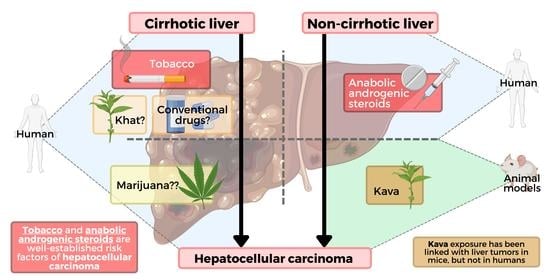
Recreational Drugs and the Risk of Hepatocellular Carcinoma
Simple Summary
Abstract
1. Introduction
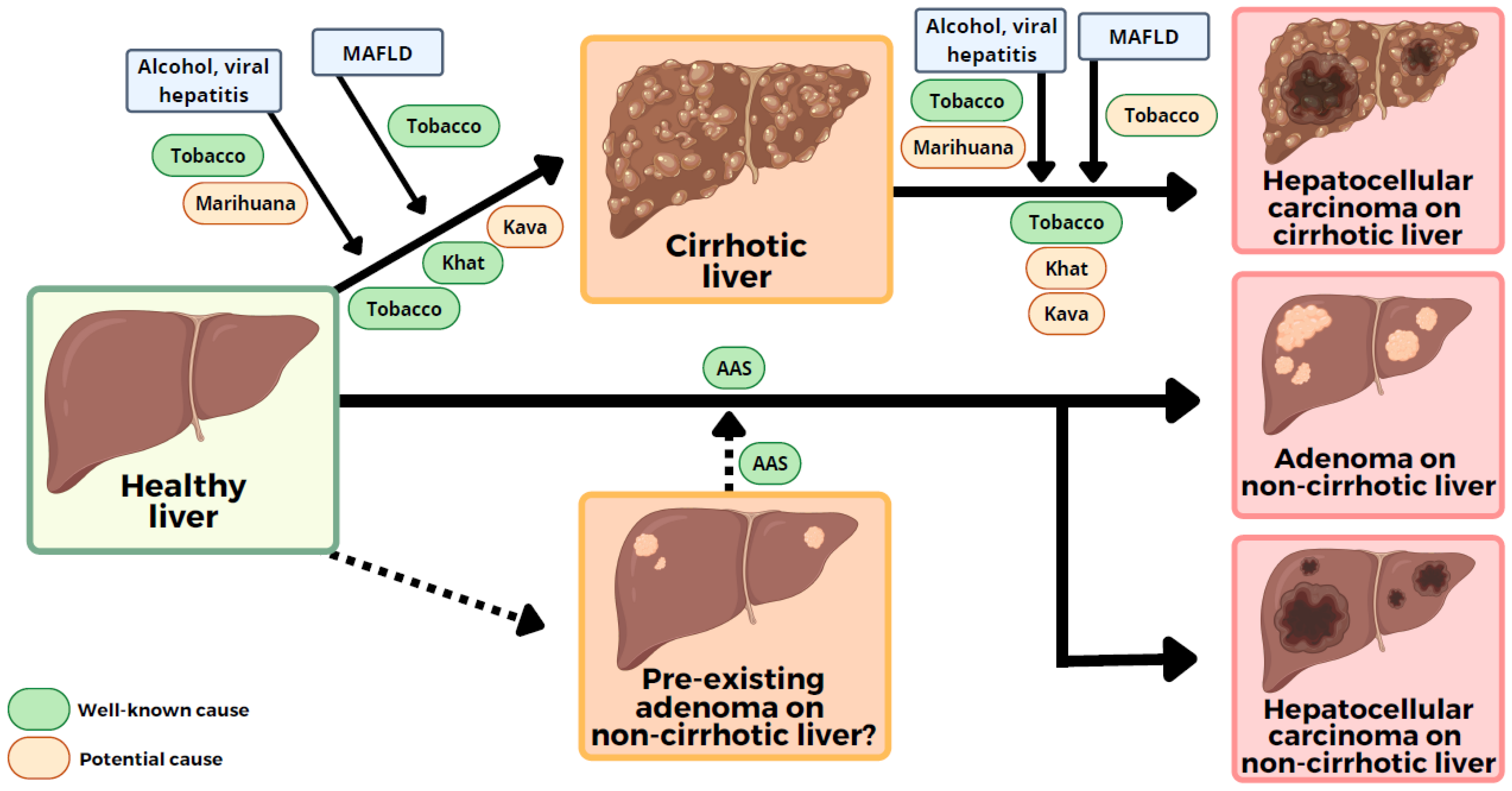
2. Conventional Drugs and Hepatocellular Carcinoma (HCC)
3. Herbal and HCC
3.1. Khat and HCC
3.2. Kava and HCC
3.3. Other Herbs and HCC
4. Cigarette Smoking and HCC
5. Cannabis and HCC
6. Other Illicit Drugs and HCC
7. Anabolic Androgenic Steroids and HCC
8. Conclusions and Future Prospects
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, M.H.; Afshin, A.; Alexaner, L.T.; Anderson, H.R.; Bhutta, Z.A.; Biryukov, S.; Brauer, M.; Burnett, R.; Cercy, K.; Charlson, F.J.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks of clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 3388, 1659–1724. [Google Scholar] [CrossRef]
- White, D.L.; Thrift AP: Kanwal, F.; Davila, J.; El-Serag, H.B. Incidence of Hepatocellular Carcinoma in All 50 United States, From 2000 Through 2012. Gastroenterology 2017, 152, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 223–238. [Google Scholar] [CrossRef]
- Sangiovanni, A.; Prati, G.M.; Fasani, P.; Ronchi, P.; Romeo, R.; Manini, M.; Del Nino, E.; Morabito, A.; Colombo, M. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology 2006, 43, 1303–1310. [Google Scholar] [CrossRef]
- Björnsson, E.S.; Andrade, R.J. Long-term sequelae of drug induced liver injury. J. Hepatol. 2022, 76, 435–445. [Google Scholar] [CrossRef]
- Degenhardt, L.; Whiteford, H.A.; Ferrari, A.J.; Baxter, A.J.; Charlson, F.J.; Hall, W.D.; Freedman, G.; Burstein, R.; Johns, N.; Engell, R.E.; et al. Global burden of disease attributable to illicit drug use and dependence: Findings from the Global Buden of Disease Study 2010. Lancet 2013, 382, 1564–1574. [Google Scholar] [CrossRef]
- Machii, R.; Saika, K. Mortality attributable to tobacco by region based on the WHO Global Report. Jpn. J. Clin. Oncol. 2012, 42, 464–465. [Google Scholar] [CrossRef]
- Degenhardt, L.; Stockings, E.; Patton, G.; Hall, W.D.; Lynskey, M. The increasing global health priority of substance use in young people. Lancet Psychiatr. 2016, 3, 251–264. [Google Scholar] [CrossRef]
- Guvendeger, D.N.; Zahmacioglu, O.; Ciftci, D.A.; Kocaman, G.M.; Erdogan, A. Association of suicide attempts and non-suicidal self-injury behaviors with substance use and family characteristics among children and adolescents seeking treatment for substance use disorder. Subst. Use Misuse 2017, 52, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Reimer, J.; Lorenzen, J.; Baetz, B.; Fischer, B.; Rehm, J.; Haasen, C.; Backmund, M. Multiple viral hepatitis in injection drug users and associated risk factors. J. Gastroenterol. Hepatol. 2007, 22, 80–85. [Google Scholar] [CrossRef]
- Brennan, R.; Wells, J.S.G.; Van Hout, M.C. The injecting use of image and performance-enhancing drugs (IPED) in the general population: A systematic review. Health Soc. Care Community 2017, 25, 1459–1531. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, N.T. A review of the chemistry, biological action, and clinical applications of anabolic-androgenic steroids. Clin. Ther. 2001, 23, 1355–1390. [Google Scholar] [CrossRef]
- Mullen, C.; Whalley, B.J.; Schifano, F.; Baker, J.S. Anabolic androgenic steroid abuse in the United Kingdom: An update. Br. J. Pharmacol. 2020, 177, 2180–2198. [Google Scholar] [CrossRef] [PubMed]
- Ip, E.J.; Barnett, M.J.; Tenerowicz, M.J.; Perry, P.J. The Anabolic 500 survey: Characteristics of male users versus nonusers of anabolic-androgenic steroids for strength training. Pharmacotherapy 2011, 31, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Medina-Caliz, I.; Robles-Díaz, M.; García-Muñoz, B.; Stephens, C.; Ortega-Alonso, A.; García-Cortés, M.; González-Jiménez, A.; Sanabria-Cabrera, J.; Moreno, I.; Fernández, M.C.; et al. Definition and risk factors for chronicity following acute idiosyncratic drug-induced liver injury. J. Hepatol. 2016, 65, 532–542. [Google Scholar] [CrossRef]
- Pineda, J.A.; Larrauri, J.; Macias, J.; Hernández, A.; Guijarro, J.; Sayago, M.; Gavilán, F.; Aguilar, J.; Lissen, E. Rapid progression to liver cirrhosis of toxic hepatitis due to ebrotidine. J. Hepatol. 1999, 31, 777–778. [Google Scholar] [CrossRef]
- Lewis, J.H.; Ranard, R.C.; Caruso, A.; Jackson, L.K.; Mullick, F.; Ishak, K.G.; Seeff, L.B.; Zimmerman, H.R. Amiodarone hepatotoxicity: Prevalence and clinicopathologic correlations among 104 patients. Hepatology 1989, 9, 679–685. [Google Scholar] [CrossRef]
- Aithal, G. Hepatotoxicity related to methotrexate. In Drug-Induced Liver Disease, 3rd ed.; Kaplowitz, N., Deleve, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 593–604. [Google Scholar]
- Dawwas, M.F.; Aithal, G.P. End-stage methotrexate-related liver disease is a rare and associated with features of metabolic syndrome. Aliment. Pharmacol. Ther. 2014, 40, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, E.S.; Hoofnagle, J.H. Categorization of drugs implicated in causing liver injury: Critical assessment based upon published case reports. Hepatology 2016, 63, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, E.S.; Bergmann, O.M.; Bjornsson, H.K.; Kvaran, R.B.; Olafsson, S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013, 144, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Lan, S.H.; Wu, S.R.; Chiu, Y.C.; Lin, X.Z.; Su, I.J.; Tsai, T.F.; Yen, C.J.; Lu, T.H.; Liang, F.W.; et al. Hepatocellular carcinoma-related cyclin D1 is selectively regulated by autophagy degradation system. Hepatology 2018, 68, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.T.; Hsu, W.F.; Huang, H.S.; Yen, J.H.; Lin, M.C.; Peng, C.Y.; Yen, H.R. Improved Survival in Hepatocellular Carcinoma Patients with Cardiac Arrhythmia by Amiodarone Treatment through Autophagy. Int. J. Mol. Sci. 2019, 20, 3978. [Google Scholar] [CrossRef]
- Coton, T.; Simon, F.; Oliver, M.; Kraemer, P. Hepatotoxicity of khat chewing. Liver Int. 2011, 31, 434. [Google Scholar] [CrossRef]
- Patel, H.; Kumar, K.; Essrani, R.K.; Niazi, M.; Makker, J.; Nayudu, S.K. Acute Hepatitis in a Yemeni Immigrant Associated with Khat: A “Biological Amphetamine” Carried in Cultures. Clin. Pract. 2021, 11, 167–173. [Google Scholar] [CrossRef]
- Riyaz, S.; Imran, M.; Gleeson, D.; Karajeh, M.A. Khat (Catha Edulis) as a possible cause of autoimmune hepatitis. World J. Hepatol. 2014, 6, 150–154. [Google Scholar] [CrossRef]
- Alhaddad, O.M.; Elsabaawy, M.M.; Rewisha, E.A.; Salman, T.A.; Kohla, M.A.; Ehsan, N.A.; Waked, I.A. Khat-induced liver injuries: A report of two cases. Arab. J. Gastroenterol. 2016, 17, 45–48. [Google Scholar] [CrossRef]
- Peevers, C.G.; Moorghen, M.; Collins, P.L.; Gordon, F.H.; McCune, C.A. Liver disease and cirrhosis because of Khat chewing in UK Somali men: A case series. Liver Int. 2010, 30, 1242–1243. [Google Scholar] [CrossRef]
- Stuyt, R.J.; Willems, S.M.; Wagtmans, M.J.; Van Hoek, B. Chewing khat and chronic liver disease. Liver Int. 2011, 31, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Orlien, S.M.S.; Ismael, N.Y.; Ahmed, T.A.; Berhe, N.; Lauritzen, T.; Roald, B.; Goldin, R.D.; Sten-Johansen, K.; Dyrhol-Riise, A.M.; Gundersen, S.G.; et al. Unexplained chronic liver disease in Ethiopia: A cross-sectional study. BMC Gastroenterol. 2018, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Mahamoud, H.D.; Muse, S.M.; Roberts, L.R.; Fischer, P.R.; Torbenson, M.S.; Fader, T. Khat chewing and cirrhosis in Somaliland: Case series. Afr. J. Prim. Health Care Fam. Med. 2016, 8, e1–e4. [Google Scholar] [CrossRef]
- Jenkins, M.G.; Handslip, R.; Kumar, M.; Mahadeva, U.; Lucas, S.; Yamamoto, T.; Wood, D.M.; Wong, T.; Dargan, P.I. Reversible khat-induced hepatitis: Two case reports and review of the literature. Frontline Gastroenterol. 2013, 4, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.H.; Kajihara, M.; Borges, G.; O´Beirne, J.; Patch, D.; Dhillon, A.P.; Crozier, A.; Morgan, M.Y. Severe, acute liver injury and khat leaves. N. Engl. J. Med. 2010, 362, 1642–1644. [Google Scholar] [CrossRef] [PubMed]
- Forbes, M.P.; Raj, A.S.; Martin, J.; Lampe, G.; Powell, E.E. Khat-associated hepatitis. Med. J. Aust. 2013, 199, 498–499. [Google Scholar] [CrossRef]
- Teisen, E.; Vainer, B.; Ytting, H. Hepatitis after chewing of khat leaves. Ugeskr. Laeger. 2016, 178, V02160124. [Google Scholar]
- Teshke, R. Kava hepatotoxicity. A clinical review. Ann. Hepatol. 2010, 9, 251–265. [Google Scholar] [CrossRef]
- Behl, M.; Nyska, A.; Chhabra, R.S.; Travlos, G.S.; Fomby, L.M.; Sparrow, B.R.; Hejtmanicik, M.R.; Chan, P.C. Liver toxicity and carcinogenicity in F344/N rats and B6C3F1 mice exposed to Kava Kava. Food Chem. Toxicol. 2011, 49, 2820–2829. [Google Scholar] [CrossRef]
- National Toxicology Program. Toxicology and carcinogenesis studies of kava kava extract (CAS No. 9000-38-8) in F344/N rats and B6C3F1 mice (Gavage Studies). Natl. Toxicol. Progr. Tech. Rep. Ser. 2012, 571, 1–186. [Google Scholar]
- Strahl, S.; Ehret, V.; Dahm, H.H.; Maier, K.P. Necrotising hepatitis after taking herbal remedies. Dtsch. Med. Wschr. 1998, 123, 1410–1414. [Google Scholar] [CrossRef] [PubMed]
- Escher, M.; Desmeules, J.; Giostra, E.; Mentha, G. Hepatitis associated with kava, a herbal remedy for anxiety. Br. Med. J. 2001, 322, 139. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.; Spahn, T.W.; Menzel, J.; Senninger, N.; Dietl, K.H.; Herbst, H.; Domschke, W.; Lerch, M.M. Fulminant liver failure after administration of the herbal antidepressant Kava-Kava. Dtsch. Med. Wschr. 2001, 126, 970–972. [Google Scholar] [CrossRef] [PubMed]
- Russmann, S.; Lauterburg, B.H.; Helbling, A. Kava hepatotoxicity. Ann. Intern. Med. 2001, 135, 68–69. [Google Scholar] [CrossRef]
- Bujanda, L.; Palacios, A.; Silvariño, R.; Sánchez, A.; Muñoz, C. Hepatitis aguda icterica secundaria a kava. Gastroenterol. Hepatol. 2002, 25, 434–435. [Google Scholar] [CrossRef]
- Denham, A.; McIntyre, M.; Whitehouse, J. Kava—The unfolding story: Report on a work-in-progress. J. Altern. Complement. Med. 2002, 8, 237–263. [Google Scholar] [CrossRef]
- Gow, P.J.; Connelly, N.J.; Hill, R.L.; Crowley, P.; Angus, P.W. Fatal fulminant hepatic failure induced by a natural therapy containing kava. Med. J. Aust. 2003, 178, 442–443. [Google Scholar] [CrossRef]
- Humberston, C.L.; Akhtar, J.; Krenzelok, E.P. Acute hepatitis induced by kava kava. J. Toxicol./Clin. Toxicol. 2003, 41, 109–113. [Google Scholar] [CrossRef]
- Russmann, S.; Barguil, Y.; Cabalion, P.; Kritsanida, M.; Duhet, D.; Lauterburg, B.H. Hepatic injury due to traditional aqueous extracts of kava root in New Caledonia. Eur. J. Gastroenterol. Hepatol. 2003, 15, 1033–1036. [Google Scholar] [CrossRef]
- Stickel, F.; Baumu¨ller, H.M.; Seitz, K.; Vasilakis, D.; Seitz, G.; Seitz, H.K.; Schuppan, D. Hepatitis induced by kava (Piper methysticum rhizoma). J. Hepatol. 2003, 39, 62–67. [Google Scholar] [CrossRef]
- Teschke, R.; Schwarzenboeck, A.; Hennermann, K.H. Kava hepatotoxicity: A clinical survey and critical analysis of 26 suspected cases. Eur. J. Gastroenterol. Hepatol. 2008, 20, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Christl, S.U.; Seifert, A.; Seeler, D. Toxic hepatitis after consumption of traditional kava preparation. Int. Soc. Travel Med. 2009, 16, 55–56. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.; Gasca, J.; Parana, R.; Nunes, V.; Schinnoni, M.; Medina-Caliz, I.; Cabello, M.R.; Lucena, M.I.; Andrade, R.J. Profile of herbal and dietary supplements induced liver injury in Latin America: A systematic review of published reports. Phytother. Res. 2021, 35, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Zhuge, Y.; Liu, Y.; Xie, W. Chinese Society of Gastroenterology Committee of Hepatobiliary Disease. Expert consensus on the clinical management of pyrrolizidine alkaloid-induced hepatic sinusoidal obstruction syndrome. J. Gastroenterol. Hepatol. 2019, 34, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Marti-Aguado, D.; Clemente-Sanchez, A.; Bataller, R. Cigarrette smoking and liver diseases. J. Hepatol. 2022, 77, 191–205. [Google Scholar] [CrossRef]
- Lee, Y.C.; Cohet, C.; Yang, Y.C.; Stayner, L.; Hashibe, M.; Straif, K. Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. Int. J. Epidemiol. 2009, 38, 1497–1511. [Google Scholar] [CrossRef]
- Abdel-Rahman, O.; Helbling, D.; Schöb, O.; Eltogby, M.; Mohamed, H.; Schimdt, J.; Giryes, A.; Mehrabi, A.; Iype, S.; John, H.; et al. Cigarette smoking as a risk factor for the development of and mortality from hepatocellular carcinoma: An updated systematic review of 81 epidemiological studies. J. Evid. Based. Med. 2017, 10, 245–254. [Google Scholar] [CrossRef]
- Petrick, J.L.; Campbell, P.T.; Koshiol, J.; Thistle, J.E.; Andreotti, G.; Beane-Freeman, L.E.; Buring, J.E.; Chan, A.T.; Chong, D.Q.; Doody, M.M.; et al. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: The Liver Cancer Pooling Project. Br. J. Cancer 2018, 118, 1005–1021. [Google Scholar] [CrossRef]
- Hézode, C.; Roudot-Thoraval, F.; Nguyen, S.; Grenard, P.; Julien, B.; Zafrani, E.S.; Pawlotsky, J.M.; Dhumeaux, D.; Lotersztajn, S.; Mallat, A. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic C hepatitis. Hepatology 2005, 42, 63–71. [Google Scholar] [CrossRef]
- Brunet, L.; Moodie, E.E.; Rollet, K.; Cooper, C.; Walmsley, S.; Potter, M.; Klein, M.B. Marijuana smoking dose not accelerate progression of liver disease in HIV-hepatitis C coinfection: A longitudinal cohort analysis. Clin. Infect. Dis. 2013, 57, 663–670. [Google Scholar] [CrossRef]
- Kelly, E.M.; Dodge, J.L.; Sarkar, M.; French, A.L.; Tien, P.C.; Glesby, M.J.; Golub, E.T.; Augenbraun, M.; Plankey, M.; Peters, M.G. Marijuana use is not associated with progression to advanced liver fibrosis in HIV/Hepatitis C virus-coinfected women. Clin. Infect. Dis. 2016, 63, 512–518. [Google Scholar] [CrossRef]
- Farooqui, M.T.; Khan, M.A.; Cholankeril, G.; Khan, Z.; Abdul, M.K.M.; Li, A.A.; Shah, N.; Haq, K.; Solanki, S.; Kim, D.; et al. Marijuana is not associated with progression of hepatic fibrosis in liver disease: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2019, 31, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, M.S.; Hunter, R.L.; Yachnin, S. Hepatoma and peliosis hepatis developing in a patient with Fanconi´s anemia. N. Engl. J. Med. 1971, 284, 1135–1136. [Google Scholar] [CrossRef] [PubMed]
- Johnson, F.L.; Lerer, K.G.; Siegel, M.; Feagler, J.R.; Majerus, P.W.; Hartmann, J.R.; Thomas, E.D. Assiciation of androgenic-anabolic steroid therapy with development of hepatocellular carcinoma. Lancet 1972, 2, 1273–1276. [Google Scholar] [CrossRef]
- Henderson, J.T.; Richmond, J.; Sumerling, M.D. Androgenic-anabolic steroid therapy and hepatocellular carcinoma. Lancet 1973, 1, 934. [Google Scholar] [CrossRef]
- Farrell, G.C.; Joshua, D.E.; Uren, R.F.; Baird, P.J.; Perkins, K.W.; Kronenberg, H. Androgen-induced hepatoma. Lancet 1975, 1, 430–432. [Google Scholar] [CrossRef]
- Hernandez-Nieto, L.; Bruguera, M.; Bombi, J.; Camacho, L.; Rozman, C. Benign liver-cell adenoma associated with long-term administration of an androgenic-anabolic steroid (methandienone). Cancer 1977, 40, 1761–1764. [Google Scholar] [CrossRef]
- Shapiro, P.; Ikeda, R.M.; Ruebnder, B.H.; Connors, M.H.; Halsted, C.C.; Abildgraad, C.F. Multiple hepatic tumors and peliosis hepatis in Fanconi´s anemia treated with androgens. Am. J. Dis. Child. 1977, 131, 1104–1106. [Google Scholar]
- Lopez, R.; Cantu, J.G. Androgenic therapy and hepatocellular carcinoma. Rev. Gastroenterol. Mex. 1979, 44, 35–40. [Google Scholar]
- Carrasco, D.; Prieto, M.; Pallardó, L.; Moll, J.L.; Cruz, J.M.; Muñoz, C.; Berenguer, J. Multiple hepatic adenomas after long-term therapy with testosterone enanthate. Review of the literature. J. Hepatol. 1985, 1, 573–578. [Google Scholar] [CrossRef]
- Linares, M.; Pastor, E.; Gomez, A.; Grau, E. Hepatocellular carcinoma and squamous cell carcinoma in a patient with Fanconi´s anemia. Ann. Hematol. 1991, 63, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Bork, K.; Pitton, M.; Harten, P.; Koch, P. Hepatocellular adenomas in patients taking danazol for hereditary angio-oedema. Lancet 1999, 353, 1066–1067. [Google Scholar] [CrossRef]
- Socas, L.; Zumbado, M.; Pérez-Luzardo, O.; Ramos, A.; Pérez, C.; Hernández, J.R.; Boada, L.D. Hepatocellular adenomas associated with anabolic androgenic steroid abuse in bodybulders: A report of two cases and a review of literature. Br. J. Sports Med. 2005, 39, e27. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.M.; Abu Dayyeh, B.K.; Chung, R.T. Anabolic steroid abuse causing recurrent hepatic adenomas and hemorrhage. World J. Gastroenterol. 2008, 14, 4573–4575. [Google Scholar] [CrossRef] [PubMed]
- Hardt, A.; Stippel, D.; Odenthal, M.; Hölscher, A.H.; Diene, H.P.; Drebber, U. Development of hepatocellular carcinoma associated with anabolic androgenic steroid abuse in a young bodybuilder: A case report. Case Rep. Pathol. 2012, 2012, 195607. [Google Scholar] [CrossRef]
- Pais-Costa, S.R.; Lima, O.A.; Soares, A.F. Giant hepatic adenoma associated with anabolic-androgenic steroid abuse: Case report. Arq. Bras. Cir. Dig. 2012, 25, 180–182. [Google Scholar] [CrossRef]
- Kesler, T.; Sandhu, R.S.; Krishnamoorthy, S. Hepatology: Hepatocellular carcinoma in a young man secondary to androgenic anabolic steroid abuse. J. Gastroenterol. Hepatol. 2014, 29, 1852. [Google Scholar] [CrossRef]
- Solbach, P.; Potthof, A.; Raatschen, H.J.; Soudah, B.; Lehman, U.; Schneider, A.; Gebel, M.J.; Manns, M.P.; Vogel, A. Testosterone-receptor positive hepatocellular carcinoma in a 29-year old bodybuilder with a history of anabolic androgenic steroid abuse: A case report. BMC Gastroenterol. 2015, 15, 60. [Google Scholar] [CrossRef]
- Kato, K.; Abe, H.; Hanawa, N.; Fukuzawa, J.; Matsuo, R.; Yonezawa, T.; Itoh, S.; Ika, M.; Shimizu, S.; Endo, S.; et al. Hepatocellular adenoma in a woman who was undergoing testosterone treatment for gender identify disorder. Clin. J. Gastroenterol. 2018, 11, 401–410. [Google Scholar] [CrossRef]
- Woodward, C.; Smith, J.; Acreman, D.; Kumar, N. Hepatocellular carcinoma in body builders; an emerging rare but serious complication of androgenic anabolic steroid use. Ann. Hepato-Biliary-Pancreat. Surg. 2019, 23, 174–177. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Li, W.; Meng, F.; Li, Y.; Fan, H.; Zhou, Y.; Bharathi, G.; Yang, Y. Multiple hepatocellular adenomas associatted with long-term administration of androgenic steroids for aplastic anemia. A case report and literature review. Medicine 2020, 99, e20829. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.J.; Baranski, T.; Chaterjee, D.; Chapman, W.; Foltz, G.; Kim, H. Androgen-receptor-positive hepatocellular carcinoma in a transgender teeneger taking exogenous testosterone. Lancet 2020, 396, 198. [Google Scholar] [CrossRef]
- Andrade, R.J.; Chalasani, N.; Björnsson, E.S.; Suzuki, A.; Kullak-Ublick, G.A.; Watkins, P.B.; Devarbhavi, H.; Merz, M.; Lucena, M.I.; Kaploowitz, N.; et al. Drug-induced liver injury. Nat. Rev. Dis. Primers 2019, 5, 58. [Google Scholar] [CrossRef]
- Balint, E.E.; Falkay, G.; Balint, G.A. Khat—A controversial plant. Wien. Klin. Wochenschr. 2009, 121, 604–614. [Google Scholar] [CrossRef]
- El-Menyar, A.; Mekkodathil, A.; Al-Thani, H.; Al-Motarreb, A. Khat use: History and heart failure. Oman Med. J. 2015, 30, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.B. “Natural Amphetamine” Khat: A cultural tradition or a drug of abuse? Int. Rev. Neurobiol. 2015, 120, 235–255. [Google Scholar] [PubMed]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Disease: Bethesda, MD, USA, 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548707 (accessed on 17 June 2022).
- Valente, M.J.; Araújo, A.M.; Bastos, M.L.; Fernandes, E.; Carvalho, F.; De Pinho, P.G.; Carvalho, M. Editor’s Highlight: Characterization of Hepatotoxicity Mechanisms Triggered by Designer Cathinone Drugs (β-Keto Amphetamines). Toxicol. Sci. 2016, 153, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Alkadi, H.O.; Al-Kamarany, M.; Al-Kadi, H.; Lyoussi, B.; Khalil, K.A. Khat-aspirin interaction. Yemen J. Pharm. Biol. Sci. 2008, 2, e39. [Google Scholar]
- Björnsson, E.S.; Medina-Caliz, I.; Andrade, R.J.; Lucena, M.I. Setting up criteria for drug-induced autoimmune-like hepatitis through a systematic analysis of published reports. Hepatol. Commun. 2022, 6, 1895–1909. [Google Scholar] [CrossRef]
- Sarris, J. Herbal medicines in the treatment of psychiatric disorders. 10-year updated review. Phytother. Res. 2018, 32, 1147–1162. [Google Scholar] [CrossRef]
- Pantano, F.; Tittarelli, R.; Mannocchi, G.; Zaami, S.; Ricci, S.; Giorgetti, R.; Terranova, D.; Busardo, F.P.; Marinelli, E. Hepatotoxicity Induced by “the 3Ks”: Kava, Kratom and Khat. Int. J. Mol. Sci. 2016, 17, 580. [Google Scholar] [CrossRef] [PubMed]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Disease: Bethesda, MD, USA, 2012. Available online: www.ncbi.nlm.nih.gov/books/NBK548637/ (accessed on 20 June 2022).
- Jorge, O.A.; Jorge, A.D. Hepatotoxicity associated with the ingestion of Centella asiatica. Rev. Esp. Enf. Dig. 2005, 97, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Wu, L.; Wu, Y.; Zhang, C.; Qin, L.; Hayashi, M.; Kudo, M.; Gao, M.; Liu, T. Therapeutic Potential of Centella asiatica and Its Triterpenes: A Review. Front. Pharmacol. 2020, 11, 568032. [Google Scholar] [CrossRef] [PubMed]
- Lyford, C.L.; Vergara, G.G.; Moeller, D.D. Hepatic venoocclusive disease originating in Ecuador. Gastroenterology 1976, 70, 105–108. [Google Scholar] [CrossRef]
- Sugarmori, K.S.; Brenneman, D.; Sanchez, O.; Doll, M.A.; Hein, D.W.; Pierce, W.M., Jr.; Grant, D.M. Reduced 4-aminobiphenyl-induced liver tumorigenicity but not DNA damage in arylamine N-acetyltransferase null mice. Cancer Lett. 2021, 318, 206–213. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, S.Y.; Wang, L.Y.; Tsai, W.Y.; Tsai, W.Y.; Lee, P.H.; Lee, C.S.; Ahsan, H.; Zhang, Y.J.; Chen, C.J.; Santella, R.M. Polycyclic aromatic hydrocarbon-DNA adducts in liver tissues of hepatocellular carcinoma patients and controls. Int. J. Cancer 2002, 99, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S. Microsomal ethanol-oxiding system (MEOS): The first 30 years (1968–1998)—A review. Alcohol. Clin. Exp. Res. 1999, 23, 991–1007. [Google Scholar] [PubMed]
- Marrero, J.A.; Fontana, R.J.; Fu, S.; Conjeevaram, H.S.; Su, G.L.; Lok, A.S. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J. Hepatol. 2005, 42, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Baecker, A.; Liu, X.; La Vecchia, C.; Zhang, Z.F. Worldwide incidence of hepatocellular carcinoma cases attributable to major risk factors. Eur. J. Cancer Prev. 2018, 27, 205–212. [Google Scholar] [CrossRef]
- World Drug Report 2021. In Drug Market Trends: Cannabis, Opioids; United Nations Office on Drugs and Crime: Viena, Austria, 1997; Available online: https://unodc.org/res/wdr2021/field/WDR21_Booklet_3.pdf (accessed on 20 June 2022).
- Yang, J.; Zhang, Y.; Luo, L.; Meng, R.; Yu, C. Global mortality burden of cirrhosis and liver cancer attributable to injection drug use, 1990-2016: An age-period-cohort and spatial autocorrelation analysis. Int. J. Environ. Res. Public Health 2018, 15, 170. [Google Scholar] [CrossRef]
- Kanel, G.C.; Cassidy, W.; Shuster, L.; Reynolds, T.B. Cocaine-induced liver cell injury: Comparison of morphological features in man and in experimental models. Hepatology 1990, 11, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Guollo, F.; Narciso-Schiavon, J.L.; Barotto, A.M.; Zannin, M.; Schiavon, L.L. Significance of alanine aminotransferase levels in patients admitted for cocaine intoxication. J. Clin. Gastroenterol. 2015, 49, 250–255. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Cao, H.; Wang, Q.; Zhou, Z.; Zhao, H.; Kuang, H. Determination of metabolic phenotype and potential biomarkers in the liver of heroin addicted mice with hepatotoxicity. Life Sci. 2021, 287, 120103. [Google Scholar] [CrossRef] [PubMed]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547864/ (accessed on 30 August 2022).
- Halpin, L.E.; Gunning, W.T.; Yamamoto, B.K. Methamphetamine causes acute hyperthermia-dependent liver damage. Pharmacol. Res. Perspect. 2013, 1, e00008. [Google Scholar] [CrossRef]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548941/ (accessed on 30 August 2022).
- Leifman, H.; Rehnman, C.; Sjöblom, E.; Holgersson, S. Anabolic androgenic steroids—Use and correlates among gym users—An assessment study using quesionnaires and observations at gyms in the Stockholm region. Int. J. Environ. Res. Public Health 2011, 8, 2656–2674. [Google Scholar] [CrossRef] [PubMed]
- Börjesson, A.; Garevik, N.; Dahl, M.L.; Rane, A.; Ekström, L. Recruitment to doping and help-seeking behavior of eight female users. Subst. Abus. Treat. Prev. Policy 2016, 11, 11. [Google Scholar] [CrossRef]
- Kanayama, G.; Hudson, J.I.; Pope, H.G. Illicit Anabolic-Androgenic Steroid Use. Horm. Behav. 2010, 58, 111–121. [Google Scholar] [CrossRef]
- Navarro, V.J.; Khan, I.; Björnsson, E.; Seeff, L.B.; Serrano, J.; Hoofnagle, J.H. Liver injury from herbal and dietary supplements. Hepatology 2017, 65, 363–373. [Google Scholar] [CrossRef]
- Medina-Caliz, I.; Garcia-Cortes, M.; Gonzalez-Jimenez, A.; Cabello, M.R.; Robles-Díaz, M.; Sanabria-Cabrera, J.; Sanjuan-Jimenez, R.; Ortega-Alonso, A.; García-Muñoz, B.; Moreno, I.; et al. Herbal and dietary supplement-induced liver injuries in the Spanish DILI Registry. Clin. Gastroenterol. Hepatol. 2018, 16, 1495–1502. [Google Scholar] [CrossRef]
- Bessone, F.; García-Cortés, M.; Medina-Caliz, I.; Hernández, N.; Parana, R.; Mendizabal, M.; Schinoni, M.I.; Ridruejo, E.; Nunes, V.; Peralta, M.; et al. Herbal and Dietary Supplements-Induced liver injury in Latin America: Experience from the LATINDILI Network. Clin. Gastroenterol. Hepatol. 2022, 20, e548–e563. [Google Scholar] [CrossRef]
- Robles-Diaz, M.; Gonzalez-Jimenez, A.; Medina-Caliz, I.; Stephens, C.; García-Cortés, M.; García-Cortés, B.; Ortega-Alonso, A.; Blanco-Reina, E.; Gonzalez-Grande, R.; Jimenez-Pérez, M.; et al. Distinct phenotype of hepatotoxicity associated with illicit use of anabolic androgenic steroids. Aliment. Pharmacol. Ther. 2015, 41, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Stolz, A.; Navarro, V.; Hayashi, P.H.; Fontana, R.J.; Barnhart, H.X.; Gu, J.; Chalasani, N.P.; Vega, M.M.; Bonkovsky, H.L.; Seeff, L.B.; et al. Severe and protracted cholestasis in 44 young men taking bodybuilding supplements: Assessment of genetic, clinical and chemical risk factors. Aliment. Pharmacol. Ther. 2019, 49, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Disease: Bethesda, MD, USA, 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548931 (accessed on 20 June 2022).
- Jasirwan, C.O.M.; Fahira, A.; Sirega, L.; Loho, I. The alpha-fetoprotein serum is still reliable as a biomarker for the surveillance of hepatocellular carcinoma in Indonesia. BMC Gastroenterol. 2020, 20, 215. [Google Scholar] [CrossRef] [PubMed]
- Pilati, C.; Letouze, E.; Nault, J.C.; Imbeaud, S.; Boulai, A.; Calderaro, J.; Poussin, K.; Franconi, A.; Couchy, G.; Mocrette, G.; et al. Genomic profiling of hepatocellular adenomas reveals recurrent FRK activating mutations and the mechanisms of malignant transformation. Cancer Cell 2014, 25, 428–441. [Google Scholar] [CrossRef]
- Stoot, J.H.; Coelen, R.J.; De Jong, M.C.; Dejong, C.H. Malignant transformation of hepatocellular carcinomas: A systematic review including more than 1600 adenomas cases. HPB 2010, 12, 509–522. [Google Scholar] [CrossRef]
| Drugs | Carcinogenic Potential Related to HCC | Physiopathology | Literature Support | References | |
|---|---|---|---|---|---|
| Conventional drugs (ebrotidine, amiodarone, methotrexate and nitrofurantoin) | Presumed 1 | Cirrhosis (cirrhosis–HCC sequence) Amiodarone: protective factor | No evidence Amiodarone data from retrospective observational study | [18,19,20,21,22,23,24] [25,26] | |
| Herbal & Dietary Supplements | Khat Kava Pyrrolizidine alkaloids | Presumed Moderate 2 Presumed | Cirrhosis (cirrhosis–HCC sequence) Unknown Veno-occlusive syndrome (cirrhosis–HCC sequence) | No evidence Evidence in vivo, animal (case series) studies. None in humans No evidence | [27,28,29,30,31,32,33,34,35,36,37,38] [39,40,41,42,43,44,45,46,47,48,49,50,51,52,53] [54,55] |
| Tobacco | Strong 3 | Carcinogenic substances after hepatic metabolism (via CYP2E1) | Evidence from retrospective/prospective observational studies and meta-analysis | [56,57,58,59] | |
| Cannabis | Controversial 4 | Fast progression of liver fibrosis in chronic C hepatitis | Evidence for: retrospective observational study; evidence against: prospective observational studies and meta-analysis | [60] [61,62,63] | |
| Cocaine | Absent 5 | NA | No evidence | NA | |
| Heroin | Absent | NA | No evidence | NA | |
| Amphetamines | Absent | NA | No evidence | NA | |
| AAS | Strong | Genetic predisposition (?) Unknown liver-adenoma-like underlying disease (?) | Evidence based on cases and case series | [64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83] | |
| Authors | AAS | Duration of Treatment * | Indication | Single or Multiple Tumor | Initial Symptoms/ Signs | α-Fetoprotein (ng/mL) | Other Histological Findings |
|---|---|---|---|---|---|---|---|
| Bernstein et al. [64] | Oxymetholone | 11 | Fanconi´s anemia | Multiple | Yes | NA | Peliosis hepatis (tumoral and non-tumoral tissues) |
| Johnson et al. [65] (4 cases) | Oxymetholone Oxymetholone Methyltestosterone Methandienone | 36 15 51 NA | Aplastic anemia Aplastic anemia Fanconi´s anemia Fanconi´s anemia | Multiple Multiple Multiple Single | Yes Yes Yes Yes | Not increased Not increased NA Not increased | NA NA NA NA |
| Henderson et al. [66] | Methyltestosterone Norethandrolone Stanozolol Oxymetholone ** | 90 ** | Hypoplastic anemia | Multiple | Yes | High * | No |
| Farrell GC et al. [67] (3 cases) | Oxymetholone Methyltestosterone Methyltestosterone Testosterone ** | 65 72 96 ** | PNH Hypopituitarism Cryptorchidism | Multiple Multiple Multiple | Yes Yes Yes | Not increased Not increased Not increased | NA NA NA |
| Hernández et al. [68] | Methandienone | 36 | PNH | Multiple | Yes | Not increased | Peliosis hepatis (in non-tumoral tissue) |
| Shapiro et al. [69] | Testosterone propionate Oxymetholone ** | 108 ** | Fanconi´s anemia | Multiple | Yes | Not increased | Cholestasis, peliosis hepatis (in tumoral and non-tumoral tissues) |
| Lopez et al. [70] | NA | 8 | Aplastic anemia | Multiple | Yes | NA | NA |
| Carrasco et al. [71] | Nandrolone Testosterone enanthate ** | 132 ** | Alport´s syndrome | Multiple | No | Not increased | Tumor cells in pseudo-acinar pattern. No peliosis hepatis |
| Linares et al. [72] | Oxymetholone | 120 | Fanconi´s anemia | Multiple | Yes | Not increased | Peliosis hepatis (in tumoral tissue) |
| Bork et al. [73] (3 cases) | Danazol Danazol Danazol | 240 156 192 | Hereditary angioedema Hereditary angioedema Hereditary angioedema | Single Multiple Single | No Yes No | NA NA NA | NA NA NA |
| Socas et al. [74] (2 cases) | Stanozolol Oxymetholone Nandrolone Testosterone Methenolone ** Stanozolol Oxymetholone Nandrolone Testosterone Boldenone ** | 180 ** 6 ** | Recreational (bodybuilding) Recreational (bodybuilding) | Multiple Multiple | Yes Yes | Not increased Not increased | NA NA |
| Martin et al. [75] | Androstendione Nandrolone ** | 60 ** | Recreational (bodybuilding) | Multiple | Yes | NA | Peliosis hepatis (in tumoral tissue) |
| Hardt et al. [76] | Testosterone Trenbolone acetate Androstanediol Boldenone Methandriol Letrozole Oxymetholone Methandienone ** | 60 ** | Recreational (bodybuilding) | Single | Yes | Not increased | IHC: cytoplasmic CK8 (+), Hep-Par1 (+). Canalicular CD10 (+), CEA (+). Nuclear β-catenin, progesteron and estrogen receptors (weakly +). |
| Pais-Costa et al. [77] | Andronstendiona Nandrolone ** | 72 ** | Recreational (bodybuilding) | Multiple | Yes | Not increased | NA |
| Kesler et al. [78] | Testosterone | 84 | Recreational (bodybuilding) | Multiple | Yes | High (366) | IHC: arginase, glypican 3, heat shock protein 70, glutamine synthetase, β-catenin and CD34 (+) |
| Solbach et al. [79] | Nandrolone Sustanon Methandienone Stanozolol ** | 72 ** | Recreational (bodybuilding) | Multiple | Yes | NA | IHC: glutamine synthetase, androgen-receptor nuclear, β-catenin and CD34 (+) |
| Kato K et al. [80] | Testosterone enanthate | 144 | F-to-M gender identity disorder | Multiple | Yes | Not increased | IHC: β-catenin and GS (+) |
| Woodward et al. [81] (2 cases) | NA NA | NA 60 | Recreational (bodybuilding) Recreational (bodybuilding) | Single Multiple | Yes Yes | NA NA | NA NA |
| Wang et al. [82] | Stanozolol | 48 | Aplastic anemia | Multiple | Yes | Not increased | IHC: β-catenin and CD34 (+) |
| Lin et al. [83] | Testosterone cypionate | 14 | F-to-M gender identity disorder | Multiple | Yes | High (4320) | IHC: androgen-receptor (+) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinazo-Bandera, J.M.; García-Cortés, M.; Segovia-Zafra, A.; Lucena, M.I.; Andrade, R.J. Recreational Drugs and the Risk of Hepatocellular Carcinoma. Cancers 2022, 14, 5395. https://doi.org/10.3390/cancers14215395
Pinazo-Bandera JM, García-Cortés M, Segovia-Zafra A, Lucena MI, Andrade RJ. Recreational Drugs and the Risk of Hepatocellular Carcinoma. Cancers. 2022; 14(21):5395. https://doi.org/10.3390/cancers14215395
Chicago/Turabian StylePinazo-Bandera, José M., Miren García-Cortés, Antonio Segovia-Zafra, María Isabel Lucena, and Raúl J. Andrade. 2022. "Recreational Drugs and the Risk of Hepatocellular Carcinoma" Cancers 14, no. 21: 5395. https://doi.org/10.3390/cancers14215395
APA StylePinazo-Bandera, J. M., García-Cortés, M., Segovia-Zafra, A., Lucena, M. I., & Andrade, R. J. (2022). Recreational Drugs and the Risk of Hepatocellular Carcinoma. Cancers, 14(21), 5395. https://doi.org/10.3390/cancers14215395