019, 12, 561–575. [Google Scholar] [CrossRef]
- Azuma, K.; Ikeda, K.; Suzuki, T.; Aogi, K.; Horie-Inoue, K.; Inoue, S. TRIM47 activates NF-κB signaling via PKC-ε/PKD3 stabilization and contributes to endocrine therapy resistance in breast cancer. Proc. Natl. Acad. Sci USA 2021, 118, e2100784118. [Google Scholar] [CrossRef] [PubMed]
- Basu, A. Regulation of autophagy by protein kinase C-ε in breast cancer cells. Int. J. Mol. Sci. 2020, 21, 4247. [Google Scholar] [CrossRef]
- Wu, B.; Zhou, H.; Hu, L.; Mu, Y.; Wu, Y. Involvement of PKCα activation in TF/VIIa/PAR2-induced proliferation, migration, and survival of colon cancer cell SW620. Tumor Biol. 2013, 34, 837–846. [Google Scholar] [CrossRef]
- Lee, S.K.; Shehzad, A.; Jung, J.C.; Sonn, J.K.; Lee, J.T.; Park, J.W.; Lee, Y.S. Protein kinase Cα protects against multidrug resistance in human colon cancer cells. Mol. Cells 2012, 34, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xia, L.; Zhou, H.; Wu, B.; Mu, Y.; Wu, Y.; Yan, J. TF/FVIIa/PAR2 promotes cell proliferation and migration via PKCα and ERK-dependent c-Jun/AP-1 pathway in colon cancer cell line SW620. Tumor Biol. 2013, 34, 2573–2581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, B.; Zhu, N.; Tao, M.; Jun, Y.; Chen, X.; Wang, Q.; Luo, C. Mitotic checkpoint kinase Mps1/TTK predicts prognosis of colon cancer patients and regulates tumor proliferation and differentiation via PKCα/ERK1/2 and PI3K/Akt pathway. Med. Oncol. 2019, 37, 5. [Google Scholar] [CrossRef]
- Fang, J.Y.; Li, Z.H.; Li, Q.; Huang, W.S.; Kang, L.; Wang, J.P. Resveratrol affects protein kinase C activity and promotes apoptosis in human colon carcinoma cells. Asian Pac. J. Cancer Prev. 2012, 13, 6017–6022. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhu, Y.; Xu, W.; Xu, J.; Yang, M.; Chen, P.; Zhao, J.; Geng, L.; Gong, S. PKCα in colon cancer cells promotes M1 macrophage polarization via MKK3/6-P38 MAPK pathway. Mol. Carcinog. 2018, 57, 1017–1029. [Google Scholar] [CrossRef]
- Gwak, J.; Jung, S.J.; Kang, D.I.; Kim, E.Y.; Kim, D.E.; Chung, Y.H.; Shin, J.G.; Oh, S. Stimulation of protein kinase C-α suppresses colon cancer cell proliferation by down-regulation of β-catenin. J. Cell. Mol. Med. 2009, 13, 2171–2180. [Google Scholar] [CrossRef]
- Oster, H.; Leitges, M. Protein kinase C α but not PKCζ suppresses intestinal tumor formation in ApcMin/+ mice. Cancer Res. 2006, 66, 6955–6963. [Google Scholar] [CrossRef]
- Suga, K.; Sugimoto, I.; Ito, H.; Hashimoto, E. Down-regulation of protein kinase C-α detected in human colorectal cancer. Biochem. Mol. Biol. Int. 1998, 44, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Dupasquier, S.; Blache, P.; Picque Lasorsa, L.; Zhao, H.; Abraham, J.D.; Haigh, J.J.; Ychou, M.; Prévostel, C. Modulating PKCα activity to target Wnt/β-catenin signaling in colon cancer. Cancers 2019, 11, 693. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, Y.; Zhang, Y.; Wan, Y. Low expression of PKCα and high expression of KRAS predict poor prognosis in patients with colorectal cancer. Oncol. Lett. 2016, 12, 1655–1660. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Islam, S.M.A.; Dey, A.; Patel, R.; Smalley, T.; Acevedo-Duncan, M. Atypical protein kinase-C inhibitors exhibit a synergistic effect in facilitating DNA damaging effect of 5-fluorouracil in colorectal cancer cells. Biomed. Pharmacother. 2020, 121, 109665. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Cheng, Q.; Ma, Z.; Gong, G.; Deng, Z.; Xu, K.; Wang, G.; Wei, Y.; Zou, X. Silencing protein kinase C ζ by microRNA-25-5p activates AMPK signaling and inhibits colorectal cancer cell proliferation. Oncotarget 2017, 8, 65329–65338. [Google Scholar] [CrossRef]
- Umemori, Y.; Kuribayashi, K.; Nirasawa, S.; Kondoh, T.; Tanaka, M.; Kobayashi, D.; Watanabe, N. Protein kinase C ζ regulates survivin expression and inhibits apoptosis in colon cancer. Int. J. Oncol. 2014, 45, 1043–1050. [Google Scholar] [CrossRef]
- Islam, S.M.A.; Patel, R.; Acevedo-Duncan, M. Protein kinase C-ζ stimulates colorectal cancer cell carcinogenesis via PKC-ζ/Rac1/Pak1/β-Catenin signaling cascade. Biochim. Biophys. Acta Mol. Cell. Res. 2018, 1865, 650–664. [Google Scholar] [CrossRef]
- Yeo, M.K.; Kim, J.Y.; Seong, I.O.; Kim, J.M.; Kim, K.H. Phosphorylated protein kinase C (Zeta/Lambda) expression in colorectal adenocarcinoma and its correlation with clinicopathologic characteristics and prognosis. J. Cancer 2017, 8, 3371–3377. [Google Scholar] [CrossRef]
- Dowling, C.M.; Phelan, J.; Callender, J.A.; Cathcart, M.C.; Mehigan, B.; McCormick, P.; Dalton, T.; Coffey, J.C.; Newton, A.C.; O’Sullivan, J.; et al. Protein kinase beta II suppresses colorectal cancer by regulating IGF-1 mediated cell survival. Oncotarget 2016, 7, 20919–20933. [Google Scholar] [CrossRef]
- Spindler, K.L.; Lindebjerg, J.; Lahn, M.; Kjaer-Frifeldt, S.; Jakobsen, A. Protein kinase C-beta II (PKC-βII) expression in patients with colorectal cancer. Int. J. Colorectal. Dis. 2009, 24, 641–645. [Google Scholar] [CrossRef]
- Kahl-Rainer, P.; Sedivy, R.; Marian, B. Protein kinase C tissue localization in human colonic tumors suggests a role for adenoma growth control. Gastroenterology 1996, 110, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Serova, M.; Astorgues-Xerri, L.; Bieche, I.; Albert, S.; Vidaud, M.; Benhadji, K.A.; Emami, S.; Vidaud, D.; Hammel, P.; Theou-Anton, N.; et al. Epithelial-to-mesenchymal transition and oncogenic Ras expression in resistance to the protein kinase Cβ inhibitor enzastaurin in colon cancer cells. Mol. Cancer Ther. 2010, 9, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Perletti, G.; Marras, E.; Dondi, D.; Osti, D.; Congiu, T.; Ferrarese, R.; de Eguileor, M.; Tashjian, A.H., Jr. p21Waf1/Cip1 and p53 are downstream effectors of protein kinase Cδ in tumor suppression and differentiation in human colon cancer cells. Int. J. Cancer 2005, 113, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Mhaidat, N.M.; Bouklihacene, M.; Thorne, R.F. 5-Fluorouracil-induced apoptosis in colorectal cancer cells is caspase-9-dependent and mediated by activation of protein kinase C-δ. Oncol. Lett. 2014, 8, 699–704. [Google Scholar] [CrossRef]
- Zhou, B.; Lu, Y.; Zhao, Z.; Shi, T.; Wu, H.; Chen, W.; Zhang, L.; Zhang, X. B7-H4 expression is upregulated by PKCδ activation and contributes to PKCδ-induced cell motility in colorectal cancer. Cancer Cell Int. 2022, 22, 147. [Google Scholar] [CrossRef]
- Su, C.M.; Weng, Y.S.; Kuan, L.Y.; Chen, J.H.; Hsu, F.T. Suppression of PKCδ/NF-κB signaling and apoptosis induction through extrinsic/intrinsic pathways are associated magnolol-inhibited tumor progression in colorectal cancer in vitro and in vivo. Int. J. Mol. Sci. 2020, 21, 3527. [Google Scholar] [CrossRef]
- Cheng, J.; He, S.; Wang, M.; Zhou, L.; Zhang, Z.; Feng, X.; Yu, Y.; Ma, J.; Dai, C.; Zhang, S.; et al. The caspase-3/PKCδ/Akt/VEGF-A signaling pathway mediates tumor repopulation during radiotherapy. Clin. Cancer Res. 2019, 25, 3732–3743. [Google Scholar] [CrossRef]
- Du, G.S.; Qiu, Y.; Wang, W.S.; Peng, K.; Zhang, Z.C.; Li, X.S.; Xiao, W.D.; Yang, H. Knockdown on aPKC-ι inhibits epithelial-mesenchymal transition, migration and invasion of colorectal cancer cells through Rac1-JNK pathway. Exp. Mol. Pathol. 2019, 107, 57–67. [Google Scholar] [CrossRef]
- Linares, J.F.; Zhang, X.; Martinez-Ordoñez, A.; Duran, A.; Kinoshita, H.; Kasashima, H.; Nakanishi, N.; Nakanishi, Y.; Carelli, R.; Cappelli, L.; et al. PKCλ/ι inhibition activates an ULK2-mediated interferon response to repress tumorigenesis. Mol. Cell 2021, 81, 4509–4526. [Google Scholar] [CrossRef]
- Lin, K.Y.; Fang, C.L.; Uen, Y.H.; Chang, C.C.; Lou, H.Y.; Hsieh, C.R.; Tiong, C.; Pan, S.; Chen, S.H. Overexpression of protein kinase Cα mRNA may be an independent prognostic marker for gastric carcinoma. J. Surg. Oncol. 2008, 97, 538–543. [Google Scholar] [CrossRef]
- Lin, S.C.; Chen, W.Y.; Lin, K.Y.; Chen, S.H.; Chang, C.C.; Lin, S.E.; Fang, C.L. Clinicopathological correlation and prognostic significance of protein kinase Cα overexpression in human gastric carcinoma. PLoS ONE 2013, 8, e56675. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.L.; Sui, F.Y.; Du, C.; Zhang, C.W.; Hui, B.; Xu, S.L.; Lu, H.Z.; Song, G.J. Antisense e expression of PKCα improved sensitivity of SGC7901/VCR cells to doxorubicin. World J. Gastroenterol. 2009, 15, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Han, Z.Y.; Zhou, X.M.; Shi, R.; Zheng, Y.; Shi, Y.Q.; Miao, J.Y.; Pan, B.R.; Fan, D.M. Expression and function of classical protein kinase C isoenzymes in gastric cancer cell line and its drug-resistant sublines. World J. Gastroenterol. 2002, 8, 441–445. [Google Scholar] [CrossRef]
- Tseng, L.L.; Cheng, H.H.; Yeh, T.S.; Huang, S.C.; Syu, Y.Y.; Chuu, C.P.; Yuh, C.H.; Kung, H.J.; Wang, W.C. Targeting the histone demethylase PHF8-mediated PKCα-Src-PTEN axis in HER2-negative gastric cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 24859–24866. [Google Scholar] [CrossRef]
- Hashimoto, I.; Sakamaki, K.; Oue, N.; Kimura, Y.; Hiroshima, Y.; Hara, K.; Maezawa, Y.; Kano, K.; Aoyama, T.; Yamada, T.; et al. Clinical significance of PRKCI gene expression in cancerous tissue in patients with gastric cancer. Anticancer Res. 2019, 39, 5715–5720. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, Y.; Xu, X.M.; Zhu, X. Circular RNA circ-PRKCI promotes cell proliferation and invasion by binding to microRNA-545 in gastric cancer. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9418–9426. [Google Scholar] [PubMed]
- Batsaikhan, B.E.; Yoshikawa, K.; Kurita, N.; Iwata, T.; Takasu, C.; Kashihara, H.; Shimada, M. Expression of Stathmin1 in gastric adenocarcinoma. Anticancer Res. 2014, 34, 4217–4421. [Google Scholar]
- Takagawa, R.; Akimoto, K.; Ichikawa, Y.; Akiyama, H.; Kojima, Y.; Ishiguro, H.; Inayama, Y.; Aoki, I.; Kunisaki, C.; Endo, I.; et al. High expression of atypical protein kinase C λ/ι in gastric cancer as a prognostic factor for recurrence. Ann. Surg. Oncol. 2010, 17, 81–88. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Martínez-Gimeno, C.; Díaz-Meco, M.T.; Domínguez, I.; Moscat, J. Alterations in levels of different protein kinase C isotypes and their influence on behavior of squamous cell carcinoma of the oral cavity: εPKC, a novel prognostic factor for relapse and survival. Head Neck. 1995, 17, 516–525. [Google Scholar] [CrossRef]
- Chu, P.Y.; Hsu, N.C.; Lin, S.H.; Hou, M.F.; Yeh, K.T. High nuclear protein kinase CβII expression is a marker of disease recurrence in oral squamous cell carcinoma. Anticancer Res. 2012, 32, 3987–3991. [Google Scholar] [PubMed]
- Gao, W.; Guo, H.; Niu, M.; Zheng, X.; Zhang, Y.; Xue, X.; Bo, Y.; Guan, X.; Li, Z.; Guo, Y.; et al. circPARD3 drives malignant progression and chemoresistance of laryngeal squamous cell carcinoma by inhibiting autophagy through the PRKCI-Akt-mTOR pathway. Mol. Cancer 2020, 19, 166. [Google Scholar] [CrossRef] [PubMed]
- Parzefall, T.; Schnoell, J.; Monschein, L.; Foki, E.; Liu, D.T.; Frohne, A.; Grasl, S.; Pammer, J.; Lucas, T.; Kadletz, L.; et al. PRKCA overexpression is frequent in young oral tongue squamous cell carcinoma patients and is associated with poor prognosis. Cancers 2021, 13, 2082. [Google Scholar] [CrossRef] [PubMed]
- Zhen-jin, Z.; Peng, L.; Fa-yu, L.; Liyan, S.; Chang-fu, S. PKCα take part in CCR7/NF-κB autocrine signaling loop in CCR7-positive squamous cell carcinoma of head and neck. Mol. Cell Biochem. 2011, 357, 181–187. [Google Scholar] [CrossRef]
- Cohen, E.E.; Zhu, H.; Lingen, M.W.; Martin, L.E.; Kuo, W.L.; Choi, E.A.; Kocherginsky, M.; Parker, J.S.; Chung, C.H.; Rosner, M.R. A feed-forward loop involving protein kinase Cα and microRNAs regulates tumor cell cycle. Cancer Res. 2009, 69, 65–74. [Google Scholar] [CrossRef]
- Baba, J.; Kioi, M.; Akimoto, K.; Nagashima, Y.; Taguri, M.; Inayama, Y.; Aoki, I.; Ohno, S.; Mitsudo, K.; Tohnai, I. Atypical protein Kinase Cλ/ι expression is associated with malignancy of oral squamous cell carcinoma. Anticancer Res. 2018, 38, 6291–6297. [Google Scholar] [CrossRef]
- Chu, P.Y.; Hsu, N.C.; Tai, H.C.; Yeh, C.M.; Lin, S.H.; Hou, M.F.; Yeh, K.T. High nuclear protein kinase Cθ expression may correlate with disease recurrence and poor survival in oral squamous cell carcinoma. Hum. Pathol. 2012, 43, 276–281. [Google Scholar] [CrossRef]
- Caspa Gokulan, R.; Devaraj, H. Stem cell markers CXCR-4 and CD133 predict aggressive phenotype and their double positivity indicates poor prognosis of oral squamous cell carcinoma. Cancers 2021, 13, 5895. [Google Scholar] [CrossRef]
- Tsai, J.H.; Tsai, M.T.; Su, W.W.; Chen, Y.L.; Wu, T.T.; Hsieh, Y.S.; Huang, C.Y.; Yeh, K.T.; Liu, J.Y. Expression of protein kinase Cα in biopsies and surgical specimens of human hepatocellular carcinoma. Chin. J. Physiol. 2005, 48, 139–143. [Google Scholar]
- Wu, T.T.; Hsieh, Y.H.; Wu, C.C.; Hsieh, Y.S.; Huang, C.Y.; Liu, J.Y. Overexpression of protein kinase Cα mRNA in human hepatocellular carcinoma: A potential marker of disease prognosis. Clin. Chim. Acta 2007, 382, 54–58. [Google Scholar] [CrossRef]
- Wu, T.T.; Hsieh, Y.H.; Hsieh, Y.S.; Liu, J.Y. Reduction of PKCα decreases cell proliferation, migration, and invasion of human malignant hepatocellular carcinoma. J. Cell. Biochem. 2008, 103, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Liu, Y.; Ding, X.; Ke, Q.; Shi, J.; Ma, Z.; Gu, H.; Wang, H.; Zhang, C.; Yang, C.; et al. E2F1 transactivates IQGAP3 and promotes proliferation of hepatocellular carcinoma cells through IQGAP3-mediated PKC-alpha activation. Am. J. Cancer Res. 2019, 9, 285–299. [Google Scholar] [PubMed]
- Wang, J.; Shao, M.; Liu, M.; Peng, P.; Li, L.; Wu, W.; Wang, L.; Duan, F.; Zhang, M.; Song, S.; et al. PKCα promotes generation of reactive oxygen species via DUOX2 in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2015, 463, 839–845. [Google Scholar] [CrossRef]
- Wei, C.Y.; Zhu, M.X.; Zhang, P.F.; Huang, X.Y.; Wan, J.K.; Yao, X.Z.; Hu, Z.T.; Chai, X.Q.; Peng, R.; Yang, X.; et al. PKCα/ZFP64/CSF1 axis resets the tumor microenvironment and fuels anti-PD1 resistance in hepatocellular carcinoma. J. Hepatol. 2022, 77, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Hsu, F.T.; Chao, T.L.; Lee, Y.H.; Kuo, Y.C. Revealing the suppressive role of protein kinase Cδ and p38 mitogen-activated protein kinase (MAPK)/NF-κB axis associates with lenvatinib-inhibited progression in hepatocellular carcinoma in vitro and in vivo. Biomed. Pharmacother. 2022, 145, 112437. [Google Scholar] [CrossRef]
- Takai, S.; Matsushima-Nishiwaki, R.; Tokuda, H.; Yasuda, E.; Toyoda, H.; Kaneoka, Y.; Yamaguchi, A.; Kumada, T.; Kozawa, O. Protein kinase Cδ regulates the phosphorylation of heat shock protein 27 in human hepatocellular carcinoma. Life Sci. 2007, 81, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Yang, H.; Jeong, S.I.; Jin, Y.H.; Park, C.S.; Park, Y.S. Induction of heme oxygenase-1 inhibits cell death in crotonaldehyde-stimulated HepG2 cells via the PKC-δ-p38-Nrf2 pathway. PLoS ONE 2012, 7, e41676. [Google Scholar] [CrossRef] [PubMed]
- Mandal, J.P.; Shiue, C.N.; Chen, Y.C.; Lee, M.C.; Yang, H.H.; Chang, H.H.; Hu, C.T.; Liao, P.C.; Hui, L.C.; You, R.I.; et al. PKCδ mediates mitochondrial ROS generation and oxidation of HSP60 to relieve RKIP inhibition on MAPK pathway for HCC progression. Free Radic. Biol. Med. 2021, 163, 69–87. [Google Scholar] [CrossRef]
- Cao, M.; Gao, J.; Zhou, H.; Huang, J.; You, A.; Guo, Z.; Fang, F.; Zhang, W.; Song, T.; Zhang, T. HIF-2α regulates CDCP1 to promote PKCδ-mediated migration in hepatocellular carcinoma. Tumor Biol. 2016, 37, 1651–1662. [Google Scholar] [CrossRef]
- Kudo, Y.; Sugimoto, M.; Arias, E.; Kasashima, H.; Cordes, T.; Linares, J.F.; Duran, A.; Nakanishi, Y.; Nakanishi, N.; L’Hermitte, A.; et al. PKCλ/ι loss induces autophagy, oxidative phosphorylation, and NRF2 to promote liver cancer progression. Cancer Cell 2020, 38, 247–262. [Google Scholar] [CrossRef]
- Moscat, J.; Diaz-Meco, M.T. The interplay between PRKCI/PKCλ/ι, SQSTM1/p62, and autophagy orchestrates the oxidative metabolic response that drives liver cancer. Autophagy 2020, 16, 1915–1917. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.C.; Chou, F.P.; Yeh, K.T.; Chang, Y.S.; Hsu, N.C.; Chang, J.G. Expression of protein kinase C family in human hepatocellular carcinoma. Pathol. Oncol. Res. 2010, 16, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Mehta, D.; Sif, S.; Kent, L.N.; Jacob, S.T.; Ghoshal, K.; Mehta, K.D. Dietary fat/cholesterol-sensitive PKCβ-RB signaling: Potential role in NASH/HCC axis. Oncotarget 2017, 8, 73757–73765. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Li, Y.; Kang, X.; Sun, L.; Cui, J.; Gao, D.; Liu, Y. Role of PKCβ in hepatocellular carcinoma cells migration and invasion in vitro: A potential therapeutic target. Clin. Exp. Metastasis 2009, 26, 189–195. [Google Scholar] [CrossRef]
- Lu, H.C.; Chou, F.P.; Yeh, K.T.; Chang, Y.S.; Hsu, N.C.; Chang, J.G. Analysing the expression of protein kinase Cη in human hepatocellular carcinoma. Pathology 2009, 41, 626–629. [Google Scholar] [CrossRef]
- Singhal, S.S.; Wickramarachchi, D.; Singhal, J.; Yadav, S.; Awasthi, Y.C.; Awasthi, S. Determinants of differential doxorubicin sensitivity between SCLC and NSCLC. FEBS Lett. 2006, 580, 2258–2264. [Google Scholar] [CrossRef]
- Lang, W.; Wang, H.; Ding, L.; Xiao, L. Cooperation between PKC-α and PKC-ε in the regulation of JNK activation in human lung cancer cells. Cell. Signal. 2004, 16, 457–467. [Google Scholar] [CrossRef]
- Lahn, M.; Su, C.; Li, S.; Chedid, M.; Hanna, K.R.; Graff, J.R.; Sandusky, G.E.; Ma, D.; Niyikiza, C.; Sundell, K.L.; et al. Expression levels of protein kinase C-α in non-small-cell lung cancer. Clin. Lung Cancer 2004, 6, 184–189. [Google Scholar] [CrossRef]
- Tzeng, H.T.; Li, T.H.; Tang, Y.A.; Tsai, C.H.; Frank Lu, P.J.; Lai, W.W.; Chiang, C.W.; Wang, Y.C. Phosphorylation of Rab37 by protein kinase Cα inhibits the exocytosis function and metastasis suppression activity of Rab37. Oncotarget 2017, 8, 108556–108570. [Google Scholar] [CrossRef][Green Version]
- Salama, M.F.; Liu, M.; Clarke, C.J.; Espaillat, M.P.; Haley, J.D.; Jin, T.; Wang, D.; Obeid, L.M.; Hannun, Y.A. PKCα is required for Akt-mTORC1 activation in non-small cell lung carcinoma (NSCLC) with EGFR mutation. Oncogene 2019, 38, 7311–7328. [Google Scholar] [CrossRef]
- Gao, X.; Xu, F.; Zhang, H.T.; Chen, M.; Huang, W.; Zhang, Q.; Zeng, Q.; Liu, L. PKCα-GSK3β-NF-κB signaling pathway and the possible involvement of TRIM21 in TRAIL-induced apoptosis. Biochem. Cell Biol. 2016, 94, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.D.; Gu, J.F.; Yuan, J.R.; Feng, L.; Jia, X.B. Suppression of A549 cell proliferation and metastasis by calycosin via inhibition of the PKC-α/ERK1/2 pathway: An in vitro investigation. Mol. Med. Rep. 2015, 12, 7992–8002. [Google Scholar] [CrossRef] [PubMed]
- Abera, M.B.; Kazanietz, M.G. Protein kinase Cα mediates erlotinib resistance in lung cancer cells. Mol. Pharmacol. 2015, 87, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H. Protein kinase C (PKC) isozymes and cancer. New J. Sci. 2014, 2014, 231418. [Google Scholar]
- Halvorsen, A.R.; Haugen, M.H.; Öjlert, Å.K.; Lund-Iversen, M.; Jørgensen, L.; Solberg, S.; Mælandsmo, G.M.; Brustugun, O.T.; Helland, Å. Protein kinase C isozymes associated with relapse free survival in non-small cell lung cancer patients. Front. Oncol. 2020, 10, 590755. [Google Scholar] [CrossRef]
- Hill, K.S.; Erdogan, E.; Khoor, A.; Walsh, M.P.; Leitges, M.; Murray, N.R.; Fields, A.P. Protein kinase Cα suppresses Kras-mediated lung tumor formation through activation of a p38 MAPK-TGFβ signaling axis. Oncogene 2014, 33, 2134–2144. [Google Scholar] [CrossRef]
- Tsai, J.Y.; Rédei, D.; Hohmann, J.; Wu, C.C. 12-Deoxyphorbol esters induce growth arrest and apoptosis in human lung cancer A549 cells via activation of PKC-δ/PKD/ERK signaling pathway. Int. J. Mol. Sci. 2020, 21, 7579. [Google Scholar] [CrossRef]
- Iitaka, D.; Moodley, S.; Shimizu, H.; Bai, X.H.; Liu, M. PKCδ-iPLA2-PGE2-PPARγ signaling cascade mediates TNF-α induced Claudin 1 expression in human lung carcinoma cells. Cell. Signal. 2015, 27, 568–577. [Google Scholar] [CrossRef]
- Zhang, H.; Okamoto, M.; Panzhinskiy, E.; Zawada, W.M.; Das, M. PKCδ/midkine pathway drives hypoxia-induced proliferation and differentiation of human lung epithelial cells. Am. J. Physiol. Cell Physiol. 2014, 306, C648–C658. [Google Scholar] [CrossRef][Green Version]
- Yueh, P.F.; Lee, Y.H.; Chiang, I.T.; Chen, W.T.; Lan, K.L.; Chen, C.H.; Hsu, F.T. Suppression of EGFR/PKC-δ/NF-κB signaling associated with imipramine-inhibited progression of non-small cell lung cancer. Front. Oncol. 2021, 11, 735183. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Yun, H.S.; Kwon, G.T.; Lee, J.; Kim, J.Y.; Jo, Y.; Cho, J.M.; Lee, C.W.; Song, J.Y.; Ahn, J.; et al. PLOD3 suppression exerts an anti-tumor effect on human lung cancer cells by modulating the PKC-delta signaling pathway. Cell Death Dis. 2019, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Fang, Y.F.; Yamaguchi, H.; Wang, W.J.; Chen, T.C.; Hong, X.L.; Ke, B.; Xia, W.; Wei, Y.; Zha, Z.; et al. Targeting PKCδ as a therapeutic strategy against heterogeneous mechanisms of EGFR inhibitor resistance in EGFR-mutant lung cancer. Cancer Cell 2018, 34, 954–969. [Google Scholar] [CrossRef]
- Bae, K.M.; Wang, H.; Jiang, G.; Chen, M.G.; Lu, L.; Xiao, L. Protein kinase Cε is overexpressed in primary human non-small cell lung cancers and functionally required for proliferation of non-small cell lung cancer cells in a p21/Cip1-dependent manner. Cancer Res. 2007, 67, 6053–6063. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Cooke, M.; Benavides, F.; Abba, M.C.; Cicchini, M.; Feldser, D.M.; Kazanietz, M.G. PKC ε is required for KRAS-driven lung tumorigenesis. Cancer Res. 2020, 80, 5166–5173. [Google Scholar] [CrossRef] [PubMed]
- Caino, M.C.; Lopez-Haber, C.; Kim, J.; Mochly-Rosen, D.; Kazanietz, M.G. Proteins kinase Cɛ is required for non-small cell lung carcinoma growth and regulates the expression of apoptotic genes. Oncogene 2012, 31, 2593–2600. [Google Scholar] [CrossRef]
- Pardo, O.E.; Wellbrock, C.; Khanzada, U.K.; Aubert, M.; Arozarena, I.; Davidson, S.; Bowen, F.; Parker, P.J.; Filonenko, V.V.; Gout, I.T.; et al. FGF-2 protects small cell lung cancer cells from apoptosis through a complex involving PKCε, B-Raf and S6K2. EMBO J. 2006, 25, 3078–3088. [Google Scholar] [CrossRef]
- Liu, L.; Lei, B.; Wang, L.; Chang, C.; Yang, H.; Liu, J.; Huang, G.; Xie, W. Protein kinase C-iota-mediated glycolysis promotes non-small-cell lung cancer progression. Onco Targets Ther. 2019, 12, 5835–5848. [Google Scholar] [CrossRef]
- Krasnitsky, E.; Baumfeld, Y.; Freedman, J.; Sion-Vardy, N.; Ariad, S.; Novack, V.; Livneh, E. PKCη is a novel prognostic marker in non-small cell lung cancer. Anticancer Res. 2012, 32, 1507–1513. [Google Scholar]
- Lemjabbar-Alaoui, H.; Sidhu, S.S.; Mengistab, A.; Gallup, M.; Basbaum, C. TACE/ADAM-17 phosphorylation by PKC-epsilon mediates premalignant changes in tobacco smoke-exposed lung cells. PLoS ONE 2011, 6, e17489. [Google Scholar] [CrossRef]
- Jin, Z.; Xin, M.; Deng, X. Survival function of protein kinase Cι as a novel nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-activated Bad kinase. J. Biol. Chem. 2005, 280, 16045–16052. [Google Scholar] [CrossRef]
- Zhao, L.J.; Xu, H.; Qu, J.W.; Zhao, W.Z.; Zhao, Y.B.; Wang, J.H. Modulation of drug resistance in ovarian cancer cells by inhibition of protein kinase C-alpha (PKC-α) with small interference RNA (siRNA) agents. Asian Pac. J. Cancer Prev. 2012, 13, 3631–3636. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.N.; Zhao, L.J.; Wu, L.N.; He, M.F.; Qu, J.W.; Zhao, Y.B.; Zhao, W.Z.; Li, J.S.; Wang, J.H. Mechanistic analysis of taxol-induced multidrug resistance in an ovarian cancer cell line. Asian Pac. J. Cancer Prev. 2013, 14, 4983–4988. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Sun, B.; Zhao, X.; Du, J.; Gu, Q.; Liu, Y.; Cheng, R.; Dong, X. Wnt5a promotes vasculogenic mimicry and epithelial-mesenchymal transition via protein kinase Cα in epithelial ovarian cancer. Oncol. Rep. 2014, 32, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Mahanivong, C.; Chen, H.M.; Yee, S.W.; Pan, Z.K.; Dong, Z.; Huang, S. Protein kinase Cα-CARMA3 signaling axis links Ras to NF-κB for lysophosphatidic acid-induced urokinase plasminogen activator expression in ovarian cancer cells. Oncogene 2008, 27, 1273–1280. [Google Scholar] [CrossRef]
- Lili, X.; Xiaoyu, T. Expression of PKCα, PKCε, and P-gp in epithelial ovarian carcinoma and the clinical significance. Eur. J. Gynaecol. Oncol. 2015, 36, 181–185. [Google Scholar]
- Weichert, W.; Gekeler, V.; Denkert, C.; Dietel, M.; Hauptmann, S. Protein kinase C isoform expression in ovarian carcinoma correlates with indicators of poor prognosis. Int. J. Oncol. 2003, 23, 633–639. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, J.; Yang, N.; Liang, S.; Barchetti, A.; Giannakakis, A.; Cadungog, M.G.; O’Brien-Jenkins, A.; Massobrio, M.; Roby, K.F.; et al. Integrative genomic analysis of protein kinase C (PKC) family identifies PKCι as a biomarker and potential oncogene in ovarian carcinoma. Cancer Res. 2006, 66, 4627–4635. [Google Scholar] [CrossRef]
- Eder, A.M.; Sui, X.; Rosen, D.G.; Nolden, L.K.; Cheng, K.W.; Lahad, J.P.; Kango-Singh, M.; Lu, K.H.; Warneke, C.L.; Atkinson, E.N.; et al. Atypical PKCι contributes to poor prognosis through loss of apical-basal polarity and cyclin E overexpression in ovarian cancer. Proc. Natl. Acad. Sci USA 2005, 102, 12519–12524. [Google Scholar] [CrossRef]
- Rehmani, H.; Li, Y.; Li, T.; Padia, R.; Calbay, O.; Jin, L.; Chen, H.; Huang, S. Addiction to protein kinase Cι due to PRKCI gene amplification can be exploited for an aptamer-based targeted therapy in ovarian cancer. Signal. Transduct. Target Ther. 2020, 5, 140. [Google Scholar] [CrossRef]
- Sarkar, S.; Bristow, C.A.; Dey, P.; Rai, K.; Perets, R.; Ramirez-Cardenas, A.; Malasi, S.; Huang-Hobbs, E.; Haemmerle, M.; Wu, S.Y.; et al. PRKCI promotes immune suppression in ovarian cancer. Genes Dev. 2017, 31, 1109–1121. [Google Scholar] [CrossRef]
- Wang, Y.; Justilien, V.; Brennan, K.I.; Jamieson, L.; Murray, N.R.; Fields, A.P. PKCι regulates nuclear YAP1 localization and ovarian cancer tumorigenesis. Oncogene 2017, 36, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Nazarenko, I.; Jenny, M.; Keil, J.; Gieseler, C.; Weisshaupt, K.; Sehouli, J.; Legewie, S.; Herbst, L.; Weichert, W.; Darb-Esfahani, S.; et al. Atypical protein kinase C ζ exhibits a proapoptotic function in ovarian cancer. Mol. Cancer Res. 2010, 8, 919–934. [Google Scholar] [CrossRef]
- Smalley, T.; Metcalf, R.; Patel, R.; Islam, S.M.A.; Bommareddy, R.R.; Acevedo-Duncan, M. The atypical protein kinase C small molecule inhibitor ζ-Stat, and its effects on invasion through decreases in PKC-ζ protein expression. Front. Oncol. 2020, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Baffi, T.R.; Van, A.N.; Zhao, W.; Mills, G.B.; Newton, A.C. Protein kinase C quality control by phosphatase PHLPP1 unveils loss-of-function mechanism in cancer. Mol. Cell. 2019, 74, 378–392.e5. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Bao, X.; Wang, H.; Zhu, S.; Liu, Z.; Chen, Q.; Ai, K.; Shi, B. TRPM2 promotes pancreatic cancer by PKC/MAPK pathway. Cell Death Dis. 2021, 12, 585. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, S.; Yoo, S.; Rho, J.K.; Jun, E.S.; Chang, S.; Kim, K.K.; Kim, S.C.; Kim, I. Downregulation of X-linked inhibitor of apoptosis protein by ′7-Benzylidenenaltrexone maleate′ sensitizes pancreatic cancer cells to TRAIL-induced apoptosis. Oncotarget 2017, 8, 61057–61071. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.Y.; Dong, H.; Quach, K.T.; Van Nguyen, P.N.; Chen, K.; Carethers, J.M. TGF-β mediates PTEN suppression and cell motility through calcium-dependent PKC-α activation in pancreatic cancer cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G899–G905. [Google Scholar] [CrossRef]
- Ma, J.; Xue, H.; He, L.H.; Wang, L.Y.; Wang, X.J.; Li, X.; Zhang, L. The role and mechanism of autophagy in pancreatic cancer: An update review. Cancer Manag. Res. 2021, 13, 8231–8240. [Google Scholar] [CrossRef]
- Jia, S.; Xu, X.; Zhou, S.; Chen, Y.; Ding, G.; Cao, L. Fisetin induces autophagy in pancreatic cancer cells via endoplasmic reticulum stress- and mitochondrial stress-dependent pathways. Cell Death Dis. 2019, 10, 142. [Google Scholar] [CrossRef]
- Kyuno, D.; Kojima, T.; Ito, T.; Yamaguchi, H.; Tsujiwaki, M.; Takasawa, A.; Murata, M.; Tanak, A.S.; Hirata, K.; Sawada, N. Protein kinase Cα inhibitor enhances the sensitivity of human pancreatic cancer HPAC cells to Clostridium perfringens enterotoxin via claudin-4. Cell Tissue Res. 2011, 346, 369–381. [Google Scholar] [CrossRef]
- Taniuchi, K.; Yokotani, K.; Saibara, T. BART inhibits pancreatic cancer cell invasion by PKCα inactivation through binding to ANX7. PLoS ONE 2012, 7, e35674. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Wu, M.Y.; Shou, L.M.; Chen, L.P.; Gong, F.R.; Chen, K.; Li, D.M.; Duan, W.M.; Xie, Y.F.; Mao, Y.X.; et al. Tamoxifen enhances the anticancer effect of cantharidin and norcantharidin in pancreatic cancer cell lines through inhibition of the protein kinase C signaling pathway. Oncol. Lett. 2015, 9, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, S.; Peng, B.; Shen, L.; Yu, T.; Lafontant, J.; Li, P.; Xiong, R.; Makriyannis, A.; Chen, C. Suppression of PKC causes oncogenic stress for triggering apoptosis in cancer cells. Oncotarget 2017, 8, 30992–31002. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Akimoto, K.; Nagashima, Y.; Ishiguro, H.; Kubota, K.; Kobayashi, N.; Hosono, K.; Watanabe, S.; Sekino, Y.; Sato, T.; et al. aPKCλ/ι is a beneficial prognostic marker for pancreatic neoplasms. Pancreatology 2013, 13, 360–368. [Google Scholar] [CrossRef]
- Abdelatty, A.; Fang, D.; Wei, G.; Wu, F.; Zhang, C.; Xu, H.; Yao, C.; Wang, Y.; Xia, H. PKCι is a promising prognosis biomarker and therapeutic target for pancreatic cancer. Pathobiology 2022, 4, 1–12. [Google Scholar] [CrossRef]
- Scotti, M.L.; Bamlet, W.R.; Smyrk, T.C.; Fields, A.P.; Murray, N.R. Protein kinase Cι is required for pancreatic cancer cell transformed growth and tumorigenesis. Cancer Res. 2010, 70, 2064–2074. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, H.; Yang, J.; Li, Z.; Wang, Y.; Leng, X.; Ganapathy, S.; Isakson, P.; Chen, C.; Zhu, T. Mu-KRAS attenuates Hippo signaling pathway through PKCι to sustain the growth of pancreatic cancer. J. Cell. Physiol. 2020, 235, 408–420. [Google Scholar] [CrossRef]
- Butler, A.M.; Scotti Buzhardt, M.L.; Erdogan, E.; Li, S.; Inman, K.S.; Fields, A.P.; Murray, N.R. A small molecule inhibitor of atypical protein kinase C signaling inhibits pancreatic cancer cell transformed growth and invasion. Oncotarget 2015, 6, 15297–15310. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Zhang, H.; Li, C.; Chen, C.; Zhu, T. Transcription factor Sp1 is upregulated by PKCι to drive the expression of YAP1 during pancreatic carcinogenesis. Carcinogenesis 2021, 42, 344–356. [Google Scholar] [CrossRef]
- Laudanna, C.; Sorio, C.; Tecchio, C.; Butcher, E.C.; Bonora, A.; Bassi, C.; Scarpa, A. Motility analysis of pancreatic adenocarcinoma cells reveals a role for the atypical ζ isoform of protein kinase C in cancer cell movement. Lab. Invest. 2003, 83, 1155–1163. [Google Scholar] [CrossRef][Green Version]
- Ryota, H.; Ishida, M.; Ebisu, Y.; Yanagimoto, H.; Yamamoto, T.; Kosaka, H.; Hirooka, S.; Yamaki, S.; Kotsuka, M.; Matsui, Y.; et al. Clinicopathological characteristics of pancreatic ductal adenocarcinoma with invasive micropapillary carcinoma component with emphasis on the usefulness of PKCζ immunostaining for detection of reverse polarity. Oncol. Lett. 2021, 22, 525. [Google Scholar] [CrossRef] [PubMed]
- Mauro, L.V.; Grossoni, V.C.; Urtreger, A.J.; Yang, C.; Colombo, L.L.; Morandi, A.; Pallotta, M.G.; Kazanietz, M.G.; Bal de Kier Joffé, E.D.; Puricelli, L.L. PKC delta (PKCδ) promotes tumoral progression of human ductal pancreatic cancer. Pancreas 2010, 39, e31–e41. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jin, W.; Zhou, X.; Li, J.; Xu, C.; Ma, Z.; Wang, J.; Qin, L.; Zhou, B.; Ding, W.; et al. Identification, structure-activity relationships of marine-derived indolocarbazoles, and a dual PKCθ/δ inhibitor with potent antipancreatic cancer efficacy. J. Med. Chem. 2020, 63, 12978–12991. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Wu, H.Y.; Chu, P.C.; Lai, I.L.; Huang, P.H.; Kulp, S.K.; Pan, S.L.; Teng, C.M.; Chen, C.S. Role of integrin-linked kinase in regulating the protein stability of the MUC1-C oncoprotein in pancreatic cancer cells. Oncogenesis 2017, 6, e359. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Zhu, L.; Sun, Y.; Bettencourt, R.; Damsz, B.; Hruban, R.H.; Konieczny, S.F. Loss of the acinar-restricted transcription factor Mist1 accelerates Kras-induced pancreatic intraepithelial neoplasia. Gastroenterology 2009, 136, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.L.; Peat, J.M.; Volante, S.N.; Wang, R.; McLean, C.A.; Pin, C.L. Activation of protein kinase Cδ leads to increased pancreatic acinar cell dedifferentiation in the absence of MIST1. J. Pathol. 2012, 228, 351–365. [Google Scholar] [CrossRef]
- Cheng, J.; Tian, L.; Ma, J.; Gong, Y.; Zhang, Z.; Chen, Z.; Xu, B.; Xiong, H.; Li, C.; Huang, Q. Dying tumor cells stimulate proliferation of living tumor cells via caspase-dependent protein kinase Cδ activation in pancreatic ductal adenocarcinoma. Mol. Oncol. 2015, 9, 105–114. [Google Scholar] [CrossRef]
- Singh, B.N.; Kumar, D.; Shankar, S.; Srivastava, R.K. Rottlerin induces autophagy which leads to apoptotic cell death through inhibition of PI3K/Akt/mTOR pathway in human pancreatic cancer stem cells. Biochem. Pharmacol. 2012, 84, 1154–1163. [Google Scholar] [CrossRef]
- Sorescu, G.P.; Forman, L.W.; Faller, D.V. Effect of inhibition of protein kinase C delta (PKCδ) on pancreatic cancer cells. J. Clin. Oncol. 2012, 30, e14591. [Google Scholar] [CrossRef]
- Takahashi, T.; Uehara, H.; Ogawa, H.; Umemoto, H.; Bando, Y.; Izumi, K. Inhibition of EP2/EP4 signaling abrogates IGF-1R-mediated cancer cell growth: Involvement of protein kinase C-θ activation. Oncotarget 2015, 6, 4829–4844. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, J.M.; Liu, C.; Xiao, B.L.; Lu, J.X.; Zou, S.Q. Correlation of aPKC-iota and E-cadherin expression with invasion and prognosis of cholangiocarcinoma. Hepatobiliary Pancreat. Dis. Int. 2008, 7, 70–75. [Google Scholar] [PubMed]
- Qian, Y.; Yao, W.; Yang, T.; Yang, Y.; Liu, Y.; Shen, Q.; Zhang, J.; Qi, W.; Wang, J. aPKC-ι/P-Sp1/Snail signaling induces epithelial-mesenchymal transition and immunosuppression in cholangiocarcinoma. Hepatology 2017, 66, 1165–1182. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; He, J.C.; Wang, J.M.; Schemmer, P.; Ma, C.Q.; Qian, Y.W.; Yao, W.; Zhang, J.; Qi, W.P.; et al. 14-3-3ζ and aPKC-ι synergistically facilitate epithelial-mesenchymal transition of cholangiocarcinoma via GSK-3β/Snail signaling pathway. Oncotarget 2016, 7, 55191–55210. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Okumura, K.; Gogna, S.; Gachabayov, M.; Felsenreich, D.M.; McGuirk, M.; Rojas, A.; Quintero, L.; Seshadri, R.; Gu, K.; Dong, X.D. Gallbladder cancer: Historical treatment and new management options. World J. Gastrointest. Oncol. 2021, 13, 1317–1335. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Lu, Y.; Yang, T.; Deng, Z.; Xu, L.; Yao, W.; Ma, C.; Li, X.; Zhang, J.; Liu, Y.; et al. aPKCι promotes gallbladder cancer tumorigenesis and gemcitabine resistance by competing with Nrf2 for binding to Keap1. Redox. Biol. 2019, 22, 101149. [Google Scholar] [CrossRef]
- Tian, L.; Deng, Z.; Xu, L.; Yang, T.; Yao, W.; Ji, L.; Lu, Y.; Zhang, J.; Liu, Y.; Wang, J. Downregulation of ASPP2 promotes gallbladder cancer metastasis and macrophage recruitment via aPKC-ι/GLI1 pathway. Cell Death Dis. 2018, 9, 1115. [Google Scholar] [CrossRef]
- Zhang, G.F.; Wu, J.C.; Wang, H.Y.; Jiang, W.D.; Qiu, L. Overexpression of microRNA-205-5p exerts suppressive effects on stem cell drug resistance in gallbladder cancer by down-regulating PRKCE. Biosci. Rep. 2020, 40, BSR20194509. [Google Scholar] [CrossRef]
- Wang, H.; Zhan, M.; Xu, S.W.; Chen, W.; Long, M.M.; Shi, Y.H.; Liu, Q.; Mohan, M.; Wang, J. miR-218-5p restores sensitivity to gemcitabine through PRKCE/MDR1 axis in gallbladder cancer. Cell Death Dis. 2017, 8, e2770. [Google Scholar] [CrossRef]
- Koren, R.; Ben Meir, D.; Langzam, L.; Dekel, Y.; Konichezky, M.; Baniel, J.; Livne, P.M.; Gal, R.; Sampson, S.R. Expression of protein kinase C isoenzymes in benign hyperplasia and carcinoma of prostate. Oncol. Rep. 2004, 11, 321–326. [Google Scholar] [CrossRef]
- Cornford, P.; Evans, J.; Dodson, A.; Parsons, K.; Woolfenden, A.; Neoptolemos, J.; Foster, C.S. Protein kinase C isoenzyme patterns characteristically modulated in early prostate cancer. Am. J. Pathol. 1999, 154, 137–144. [Google Scholar] [CrossRef]
- Villar, J.; Arenas, M.I.; MacCarthy, C.M.; Blánquez, M.J.; Tirado, O.M.; Notario, V. PCPH/ENTPD5 expression enhances the invasiveness of human prostate cancer cells by a protein kinase Cδ-dependent mechanism. Cancer Res. 2007, 67, 10859–10868. [Google Scholar] [CrossRef] [PubMed]
- Castilla, C.; Chinchón, D.; Medina, R.; Torrubia, F.J.; Japón, M.A.; Sáez, C. PTPL1 and PKCδ contribute to proapoptotic signalling in prostate cancer cells. Cell Death Dis. 2013, 4, e576. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, Y.L.; Vallentin, A.; Hunrichs, B.S.; Hellerstein, M.K.; Peehl, D.M.; Mochly-Rosen, D. Centrosomal PKCβII and pericentrin are critical for human prostate cancer growth and angiogenesis. Cancer Res. 2008, 68, 6831–6839. [Google Scholar] [CrossRef] [PubMed]
- Paone, A.; Starace, D.; Galli, R.; Padula, F.; De Cesaris, P.; Filippini, A.; Ziparo, E.; Riccioli, A. Toll-like receptor 3 triggers apoptosis of human prostate cancer cells through a PKC-α-dependent mechanism. Carcinogenesis 2008, 29, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Tsuji, T.; Chen, C. Roles of PKC isoforms in the induction of apoptosis elicited by aberrant Ras. Oncogene 2010, 29, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Quadri, H.S.; Song, I.; Tomita, Y.; Tirado, O.M.; Notario, V. PCPH/ENTPD5 expression confers to prostate cancer cells resistance against cisplatin-induced apoptosis through protein kinase Cα-mediated Bcl-2 stabilization. Cancer Res. 2009, 69, 102–110. [Google Scholar] [CrossRef]
- Truman, J.P.; Rotenberg, S.A.; Kang, J.H.; Lerman, G.; Fuks, Z.; Kolesnick, R.; Marquez, V.E.; Haimovitz-Friedman, A. PKCα activation downregulates ATM and radio-sensitizes androgen-sensitive human prostate cancer cells in vitro and in vivo. Cancer Biol. Ther. 2009, 8, 54–63. [Google Scholar] [CrossRef]
- Gurbuz, N.; Park, M.A.; Dent, P.; Abdel Mageed, A.B.; Sikka, S.C.; Baykal, A. Cystine dimethyl ester induces apoptosis through regulation of PKC-δ and PKC-ε in prostate cancer cells. Anticancer Agents Med. Chem. 2015, 15, 217–227. [Google Scholar] [CrossRef]
- Von Burstin, V.A.; Xiao, L.; Kazanietz, M.G. Bryostatin 1 inhibits phorbol ester-induced apoptosis in prostate cancer cells by differentially modulating protein kinase C (PKC)δ translocation and preventing PKCδ-mediated release of tumor necrosis factor-α. Mol. Pharmacol. 2010, 78, 325–332. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, L.; Kazanietz, M.G. p23/Tmp21 associates with protein kinase Cδ (PKCδ) and modulates its apoptotic function. J. Biol. Chem. 2011, 286, 15821–15831. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.S.; Lee, H.J.; Sim, D.Y.; Im, E.; Park, J.E.; Park, W.Y.; Koo, J.I.; Shim, B.S.; Kim, S.H. Moracin D induces apoptosis in prostate cancer cells via activation of PPARγ/PKCδ and inhibition of PKCα. Phytother. Res. 2021, 35, 6944–6953. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.H.; Yu, C.C.; Chiang, P.C.; Chen, Y.C.; Ho, Y.F.; Kung, F.L.; Guh, J.H. Paclitaxel induces apoptosis through activation of nuclear protein kinase C-δ and subsequent activation of Golgi associated Cdk1 in human hormone refractory prostate cancer. J. Urol. 2011, 186, 2434–2441. [Google Scholar] [CrossRef] [PubMed]
- Benavides, F.; Blando, J.; Perez, C.J.; Garg, R.; Conti, C.J.; DiGiovanni, J.; Kazanietz, M.G. Transgenic overexpression of PKCε in the mouse prostate induces preneoplastic lesions. Cell Cycle. 2011, 10, 268–277. [Google Scholar] [CrossRef]
- Garg, R.; Blando, J.M.; Perez, C.J.; Abba, M.C.; Benavides, F.; Kazanietz, M.G. Protein kinase Cε cooperates with PTEN loss for prostate tumorigenesis through the CXCL13-CXCR5 pathway. Cell Rep. 2017, 19, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.H.; Hafeez, B.B.; Sand, J.M.; Pierce, D.B.; Aziz, S.W.; Dreckschmidt, N.E.; Verma, A.K. Protein kinase Cε mediates Stat3Ser727 phosphorylation, Stat3-regulated gene expression, and cell invasion in various human cancer cell lines through integration with MAPK cascade (RAF-1, MEK1/2, and ERK1/2). Oncogene 2010, 29, 3100–3109. [Google Scholar] [CrossRef]
- Hafeez, B.B.; Zhong, W.; Weichert, J.; Dreckschmidt, N.E.; Jamal, M.S.; Verma, A.K. Genetic ablation of PKCε inhibits prostate cancer development and metastasis in transgenic mouse model of prostate adenocarcinoma. Cancer Res. 2011, 71, 2318–2327. [Google Scholar] [CrossRef]
- Yao, S.; Bee, A.; Brewer, D.; Dodson, A.; Beesley, C.; Ke, Y.; Ambroisine, L.; Fisher, G.; Møller, H.; Dickinson, T.; et al. PRKC-ζ expression promotes the aggressive phenotype of human prostate cancer cells and is a novel target for therapeutic intervention. Genes Cancer 2010, 1, 444–464. [Google Scholar] [CrossRef]
- Apostolatos, A.H.; Apostolatos, C.A.; Ratnayake, W.S.; Neuger, A.; Sansil, S.; Bourgeois, M.; Acevedo-Duncan, M. Preclinical testing of 5-amino-1-((1R,2S,3S,4R)-2,3-dihydroxy-4-methylcyclopentyl)-1H-imidazole-4-carboxamide: A potent protein kinase C-ι inhibitor as a potential prostate carcinoma therapeutic. Anticancer Drugs 2019, 30, 65–671. [Google Scholar] [CrossRef]
- Apostolatos, A.H.; Ratnayake, W.S.; Win-Piazza, H.; Apostolatos, C.A.; Smalley, T.; Kang, L.; Salup, R.; Hill, R.; Acevedo-Duncan, M. Inhibition of atypical protein kinase C-ι effectively reduces the malignancy of prostate cancer cells by downregulating the NF-κB signaling cascade. Int. J. Oncol. 2018, 53, 1836–1846. [Google Scholar] [CrossRef]
- Hamshaw, I.; Ajdarirad, M.; Mueller, A. The role of PKC and PKD in CXCL12 directed prostate cancer migration. Biochem. Biophys. Res. Commun. 2019, 519, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, W.S.; Apostolatos, C.A.; Breedy, S.; Dennison, C.L.; Hill, R.; Acevedo-Duncan, M. Atypical PKCs activate Vimentin to facilitate prostate cancer cell motility and invasion. Cell Adh. Migr. 2021, 15, 37–57. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, S.; Inoue, T.; Ogawa, O.; Gleave, M.E. Clinical and molecular features of treatment-related neuroendocrine prostate cancer. Int. J. Urol. 2018, 25, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Reina-Campos, M.; Linares, J.F.; Duran, A.; Cordes, T.; L’Hermitte, A.; Badur, M.G.; Bhangoo, M.S.; Thorson, P.K.; Richards, A.; Rooslid, T.; et al. Increased serine and one-carbon pathway metabolism by PKCλ/ι deficiency promotes. Cancer Cell 2019, 35, 385–400. [Google Scholar] [CrossRef]
- Hashemi, M.; Shahkar, G.; Simforoosh, N.; Basiri, A.; Ziaee, S.A.; Narouie, B.; Taheri, M. Association of polymorphisms in PRKCI gene and risk of prostate cancer in a sample of Iranian Population. Cell. Mol. Biol. 2015, 61, 16–21. [Google Scholar]
- Li, Q.; Gu, C.; Zhu, Y.; Wang, M.; Yang, Y.; Wang, J.; Jin, L.; Zhu, M.L.; Shi, T.Y.; He, J.; et al. Two novel PRKCI polymorphisms and prostate cancer risk in an Eastern Chinese Han population. Mol. Carcinog. 2015, 54, 632–641. [Google Scholar] [CrossRef]
- Cairns, P. Renal Cell Carcinoma. Cancer Biomark. 2010, 9, 461–473. [Google Scholar] [CrossRef]
- Hsieh, J.J.; Le, V.; Cao, D.; Cheng, E.H.; Creighton, C.J. Genomic classifications of renal cell carcinoma: A critical step towards the future application of personalized kidney cancer care with pan-omics precision. J. Pathol. 2018, 244, 525–537. [Google Scholar] [CrossRef]
- Brenner, W.; Färber, G.; Herget, T.; Wiesner, C.; Hengstler, J.G.; Thüroff, J.W. Protein kinase Cη is associated with progression of renal cell carcinoma (RCC). Anticancer Res. 2003, 23, 4001–4006. [Google Scholar]
- Pu, Y.S.; Huang, C.Y.; Chen, J.Y.; Kang, W.Y.; Lin, Y.C.; Shiu, Y.S.; Chuang, S.J.; Yu, H.J.; Lai, M.K.; Tsai, Y.C.; et al. Down-regulation of PKCζ in renal cell carcinoma and its clinicopathological implications. J. Biomed. Sci. 2012, 19, 39. [Google Scholar] [CrossRef]
- Von Brandenstein, M.; Pandarakalam, J.J.; Kroon, L.; Loeser, H.; Herden, J.; Braun, G.; Wendland, K.; Dienes, H.P.; Engelmann, U.; Fries, J.W. MicroRNA 15a, inversely correlated to PKCα, is a potential marker to differentiate between benign and malignant renal tumors in biopsy and urine samples. Am. J. Pathol. 2012, 180, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Razorenova, O.V.; Finger, E.C.; Colavitti, R.; Chernikova, S.B.; Boiko, A.D.; Chan, C.K.; Krieg, A.; Bedogni, B.; LaGory, E.; Weissman, I.L.; et al. VHL loss in renal cell carcinoma leads to up-regulation of CUB domain-containing protein 1 to stimulate PKCδ-driven migration. Proc. Natl. Acad. Sci. USA 2011, 108, 1931–1936. [Google Scholar] [CrossRef] [PubMed]
- Brenner, W.; Greber, I.; Gudejko-Thiel, J.; Beitz, S.; Schneider, E.; Walenta, S.; Peters, K.; Unger, R.; Thüroff, J.W. Migration of renal carcinoma cells is dependent on protein kinase Cδ via β1 integrin and focal adhesion kinase. Int. J. Oncol. 2008, 32, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Brenner, W.; Benzing, F.; Gudejko-Thiel, J.; Fischer, R.; Färber, G.; Hengstler, J.G.; Seliger, B.; Thüroff, J.W. Regulation of β1 integrin expression by PKCε in renal cancer cells. Int. J. Oncol. 2004, 25, 1157–1163. [Google Scholar] [PubMed]
- Huang, B.; Cao, K.; Li, X.; Guo, S.; Mao, X.; Wang, Z.; Zhuang, J.; Pan, J.; Mo, C.; Chen, J.; et al. The expression and role of protein kinase C (PKC)ε in clear cell renal cell carcinoma. J. Exp. Clin. Cancer Res. 2011, 30, 88. [Google Scholar] [CrossRef]
- Huang, B.; Fu, S.J.; Fan, W.Z.; Wang, Z.H.; Chen, Z.B.; Guo, S.J.; Chen, J.X.; Qiu, S.P. PKCε inhibits isolation and stemness of side population cells via the suppression of ABCB1 transporter and PI3K/Akt, MAPK/ERK signaling in renal cell carcinoma cell line 769P. Cancer Lett. 2016, 376, 148–154. [Google Scholar] [CrossRef]
- Owari, T.; Sasaki, T.; Fujii, K.; Fujiwara-Tani, R.; Kishi, S.; Mori, S.; Mori, T.; Goto, K.; Kawahara, I.; Nakai, Y.; et al. Role of nuclear claudin-4 in renal cell carcinoma. Int. J. Mol. Sci. 2020, 21, 8340. [Google Scholar] [CrossRef]
- Engers, R.; Mrzyk, S.; Springer, E.; Fabbro, D.; Weissgerber, G.; Gernharz, C.D.; Gabbert, H.E. Protein kinase C in human renal cell carcinomas: Role in invasion and differential isoenzyme expression. Br. J. Cancer 2000, 82, 1063–1069. [Google Scholar] [CrossRef]
- Ciążyńska, M.; Kamińska-Winciorek, G.; Lange, D.; Lewandowski, B.; Reich, A.; Sławińska, M.; Pabianek, M.; Szczepaniak, K.; Hankiewicz, A.; Ułańska, M.; et al. The incidence and clinical analysis of non-melanoma skin cancer. Sci. Rep. 2021, 11, 4337. [Google Scholar] [CrossRef]
- Krasagakis, K.; Fimmel, S.; Genten, D.; Eberle, J.; Quas, P.; Ziegler, W.; Haller, H.; Orfanos, C.E. Lack of protein kinase C (PKC)-β and low PKC-α, -δ, -ε, and -ζ isozyme levels in proliferating human melanoma cells. Int. J. Oncol. 2002, 20, 865–871. [Google Scholar]
- Selzer, E.; Okamoto, I.; Lucas, T.; Kodym, R.; Pehamberger, H.; Jansen, B. Protein kinase C isoforms in normal and transformed cells of the melanocytic lineage. Melanoma Res. 2002, 12, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Gilhooly, E.M.; Morse-Gaudio, M.; Bianchi, L.; Reinhart, L.; Rose, D.P.; Connolly, J.M.; Reed, J.A.; Albino, A.P. Loss of expression of protein kinase C β is a common phenomenon in human malignant melanoma: A result of transformation or differentiation? Melanoma Res. 2001, 11, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Krasagakis, K.; Tsentelierou, E.; Chlouverakis, G.; Stathopoulos, E.N. Topography of Protein Kinase C βII in Benign and Malignant Melanocytic Lesions. Int. J. Surg. Pathol. 2017, 25, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Voris, J.P.; Sitailo, L.A.; Rahn, H.R.; Defnet, A.; Gerds, A.T.; Sprague, R.; Yadav, V.; Caroline Le Poole, I.; Denning, M.F. Functional alterations in protein kinase C beta II expression in melanoma. Pigment. Cell Melanoma Res. 2010, 23, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, L.; Andruska, N.; Mao, C.; Gruber, S.B.; Johnson, T.M.; Fullen, D.R.; Raskin, L.; Shapiro, D.J. Protein kinase C-α is upregulated by IMP1 in melanoma and is linked to poor survival. Melanoma Res. 2019, 29, 539–543. [Google Scholar] [CrossRef]
- Halder, K.; Banerjee, S.; Ghosh, S.; Bose, A.; Das, S.; Chowdhury, B.P.; Majumdar, S. Mycobacterium indicus pranii (Mw) inhibits invasion by reducing matrix metalloproteinase (MMP-9) via AKT/ERK-1/2 and PKCα signaling: A potential candidate in melanoma cancer therapy. Cancer Biol. Ther. 2017, 18, 850–862. [Google Scholar] [CrossRef]
- Putnam, A.J.; Schulz, V.V.; Freiter, E.M.; Bill, H.M.; Miranti, C.K. Src, PKCα, and PKCδ are required for αvβ3 integrin-mediated metastatic melanoma invasion. Cell Commun. Signal. 2009, 7, 10. [Google Scholar] [CrossRef]
- Halder, K.; Banerjee, S.; Bose, A.; Majumder, S.; Majumdar, S. Overexpressed PKCδ downregulates the expression of PKCα in B16F10 melanoma: Induction of apoptosis by PKCδ via ceramide generation. PLoS ONE 2014, 9, e91656. [Google Scholar]
- Vartanian, A.; Stepanova, E.; Grigorieva, I.; Solomko, E.; Baryshnikov, A.; Lichinitser, M. VEGFR1 and PKCα signaling control melanoma vasculogenic mimicry in a VEGFR2 kinase-independent manner. Melanoma Res. 2011, 21, 91–98. [Google Scholar] [CrossRef]
- Mhaidat, N.M.; Thorne, R.F.; Zhang, X.D.; Hersey, P. Regulation of docetaxel-induced apoptosis of human melanoma cells by different isoforms of protein kinase C. Mol. Cancer Res. 2007, 5, 1073–1081. [Google Scholar] [CrossRef]
- Heijkants, R.C.; Nieveen, M.; Hart, K.C.; Teunisse, A.F.A.S.; Jochemsen, A.G. Targeting MDMX and PKCδ to improve current uveal melanoma therapeutic strategies. Oncogenesis 2018, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Piperno-Neumann, S.; Larkin, J.; Carvajal, R.D.; Luke, J.J.; Schwartz, G.K.; Hodi, F.S.; Sablin, M.P.; Shoushtari, A.N.; Szpakowski, S.; Chowdhury, N.R.; et al. Genomic profiling of metastatic uveal melanoma and clinical results of a phase I study of the protein kinase C inhibitor AEB071. Mol. Cancer Ther. 2020, 19, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, W.S.; Apostolatos, C.A.; Apostolatos, A.H.; Schutte, R.J.; Huynh, M.A.; Ostrov, D.A.; Acevedo-Duncan, M. Oncogenic PKC-ι activates vimentin during epithelial-mesenchymal transition in melanoma; a study based on PKC-ι and PKC-ζ specific inhibitors. Cell Adh. Migr. 2018, 12, 447–463. [Google Scholar] [PubMed]
- Ratnayake, W.S.; Apostolatos, A.H.; Ostrov, D.A.; Acevedo-Duncan, M. Two novel atypical PKC inhibitors; ACPD and DNDA effectively mitigate cell proliferation and epithelial to mesenchymal transition of metastatic melanoma while inducing apoptosis. Int. J. Oncol. 2017, 51, 1370–1382. [Google Scholar] [CrossRef] [PubMed]
- Varsano, T.; Lau, E.; Feng, Y.; Garrido, M.; Milan, L.; Heynen-Genel, S.; Hassig, C.A.; Ronai, Z.A. Inhibition of melanoma growth by small molecules that promote the mitochondrial localization of ATF2. Clin. Cancer Res. 2013, 19, 2710–2722. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.; Feng, Y.; Claps, G.; Fukuda, M.N.; Perlina, A.; Donn, D.; Jilaveanu, L.; Kluger, H.; Freeze, H.H.; Ronai, Z.A. The transcription factor ATF2 promotes melanoma metastasis by suppressing protein fucosylation. Sci. Signal. 2015, 8, ra124. [Google Scholar] [CrossRef] [PubMed]
- Pollock, P.M.; Cohen-Solal, K.; Sood, R.; Namkoong, J.; Martino, J.J.; Koganti, A.; Zhu, H.; Robbins, C.; Makalowska, I.; Shin, S.S.; et al. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat. Genet. 2003, 34, 108–112. [Google Scholar] [CrossRef]
- Marín, Y.E.; Namkoong, J.; Cohen-Solal, K.; Shin, S.S.; Martino, J.J.; Oka, M.; Chen, S. Stimulation of oncogenic metabotropic glutamate receptor 1 in melanoma cells activates ERK1/2 via PKCε. Cell. Signal. 2006, 18, 1279–1286. [Google Scholar] [CrossRef]
- Zhang, D.; Fu, M.; Li, L.; Ye, H.; Song, Z.; Piao, Y. PKC-δ attenuates the cancer stem cell among squamous cell carcinoma cells through down-regulating p63. Pathol. Res. Pract. 2017, 213, 1119–1124. [Google Scholar] [CrossRef]
- Yadav, V.; Yanez, N.C.; Fenton, S.E.; Denning, M.F. Loss of protein kinase C δ gene expression in human squamous cell carcinomas: A laser capture microdissection study. Am. J. Pathol. 2010, 176, 1091–1096. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.; Sand, J.M.; Heninger, E.; Hafeez, B.B.; Verma, A.K. Protein kinase C ε, which is linked to ultraviolet radiation-induced development of squamous cell carcinomas, stimulates rapid turnover of adult hair follicle stem cells. J. Skin Cancer 2013, 2013, 452425. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.H.; Manoharan, H.T.; Verma, A.K. Protein kinase C epsilon, which sensitizes skin to sun’s UV radiation-induced cutaneous damage and development of squamous cell carcinomas, associates with Stat3. Cancer Res. 2007, 67, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Sand, J.M.; Bin Hafeez, B.; Aziz, M.H.; Siebers, E.M.; Dreckschmidt, N.E.; Verma, A.K. Ultraviolet radiation and 12-O-tetradecanoylphorbol-13-acetate-induced interaction of mouse epidermal protein kinase Cε with Stat3 involve integration with ERK1/2. Mol. Carcinog. 2012, 51, 291–302. [Google Scholar] [CrossRef]
- Chow, R.Y.; Levee, T.M.; Kaur, G.; Cedeno, D.P.; Doan, L.T.; Atwood, S.X. MTOR promotes basal cell carcinoma growth through atypical PKC. Exp. Dermatol. 2021, 30, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.N.; Fry, M.A.; Urman, N.M.; Atwood, S.X.; Roffey, J.; Ott, G.R.; Chen, B.; Lee, A.; Brown, A.S.; Aasi, S.Z.; et al. Combined inhibition of atypical PKC and histone deacetylase 1 is cooperative in basal cell carcinoma treatment. JCI Insight 2017, 2, e97071. [Google Scholar] [CrossRef]
- Huang, K.; Cui, M.; Ye, F.; Li, Y.; Zhang, D. Global profiling of the signaling network of papillary thyroid carcinoma. Life Sci. 2016, 147, 9–14. [Google Scholar] [CrossRef]
- Knauf, J.A.; Ward, L.S.; Nikiforov, Y.E.; Nikiforova, M.; Puxeddu, E.; Medvedovic, M.; Liron, T.; Mochly-Rosen, D.; Fagin, J.A. Isozyme-specific abnormalities of PKC in thyroid cancer: Evidence for post-transcriptional changes in PKC epsilon. J. Clin. Endocrinol. Metab. 2002, 87, 2150–2159. [Google Scholar] [CrossRef]
- Afrasiabi, E.; Ahlgren, J.; Bergelin, N.; Törnquist, K. Phorbol 12-myristate 13-acetate inhibits FRO anaplastic human thyroid cancer cell proliferation by inducing cell cycle arrest in G1/S phase: Evidence for an effect mediated by PKCδ. Mol. Cell. Endocrinol. 2008, 292, 26–35. [Google Scholar] [CrossRef]
- Molè, D.; Gentilin, E.; Gagliano, T.; Tagliati, F.; Bondanelli, M.; Pelizzo, M.R.; Rossi, M.; Filieri, C.; Pansini, G.; degli Uberti, E.C.; et al. Protein kinase C: A putative new target for the control of human medullary thyroid carcinoma cell proliferation in vitro. Endocrinology 2012, 153, 2088–2098. [Google Scholar] [CrossRef]
- Zhu, Y.; Dong, Q.; Tan, B.J.; Lim, W.G.; Zhou, S.; Duan, W. The PKCα-D294G mutant found in pituitary and thyroid tumors fails to transduce extracellular signals. Cancer Res. 2005, 65, 4520–4524. [Google Scholar] [CrossRef]
- Prévostel, C.; Martin, A.; Alvaro, V.; Jaffiol, C.; Joubert, D. Protein kinase C α and tumorigenesis of the endocrine gland. Horm. Res. 1997, 47, 140–144. [Google Scholar] [PubMed]
- Assert, R.; Kötter, R.; Schiemann, U.; Goretzki, P.; Pfeiffer, A.F. Effects of the putatively oncogenic protein kinase Cα D294G mutation on enzymatic activity and cell growth and its occurrence in human thyroid neoplasias. Horm. Metab. Res. 2002, 34, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Motegi, A.; Sakurai, S.; Nakayama, H.; Sano, T.; Oyama, T.; Nakajima, T. PKC theta, a novel immunohistochemical marker for gastrointestinal stromal tumors (GIST), especially useful for identifying KIT-negative tumors. Pathol. Int. 2005, 55, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.H.; Srivastava, A.; Kim, Y.E.; Park, H.J.; Park, C.K.; Sohn, T.S.; Kim, S.; Kang, D.Y.; Kim, K.M. DOG1 and PKC-θ are useful in the diagnosis of KIT-negative gastrointestinal stromal tumors. Mod. Pathol. 2011, 24, 866–875. [Google Scholar] [CrossRef]
- Wang, C.; Jin, M.S.; Zou, Y.B.; Gao, J.N.; Li, X.B.; Peng, F.; Wang, H.Y.; Wu, Z.D.; Wang, Y.P.; Duan, X.M. Diagnostic significance of DOG-1 and PKC-θ expression and c-Kit/PDGFRA mutations in gastrointestinal stromal tumours. Scand. J. Gastroenterol. 2013, 48, 1055–1065. [Google Scholar] [CrossRef]
- Kang, J.H.; Asai, D.; Toita, R.; Kitazaki, H.; Katayama, Y. Plasma protein kinase C (PKC)α as a biomarker for the diagnosis of cancers. Carcinogenesis 2009, 30, 1927–1931. [Google Scholar] [CrossRef][Green Version]
- Kang, J.H.; Mori, T.; Kitazaki, H.; Niidome, T.; Takayama, K.; Nakanishi, Y.; Katayama, Y. Serum protein kinase Cα as a diagnostic biomarker of cancers. Cancer Biomark. 2013, 13, 99–103. [Google Scholar] [CrossRef]
- Yamada, K.; Oikawa, T.; Kizawa, R.; Motohashi, S.; Yoshida, S.; Kumamoto, T.; Saeki, C.; Nakagawa, C.; Shimoyama, Y.; Aoki, K.; et al. Unconventional secretion of PKCδ exerts tumorigenic function via stimulation of ERK1/2 signaling in liver Cancer. Cancer Res. 2021, 81, 414–425. [Google Scholar] [CrossRef]
- Kang, J.H.; Mori, T.; Kitazaki, H.; Niidome, T.; Takayama, K.; Nakanishi, Y.; Katayama, Y. Kinase activity of protein kinase Cα in serum as a diagnostic biomarker of human lung cancer. Anticancer Res. 2013, 33, 485–488. [Google Scholar]
- El-Sisi, M.G.; Radwan, S.M.; Saeed, A.M.; El-Mesallamy, H.O. Serum levels of FAK and some of its effectors in adult AML: Correlation with prognostic factors and survival. Mol. Cell. Biochem. 2021, 476, 1949–1963. [Google Scholar] [CrossRef]
- Safi, S.; Badshah, Y.; Shabbir, M.; Zahra, K.; Khan, K.; Dilshad, E.; Afsar, T.; Almajwal, A.; Alruwaili, N.W.; Al-Disi, D.; et al. Predicting 3D structure, cross talks, and prognostic significance of KLF9 in cervical cancer. Front. Oncol. 2022, 11, 797007. [Google Scholar] [CrossRef] [PubMed]
- Körner, C.; Keklikoglou, I.; Bender, C.; Wörner, A.; Münstermann, E.; Wiemann, S. MicroRNA-31 sensitizes human breast cells to apoptosis by direct targeting of protein kinase C ε (PKCε). J. Biol. Chem. 2013, 288, 8750–8761. [Google Scholar] [CrossRef] [PubMed]
- Von Brandenstein, M.; Schlosser, M.; Herden, J.; Heidenreich, A.; Störkel, S.; Fries, J.W.U. MicroRNAs as urinary biomarker for oncocytoma. Dis. Markers 2018, 2018, 6979073. [Google Scholar] [CrossRef]
- Kawano, T.; Tachibana, Y.; Inokuchi, J.; Kang, J.H.; Murata, M.; Eto, M. Identification of activated protein kinase Cα (PKCα) in the urine of orthotopic bladder cancer xenograft model as a potential biomarker for the diagnosis of bladder cancer. Int. J. Mol. Sci. 2021, 22, 9276. [Google Scholar] [CrossRef]
- Köditz, B.; Brandenstein, M.V.; Huerta-Arana, M.; Fries, J.W.U. Novel noninvasive marker of regression of clear cell renal cell carcinoma (ccRCC). Turk. J. Urol. 2022, 48, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.A.; Aymond, C.M.; Jiang, Y.H.; Turner, N.D.; Lupton, J.R.; Chapkin, R.S. Non-invasive detection of fecal protein kinase C βII and ζ messenger RNA: Putative biomarkers for colon cancer. Carcinogenesis 1998, 19, 253–257. [Google Scholar] [CrossRef]
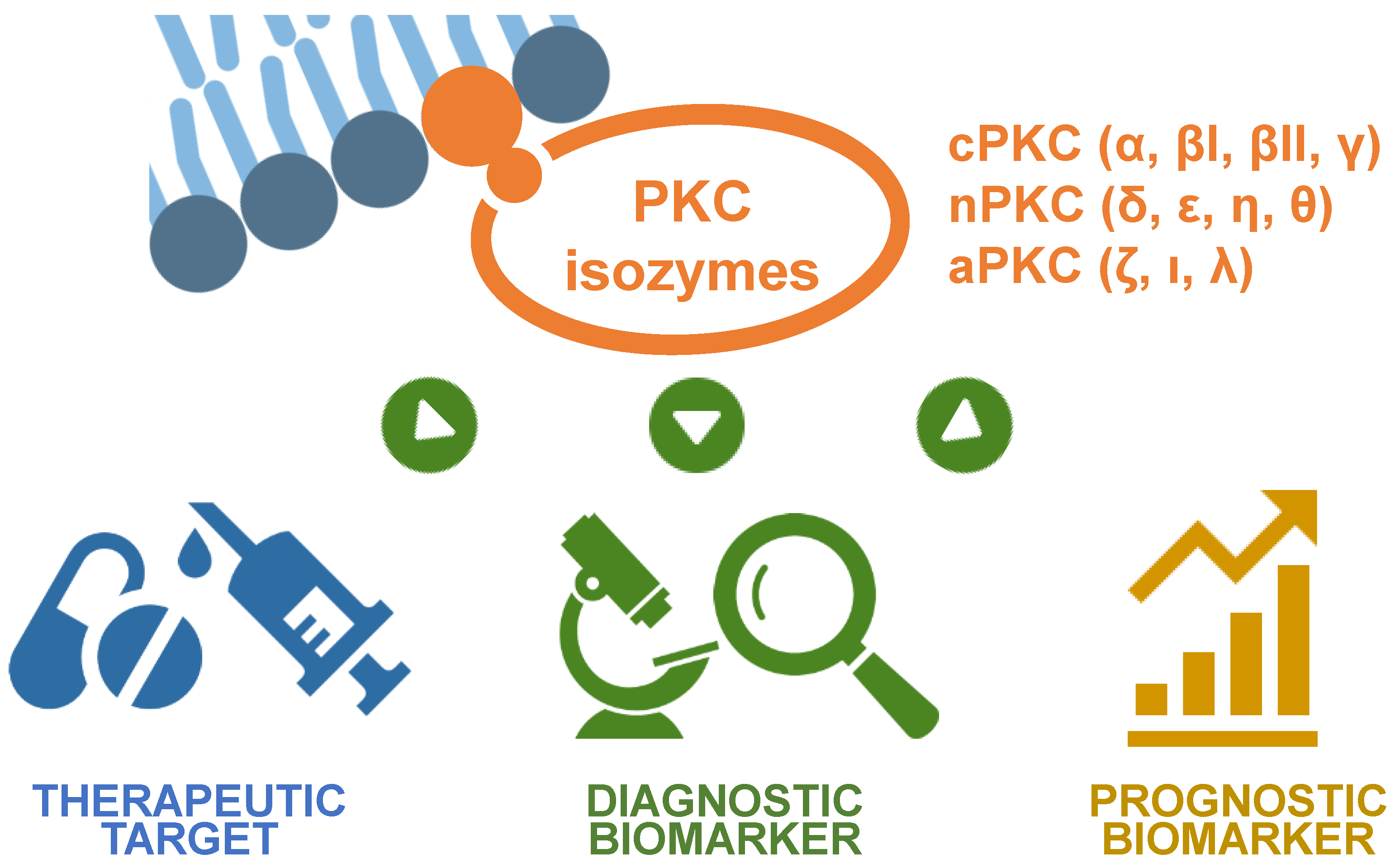
| Cancer Types | PKC Isozymes | Activity | Effect of Change in PKC Activation on the Cancer | Refs. |
|---|---|---|---|---|
| Bladder cancer | PKCα | Upregulation | Poor prognosis Increased anticancer drug resistance | [14,15] [16,18] |
| Blood and bone marrow cancer | ||||
| Multiple myeloma | PKCβ | Upregulation | Potential therapeutic target | [24] |
| Leukemia: lymphocytic leukemia | PKCα | Upregulation | Enhanced chemoresistance | [27,28] |
| PKCβ | Upregulation | Potential therapeutic target | [30] | |
| Leukemia: myeloid leukemia | PKCα | Upregulation | Poor survival Promoted anticancer drug resistance | [40] [41,51] |
| PKCβ | Upregulation | Enhanced anticancer drug resistance | [52] | |
| PKCδ | Upregulation | Increased anticancer drug-mediated apoptosis | [45,47] | |
| PKCε | Upregulation | Poor survival and increased anticancer drug resistance | [43,44] | |
| Myelodysplastic syndromes | PKCα | Upregulation (1) | Induced erythropoiesis | [56] |
| Lymphoma | PKCβII | Upregulation | Poor prognostic marker and chemotherapeutic target | [63,66] |
| PKCδ | Upregulation | Increased anticancer drug-mediated apoptosis | [68,69] | |
| Brain cancer (glioblastoma) | PKCα | Upregulation | Potential therapeutic target Potential prognostic marker | [73,79] [72] |
| PKCδ | Upregulation | Antiproliferative and proapoptotic | [90,91] | |
| PKCε | Upregulation | Potential therapeutic target | [93] | |
| PKCι | Upregulation | Potential therapeutic target | [83,84] | |
| Breast cancer | PKCα | Upregulation | Poor survival and prognosis Maintenance of migratory and invasive behavior Decreased ER levels and increased antiestrogen resistance Enhanced anti-ErbB-1 sensitivity in ErbB-2-positive breast cancer | [100] [101] [102,103] [111] |
| PKCδ | Upregulation | Enhanced mammary tumorigenesis | [114,115] | |
| PKCθ | Upregulation | Increased migratory and invasive behavior | [122,123] | |
| PKCε | Upregulation | Decreased disease-free survival | [131] | |
| PKCη | Upregulation | Enhanced breast cancer malignancy Poor survival following anticancer treatment | [125] [126] | |
| PKCζ | Upregulation | Increased invasive behavior Poor prognosis, disease-free survival, and survival rate | [121] [120] | |
| PKCλ | Upregulation | Poor prognosis | [130] | |
| Colorectal (colon) cancer | PKCα (2) | Downregulation Upregulation | Potential therapeutic target Enhanced anticancer drug resistance | [144] [135] |
| PKCδ | Upregulation (1) | Increased cancer progression and poor prognosis | [156,158] | |
| PKCζ | Upregulation | Potential therapeutic target | [145,146] | |
| PKCι | Upregulation | Potential therapeutic target | [159] | |
| Gastric (stomach) cancer | PKCα | Upregulation | Poor prognosis and increased anticancer drug resistance | [162,164] |
| PKCι | Upregulation | Enhanced recurrence of cancer and poor survival | [166,169] | |
| Head and neck squamous cell carcinoma | PKCα | Upregulation | Poor prognosis and survival | [174,175] |
| PKCβII | Upregulation (1) | Poor survival and rapid recurrence | [172] | |
| PKCθ | Upregulation (1) | Poor survival and rapid recurrence | [179] | |
| PKCι | Upregulation | Increased malignancy and poor survival | [177] | |
| Liver cancer (hepatocellular carcinoma) | PKCα | Upregulation | Poor prognosis and survival Immune escape and anti-PD1 tolerance | [181,184] [185] |
| PKCβ | Upregulation | Potential tumor suppressor | [194] | |
| PKCδ | Upregulation | Potential prognostic marker Poor disease-free survival | [186,189] [190] | |
| PKCλ/ι | Upregulation | Potential tumor suppressor | [191] | |
| PKCη | Downregulation | Poor long-term survival | [196] | |
| Lung cancer | PKCα | Upregulation | Potential therapeutic target and poor survival | [200] |
| PKCδ | Upregulation | Increased cell survival Increased anticancer drug resistance and potential therapeutic target | [208] [213] | |
| PKCε | Upregulation | Potential therapeutic target Elevated survival and anticancer drug resistance | [215] [215,217] | |
| PKCη | Upregulation | Poor prognosis and survival | [219] | |
| PKCι | Upregulation | Poor prognosis | [218] | |
| Ovarian cancer | PKCα | Upregulation | Poor prognosis and survival Increased anticancer drug resistance | [226] [222,223] |
| PKCι | Upregulation | Poor prognosis and survival Potential therapeutic target | [227,228] [230] | |
| PKCζ | Upregulation | Poor prognosis | [233,234] | |
| Pancreatic, bile duct, and gallbladder cancer | ||||
| Pancreatic cancer | PKCα | Upregulation | Potential therapeutic target Potential prognostic marker | [241,244] [236] |
| PKCδ | Upregulation | Enhanced cancer progression and poor survival Potential therapeutic target | [254,255] [259,260] | |
| PKCθ | Upregulation | Poor survival and therapeutic target | [254,261] | |
| PKCι | Upregulation | Potential prognostic marker and therapeutic target | [246,249] | |
| PKCζ | Upregulation | Enhanced worse prognosis | [251,252] | |
| Bile duct cancer | PKCι | Upregulation | Potential prognostic marker and therapeutic target | [263,264] |
| Gallbladder cancer | PKCι | Upregulation | Poor prognosis Enhanced cell growth, migration, and anticancer drug resistance | [267] [268,269] |
| PKCε | Upregulation | Enhanced anticancer drug resistance, proliferation, and colony formation rate | [269,270] | |
| Prostate cancer | PKCα | Upregulation | Promoted cell growth and anticancer drug resistance | [275,276] |
| PKCδ | Upregulation | Enhanced anticancer drug-induced cell apoptosis | [280,284] | |
| PKCε | Upregulation | Potential therapeutic target | [288] | |
| PKCζ | Upregulation | Worse survival and poor overall survival Potential preventive and therapeutic target | [289] [290,292] | |
| PKCι | Upregulation | Potential preventive and therapeutic target | [290,291] | |
| Renal cell carcinoma | PKCδ | Upregulation | Promoted cancer cell migration | [303] |
| PKCε | Upregulation | Potential therapeutic target | [307] | |
| Skin cancer | ||||
| Melanoma | PKCα | Upregulation | Poor prognosis and survival Potential therapeutic target for pancreatic cancer stem cells | [316] [317] |
| PKCδ | Upregulation | Enhanced proapoptotic response | [319,321] | |
| PKCζ and ι | Upregulation | Potential therapeutic target | [324] | |
| PKCε | Upregulation | Potential therapeutic target | [326,327] | |
| Non-melanoma | PKCδ | Upregulation | Protective role in squamous cell carcinomas | [330,331] |
| PKCε | Upregulation | Enhanced development of squamous cell carcinomas | [332,334] | |
| Thyroid carcinoma | PKCα | Mutation | Loss of function | [341,343] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawano, T.; Inokuchi, J.; Eto, M.; Murata, M.; Kang, J.-H. Protein Kinase C (PKC) Isozymes as Diagnostic and Prognostic Biomarkers and Therapeutic Targets for Cancer. Cancers 2022, 14, 5425. https://doi.org/10.3390/cancers14215425
Kawano T, Inokuchi J, Eto M, Murata M, Kang J-H. Protein Kinase C (PKC) Isozymes as Diagnostic and Prognostic Biomarkers and Therapeutic Targets for Cancer. Cancers. 2022; 14(21):5425. https://doi.org/10.3390/cancers14215425
Chicago/Turabian StyleKawano, Takahito, Junichi Inokuchi, Masatoshi Eto, Masaharu Murata, and Jeong-Hun Kang. 2022. "Protein Kinase C (PKC) Isozymes as Diagnostic and Prognostic Biomarkers and Therapeutic Targets for Cancer" Cancers 14, no. 21: 5425. https://doi.org/10.3390/cancers14215425
APA StyleKawano, T., Inokuchi, J., Eto, M., Murata, M., & Kang, J.-H. (2022). Protein Kinase C (PKC) Isozymes as Diagnostic and Prognostic Biomarkers and Therapeutic Targets for Cancer. Cancers, 14(21), 5425. https://doi.org/10.3390/cancers14215425






