Application of Electronic Health Record Text Mining: Real-World Tolerability, Safety, and Efficacy of Adjuvant Melanoma Treatments
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
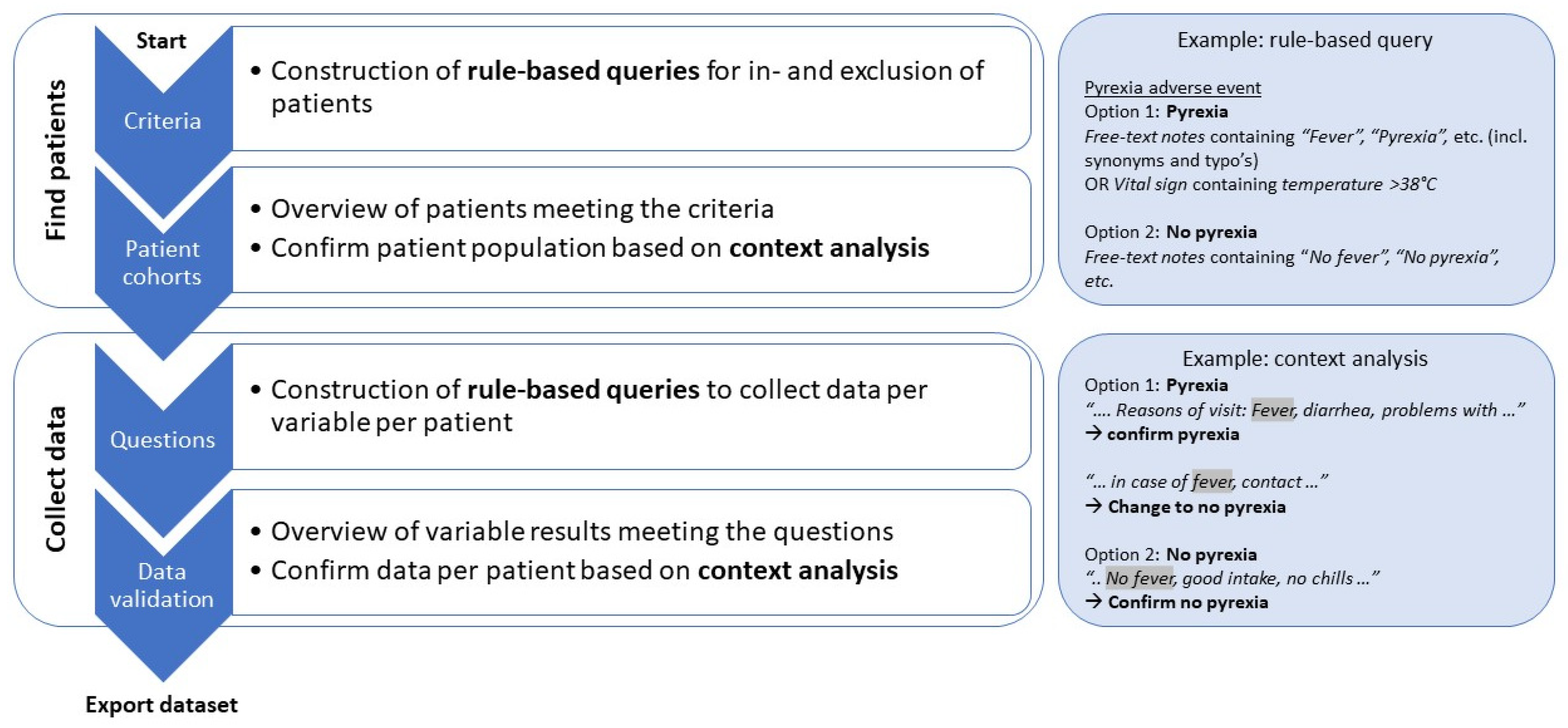
2.1. Electronic Health Record Text Mining
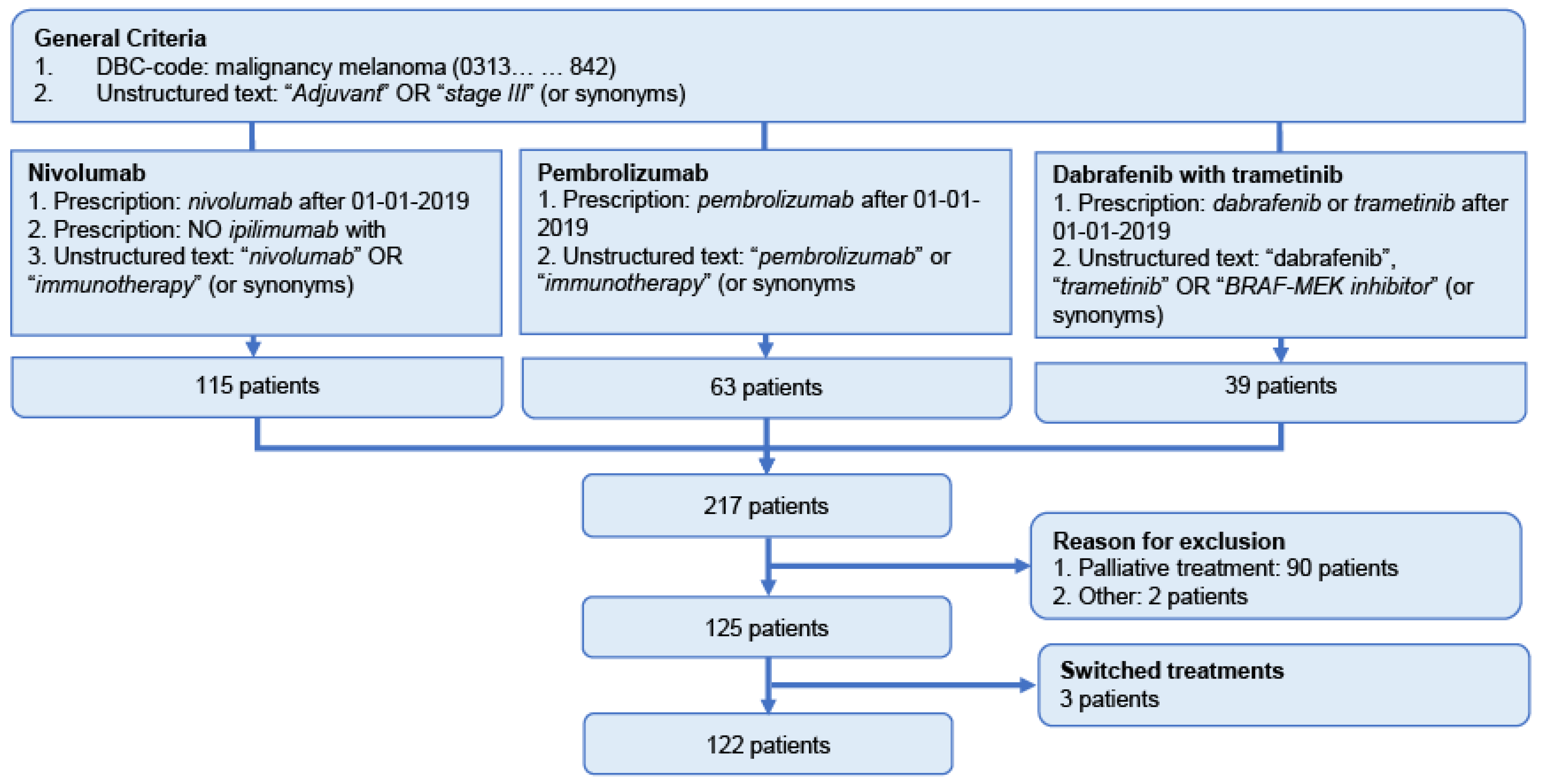
2.2. Patient Population
2.3. Data Collection
2.4. Statistical Analysis
3. Results
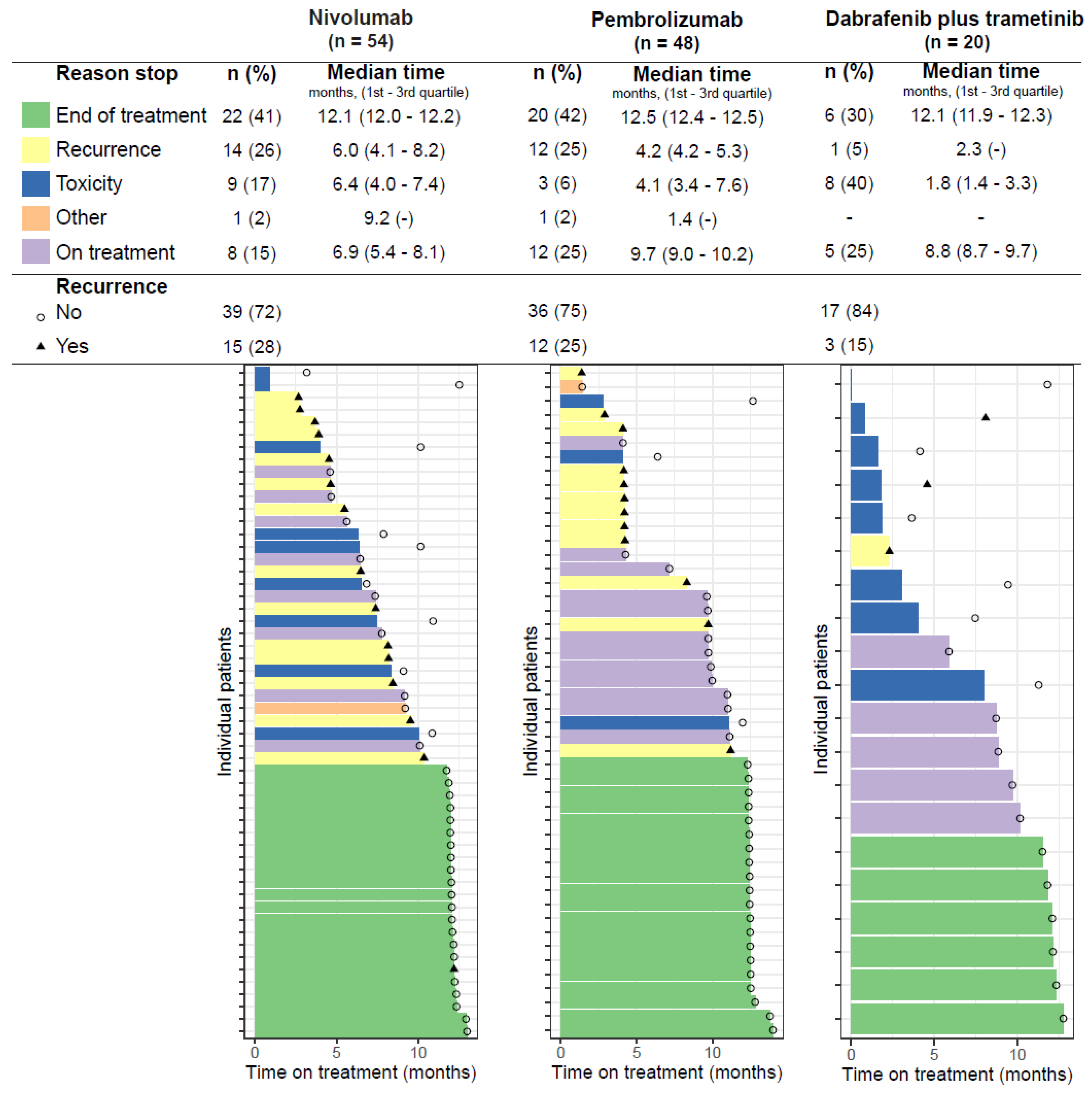
3.1. Time on Treatment
3.2. Adverse Events
3.3. Treatment-Limiting Toxicity
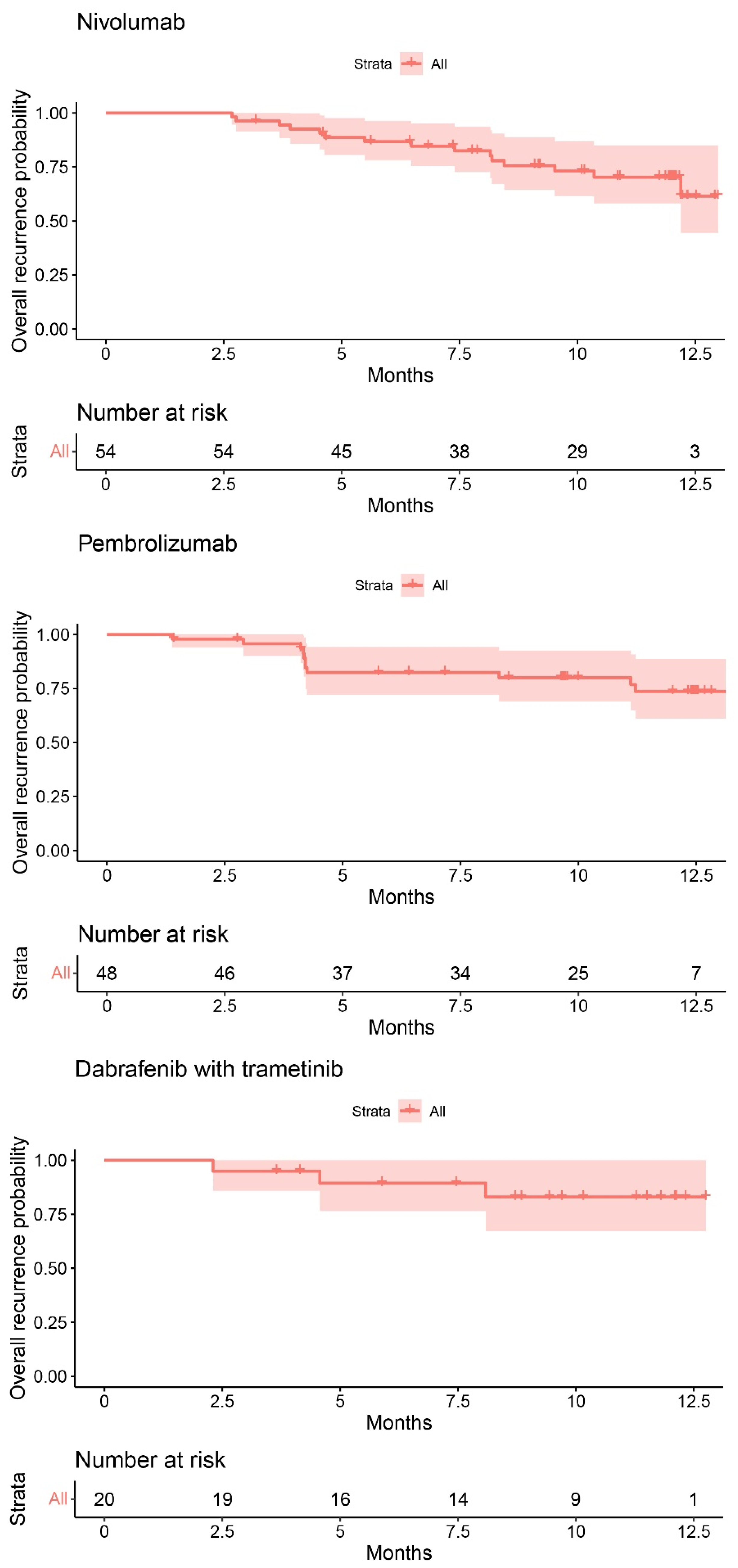
3.4. Recurrence
4. Discussion
4.1. Tolerability
4.2. Safety
4.2.1. Immune Checkpoint Inhibitors
4.2.2. Dabrafenib Plus Trametinib
4.3. Efficacy
4.4. Eligibility Criteria
4.5. Need for Real-World Data
4.6. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- NKR-cijfers/IKNL. Incidentie, Melanoom van de Huid en Externe Geslachtsorganen, CR. Available online: https://iknl.nl/nkr-cijfers?fs%7Cepidemiologie_id=526&fs%7Ctumor_id=243&fs%7Cregio_id=550&fs%7Cperiode_id=564%2C565%2C566%2C567%2C568%2C569%2C570%2C571%2C572%2C573%2C574%2C575%2C576%2C577%2C578%2C579%2C580%2C581%2C582%2C583%2C584%2C585%2C586%2C587%2C588%2C589%2C590%2C591%2C592%2C593%2C563%2C562%2C561&fs%7Cgeslacht_id=644&fs%7Cleeftijdsgroep_id=677&fs%7Cjaren_na_diagnose_id=687&fs%7Ceenheid_id=702&cs%7Ctype=line&cs%7CxAxis=periode_id&cs%7Cseries=epidemiologie_id&ts%7CrowDimensions=&ts%7CcolumnDimensions=periode_id&lang%7Clanguage=nl (accessed on 14 June 2022).
- Curti, B.D.; Faries, M.B. Recent Advances in the Treatment of Melanoma. N. Engl. J. Med. 2021, 384, 2229–2240. [Google Scholar] [CrossRef]
- European Medicines Agency. CHMP Post-Authorisation Summary of Positive Opinion for Keytruda. 2018. Available online: https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-keytruda-ii-47_en.pdf (accessed on 14 June 2022).
- European Medicines Agency. CHMP Post-Authorisation Summary of Positive Opinion for Opdivo. 2018. Available online: https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-opdivo_en-1.pdf (accessed on 14 June 2022).
- European Medicines Agency. CHMP Post-Authorisation Summary of Positive Opinion for Mekinist. 2018. Available online: https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-mekinist-ws/1274_en.pdf (accessed on 14 June 2022).
- European Medicines Agency. CHMP Post-Authorisation Summary of Positive Opinion for Tafinlar. 2018. Available online: https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-tafinlar-ws-1274_en.pdf (accessed on 14 June 2022).
- Weber, J.; Mandala, M.; del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef]
- Long, G.V.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Haydon, A.; et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N. Engl. J. Med. 2017, 377, 1813–1823. [Google Scholar] [CrossRef]
- Ascierto, P.A.; del Vecchio, M.; Mandalá, M.; Gogas, H.; Arance, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.J.; Chiarion-Sileni, V.; et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1465–1477. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): Distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 643–654. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef]
- Dummer, R.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Kirkwood, J.M.; Sileni, V.C.; Larkin, J.; Nyakas, M.; Dutriaux, C.; et al. Five-Year Analysis of Adjuvant Dabrafenib plus Trametinib in Stage III Melanoma. N. Engl. J. Med. 2020, 383, 1139–1148. [Google Scholar] [CrossRef]
- Wright, J.J.; Powers, A.C.; Johnson, D.B. Endocrine toxicities of immune checkpoint inhibitors. Nat. Rev. Endocrinol. 2021, 17, 389–399. [Google Scholar] [CrossRef]
- Dimitriou, F.; Long, G.V.; Menzies, A.M. Novel adjuvant options for cutaneous melanoma. Ann. Oncol. 2021, 32, 854–865. [Google Scholar] [CrossRef]
- Wang, S.V.; Schneeweiss, S.; Gagne, J.J.; Evers, T.; Gerlinger, C.; Desai, R.; Najafzadeh, M. Using Real-World Data to Extrapolate Evidence From Randomized Controlled Trials. Clin. Pharmacol. Ther. 2019, 105, 1156–1163. [Google Scholar] [CrossRef]
- Casey, J.A.; Schwartz, B.S.; Stewart, W.F.; Adler, N.E. Using Electronic Health Records for Population Health Research: A Review of Methods and Applications. Annu. Rev. Public Health 2016, 37, 61–81. [Google Scholar] [CrossRef]
- van Laar, S.A.; Gombert-Handoko, K.B.; Guchelaar, H.-J.; Zwaveling, J. An Electronic Health Record Text Mining Tool to Collect Real-World Drug Treatment Outcomes: A Validation Study in Patients With Metastatic Renal Cell Carcinoma. Clin. Pharmacol. Ther. 2020, 108, 644–652. [Google Scholar] [CrossRef]
- van Laar, S.A.; Gombert-Handoko, K.B.; Wassenaar, S.; Kroep, J.R.; Guchelaar, H.-J.; Zwaveling, J. Real-world evaluation of supportive care using an electronic health record text-mining tool: G-CSF use in breast cancer patients. Support. Care Cancer 2022. [Google Scholar] [CrossRef]
- van Laar, S.A.; Gombert-Handoko, K.B.; Groenwold, R.H.H.; van der Hulle, T.; Visser, L.E.; Houtsma, D.; Guchelaar, H.J.; Zwaveling, J. Real-World Metastatic Renal Cell Carcinoma Treatment Patterns and Clinical Outcomes in The Netherlands. Front. Pharmacol. 2022, 13, 803935. [Google Scholar] [CrossRef]
- de Meza, M.M.; Ismail, R.K.; Rauwerdink, D.; van Not, O.J.; van Breeschoten, J.; Blokx, W.A.M.; de Boer, A.; van Dartel, M.; Hilarius, D.L.; Ellebaek, E.; et al. Adjuvant treatment for melanoma in clinical practice – Trial versus reality. Eur. J. Cancer 2021, 158, 234–245. [Google Scholar] [CrossRef]
- Hoffmann, M.; Hayoz, S.; Özdemir, B.C. Prescription Patterns, Recurrence, and Toxicity Rates of Adjuvant Treatment for Stage III/IV Melanoma-A Real World Single-Center Analysis. Biology 2022, 11, 422. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Khan, S.; Gerber, D.E. Autoimmunity, checkpoint inhibitor therapy and immune-related adverse events: A review. Semin. Cancer Biol. 2020, 64, 93–101. [Google Scholar] [CrossRef]
- Mor, A.; Strazza, M. Bridging the Gap: Connecting the Mechanisms of Immune-Related Adverse Events and Autoimmunity Through PD-1. Front. Cell Dev. Biol. 2022, 9, 790386. [Google Scholar] [CrossRef]
- Darnell, E.P.; Mooradian, M.J.; Baruch, E.N.; Yilmaz, M.; Reynolds, K.L. Immune-Related Adverse Events (irAEs): Diagnosis, Management, and Clinical Pearls. Curr. Oncol. Rep. 2020, 22, 39. [Google Scholar] [CrossRef]
- Patrinely, J.R.; Johnson, R., Jr.; Lawless, A.R.; Bhave, P.; Sawyers, A.; Dimitrova, M.; Yeoh, H.L.; Palmeri, M.; Ye, F.; Fan, R.; et al. Chronic Immune-Related Adverse Events Following Adjuvant Anti–PD-1 Therapy for High-risk Resected Melanoma. JAMA Oncol. 2021, 7, 744–748. [Google Scholar] [CrossRef]
- Santini, F.C.; Rizvi, H.; Wilkins, O.; van Voorthuysen, M.; Panora, E.; Halpenny, D.; Kris, M.G.; Rudin, C.M.; Chaft, J.E.; Hellmann, M.D. Safety of retreatment with immunotherapy after immune-related toxicity in patients with lung cancers treated with anti-PD(L)-1 therapy. J. Clin. Oncol. 2017, 35, 9012. [Google Scholar] [CrossRef]
- Abou Alaiwi, S.; Xie, W.; Nassar, A.H.; Dudani, S.; Martini, D.; Bakouny, Z.; Steinharter, J.A.; Nuzzo, P.V.; Flippot, R.; Martinez-Chanza, N.; et al. Safety and efficacy of restarting immune checkpoint inhibitors after clinically significant immune-related adverse events in metastatic renal cell carcinoma. J. Immunother. Cancer 2020, 8, e000144. [Google Scholar] [CrossRef]
- Heinzerling, L.; Eigentler, T.K.; Fluck, M.; Hassel, J.C.; Heller-Schenck, D.; Leipe, J.; Pauschinger, M.; Vogel, A.; Zimmer, L.; Gutzmer, R. Tolerability of BRAF/MEK inhibitor combinations: Adverse event evaluation and management. ESMO Open 2019, 4, e000491. [Google Scholar] [CrossRef]
- Atkinson, V.; Robert, C.; Grob, J.J.; Gogas, H.; Dutriaux, C.; Demidov, L.; Gupta, A.; Menzies, A.M.; Ryll, B.; Miranda, F.; et al. Improved pyrexia-related outcomes associated with an adapted pyrexia adverse event management algorithm in patients treated with adjuvant dabrafenib plus trametinib: Primary results of COMBI-APlus. Eur. J. Cancer 2022, 163, 79–87. [Google Scholar] [CrossRef]
- Mansfield, C.; Myers, K.; Klein, K.; Patel, J.; Nakasato, A.; Ling, Y.-L.; Tarhini, A.A. Risk tolerance in adjuvant and metastatic melanoma settings: A patient perspective study using the threshold technique. Future Oncol. 2021, 17, 2151–2167. [Google Scholar] [CrossRef]
- Suciu, S.; Eggermont, A.M.M.; Lorigan, P.; Kirkwood, J.M.; Markovic, S.N.; Garbe, C.; Cameron, D.; Kotapati, S.; Chen, T.T.; Wheatley, K.; et al. Relapse-Free Survival as a Surrogate for Overall Survival in the Evaluation of Stage II-III Melanoma Adjuvant Therapy. J. Natl. Cancer Inst. 2018, 110. [Google Scholar] [CrossRef]
- Koelblinger, P.; Hoellwerth, M.; Dernoscheg, M.-T.; Koch, L.; Richtig, E.; Wanner, M.; Nguyen, V.-A.; Ostermann, H.; Bauer, J.W.; Laimer, M. Adjuvant anti-PD-1 antibody treatment in stage III/IV melanoma: Real-world experience and health economic considerations. J. Dtsch. Dermatol. Ges. 2021, 19, 1186–1198. [Google Scholar] [CrossRef]
- van Zeijl, M.C.T.; Ismail, R.K.; de Wreede, L.C.; van den Eertwegh, A.J.M.; de Boer, A.; van Dartel, M.; Hilarius, D.L.; Aarts, M.J.B.; van den Berkmortel, F.; Boers-Sonderen, M.J.; et al. Real-world outcomes of advanced melanoma patients not represented in phase III trials. Int. J. Cancer 2020, 147, 3461–3470. [Google Scholar] [CrossRef]
- Board, R.; Smittenaar, R.; Lawton, S.; Liu, H.; Juwa, B.; Chao, D.; Corrie, P. Metastatic melanoma patient outcomes since introduction of immune checkpoint inhibitors in England between 2014 and 2018. Int. J. Cancer 2021, 148, 868–875. [Google Scholar] [CrossRef]
- Moser, J.C.; Chen, D.; Hu-Lieskovan, S.; Grossmann, K.F.; Patel, S.; Colonna, S.V.; Ying, J.; Hyngstrom, J.R. Real-world survival of patients with advanced BRAF V600 mutated melanoma treated with front-line BRAF/MEK inhibitors, anti-PD-1 antibodies, or nivolumab/ipilimumab. Cancer Med. 2019, 8, 7637–7643. [Google Scholar] [CrossRef]
- Reijers, I.L.M.; Menzies, A.M.; van Akkooi, A.C.J.; Versluis, J.M.; van den Heuvel, N.M.J.; Saw, R.P.M.; Pennington, T.E.; Kapiteijn, E.; van der Veldt, A.A.M.; Suijkerbuijk, K.P.M.; et al. Personalized response-directed surgery and adjuvant therapy after neoadjuvant ipilimumab and nivolumab in high-risk stage III melanoma: The PRADO trial. Nat. Med. 2022, 28, 1178–1188. [Google Scholar] [CrossRef]
- Weilandt, J.; Diehl, K.; Schaarschmidt, M.L.; Kieker, F.; Sasama, B.; Pronk, M.; Ohletz, J.; Könnecke, A.; Müller, V.; Utikal, J.; et al. Patient Preferences in Adjuvant and Palliative Treatment of Advanced Melanoma: A Discrete Choice Experiment. Acta Derm. Venereol. 2020, 100, adv00083. [Google Scholar] [CrossRef]
- Livingstone, A.; Dempsey, K.; Stockler, M.R.; Howard, K.; Long, G.V.; Carlino, M.S.; Menzies, A.M.; Morton, R.L. Adjuvant immunotherapy recommendations for stage III melanoma: Physician and nurse interviews. BMC Cancer 2021, 21, 1014. [Google Scholar] [CrossRef]
- Bica, I.; Alaa, A.M.; Lambert, C.; van der Schaar, M. From Real-World Patient Data to Individualized Treatment Effects Using Machine Learning: Current and Future Methods to Address Underlying Challenges. Clin. Pharmacol. Ther. 2021, 109, 87–100. [Google Scholar] [CrossRef]
- Haneuse, S.; Bogart, A.; Jazic, I.; Westbrook, E.O.; Boudreau, D.; Theis, M.K.; Simon, G.E.; Arterburn, D. Learning About Missing Data Mechanisms in Electronic Health Records-based Research: A Survey-based Approach. Epidemiology 2016, 27, 82–90. [Google Scholar] [CrossRef]
- Percha, B. Modern Clinical Text Mining: A Guide and Review. Annu. Rev. Biomed. Data Sci. 2021, 4, 165–187. [Google Scholar] [CrossRef]
| Nivolumab | Pembrolizumab | Dabrafenib Plus Trametinib | |
|---|---|---|---|
| Phase III trial | Checkmate 238 [8,10] | EORTC/KEYNOTE-054 [11,12] | COMBI-AD [9,13] |
| Comparator | Ipilimumab | Placebo | Placebo |
| Eligibility criteria | Resected stage IIIb, IIIc and IV melanoma, ECOG PS: 0 or 1 | Resected stage IIIa, IIIb, and IIIc melanoma, ECOG PS: 0 or 1 | Resected stage IIIa, IIIb, or IIIc melanoma with a BRAF V600E or V600K mutation, ECOG PS: 0 or 1. |
| Recurrence-free survival | 1-year | 1-year | 1-year |
| NIV: 71% | PEM: 75% | D + T: 88% | |
| IPI: 61% | PLA: 61% | PLA: 56% | |
| 4-year | 3.5-year | 5-year | |
| NIV: 51.7% | PEM: 60% | D + T: 52% | |
| IPI: 41.2% | PLA: 41% | PLA: 36% | |
| HR: 0.71 | HR: 0.59 | HR: 0.51 | |
| 95% CI = 0.60–0.86 | 95% CI = 0.49–0.70 | 95% CI = 0.42–0.61 | |
| Overall survival | 4-year | - | 3-year |
| NIV: 77.9% | D + T: 86% | ||
| IPI: 76.6% | PLA: 77% | ||
| HR: 0.87 | HR: 0.57 | ||
| 95% CI = 0.66–1.14 | 95% CI = 0.42–0.79 * | ||
| ≥Grade 3 AE | NIV: 25.4% | PEM: 31.6% | D + T: 41% |
| IPI: 55.2% | PLA: 18.5% | PLA: 14% | |
| Most common AE | Skin reactions: 44.5% | All immune-related AE: 37.3% | Pyrexia: 63% |
| Fatigue: 34.5% | Fatigue or asthenia: 37.1% | Fatigue: 47% | |
| Gastrointestinal: 25.2% | Skin reactions: 28.3% | Nausea: 40% | |
| AE leading to discontinuation | NIV: 9.7% | PEM: 13.8% | D + T: 26% |
| IPI: 42.6% | PLA: 2.2% | PLA: 3% |
| Characteristics | Nivolumab (n = 54) | Pembrolizumab (n = 48) | Dabrafenib Plus Trametinib (n = 20) | All Patients (n = 122) |
|---|---|---|---|---|
| Median age (range)—year | 61 (21–84) | 57.5 (23–80) | 57.5 (31–73) | 59 (21–84) |
| Sex, no. (%) | ||||
| Male | 35 (64.8) | 31 (64.6) | 9 (45.0) | 75 (61.5) |
| Female | 19 (35.2) | 17 (35.4) | 11 (55.0) | 47 (38.5) |
| ECOG performance status, no. (%) | ||||
| 0 | 32 (59.3) | 24 (50.0) | 9 (45.0) | 65 (53.3) |
| 1 | 7 (13.0) | 5 (10.4) | 1 (5.0) | 13 (10.7) |
| Unknown | 15 (27.8) | 18 (37.5) | 10 (50.0) | 43 (35.2) |
| Disease stage, no. (%) AJCC 7 | ||||
| III | 34 (63.0) | 48 (100) | 20 (100) | 102 (83.6) |
| Unspecified | 3 (5.6) | 1 (2.1) | 1 (5.0) | 5 (4.1) |
| IIIa | 1 (1.9) | 20 (41.7) | 5 (25.0) | 26 (21.3) |
| IIIb | 10 (18.5) | 19 (39.6) | 9 (45.0) | 38 (31.1) |
| IIIc | 20 (37.0) | 8 (16.7) | 5 (25.0) | 33 (27.0) |
| Resected IV | 20 (37.0) | - | - | 20 (16.4) |
| Primary tumor location, no. (%) | ||||
| Head or neck | 6 (11.1) | 6 (12.5) | 2 (10.0) | 14 (11.5) |
| Body | 19 (35.2) | 22 (45.8) | 8 (40.0) | 49 (40.2) |
| Extremities | 17 (31.5) | 13 (27.1) | 6 (30.0) | 36 (29.5) |
| Acral | 1 (1.9) | 3 (6.3) | 1 (5.0) | 5 (4.1) |
| Mucosal | 1 (1.9) | - | - | 1 (0.8) |
| Unknown primary | 9 (16.7) | 2 (4.2) | 3 (15.0) | 14 (11.5) |
| Unknown | 1 (1.9) | 2 (4.2) | - | 3 (2.5) |
| Subtype, no. (%) | ||||
| Superficial spreading | 16 (29.6) | 25 (52.1) | 11 (55.0) | 52 (42.6) |
| Nodular | 9 (16.7) | 8 (16.7) | 5 (25.0) | 22 (18.0) |
| Acral lentiginous | - | 2 (4.2) | 1 (5.0) | 3 (2.5) |
| Spindle cell | - | 3 (6.3) | - | 3 (2.5) |
| Unclear | 29 (53.7) | 10 (20.8) | 3 (15.0) | 42 (34.4) |
| LDH above ULN, no. (%) | ||||
| Yes | 5 (9.3) | - | - | 5 (4.1) |
| No | 43 (79.6) | 44 (91.7) | 20 (100.0) | 107 (87.7) |
| Unknown | 6 (11.1) | 4 (8.3) | - | 10 (8.2) |
| Ulceration, no. (%) | ||||
| Yes | - | 15 (31.3) | 1 (5.0) | 16 (13.1) |
| No | 14 (25.0) | 25 (52.1) | 5 (25.0) | 44 (36.1) |
| Unknown | 40 (74.1) | 8 (16.7) | 14 (70.0) | 62 (50.8) |
| BRAF mutation, no. (%) | ||||
| Yes | 28 (51.9) | 17 (35.4) | 20 (100.0) | 65 (53.3) |
| No | 21 (38.9) | 20 (41.7) | - | 41 (33.6) |
| Unknown | 5 (9.3) | 11 (22.9) | - | 16 (13.1) |
| NRAS mutation, no. (%) | ||||
| Yes | 18 (33.3) | 12 (25.0) | 1 (5.0) | 31 (25.4) |
| No | 28 (51.9) | 13 (27.1) | 15 (75.0) | 56 (45.9) |
| Unknown | 8 (14.8) | 23 (47.9) | 4 (20.0) | 35 (28.7) |
| KIT mutation, no. (%) | ||||
| Yes | - | 1 (2.1) | - | 1 (0.8) |
| No | 45 (83.3) | 31 (64.6) | 17 (85.0) | 93 (76.2) |
| Unknown | 9 (16.7) | 16 (33.3) | 3 (15.0) | 28 (23.0) |
| Previous systemic therapy for melanoma, no. (%) | ||||
| Adjuvant | 1 (1.9) | 1 (2.1) | 1 (5.0) | 3 (2.5) |
| Neo-adjuvant | 1 (1.9) | - | 3 (15.0) | 4 (3.3) |
| Adverse Events, No. (%) | Nivolumab | Pembrolizumab | Dabrafenib Plus Trametinib | |||
|---|---|---|---|---|---|---|
| (n = 54) | (n = 48) | (n = 20) | ||||
| Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | |
| Abdominal pain | 2 (3.7) | - | 4 (8.3) | - | 1 (5.0) | - |
| Arthralgia | 2 (3.7) | - | 2 (4.2) | - | 2 (10.0) | - |
| Chills | 2 (3.7) | - | - | - | 9 (45.0) | - |
| Cough | 12 (22.2) | - | 7 (14.6) | - | 4 (20.0) | - |
| Diarrhea | 22 (42.6) | 1 (1.9) | 9 (18.8) | 2 (4.2) | 1 (5.0) | - |
| Dyspnea | 6 (11.1) | - | 4 (8.3) | - | - | - |
| Fatigue or asthenia | 43 (79.6) | - | - | 16 (80.0) | - | |
| Fatigue | 42 (77.8) | - | 36 (75.0) | - | 16 (80.0) | - |
| Asthenia | 1 (1.9) | 1 (1.9) | 1 (2.1) | - | 1 (5.0) | - |
| Headache | 16 (29.6) | - | 8 (16.7) | - | 10 (50.0) | - |
| Nausea, incl. vomiting | 13 (24.1) | - | 9 (18.8) | - | 10 (50.0) | 1 (5.0) |
| Pyrexia | 15 (27.8) | - | 4 (8.3) | - | 13 (65.0) | - |
| Skin reaction | 17 (31.5) | - | 9 (18.8) | - | 6 (30.0) | - |
| Pruritus | 13 (24.1) | - | 9 18.8) | - | 3 (15.0) | - |
| Rash | 9 (16.7) | - | 2 (4.2) | - | 5 (25.0) | - |
| Immune-related adverse events | ||||||
| Colitis | 3 (5.6) | - | 1 (2.1) | 1 (2.1) | - | - |
| Diabetes Mellitus type 1 | - | - | - | - | - | - |
| Hepatitis | - | 2 (3.7) | - | - | - | - |
| Hyperthyroidism | 8 (14.8) | - | 6 (12.5) | - | - | - |
| Hypothyroidism | 11 (20.4) | - | 9 (18.8) | - | - | - |
| Pneumonitis | - | - | 2 (4.2) | - | - | - |
| Adverse Events, No. | Nivolumab n = 9 | Pembrolizumab n = 3 | Dabrafenib Plus Trametinib n = 8 |
|---|---|---|---|
| Allergic reaction | - | - | 1 |
| Arthalgia | 1 | 1 | 1 |
| Chills | - | - | 3 |
| Decreased appetite | - | - | 1 |
| Fatigue | - | - | 2 |
| Fever | - | - | 5 |
| Headache | - | - | 1 |
| Liver function disorders | - | - | 1 |
| Malaise | - | - | 2 |
| Myalgia | - | 1 | 2 |
| Nausea | - | - | 3 |
| Skin disorder | - | - | 4 |
| Syncope | - | - | 1 |
| Tachycardia | - | - | 1 |
| Immune-related adverse events | 8 | 2 | - |
| Adrenalitis | 1 | - | - |
| Colitis | 2 | 1 | - |
| Hepatitis | 2 | - | - |
| Hypocortisolism | 1 | - | - |
| Meningitis | 1 | - | - |
| Myocarditis, no. | 1 | - | - |
| Myositis, no. | 1 | - | - |
| Polymyalgia rheumatica, no. | 1 | - | - |
| Pneumonitis, no. | 2 | 1 | - |
| Thyroiditis, no. | 2 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Laar, S.A.; Kapiteijn, E.; Gombert-Handoko, K.B.; Guchelaar, H.-J.; Zwaveling, J. Application of Electronic Health Record Text Mining: Real-World Tolerability, Safety, and Efficacy of Adjuvant Melanoma Treatments. Cancers 2022, 14, 5426. https://doi.org/10.3390/cancers14215426
van Laar SA, Kapiteijn E, Gombert-Handoko KB, Guchelaar H-J, Zwaveling J. Application of Electronic Health Record Text Mining: Real-World Tolerability, Safety, and Efficacy of Adjuvant Melanoma Treatments. Cancers. 2022; 14(21):5426. https://doi.org/10.3390/cancers14215426
Chicago/Turabian Stylevan Laar, Sylvia A., Ellen Kapiteijn, Kim B. Gombert-Handoko, Henk-Jan Guchelaar, and Juliette Zwaveling. 2022. "Application of Electronic Health Record Text Mining: Real-World Tolerability, Safety, and Efficacy of Adjuvant Melanoma Treatments" Cancers 14, no. 21: 5426. https://doi.org/10.3390/cancers14215426
APA Stylevan Laar, S. A., Kapiteijn, E., Gombert-Handoko, K. B., Guchelaar, H.-J., & Zwaveling, J. (2022). Application of Electronic Health Record Text Mining: Real-World Tolerability, Safety, and Efficacy of Adjuvant Melanoma Treatments. Cancers, 14(21), 5426. https://doi.org/10.3390/cancers14215426








