Cardiotoxicity Induced by Immune Checkpoint Inhibitors: What a Cardio-Oncology Team Should Know and Do
Abstract
Simple Summary
Abstract
1. Introduction
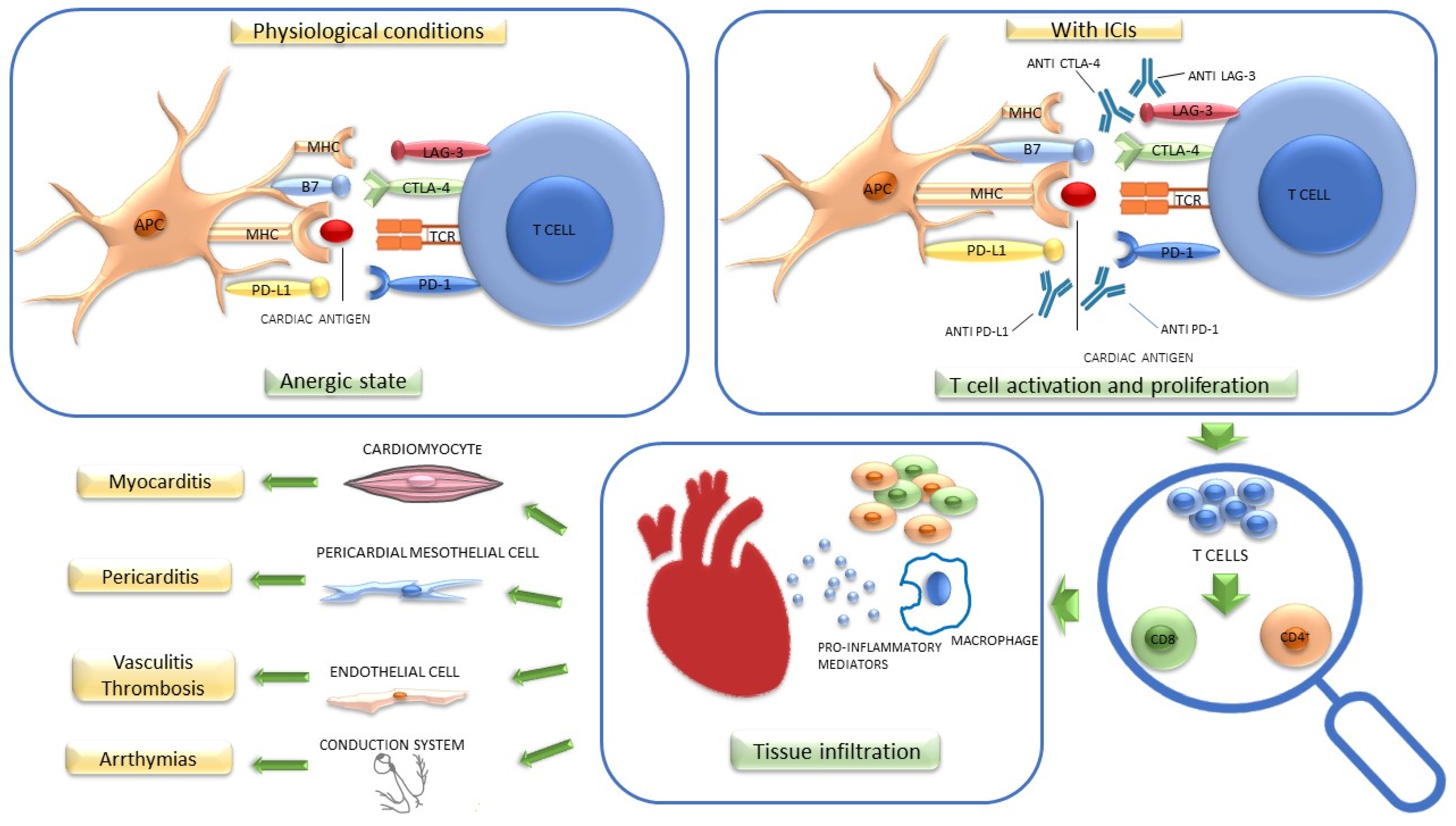
2. ICI-Related Cardiotoxicity
2.1. Pathogenetic Mechanisms
2.1.1. Myocarditis
2.1.2. Pericardial Disease
2.1.3. Takotsubo-Like Cardiomyopathy
2.1.4. Myocardial Infarction
2.1.5. Arrhythmias and Conduction Disorders
2.2. Risk Factors
3. Management of ICI-Related Cardiotoxicity
3.1. Identifying and Evaluating the Type and Severity of the Cardiotoxicity
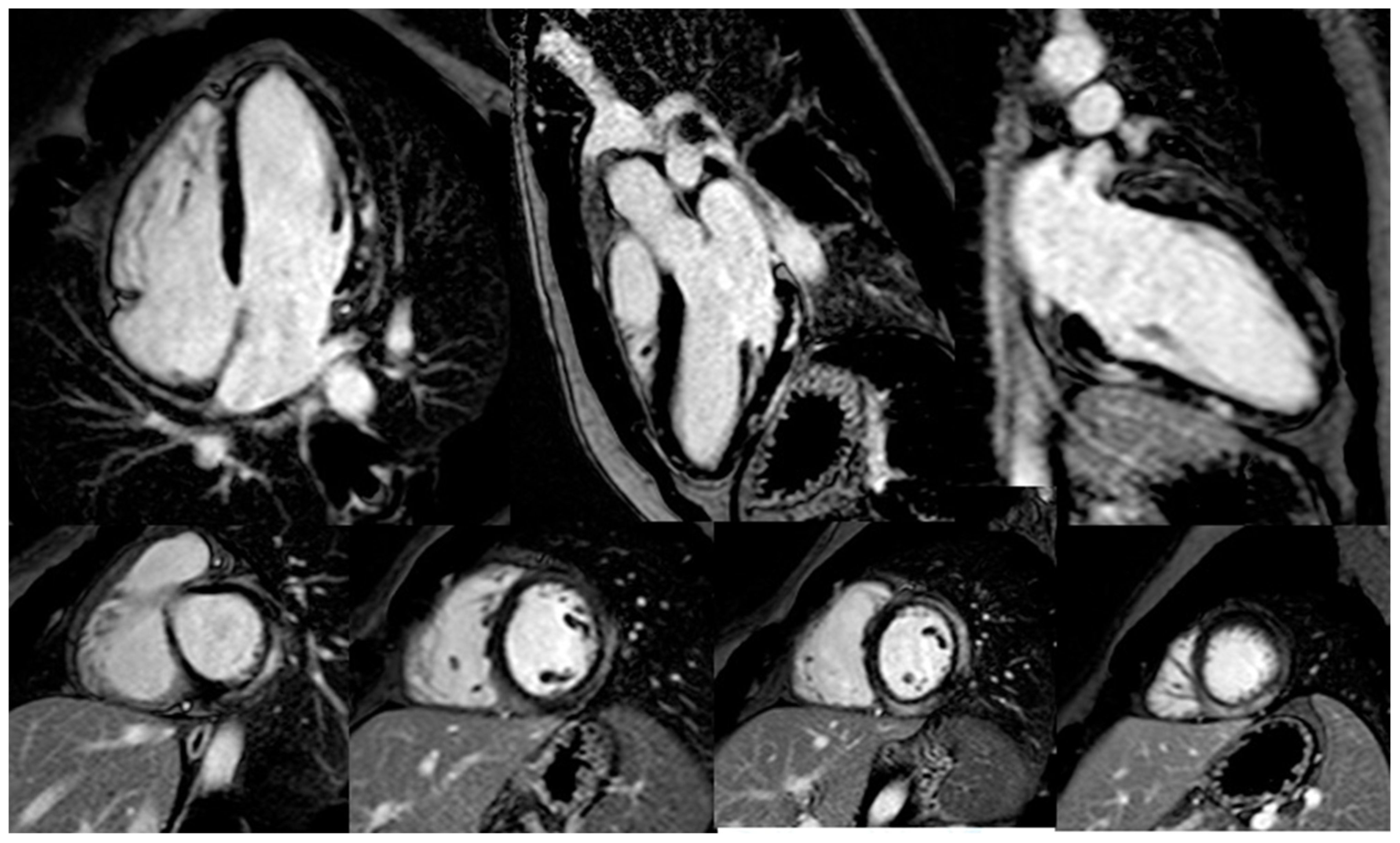
3.1.1. Myocarditis
3.1.2. Pericardial Disease
3.1.3. Takotsubo-Like Syndrome
3.1.4. Acute Myocardial Infarction
3.1.5. Arrhythmias and Conduction Disorders
3.2. Deciding Whether to Withhold ICI Therapy
3.3. Initiating Steroid and Immunosuppressive Therapy
Management of ICI Related Myocarditis
3.4. Conventional Cardiac Treatment of Cardiac Complications
3.5. Restarting ICI Therapy
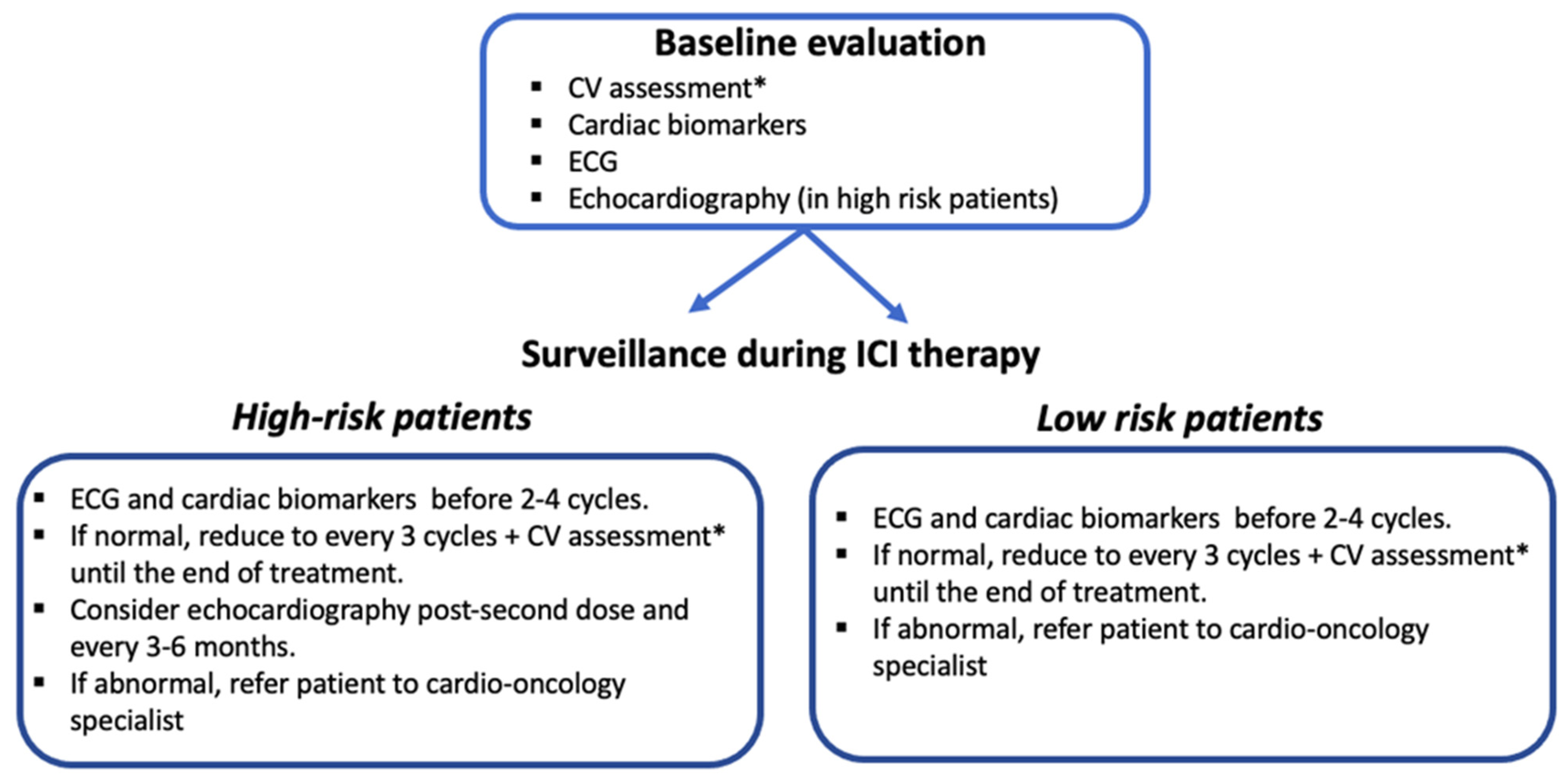
4. Surveillance for ICI-Related Cardiotoxicity
5. Long-Term Cardiotoxicity
6. Concurrent irAEs
7. The Role of the Cardio-Oncologist in the Molecular Tumor Board
8. Roadmap to Prevent and Mitigate ICI-Related Cardiotoxicity: Potential Strategies and Ongoing Research Developments
8.1. Development and Validating of Prognostic Biomarkers and Cardiac Imaging Findings for Cardiac irAEs
8.2. Utilization of Immune Checkpoint Inhibitors with Reduced Cardiotoxicity
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Michel, L.; Rassaf, T.; Totzeck, M. Cardiotoxicity from immune checkpoint inhibitors. Int. J. Cardiol. Heart Vasc. 2019, 25, 100420. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, A.H.; Pauken, K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018, 18, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.R.; Florido, R.; Lipson, E.J.; Naidoo, J.; Ardehali, R.; Tocchetti, C.G.; Lyon, A.R.; Padera, R.F.; Johnson, D.B.; Moslehi, J. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc. Res. 2019, 115, 854–868. [Google Scholar] [CrossRef] [PubMed]
- Durham, N.M.; Nirschl, C.J.; Jackson, C.M.; Elias, J.; Kochel, C.M.; Anders, R.A.; Drake, C.G. Lymphocyte Activation Gene 3 (LAG-3) modulates the ability of CD4 T-cells to be suppressed in vivo. PLoS ONE 2014, 9, e109080. [Google Scholar] [CrossRef]
- Gedye, C.; van der Westhuizen, A.; John, T. Checkpoint immunotherapy for cancer: Superior survival, unaccustomed toxicities. Intern. Med. J. 2015, 45, 696–701. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef]
- Tocchetti, C.G.; Cadeddu, C.; Di Lisi, D.; Femmino, S.; Madonna, R.; Mele, D.; Monte, I.; Novo, G.; Penna, C.; Pepe, A.; et al. From Molecular Mechanisms to Clinical Management of Antineoplastic Drug-Induced Cardiovascular Toxicity: A Translational Overview. Antioxid. Redox Signal. 2019, 30, 2110–2153. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Inno, A.; Metro, G.; Bironzo, P.; Grimaldi, A.M.; Grego, E.; Di Nunno, V.; Picasso, V.; Massari, F.; Gori, S. Pathogenesis, clinical manifestations and management of immune checkpoint inhibitors toxicity. Tumori J. 2017, 103, 405–421. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef]
- Rubio-Infante, N.; Ramírez-Flores, Y.A.; Castillo, E.C.; Lozano, O.; García-Rivas, G.; Torre-Amione, G. Cardiotoxicity associated with immune checkpoint inhibitor therapy: A meta-analysis. Eur. J. Heart Fail. 2021, 23, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Dolladille, C.; Akroun, J.; Morice, P.M.; Dompmartin, A.; Ezine, E.; Sassier, M.; Da-Silva, A.; Plane, A.F.; Legallois, D.; L’Orphelin, J.M.; et al. Cardiovascular immunotoxicities associated with immune checkpoint inhibitors: A safety meta-analysis. Eur. Heart J. 2021, 42, 4964–4977. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutierrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Kouo, T.; Huang, L.; Pucsek, A.B.; Cao, M.; Solt, S.; Armstrong, T.; Jaffee, E. Galectin-3 Shapes Antitumor Immune Responses by Suppressing CD8+ T Cells via LAG-3 and Inhibiting Expansion of Plasmacytoid Dendritic Cells. Cancer Immunol. Res. 2015, 3, 412–423. [Google Scholar] [CrossRef]
- Salem, J.E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.P.; Balko, J.M.; Bonaca, M.P.; et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef]
- Patel, R.P.; Parikh, R.; Gunturu, K.S.; Tariq, R.Z.; Dani, S.S.; Ganatra, S.; Nohria, A. Cardiotoxicity of Immune Checkpoint Inhibitors. Curr. Oncol. Rep. 2021, 23, 79. [Google Scholar] [CrossRef]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 23, e333–e465. [Google Scholar] [CrossRef]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef]
- Reuben, A.; Petaccia de Macedo, M.; McQuade, J.; Joon, A.; Ren, Z.; Calderone, T.; Conner, B.; Wani, K.; Cooper, Z.A.; Tawbi, H.; et al. Comparative immunologic characterization of autoimmune giant cell myocarditis with ipilimumab. Oncoimmunology 2017, 6, e1361097. [Google Scholar] [CrossRef]
- Love, V.A.; Grabie, N.; Duramad, P.; Stavrakis, G.; Sharpe, A.; Lichtman, A. CTLA-4 ablation and interleukin-12 driven differentiation synergistically augment cardiac pathogenicity of cytotoxic T lymphocytes. Circ. Res. 2007, 101, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Okazaki, T.; Tanaka, Y.; Nakatani, K.; Hara, M.; Matsumori, A.; Sasayama, S.; Mizoguchi, A.; Hiai, H.; Minato, N.; et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001, 291, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Tanaka, Y.; Nishio, R.; Mitsuiye, T.; Mizoguchi, A.; Wang, J.; Ishida, M.; Hiai, H.; Matsumori, A.; Minato, N.; et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat. Med. 2003, 9, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Y.; Huang, W.K.; Chien-Chia Wu, V.; Chang, W.C.; Chen, J.S.; Chuang, C.K.; Chu, P.H. Cardiovascular toxicity of immune checkpoint inhibitors in cancer patients: A review when cardiology meets immuno-oncology. J. Formos. Med. Assoc. 2020, 119, 1461–1475. [Google Scholar] [CrossRef]
- Lyon, A.R.; Yousaf, N.; Battisti, N.M.L.; Moslehi, J.; Larkin, J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018, 19, e447–e458. [Google Scholar] [CrossRef]
- Lyon, A.R.; Bossone, E.; Schneider, B.; Sechtem, U.; Citro, R.; Underwood, S.R.; Sheppard, M.N.; Figtree, G.A.; Parodi, G.; Akashi, Y.J.; et al. Current state of knowledge on Takotsubo syndrome: A Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 8–27. [Google Scholar] [CrossRef]
- Fernandez, D.M.; Rahman, A.H.; Fernandez, N.F.; Chudnovskiy, A.; Amir, E.D.; Amadori, L.; Khan, N.S.; Wong, C.K.; Shamailova, R.; Hill, C.A.; et al. Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 2019, 25, 1576–1588. [Google Scholar] [CrossRef]
- Nykl, R.; Fischer, O.; Vykoupil, K.; Taborsky, M. A unique reason for coronary spasm causing temporary ST elevation myocardial infarction (inferior STEMI)—Systemic inflammatory response syndrome after use of pembrolizumab. Arch. Med. Sci. Atheroscler. Dis. 2017, 2, e100–e102. [Google Scholar] [CrossRef]
- Stein-Merlob, A.F.; Rothberg, M.V.; Holman, P.; Yang, E.H. Immunotherapy-Associated Cardiotoxicity of Immune Checkpoint Inhibitors and Chimeric Antigen Receptor T Cell Therapy: Diagnostic and Management Challenges and Strategies. Curr. Cardiol. Rep. 2021, 23, 11. [Google Scholar] [CrossRef]
- Ganatra, S.; Parikh, R.; Neilan, T.G. Cardiotoxicity of Immune Therapy. Cardiol. Clin. 2019, 37, 385–397. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in Patients Treated with Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Reynolds, K.L.; Lyon, A.R.; Palaskas, N.; Neilan, T.G. The Evolving Immunotherapy Landscape and the Epidemiology, Diagnosis, and Management of Cardiotoxicity: JACC: CardioOncology Primer. JACC CardioOncol. 2021, 3, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Zamami, Y.; Niimura, T.; Okada, N.; Koyama, T.; Fukushima, K.; Izawa-Ishizawa, Y.; Ishizawa, K. Factors Associated with Immune Checkpoint Inhibitor-Related Myocarditis. JAMA Oncol. 2019, 5, 1635–1637. [Google Scholar] [CrossRef] [PubMed]
- Ganatra, S.; Neilan, T.G. Immune Checkpoint Inhibitor-Associated Myocarditis. Oncologist 2018, 23, 879–886. [Google Scholar] [CrossRef]
- Dal’bo, N.; Patel, R.; Parikh, R.; Shah, S.P.; Guha, A.; Dani, S.S.; Ganatra, S. Cardiotoxicity of Contemporary Anticancer Immunotherapy. Curr. Treat. Options Cardiovasc. Med. 2020, 22, 62. [Google Scholar] [CrossRef]
- Groschel, C.; Sasse, A.; Rohrborn, C.; Monecke, S.; Didie, M.; Elsner, L.; Kruse, V.; Bunt, G.; Lichtman, A.H.; Toischer, K.; et al. T helper cells with specificity for an antigen in cardiomyocytes promote pressure overload-induced progression from hypertrophy to heart failure. Sci. Rep. 2017, 7, 15998. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Abu-Sbeih, H.; Ascierto, P.A.; Brufsky, J.; Cappelli, L.C.; Cortazar, F.B.; Gerber, D.E.; Hamad, L.; Hansen, E.; Johnson, D.B.; et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J. Immunother. Cancer 2021, 9, e002435. [Google Scholar] [CrossRef]
- Berg, D.D.; Vaduganathan, M.; Nohria, A.; Davids, M.S.; Alyea, E.P.; Torre, M.; Padera, R.F., Jr. Immune-related fulminant myocarditis in a patient receiving ipilimumab therapy for relapsed chronic myelomonocytic leukaemia. Eur. J. Heart Fail. 2017, 19, 682–685. [Google Scholar] [CrossRef]
- Escudier, M.; Cautela, J.; Malissen, N.; Ancedy, Y.; Orabona, M.; Pinto, J.; Monestier, S.; Grob, J.J.; Scemama, U.; Jacquier, A.; et al. Clinical Features, Management, and Outcomes of Immune Checkpoint Inhibitor-Related Cardiotoxicity. Circulation 2017, 136, 2085–2087. [Google Scholar] [CrossRef]
- Bando, S.; Soeki, T.; Matsuura, T.; Tobiume, T.; Ise, T.; Kusunose, K.; Yamaguchi, K.; Yagi, S.; Fukuda, D.; Iwase, T.; et al. Plasma brain natriuretic peptide levels are elevated in patients with cancer. PLoS ONE 2017, 12, e0178607. [Google Scholar] [CrossRef]
- Awadalla, M.; Mahmood, S.S.; Groarke, J.D.; Hassan, M.Z.O.; Nohria, A.; Rokicki, A.; Murphy, S.P.; Mercaldo, N.D.; Zhang, L.; Zlotoff, D.A.; et al. Global Longitudinal Strain and Cardiac Events in Patients with Immune Checkpoint Inhibitor-Related Myocarditis. J. Am. Coll. Cardiol. 2020, 75, 467–478. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Awadalla, M.; Mahmood, S.S.; Nohria, A.; Hassan, M.Z.O.; Thuny, F.; Zlotoff, D.A.; Murphy, S.P.; Stone, J.R.; Golden, D.L.A.; et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur. Heart J. 2020, 41, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jia, Y.; Liu, Q.; Shen, Y.; Zhu, H.; Dong, X.; Huang, J.; Lu, J.; Yin, Q. Myocarditis related to immune checkpoint inhibitors treatment: Two case reports and literature review. Ann. Palliat. Med. 2021, 10, 8512–8517. [Google Scholar] [CrossRef] [PubMed]
- Leone, O.; Veinot, J.P.; Angelini, A.; Baandrup, U.T.; Basso, C.; Berry, G.; Bruneval, P.; Burke, M.; Butany, J.; Calabrese, F.; et al. 2011 consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc. Pathol. 2012, 21, 245–274. [Google Scholar] [CrossRef]
- Palaskas, N.L.; Segura, A.; Lelenwa, L.; Siddiqui, B.A.; Subudhi, S.K.; Lopez-Mattei, J.; Durand, J.B.; Deswal, A.; Zhao, B.; Maximilian Buja, L.; et al. Immune checkpoint inhibitor myocarditis: Elucidating the spectrum of disease through endomyocardial biopsy. Eur. J. Heart Fail. 2021, 23, 1725–1735. [Google Scholar] [CrossRef]
- Champion, S.N.; Stone, J.R. Immune checkpoint inhibitor associated myocarditis occurs in both high-grade and low-grade forms. Mod. Pathol. 2020, 33, 99–108. [Google Scholar] [CrossRef]
- Upadhrasta, S.; Elias, H.; Patel, K.; Zheng, L. Managing cardiotoxicity associated with immune checkpoint inhibitors. Chronic Dis. Transl. Med. 2019, 5, 6–14. [Google Scholar] [CrossRef]
- Dasanu, C.A.; Jen, T.; Skulski, R. Late-onset pericardial tamponade, bilateral pleural effusions and recurrent immune monoarthritis induced by ipilimumab use for metastatic melanoma. J. Oncol. Pharm. Pract. 2017, 23, 231–234. [Google Scholar] [CrossRef]
- Moriyama, S.; Fukata, M.; Tatsumoto, R.; Kono, M. Refractory constrictive pericarditis caused by an immune checkpoint inhibitor properly managed with infliximab: A case report. Eur. Heart J. Case Rep. 2021, 5, ytab002. [Google Scholar] [CrossRef]
- de Almeida, D.V.P.; Gomes, J.R.; Haddad, F.J.; Buzaid, A.C. Immune-mediated Pericarditis with Pericardial Tamponade during Nivolumab Therapy. J. Immunother. 2018, 41, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Asnani, A. Cardiotoxicities associated with immune checkpoint inhibitors. Curr. Probl. Cancer 2018, 42, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lionis, C.; et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2015, 36, 2921–2964. [Google Scholar] [CrossRef] [PubMed]
- Ederhy, S.; Cautela, J.; Ancedy, Y.; Escudier, M.; Thuny, F.; Cohen, A. Takotsubo-Like Syndrome in Cancer Patients Treated with Immune Checkpoint Inhibitors. JACC Cardiovasc. Imaging 2018, 11, 1187–1190. [Google Scholar] [CrossRef] [PubMed]
- Mir, H.; Alhussein, M.; Alrashidi, S.; Alzayer, H.; Alshatti, A.; Valettas, N.; Mukherjee, S.D.; Nair, V.; Leong, D.P. Cardiac Complications Associated with Checkpoint Inhibition: A Systematic Review of the Literature in an Important Emerging Area. Can. J. Cardiol. 2018, 34, 1059–1068. [Google Scholar] [CrossRef]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Andrews, S.; Armand, P.; Bhatia, S.; Budde, L.E.; Costa, L.; Davies, M.; Dunnington, D.; et al. NCCN Guidelines Insights: Management of Immunotherapy-Related Toxicities, Version 1.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 230–241. [Google Scholar] [CrossRef]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O., 3rd; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef]
- Ball, S.; Ghosh, R.K.; Wongsaengsak, S.; Bandyopadhyay, D.; Ghosh, G.C.; Aronow, W.S.; Fonarow, G.C.; Lenihan, D.J.; Bhatt, D.L. Cardiovascular Toxicities of Immune Checkpoint Inhibitors: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 1714–1727. [Google Scholar] [CrossRef]
- Beattie, J.; Rizvi, H.; Fuentes, P.; Luo, J.; Schoenfeld, A.; Lin, I.H.; Postow, M.; Callahan, M.; Voss, M.H.; Shah, N.J.; et al. Success and failure of additional immune modulators in steroid-refractory/resistant pneumonitis related to immune checkpoint blockade. J. Immunother. Cancer 2021, 9, e001884. [Google Scholar] [CrossRef]
- Zhang, R.S.; Padegimas, A.; Murphy, K.M.; Evans, P.T.; Peters, C.J.; Domenico, C.M.; Vidula, M.K.; Mather, P.J.; Cevasco, M.; Cohen, R.B.; et al. Treatment of corticosteroid refractory immune checkpoint inhibitor myocarditis with Infliximab: A case series. Cardiooncology 2021, 7, 13. [Google Scholar] [CrossRef]
- Luo, J.; Beattie, J.A.; Fuentes, P.; Rizvi, H.; Egger, J.V.; Kern, J.A.; Leung, D.Y.M.; Lacouture, M.E.; Kris, M.G.; Gambarin, M.; et al. Beyond Steroids: Immunosuppressants in Steroid-Refractory or Resistant Immune-Related Adverse Events. J. Thorac. Oncol. 2021, 16, 1759–1764. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, K.; Buhlaiga, N.; Thebault, P.; Lapointe, R.; Johnson, N.A.; Miller, W.H., Jr. Alemtuzumab for Immune-Related Myocarditis Due to PD-1 Therapy. N. Engl. J. Med. 2019, 380, 2375–2376. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tian, R.; Gao, P.; Wang, Q.; Zhang, L. Tocilizumab for Fulminant Programmed Death 1 Inhibitor-Associated Myocarditis. J. Thorac. Oncol. 2020, 15, e31–e32. [Google Scholar] [CrossRef] [PubMed]
- Doms, J.; Prior, J.O.; Peters, S.; Obeid, M. Tocilizumab for refractory severe immune checkpoint inhibitor-associated myocarditis. Ann. Oncol. 2020, 31, 1273–1275. [Google Scholar] [CrossRef] [PubMed]
- Ingelfinger, J.R.; Schwartz, R.S. Immunosuppression--the promise of specificity. N. Engl. J. Med. 2005, 353, 836–839. [Google Scholar] [CrossRef]
- Wei, S.C.; Meijers, W.C.; Axelrod, M.L.; Anang, N.A.S.; Screever, E.M.; Wescott, E.C.; Johnson, D.B.; Whitley, E.; Lehmann, L.; Courand, P.Y.; et al. A Genetic Mouse Model Recapitulates Immune Checkpoint Inhibitor-Associated Myocarditis and Supports a Mechanism-Based Therapeutic Intervention. Cancer Discov. 2021, 11, 614–625. [Google Scholar] [CrossRef]
- Liu, X.; Wu, W.; Fang, L.; Liu, Y.; Chen, W. TNF-α Inhibitors and Other Biologic Agents for the Treatment of Immune Checkpoint Inhibitor-Induced Myocarditis. Front. Immunol. 2022, 13, 922782. [Google Scholar] [CrossRef]
- Salem, J.E.; Allenbach, Y.; Vozy, A.; Brechot, N.; Johnson, D.B.; Moslehi, J.J.; Kerneis, M. Abatacept for Severe Immune Checkpoint Inhibitor-Associated Myocarditis. New Engl. J. Med. 2019, 380, 2377–2379. [Google Scholar] [CrossRef]
- Nguyen, L.S.; Bretagne, M.; Arrondeau, J.; Zahr, N.; Ederhy, S.; Abbar, B.; Pinna, B.; Allenbach, Y.; Mira, J.P.; Moslehi, J.; et al. Reversal of immune-checkpoint inhibitor fulminant myocarditis using personalized-dose-adjusted abatacept and ruxolitinib: Proof of concept. J. Immunother. Cancer 2022, 10, e004699. [Google Scholar] [CrossRef]
- Jespersen, M.S.; Fano, S.; Stenor, C.; Moller, A.K. A case report of immune checkpoint inhibitor-related steroid-refractory myocarditis and myasthenia gravis-like myositis treated with abatacept and mycophenolate mofetil. Eur. Heart J. Case Rep. 2021, 5, ytab342. [Google Scholar] [CrossRef]
- Cautela, J.; Zeriouh, S.; Gaubert, M.; Bonello, L.; Laine, M.; Peyrol, M.; Paganelli, F.; Lalevee, N.; Barlesi, F.; Thuny, F. Intensified immunosuppressive therapy in patients with immune checkpoint inhibitor-induced myocarditis. J. Immunother. Cancer 2020, 8, e001887. [Google Scholar] [CrossRef] [PubMed]
- Medina de Chazal, H.; Del Buono, M.G.; Keyser-Marcus, L.; Ma, L.; Moeller, F.G.; Berrocal, D.; Abbate, A. Stress Cardiomyopathy Diagnosis and Treatment: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 1955–1971. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Brugada, J.; Katritsis, D.G.; Arbelo, E.; Arribas, F.; Bax, J.J.; Blomström-Lundqvist, C.; Calkins, H.; Corrado, D.; Deftereos, S.G.; Diller, G.P.; et al. 2019 ESC Guidelines for the management of patients with supraventricular tachycardiaThe Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 655–720. [Google Scholar] [CrossRef]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [CrossRef] [PubMed]
- Pollack, M.H.; Betof, A.; Dearden, H.; Rapazzo, K.; Valentine, I.; Brohl, A.S.; Ancell, K.K.; Long, G.V.; Menzies, A.M.; Eroglu, Z.; et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann. Oncol. 2018, 29, 250–255. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, J.; Xu, L.; Yang, H.; Liang, N.; Zhang, L.; Zhang, F.; Zhang, X. Safety and Efficacy of the Rechallenge of Immune Checkpoint Inhibitors after Immune-Related Adverse Events in Patients with Cancer: A Systemic Review and Meta-Analysis. Front. Immunol. 2021, 12, 730320. [Google Scholar] [CrossRef]
- Totzeck, M.; Schuler, M.; Stuschke, M.; Heusch, G.; Rassaf, T. Cardio-oncology—Strategies for management of cancer-therapy related cardiovascular disease. Int. J. Cardiol. 2019, 280, 163–175. [Google Scholar] [CrossRef]
- Poto, R.; Marone, G.; Pirozzi, F.; Galdiero, M.R.; Cuomo, A.; Formisano, L.; Bianco, R.; Della Corte, C.M.; Morgillo, F.; Napolitano, S.; et al. How can we manage the cardiac toxicity of immune checkpoint inhibitors? Expert Opin. Drug Saf. 2021, 20, 685–694. [Google Scholar] [CrossRef]
- Zito, C.; Manganaro, R.; Cusma Piccione, M.; Madonna, R.; Monte, I.; Novo, G.; Mercurio, V.; Longobardo, L.; Cadeddu Dessalvi, C.; Deidda, M.; et al. Anthracyclines and regional myocardial damage in breast cancer patients. A multicentre study from the Working Group on Drug Cardiotoxicity and Cardioprotection, Italian Society of Cardiology (SIC). Eur. Heart J. Cardiovasc. Imaging 2021, 22, 406–415. [Google Scholar] [CrossRef]
- Celutkiene, J.; Pudil, R.; Lopez-Fernandez, T.; Grapsa, J.; Nihoyannopoulos, P.; Bergler-Klein, J.; Cohen-Solal, A.; Farmakis, D.; Tocchetti, C.G.; von Haehling, S.; et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: A position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2020, 22, 1504–1524. [Google Scholar] [CrossRef] [PubMed]
- Pudil, R.; Mueller, C.; Celutkiene, J.; Henriksen, P.A.; Lenihan, D.; Dent, S.; Barac, A.; Stanway, S.; Moslehi, J.; Suter, T.M.; et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: A position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1966–1983. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Moslehi, J.J.; Bonaca, M.; Schmidinger, M.; Albiges, L.; Choueiri, T.K.; Motzer, R.J.; Atkins, M.B.; Haanen, J.; Mariani, M.; et al. Prospective Cardiovascular Surveillance of Immune Checkpoint Inhibitor-Based Combination Therapy in Patients with Advanced Renal Cell Cancer: Data From the Phase III JAVELIN Renal 101 Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Drobni, Z.D.; Alvi, R.M.; Taron, J.; Zafar, A.; Murphy, S.P.; Rambarat, P.K.; Mosarla, R.C.; Lee, C.; Zlotoff, D.A.; Raghu, V.K.; et al. Association Between Immune Checkpoint Inhibitors with Cardiovascular Events and Atherosclerotic Plaque. Circulation 2020, 142, 2299–2311. [Google Scholar] [CrossRef] [PubMed]
- Lutgens, E.; Seijkens, T.T.P. Cancer patients receiving immune checkpoint inhibitor therapy are at an increased risk for atherosclerotic cardiovascular disease. J. Immunother. Cancer 2020, 8, e000300. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, J.J.; Salem, J.E.; Sosman, J.A.; Lebrun-Vignes, B.; Johnson, D.B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018, 391, 933. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Q.; Chen, L.; Qian, K.; Xu, X. Severe immune-related hepatitis and myocarditis caused by PD-1 inhibitors in the treatment of triple-negative breast cancer: A case report. Ann. Transl. Med. 2022, 10, 424. [Google Scholar] [CrossRef]
- Valenti-Azcarate, R.; Esparragosa Vazquez, I.; Toledano Illan, C.; Idoate Gastearena, M.A.; Gallego Perez-Larraya, J. Nivolumab and Ipilimumab-induced myositis and myocarditis mimicking a myasthenia gravis presentation. Neuromuscul. Disord. 2020, 30, 67–69. [Google Scholar] [CrossRef]
- Delgado-Lazo, V.; Abdelmottaleb, W.; Popescu-Martinez, A. Pembrolizumab-Induced Myocarditis and Pancreatitis in a Patient with Colon Cancer: A Case Report. Cureus 2022, 14, e26034. [Google Scholar] [CrossRef]
- Andres, M.S.; Pan, J.; Lyon, A.R. What Does a Cardio-oncology Service Offer to the Oncologist and the Haematologist? Clin. Oncol. 2021, 33, 483–493. [Google Scholar] [CrossRef]
- Pareek, N.; Cevallos, J.; Moliner, P.; Shah, M.; Tan, L.L.; Chambers, V.; Baksi, A.J.; Khattar, R.S.; Sharma, R.; Rosen, S.D.; et al. Activity and outcomes of a cardio-oncology service in the United Kingdom-a five-year experience. Eur. J. Heart Fail. 2018, 20, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Cardona, J.A.; Ray, J.; Carver, J.; Zaha, V.; Cheng, R.; Yang, E.; Mitchell, J.D.; Stockerl-Goldstein, K.; Kondapalli, L.; Dent, S.; et al. Cardio-Oncology Education and Training: JACC Council Perspectives. J. Am. Coll. Cardiol. 2020, 76, 2267–2281. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.F.; Ky, B. Roadmap for biomarkers of cancer therapy cardiotoxicity. Heart 2016, 102, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Petricciuolo, S.; Delle Donne, M.G.; Aimo, A.; Chella, A.; De Caterina, R. Pre-treatment high-sensitivity troponin T for the short-term prediction of cardiac outcomes in patients on immune checkpoint inhibitors. Eur. J. Clin. Investig. 2021, 51, e13400. [Google Scholar] [CrossRef]
- Waliany, S.; Neal, J.W.; Reddy, S.; Wakelee, H.; Shah, S.A.; Srinivas, S.; Padda, S.K.; Fan, A.C.; Colevas, A.D.; Wu, S.M.; et al. Myocarditis Surveillance with High-Sensitivity Troponin I During Cancer Treatment with Immune Checkpoint Inhibitors. JACC CardioOncol. 2021, 3, 137–139. [Google Scholar] [CrossRef]
- Marschner, D.; Falk, M.; Javorniczky, N.R.; Hanke-Muller, K.; Rawluk, J.; Schmitt-Graeff, A.; Simonetta, F.; Haring, E.; Dicks, S.; Ku, M.; et al. MicroRNA-146a regulates immune-related adverse events caused by immune checkpoint inhibitors. JCI Insight 2020, 5, e132334. [Google Scholar] [CrossRef]
- Xia, W.; Chen, H.; Chen, D.; Ye, Y.; Xie, C.; Hou, M. PD-1 inhibitor inducing exosomal miR-34a-5p expression mediates the cross talk between cardiomyocyte and macrophage in immune checkpoint inhibitor-related cardiac dysfunction. J. Immunother. Cancer 2020, 8, e001293. [Google Scholar] [CrossRef]
- Lim, E.A.; Drake, C.G.; Mintz, A. Molecular imaging for cancer immunotherapy. Immunooncol. Technol. 2020, 5, 10–21. [Google Scholar] [CrossRef]
- Cadour, F.; Cautela, J.; Rapacchi, S.; Varoquaux, A.; Habert, P.; Arnaud, F.; Jacquier, A.; Meilhac, A.; Paganelli, F.; Lalevee, N.; et al. Cardiac MRI Features and Prognostic Value in Immune Checkpoint Inhibitor-induced Myocarditis. Radiology 2022, 303, 512–521. [Google Scholar] [CrossRef]
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 155. [Google Scholar] [CrossRef]
|
| Grade | Presentation |
|---|---|
| 1 | Asymptomatic, abnormal cardiac biomarker levels, no ECG abnormalities |
| 2 | Abnormal cardiac biomarker levels with mild symptoms or new ECG abnormalities without conduction delay |
| 3 | Abnormal cardiac biomarker levels with either moderate symptoms or new conduction delay |
| 4 | Moderate to severe decompensation requiring IV medication or other intervention, or life-threatening conditions |
| Clinical Presentation | Diagnosis | Treatment [18,25] | |
|---|---|---|---|
| Myocarditis | - Shortness of breath - Chest pain - Pulmonary edema - Cardiogenic shock | - Troponin, NT-proBNP - ECG - Echocardiography - CMR imaging | - Discontinue ICI - Immunosuppressive therapy (Methylprednisolone i.v. 500–1000 mg/day for 3–5 days, then switch to oral prednisone 1 mg/kg/day). If no response: consider second-line immunosuppression - Consider therapy for heart failure |
| Pericardial disease | - Shortness of breath - Chest pain - Cardiogenic shock (in cardiac tamponade) | - ECG - Echocardiography - CMR imaging (to evaluate concomitant myocarditis) | - Withhold ICI therapy - Immunosuppressive therapy (1 mg prednisone/kg/day)) - Consider NSAID and colchicine - Pericardiocentesis if indicated - Consider ICI rechallenge after recovery |
| Takotsubo syndrome | - Chest pain - Shortness of breath - Palpitation - Pulmonary edema - Cardiogenic shock | -Troponin, NT-proBNP - ECG - Echocardiography - CMR imaging - Exclusion of ACS according to ESC and AHA guidelines | - Withhold ICI therapy - No clear evidence on immunosuppressive therapy - Follow management algorithm of Heart Failure Association position statement - Avoid QT-prolonging drugs |
| Acute coronary syndrome | - Chest pain - Shortness of breath - Cardiogenic shock | - Troponin, NT-proBNP - ECG - Echocardiography - Diagnostic algorithm according to ESC and AHA guidelines | - Withhold ICI therapy - No clear evidence on immunosuppressive therapy - Treatment according to ESC and AHA guidelines - Consider ICI therapy rechallenge after > 30 days in stable patients |
| Grade | Pathological Features |
|---|---|
| 0 | Negative |
| 1—Myocardial inflammation | Multifocal inflammatory infiltrates without overt cardiomyocytes loss by light microscopy |
| 1A | Mild inflammatory cell score by immunohistochemistry (10–20 inflammatory cells/high power field) |
| 1B | At least moderate inflammatory cell score by immunohistochemistry (>20 inflammatory cells/high power field) |
| 2—Definite myocarditis | Multifocal inflammatory cell infiltrates (>40 inflammatory cells/high power field) |
| Palaskas et al. Grading Criteria [46] | |
| Grade | Immunoistochemistry |
| Low Grade | 50 CD3+ cells/high power field |
| High Grade | >50 CD3+ cells/high power field |
| Champion and Stone Grading Criteria [47] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zito, C.; Manganaro, R.; Ciappina, G.; Spagnolo, C.C.; Racanelli, V.; Santarpia, M.; Silvestris, N.; Carerj, S. Cardiotoxicity Induced by Immune Checkpoint Inhibitors: What a Cardio-Oncology Team Should Know and Do. Cancers 2022, 14, 5403. https://doi.org/10.3390/cancers14215403
Zito C, Manganaro R, Ciappina G, Spagnolo CC, Racanelli V, Santarpia M, Silvestris N, Carerj S. Cardiotoxicity Induced by Immune Checkpoint Inhibitors: What a Cardio-Oncology Team Should Know and Do. Cancers. 2022; 14(21):5403. https://doi.org/10.3390/cancers14215403
Chicago/Turabian StyleZito, Concetta, Roberta Manganaro, Giuliana Ciappina, Calogera Claudia Spagnolo, Vito Racanelli, Mariacarmela Santarpia, Nicola Silvestris, and Scipione Carerj. 2022. "Cardiotoxicity Induced by Immune Checkpoint Inhibitors: What a Cardio-Oncology Team Should Know and Do" Cancers 14, no. 21: 5403. https://doi.org/10.3390/cancers14215403
APA StyleZito, C., Manganaro, R., Ciappina, G., Spagnolo, C. C., Racanelli, V., Santarpia, M., Silvestris, N., & Carerj, S. (2022). Cardiotoxicity Induced by Immune Checkpoint Inhibitors: What a Cardio-Oncology Team Should Know and Do. Cancers, 14(21), 5403. https://doi.org/10.3390/cancers14215403








