What Is the Role of Body Composition Assessment in HCC Management?
Abstract
Simple Summary
Abstract
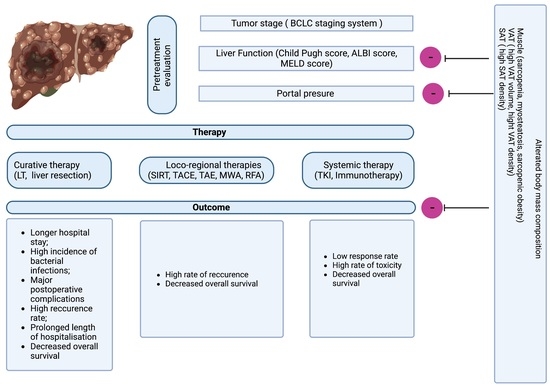
1. Introduction
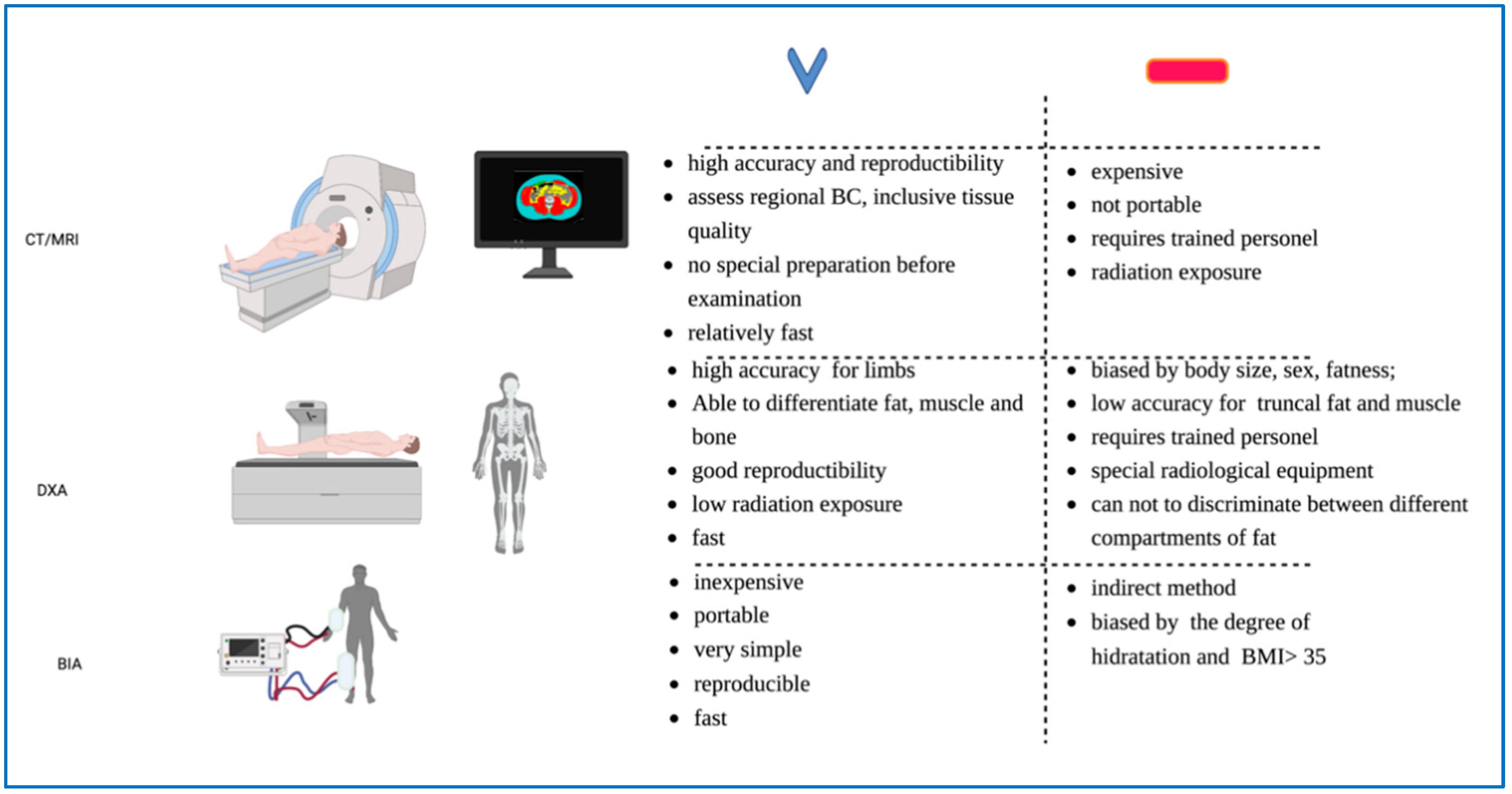
2. Current Methods Used to Evaluate Body Mass Composition
3. The Impact of Skeletal Muscle Abnormalities on HCC Outcome
3.1. The Effect of Sarcopenia on the Outcome of HCC
3.1.1. Liver Transplantation
3.1.2. Liver Resection
3.1.3. Local Ablative Therapy (RFA and MWA)
3.1.4. Chemoembolization
3.1.5. Systemic Therapy
Tyrosine Kinase Inhibitors
Immunotherapy
3.1.6. Myosteatosis
3.1.7. Sarcopenic Obesity
4. Adipose Compartment
4.1. Subcutaneous Adipose Tissue
4.2. Visceral Adipose Tissue (Volume and Radiodensity)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Perisetti, A.; Goyal, H.; Yendala, R.; Chandan, S.; Tharian, B.; Thandassery, R.B. Sarcopenia in Hepatocellular Carcinoma: Current Knowledge and Future Directions. WJG 2022, 28, 432–448. [Google Scholar] [CrossRef]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic Value of Sarcopenia in Adults with Solid Tumours: A Meta-Analysis and Systematic Review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Guo, X.; Zhang, Q.; Yang, Y. Prognostic Value of Sarcopenia in Patients with Rectal Cancer: A Meta-Analysis. PLoS ONE 2022, 17, e0270332. [Google Scholar] [CrossRef]
- Tantai, X.; Liu, Y.; Yeo, Y.H.; Praktiknjo, M.; Mauro, E.; Hamaguchi, Y.; Engelmann, C.; Zhang, P.; Jeong, J.Y.; van Vugt, J.L.A.; et al. Effect of Sarcopenia on Survival in Patients with Cirrhosis: A Meta-Analysis. J. Hepatol. 2022, 76, 588–599. [Google Scholar] [CrossRef] [PubMed]
- March, C.; Omari, J.; Thormann, M.; Pech, M.; Wienke, A.; Surov, A. Prevalence and Role of Low Skeletal Muscle Mass (LSMM) in Hepatocellular Carcinoma. A Systematic Review and Meta-Analysis. Clin. Nutr. ESPEN 2022, 49, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.-L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Mitsiopoulos, N.; Baumgartner, R.N.; Heymsfield, S.B.; Lyons, W.; Gallagher, D.; Ross, R. Cadaver Validation of Skeletal Muscle Measurement by Magnetic Resonance Imaging and Computerized Tomography. J. Appl. Physiol. 1998, 85, 115–122. [Google Scholar] [CrossRef]
- Miller, K.D.; Jones, E.; Yanovski, J.A.; Shankar, R.; Feuerstein, I.; Falloon, J. Visceral Abdominal-Fat Accumulation Associated with Use of Indinavir. Lancet 1998, 351, 871–875. [Google Scholar] [CrossRef]
- Shen, W.; Punyanitya, M.; Wang, Z.; Gallagher, D.; St.-Onge, M.-P.; Albu, J.; Heymsfield, S.B.; Heshka, S. Total Body Skeletal Muscle and Adipose Tissue Volumes: Estimation from a Single Abdominal Cross-Sectional Image. J. Appl. Physiol. 2004, 97, 2333–2338. [Google Scholar] [CrossRef]
- Achamrah, N.; Colange, G.; Delay, J.; Rimbert, A.; Folope, V.; Petit, A.; Grigioni, S.; Déchelotte, P.; Coëffier, M. Comparison of Body Composition Assessment by DXA and BIA According to the Body Mass Index: A Retrospective Study on 3655 Measures. PLoS ONE 2018, 13, e0200465. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, G.; Ponti, F.; Agostini, M.; Amadori, M.; Battista, G.; Bazzocchi, A. The Role of DXA in Sarcopenia. Aging Clin. Exp. Res. 2016, 28, 1047–1060. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.-S.; Li, H.-C.; Lu, H.-K.; Lai, C.-L.; Wang, Y.-S.; Hsieh, K.-C. Comparison of Bioelectrical Impedance Analysis and Dual Energy X-Ray Absorptiometry for Total and Segmental Bone Mineral Content with a Three-Compartment Model. Int. J. Environ. Res. Public Health 2020, 17, 2595. [Google Scholar] [CrossRef] [PubMed]
- Gallo, P.; Silletta, M.; De Vincentis, A.; Lo Prinzi, F.; Terracciani, F.; Di Fazio, G.; Flagiello, V.; Vespasiani Gentilucci, U.; Antonelli Incalzi, R.; Picardi, A. Sarcopenia in Hepatocellular Carcinoma: Pathogenesis and Management. Chemotherapy 2022, 67, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.; Bhanji, R.A.; Mazurak, V.C.; Montano-Loza, A.J. Sarcopenia in cirrhosis: From pathogenesis to interventions. J. Gastroenterol. 2019, 54, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.; Montano-Loza, A.J. Clinical relevance of skeletal muscle abnormalities in patients with cirrhosis. Dig. Liver Dis. 2019, 51, 1493–1499. [Google Scholar] [CrossRef]
- Chen, B.-B.; Liang, P.-C.; Shih, T.T.-F.; Liu, T.-H.; Shen, Y.-C.; Lu, L.-C.; Lin, Z.-Z.; Hsu, C.; Hsu, C.-H.; Cheng, A.-L.; et al. Sarcopenia and Myosteatosis Are Associated with Survival in Patients Receiving Immunotherapy for Advanced Hepatocellular Carcinoma. Eur. Radiol. 2022. [Google Scholar] [CrossRef]
- Li, S.; Wang, T.; Tong, G.; Li, X.; You, D.; Cong, M. Prognostic Impact of Sarcopenia on Clinical Outcomes in Malignancies Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 726257. [Google Scholar] [CrossRef]
- Xu, L.; Jing, Y.; Zhao, C.; Zhang, Q.; Zhao, X.; Yang, J.; Wu, L.; Yang, Y. Preoperative Computed Tomography-Assessed Skeletal Muscle Index Is a Novel Prognostic Factor in Patients with Hepatocellular Carcinoma Following Hepatectomy: A Meta-Analysis. J. Gastrointest. Oncol. 2020, 11, 1040–1053. [Google Scholar] [CrossRef]
- Nelke, C.; Dziewas, R.; Minnerup, J.; Meuth, S.G.; Ruck, T. Skeletal muscle as potential central link between sarcopenia and immune senescence. eBioMedicine 2019, 49, 381–388. [Google Scholar] [CrossRef]
- Giudice, J.; Taylor, J.M. Muscle as a Paracrine and Endocrine Organ. Curr. Opin. Pharmacol. 2017, 34, 49–55. [Google Scholar] [CrossRef]
- Choi, K.; Jang, H.Y.; Ahn, J.M.; Hwang, S.H.; Chung, J.W.; Choi, Y.S.; Kim, J.-W.; Jang, E.S.; Choi, G.H.; Jeong, S.-H. The Association of the Serum Levels of Myostatin, Follistatin, and Interleukin-6 with Sarcopenia, and Their Impacts on Survival in Patients with Hepatocellular Carcinoma. Clin. Mol. Hepatol. 2020, 26, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.K.; Das, D.; Taneja, R. Cancer Cachexia: Signaling and Transcriptional Regulation of Muscle Catabolic Genes. Cancers 2022, 14, 4258. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC Strategy for Prognosis Prediction and Treatment Recommendation: The 2022 Update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Santopaolo, F.; Lenci, I.; Milana, M.; Manzia, T.M.; Baiocchi, L. Liver Transplantation for Hepatocellular Carcinoma: Where Do We Stand? WJG 2019, 25, 2591–2602. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, Y.; Kaido, T.; Okumura, S.; Fujimoto, Y.; Ogawa, K.; Mori, A.; Hammad, A.; Tamai, Y.; Inagaki, N.; Uemoto, S. Impact of quality as well as quantity of skeletal muscle on outcomes after liver transplantation. Liver Transplant. 2014, 20, 1413–1419. [Google Scholar] [CrossRef]
- Meza-Junco, J.; Montano-Loza, A.J.; Baracos, V.E.; Prado, C.M.M.; Bain, V.G.; Beaumont, C.; Esfandiari, N.; Lieffers, J.R.; Sawyer, M.B. Sarcopenia as a Prognostic Index of Nutritional Status in Concurrent Cirrhosis and Hepatocellular Carcinoma. J. Clin. Gastroenterol. 2013, 47, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Montano-Loza, A.J. Skeletal Muscle Abnormalities and Outcomes after Liver Transplantation. Liver Transpl. 2014, 20, 1293–1295. [Google Scholar] [CrossRef] [PubMed]
- Valero, V.; Amini, N.; Spolverato, G.; Weiss, M.J.; Hirose, K.; Dagher, N.N.; Wolfgang, C.L.; Cameron, A.A.; Philosophe, B.; Kamel, I.R.; et al. Sarcopenia Adversely Impacts Postoperative Complications Following Resection or Transplantation in Patients with Primary Liver Tumors. J. Gastrointest. Surg. 2015, 19, 272–281. [Google Scholar] [CrossRef]
- Acosta, L.F.; Galuppo, R.; García, C.R.; Villacorta, E.; Dugan, A.; Castellanos, A.L.; Gedaly, R.; Lee, J.T. Association Between Sarcopenia and AFP Level in Patients Undergoing Liver Transplantation for Hepatocellular Carcinoma. J. Surg. Res. 2019, 238, 10–15. [Google Scholar] [CrossRef]
- Kim, Y.R.; Park, S.; Han, S.; Ahn, J.H.; Kim, S.; Sinn, D.H.; Jeong, W.K.; Ko, J.S.; Gwak, M.S.; Kim, G.S. Sarcopenia as a Predictor of Post-Transplant Tumor Recurrence after Living Donor Liver Transplantation for Hepatocellular Carcinoma beyond the Milan Criteria. Sci. Rep. 2018, 8, 7157. [Google Scholar] [CrossRef]
- Beumer, B.R.; van Vugt, J.L.A.; Sapisochin, G.; Yoon, P.; Bongini, M.; Lu, D.; Xu, X.; De Simone, P.; Pintore, L.; Golse, N.; et al. Impact of Muscle Mass on Survival of Patients with Hepatocellular Carcinoma after Liver Transplantation beyond the Milan Criteria. J. Cachexia Sarcopenia Muscle 2022, 13, 2373–2382. [Google Scholar] [CrossRef]
- Bhanji, R.A.; Takahashi, N.; Moynagh, M.R.; Narayanan, P.; Angirekula, M.; Mara, K.C.; Dierkhising, R.A.; Watt, K.D. The Evolution and Impact of Sarcopenia Pre- and Post-Liver Transplantation. Aliment Pharm. 2019, 49, 807–813. [Google Scholar] [CrossRef]
- Dasarathy, S. Posttransplant Sarcopenia: An Underrecognized Early Consequence of Liver Transplantation. Dig. Dis. Sci. 2013, 58, 3103–3111. [Google Scholar] [CrossRef] [PubMed]
- Kaido, T.; Ogawa, K.; Fujimoto, Y.; Ogura, Y.; Hata, K.; Ito, T.; Tomiyama, K.; Yagi, S.; Mori, A.; Uemoto, S. Impact of Sarcopenia on Survival in Patients Undergoing Living Donor Liver Transplantation: Impact of Sarcopenia on Liver Transplantation. Am. J. Transplant. 2013, 13, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Hanai, T.; Shiraki, M.; Nishimura, K.; Ohnishi, S.; Imai, K.; Suetsugu, A.; Takai, K.; Shimizu, M.; Moriwaki, H. Sarcopenia Impairs Prognosis of Patients with Liver Cirrhosis. Nutrition 2015, 31, 193–199. [Google Scholar] [CrossRef]
- Montano-Loza, A.J.; Duarte-Rojo, A.; Meza-Junco, J.; Baracos, V.E.; Sawyer, M.B.; Pang, J.X.Q.; Beaumont, C.; Esfandiari, N.; Myers, R.P. Inclusion of Sarcopenia Within MELD (MELD-Sarcopenia) and the Prediction of Mortality in Patients With Cirrhosis. Clin. Transl. Gastroenterol. 2015, 6, e102. [Google Scholar] [CrossRef] [PubMed]
- van Vugt, J.L.A.; Levolger, S.; de Bruin, R.W.F.; van Rosmalen, J.; Metselaar, H.J.; IJzermans, J.N.M. Systematic Review and Meta-Analysis of the Impact of Computed Tomography-Assessed Skeletal Muscle Mass on Outcome in Patients Awaiting or Undergoing Liver Transplantation. Am. J. Transpl. 2016, 16, 2277–2292. [Google Scholar] [CrossRef] [PubMed]
- Harimoto, N.; Shirabe, K.; Yamashita, Y.-I.; Ikegami, T.; Yoshizumi, T.; Soejima, Y.; Ikeda, T.; Maehara, Y.; Nishie, A.; Yamanaka, T. Sarcopenia as a Predictor of Prognosis in Patients Following Hepatectomy for Hepatocellular Carcinoma. Br. J. Surg. 2013, 100, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Yagi, T.; Yoshida, R.; Shinoura, S.; Umeda, Y.; Nobuoka, D.; Kuise, T.; Watanabe, N.; Fujiwara, T. Sarcopenia and American Society of Anesthesiologists Physical Status in the Assessment of Outcomes of Hepatocellular Carcinoma Patients Undergoing Hepatectomy. Acta Med. Okayama 2016, 70, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Voron, T.; Colussi, O.; Marcheteau, E.; Pernot, S.; Nizard, M.; Pointet, A.-L.; Latreche, S.; Bergaya, S.; Benhamouda, N.; Tanchot, C.; et al. VEGF-A Modulates Expression of Inhibitory Checkpoints on CD8+ T Cells in Tumors. J. Exp. Med. 2015, 212, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Dello, S.A.W.G.; Lodewick, T.M.; van Dam, R.M.; Reisinger, K.W.; van den Broek, M.A.J.; von Meyenfeldt, M.F.; Bemelmans, M.H.A.; Olde Damink, S.W.M.; Dejong, C.H.C. Sarcopenia Negatively Affects Preoperative Total Functional Liver Volume in Patients Undergoing Liver Resection. HPB 2013, 15, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-V.; Chen, J.-D.; Wu, W.-T.; Huang, K.-C.; Hsu, C.-T.; Han, D.-S. Association between Loss of Skeletal Muscle Mass and Mortality and Tumor Recurrence in Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Liver Cancer 2018, 7, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Yabusaki, N.; Fujii, T.; Yamada, S.; Suzuki, K.; Sugimoto, H.; Kanda, M.; Nakayama, G.; Koike, M.; Fujiwara, M.; Kodera, Y. Adverse Impact of Low Skeletal Muscle Index on the Prognosis of Hepatocellular Carcinoma after Hepatic Resection. Int. J. Surg. 2016, 30, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Nakagawa, H.; Kudo, Y.; Tateishi, R.; Taguri, M.; Watadani, T.; Nakagomi, R.; Kondo, M.; Nakatsuka, T.; Minami, T.; et al. Sarcopenia, Intramuscular Fat Deposition, and Visceral Adiposity Independently Predict the Outcomes of Hepatocellular Carcinoma. J. Hepatol. 2015, 63, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Yuri, Y.; Nishikawa, H.; Enomoto, H.; Ishii, A.; Iwata, Y.; Miyamoto, Y.; Ishii, N.; Hasegawa, K.; Nakano, C.; Nishimura, T.; et al. Implication of Psoas Muscle Index on Survival for Hepatocellular Carcinoma Undergoing Radiofrequency Ablation Therapy. J. Cancer 2017, 8, 1507–1516. [Google Scholar] [CrossRef]
- Yeh, W.-S.; Chiang, P.-L.; Kee, K.-M.; Chang, C.-D.; Lu, S.-N.; Chen, C.-H.; Wang, J.-H. Pre-Sarcopenia Is the Prognostic Factor of Overall Survival in Early-Stage Hepatoma Patients Undergoing Radiofrequency Ablation. Medicine 2020, 99, e20455. [Google Scholar] [CrossRef]
- Kamachi, S.; Mizuta, T.; Otsuka, T.; Nakashita, S.; Ide, Y.; Miyoshi, A.; Kitahara, K.; Eguchi, Y.; Ozaki, I.; Anzai, K. Sarcopenia Is a Risk Factor for the Recurrence of Hepatocellular Carcinoma after Curative Treatment. Hepatol. Res. 2016, 46, 201–208. [Google Scholar] [CrossRef]
- Chae, M.S.; Moon, K.U.; Jung, J.-Y.; Choi, H.J.; Chung, H.S.; Park, C.S.; Lee, J.; Choi, J.H.; Hong, S.H. Perioperative Loss of Psoas Muscle Is Associated with Patient Survival in Living Donor Liver Transplantation. Liver Transplant. 2018, 24, 623–633. [Google Scholar] [CrossRef]
- Kroh, A.; Uschner, D.; Lodewick, T.; Eickhoff, R.M.; Schöning, W.; Ulmer, F.T.; Neumann, U.P.; Binnebösel, M. Impact of Body Composition on Survival and Morbidity after Liver Resection in Hepatocellular Carcinoma Patients. Hepatobiliary Pancreat. Dis. Int. 2019, 18, 28–37. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kaido, T.; Hamaguchi, Y.; Okumura, S.; Shirai, H.; Yao, S.; Kamo, N.; Yagi, S.; Taura, K.; Okajima, H.; et al. Impact of Sarcopenic Obesity on Outcomes in Patients Undergoing Hepatectomy for Hepatocellular Carcinoma. Ann. Surg. 2019, 269, 924–931. [Google Scholar] [CrossRef]
- Hamaguchi, Y.; Kaido, T.; Okumura, S.; Kobayashi, A.; Shirai, H.; Yao, S.; Yagi, S.; Kamo, N.; Seo, S.; Taura, K.; et al. Preoperative Visceral Adiposity and Muscularity Predict Poor Outcomes after Hepatectomy for Hepatocellular Carcinoma. Liver Cancer 2019, 8, 92–109. [Google Scholar] [CrossRef] [PubMed]
- Meister, F.A.; Lurje, G.; Verhoeven, S.; Wiltberger, G.; Heij, L.; Liu, W.-J.; Jiang, D.; Bruners, P.; Lang, S.A.; Ulmer, T.F.; et al. The Role of Sarcopenia and Myosteatosis in Short- and Long-Term Outcomes Following Curative-Intent Surgery for Hepatocellular Carcinoma in a European Cohort. Cancers 2022, 14, 720. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.Y.; Choi, G.H.; Hwang, S.H.; Jang, E.S.; Kim, J.-W.; Ahn, J.M.; Choi, Y.; Cho, J.Y.; Han, H.-S.; Lee, J.; et al. Sarcopenia and Visceral Adiposity Predict Poor Overall Survival in Hepatocellular Carcinoma Patients after Curative Hepatic Resection. Transl. Cancer Res. 2021, 10, 854–866. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kawai, H.; Nakano, O.; Abe, S.; Kamimura, H.; Sakamaki, A.; Kamimura, K.; Tsuchiya, A.; Takamura, M.; Yamagiwa, S.; et al. Rapidly declining skeletal muscle mass predicts poor prognosis of hepatocellular carcinoma treated with transcatheter intra-arterial therapies. BMC Cancer 2018, 18, 756. [Google Scholar] [CrossRef]
- Fujita, M.; Takahashi, A.; Hayashi, M.; Okai, K.; Abe, K.; Ohira, H. Skeletal Muscle Volume Loss during Transarterial Chemoembolization Predicts Poor Prognosis in Patients with Hepatocellular Carcinoma. Hepatol. Res. 2019, 49, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Dodson, R.M.; Firoozmand, A.; Hyder, O.; Tacher, V.; Cosgrove, D.P.; Bhagat, N.; Herman, J.M.; Wolfgang, C.L.; Geschwind, J.-F.H.; Kamel, I.R.; et al. Impact of Sarcopenia on Outcomes Following Intra-Arterial Therapy of Hepatic Malignancies. J. Gastrointest. Surg. 2013, 17, 2123–2132. [Google Scholar] [CrossRef]
- Loosen, S.H.; Schulze-Hagen, M.; Bruners, P.; Tacke, F.; Trautwein, C.; Kuhl, C.; Luedde, T.; Roderburg, C. Sarcopenia Is a Negative Prognostic Factor in Patients Undergoing Transarterial Chemoembolization (TACE) for Hepatic Malignancies. Cancers 2019, 11, 1503. [Google Scholar] [CrossRef]
- Nishikawa, H.; Nishijima, N.; Enomoto, H.; Sakamoto, A.; Nasu, A.; Komekado, H.; Nishimura, T.; Kita, R.; Kimura, T.; Iijima, H.; et al. Prognostic Significance of Sarcopenia in Patients with Hepatocellular Carcinoma Undergoing Sorafenib Therapy. Oncol. Lett. 2017, 14, 1637–1647. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Kudo, M.; Hirooka, M.; Koizumi, Y.; Hiasa, Y.; Tajiri, K.; Toyoda, H.; Tada, T.; Ochi, H.; et al. Hepatic Function during Repeated TACE Procedures and Prognosis after Introducing Sorafenib in Patients with Unresectable Hepatocellular Carcinoma: Multicenter Analysis. Dig. Dis. 2017, 35, 602–610. [Google Scholar] [CrossRef]
- Yamashima, M.; Miyaaki, H.; Honda, T.; Shibata, H.; Miuma, S.; Taura, N.; Nakao, K. Significance of Psoas Muscle Thickness as an Indicator of Muscle Atrophy in Patients with Hepatocellular Carcinoma Treated with Sorafenib. Mol. Clin. Oncol. 2017, 7, 449–453. [Google Scholar] [CrossRef]
- Takada, H.; Kurosaki, M.; Nakanishi, H.; Takahashi, Y.; Itakura, J.; Tsuchiya, K.; Yasui, Y.; Tamaki, N.; Takaura, K.; Komiyama, Y.; et al. Impact of Pre-Sarcopenia in Sorafenib Treatment for Advanced Hepatocellular Carcinoma. PLoS ONE 2018, 13, e0198812. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, G.; Gigante, E.; Iavarone, M.; Begini, P.; Sangiovanni, A.; Iannicelli, E.; Biondetti, P.; Pellicelli, A.M.; Miglioresi, L.; Marchetti, P.; et al. Sarcopenia Is Associated with Reduced Survival in Patients with Advanced Hepatocellular Carcinoma Undergoing Sorafenib Treatment. United Eur. Gastroenterol. J. 2018, 6, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Takai, K.; Hanai, T.; Ideta, T.; Miyazaki, T.; Kochi, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M. Skeletal Muscle Depletion Predicts the Prognosis of Patients with Hepatocellular Carcinoma Treated with Sorafenib. Int. J. Mol. Sci. 2015, 16, 9612–9624. [Google Scholar] [CrossRef]
- Akce, M.; Liu, Y.; Zakka, K.; Martini, D.J.; Draper, A.; Alese, O.B.; Shaib, W.L.; Wu, C.; Wedd, J.P.; Sellers, M.T.; et al. Impact of Sarcopenia, BMI, and Inflammatory Biomarkers on Survival in Advanced Hepatocellular Carcinoma Treated With Anti-PD-1 Antibody. Am. J. Clin. Oncol. 2021, 44, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Mir, O.; Coriat, R.; Blanchet, B.; Durand, J.-P.; Boudou-Rouquette, P.; Michels, J.; Ropert, S.; Vidal, M.; Pol, S.; Chaussade, S.; et al. Sarcopenia Predicts Early Dose-Limiting Toxicities and Pharmacokinetics of Sorafenib in Patients with Hepatocellular Carcinoma. PLoS ONE 2012, 7, e37563. [Google Scholar] [CrossRef] [PubMed]
- Günther, S.L.; Trinkner, P.; von Bergwelt, M.; Cordas dos Santos, D.M.; Theurich, S. 63P Obesity and Sarcopenia as Biomarkers for Immunotherapy Outcomes: A Systematic Review and Meta-Analysis. Ann. Oncol. 2021, 32, S384. [Google Scholar] [CrossRef]
- Deng, H.-Y.; Chen, Z.-J.; Qiu, X.-M.; Zhu, D.-X.; Tang, X.-J.; Zhou, Q. Sarcopenia and Prognosis of Advanced Cancer Patients Receiving Immune Checkpoint Inhibitors: A Comprehensive Systematic Review and Meta-Analysis. Nutrition 2021, 90, 111345. [Google Scholar] [CrossRef]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E., III; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ Attenuates Tumour Response to PD-L1 Blockade by Contributing to Exclusion of T Cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef]
- Ebadi, M.; Dunichand-Hoedl, A.R.; Rider, E.; Kneteman, N.M.; Shapiro, J.; Bigam, D.; Dajani, K.; Mazurak, V.C.; Baracos, V.E.; Montano-Loza, A.J. Higher subcutaneous adipose tissue radiodensity is associated with increased mortality in patients with cirrhosis. JHEP Rep. 2022, 4, 100495. [Google Scholar] [CrossRef]
- Blaszczak, A.M.; Jalilvand, A.; Hsueh, W.A. Adipocytes, Innate Immunity and Obesity: A Mini-Review. Front. Immunol. 2021, 12, 650768. [Google Scholar] [CrossRef]
- Herold, J.; Kalucka, J. Angiogenesis in Adipose Tissue: The Interplay Between Adipose and Endothelial Cells. Front. Physiol. 2021, 11, 624903. [Google Scholar] [CrossRef] [PubMed]
- Hessen, L.; Roumet, M.; Maurer, M.H.; Lange, N.; Reeves, H.; Dufour, J.; Radu, P. High Subcutaneous Adipose Tissue Density Correlates Negatively with Survival in Patients with Hepatocellular Carcinoma. Liver Int. 2021, 41, 828–836. [Google Scholar] [CrossRef]
- Charette, N.; Vandeputte, C.; Ameye, L.; Bogaert, C.V.; Krygier, J.; Guiot, T.; Deleporte, A.; Delaunoit, T.; Geboes, K.; Van Laethem, J.-L.; et al. Prognostic Value of Adipose Tissue and Muscle Mass in Advanced Colorectal Cancer: A Post Hoc Analysis of Two Non-Randomized Phase II Trials. BMC Cancer 2019, 19, 134. [Google Scholar] [CrossRef] [PubMed]
- Veld, J.; Vossen, J.A.; De Amorim Bernstein, K.; Halpern, E.F.; Torriani, M.; Bredella, M.A. Adipose Tissue and Muscle Attenuation as Novel Biomarkers Predicting Mortality in Patients with Extremity Sarcomas. Eur. Radiol. 2016, 26, 4649–4655. [Google Scholar] [CrossRef] [PubMed]
- Petruzzelli, M.; Schweiger, M.; Schreiber, R.; Campos-Olivas, R.; Tsoli, M.; Allen, J.; Swarbrick, M.; Rose-John, S.; Rincon, M.; Robertson, G.; et al. A Switch from White to Brown Fat Increases Energy Expenditure in Cancer-Associated Cachexia. Cell Metab. 2014, 20, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.; Jacene, H.A.; Engles, J.M.; Honda, H.; Wahl, R.L. CT Hounsfield Units of Brown Adipose Tissue Increase with Activation: Preclinical and Clinical Studies. J. Nucl. Med. 2010, 51, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, A.S.; Sanna, F.; Sabharwal, N.; Thomas, S.; Oikonomou, E.K.; Herdman, L.; Margaritis, M.; Shirodaria, C.; Kampoli, A.-M.; Akoumianakis, I.; et al. Detecting Human Coronary Inflammation by Imaging Perivascular Fat. Sci. Transl. Med. 2017, 9, eaal2658. [Google Scholar] [CrossRef]
- Tsoli, M.; Swarbrick, M.M.; Robertson, G.R. Lipolytic and Thermogenic Depletion of Adipose Tissue in Cancer Cachexia. Semin. Cell Dev. Biol. 2016, 54, 68–81. [Google Scholar] [CrossRef]
- Batista, M.L.; Neves, R.X.; Peres, S.B.; Yamashita, A.S.; Shida, C.S.; Farmer, S.R.; Seelaender, M. Heterogeneous Time-Dependent Response of Adipose Tissue during the Development of Cancer Cachexia. J. Endocrinol. 2012, 215, 363–373. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kawai, H.; Nakano, O.; Abe, S.; Kamimura, H.; Sakamaki, A.; Kamimura, K.; Tsuchiya, A.; Takamura, M.; Yamagiwa, S.; et al. Prognostic Value of Subcutaneous Adipose Tissue Volume in Hepatocellular Carcinoma Treated with Transcatheter Intra-Arterial Therapy. CMAR 2018, 10, 2231–2239. [Google Scholar] [CrossRef]
- Imai, K.; Takai, K.; Miwa, T.; Taguchi, D.; Hanai, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M. Rapid Depletions of Subcutaneous Fat Mass and Skeletal Muscle Mass Predict Worse Survival in Patients with Hepatocellular Carcinoma Treated with Sorafenib. Cancers 2019, 11, 1206. [Google Scholar] [CrossRef] [PubMed]
- Ohki, T.; Tateishi, R.; Shiina, S.; Goto, E.; Sato, T.; Nakagawa, H.; Masuzaki, R.; Goto, T.; Hamamura, K.; Kanai, F.; et al. Visceral Fat Accumulation Is an Independent Risk Factor for Hepatocellular Carcinoma Recurrence after Curative Treatment in Patients with Suspected NASH. Gut 2009, 58, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Montano-Loza, A.J.; Mazurak, V.C.; Ebadi, M.; Meza-Junco, J.; Sawyer, M.B.; Baracos, V.E.; Kneteman, N. Visceral Adiposity Increases Risk for Hepatocellular Carcinoma in Male Patients with Cirrhosis and Recurrence after Liver Transplant. Hepatology 2018, 67, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Arano, T.; Nakagawa, H.; Tateishi, R.; Ikeda, H.; Uchino, K.; Enooku, K.; Goto, E.; Masuzaki, R.; Asaoka, Y.; Kondo, Y.; et al. Serum Level of Adiponectin and the Risk of Liver Cancer Development in Chronic Hepatitis C Patients. Int. J. Cancer 2011, 129, 2226–2235. [Google Scholar] [CrossRef] [PubMed]
- Lutz, C.T.; Quinn, L.S. Sarcopenia, Obesity, and Natural Killer Cell Immune Senescence in Aging: Altered Cytokine Levels as a Common Mechanism. Aging 2012, 4, 535–546. [Google Scholar] [CrossRef]
- Shan, Y.; Lu, C.; Wang, J.; Li, M.; Ye, S.; Wu, S.; Huang, J.; Bu, S.; Wang, F. IGF-1 Contributes to Liver Cancer Development in Diabetes Patients by Promoting Autophagy. Ann. Hepatol. 2022, 27, 100697. [Google Scholar] [CrossRef]
- Ebadi, M.; Moctezuma-Velazquez, C.; Meza-Junco, J.; Baracos, V.E.; DunichandHoedl, A.R.; Ghosh, S.; Sarlieve, P.; Owen, R.J.; Kneteman, N.; Montano-Loza, A.J. Visceral Adipose Tissue Radiodensity Is Linked to Prognosis in Hepatocellular Carcinoma Patients Treated with Selective Internal Radiation Therapy. Cancers 2020, 12, 356. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.; Hou, Z.-H.; Zhao, D.-X.; Li, J.-B.; Zhang, S.; Yin, Y.; Ni, C.-F.; Chen, T. High Visceral Adipose Tissue Density Correlates With Unfavorable Outcomes in Patients with Intermediate-Stage Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization. Front. Cell Dev. Biol. 2021, 9, 710104. [Google Scholar] [CrossRef]
- Parikh, N.D.; Zhang, P.; Singal, A.G.; Derstine, B.A.; Krishnamurthy, V.; Barman, P.; Waljee, A.K.; Su, G.L. Body Composition Predicts Survival in Patients with Hepatocellular Carcinoma Treated with Transarterial Chemoembolization. Cancer Res. Treat. 2018, 50, 530–537. [Google Scholar] [CrossRef]
| First Author and Year | Study Design | Cohort Characteristic | Method Used Cutoffs Used | Conclusion |
|---|---|---|---|---|
| Liver transplantation | ||||
| Kim Y.R. et al., 2018 [30] | Retrospective | Asian 92 HCC beyond MC | CT-based segmentation at L3 PMI cutoff <15.5 mm/m | Sarcopenia risk factor for recurrence (HR = 9.49 95% CI 1.18–76.32 (p = 0.034)) |
| Chae M.S. et al., 2018 [48] | Retrospective | Asian 408 (46.8% HCC) | Preoperative CT-based segmentation at L3 PMI at L3 | A PMI decrease ≤−11.7% between the before surgery and 7th day post LT was an independent predictor of patient mortality after LT |
| Acosta L. et al., 2019 [29] | Retrospective | North America 163 | Preoperative CT-based segmentation at L3 SMI 52.4 cm2/m2 in males and SMI 38.5 cm2/m2 in women | Patients in the lowest quartile of the SMI were associated with 70% increased risk of prolonged length of stay in this cohort |
| Beumer R. et al., 2022 [31] | Retrospective multicenter | European 889 HCC beyond MC | Preoperative CT-based segmentation at L3 In women, SMI: 37 cm2/m2 for BMI < 25 kg/m2, 42 cm2/m2 for BMI ≥ 25 kg/m2 In men SMI 45 cm2/m2 for BMI < 25 kg/m2, 51 cm2/m2 for BMI ≥ 25 kg/m2 | Patients with higher muscle mass had a better long-term survival |
| Liver resection | ||||
| Kroh A. et al., 2018 [49] | Retrospective | Asian 70 patients | Preoperative CT-based segmentation at L3 Sarcopenia In men, SMI < 43 for BMI < 25 kg/m2, SMI < 53 for BMI > 25 kg/m2 In women, SMI < 41 irrespective of the BMI Obesity was defined based on the top two body fat percentage quintiles for men and women, respectively | Sarcopenia, obesity, and sarcopenic obesity were not risk factors for poor postoperative survival in this study |
| Kobayashi A. et al., 2019 [50] | Retrospective | Asian 465 patients | Preoperative CT-based segmentation at L3 SMI cutoff <40.31 cm2/m2 for men <30.88 cm2/m2 for women Obesity: visceral adipose tissue area was >100 cm2 in both males and women | Sarcopenic obesity risk factor for mortality (HR = 2.504, p = 0.005) and recurrence of HCC (HR = 2.031, p = 0.006) |
| Hamaguchi Y. et al., 2019 [51] | Retrospective | Asian 606 patients | Preoperative CT-based segmentation at L3 Low SMI cutoff: <40.31 cm2/m2 for men and <30.88 cm2/m2 for women High VSR cutoff: >1.325 for males and >0.710 for women High IMAT cutoff: >–0.358 for males and >–0.229 for women | A high VRS, low SMI, and high IMAC contributed to an increased risk of death (p < 0.001) and HCC recurrence (p < 0.001) in an additive manner |
| Meister F. et al., 2022 [52] | Retrospective | European 100 patients | Preoperative CT-based segmentation at L3 SM-RA < 41 HU for patients with BMI up to 24.9 kg/m2 and <33 HU for patients with a BMI ≥ 25 kg/m2 | Myosteatosis was as an independent risk factor for perioperative morbidity (HR: 6.184, 95% CI 1.184–32.305, p = 0.031) Myosteatotic vs. non-myosteatotic (41 months vs. 60 months, p = 0.223) |
| Jang H.Y. et al., 2021 [53] | Retrospective | Asian 160 patients | Preoperative CT-based segmentation at L3: PMI cut-off: <3.33 for male, 2.38 for female VATI cut-off ≥30.39 for male, >44.70 for women | Sarcopenia and high VATI was associated with poor OS but not recurrence-free survival PMA did not predict OS |
| RFA/MWA | ||||
| Yuri Y. et al., 2017 [45] | Retrospective | Asian 182 | CT-based segmentation at L3 PMI cut-off: 6.36 cm2/m2 for men and 3.92 cm2/m2 for women | Sarcopenia was associated with a reduced OS with no effect on recurrence |
| First Author and Year | Study Design | Cohort Characteristic | Method Used Cutoffs Used | Major Finding |
|---|---|---|---|---|
| Nishikawa H. et al., 2017 [58] | Retrospective | Asian 232 | CT-based segmentation at L3 Cut off L3-SMI ≤ 36.2 cm2/m2 for male; ≤29.6 cm2/m2 for women | Sarcopenia is an independent predictor of low OS (HR 0.365; p < 0.0001) Sarcopenic patients had a lower rate of objective response rate and disease control rate |
| Hiraoka A. et al., 2017 [59] | Retrospective | Asian 93 | CT-based segmentation at L3 Cut off PSI: <4.24 cm2/m2 for male; <2.50 cm2/m2 for female | Sarcopenia is an important negative factor in patients treated with sorafenib |
| Yamashima M. et al., 2017 [60] | Retrospective | Asian 40 | CT-based segmentation at L3 TPMT was evaluated prior to treatment initiation and after 1–3 months of treatment ΔTPMT/height < 0.59 mm/m2 | Patients with mild muscle atrophy exhibited a significantly longer OS compared with patients with severe muscle atrophy (p = 0.045) |
| Takada H. et al., 2018 [61] | Retrospective | Asian 214 | CT-based segmentation at L3 Cut off L3-SMI <42 cm2/m2 for male: <38 cm2/m2 for women | Pretherapy sarcopenia in patients with two or less negative prognostic factors is an important negative prognostic factor (HR 1.6; p = 0.047) |
| Antonelli G. et al., 2018 [62] | Retrospective | Europe 96 | CT-based segmentation at L3 within 30 days from treatment start Cut off L3-SMI < 53 cm2/m2 if BMI > 25 and < 43 cm2/m2 if BMI < 25 for men <41 cm2/m2 for women | Sarcopenia is associated with reduced survival and reduced duration of sorafenib |
| Imai K. et al., 2019 [63] | Retrospective | Asian 61 | CT-based segmentation at L3 Cut off L3-SMI < 42 cm2/m2 for male: <38 cm2/m2 for women ΔL3-SMI > −5.73 cm2/m2/120 days ΔSFMI > −5.33 cm2/m2/120 days ∆VFMI > −3.95 cm2/m2/120 days | Rapid depletions in subcutaneous fat mass and skeletal muscle mass after the introduction of sorafenib indicate a poor prognosis |
| Immunotherapy | ||||
| Akce M. et al., 2021 [64] | Retrospective | American 57 | Pretreatment CT at L3 level SMI cut-off: 43 cm2/m2 for males and 39 cm2/m2 for women | Sex-specific sarcopenia does not predict OS, whereas baseline BMI and NLR together may predict OS in advanced HCC patients treated with anti-PD-1 antibody |
| Chen B.B. et al., 2022 [16] | Retrospective | Asian 138 | Pretreatment CT at L3 level SMD cutoff: <41 HU for BMI < 25 kg/m2, and <33 HU in for BMI ≥ 25 kg/m2 SMI cut-off: 40.8 cm2/m2 for men and 34.9 cm2/m2 for women Sarcopenic obesity: sarcopenia in patients with BMI > 25 kg/m2 | Sarcopenia and myosteatosis had a negative impact in patients who received immunotherapy for advanced HCC |
| First Author and Year | Study Design | Cohort Characteristic | Method Used Cutoffs Used | Conclusion |
|---|---|---|---|---|
| Kobayashi T. et al., 2018 [80] | Retrospective | Asian 100 HCC patients assigned to TACE | Preoperative CT-based segmentation at L3 | High SAT volume is associated with better survival outcomes in HCC patients treated with TACE |
| Imai K. et al., 2019 [81] | Retrospective | Asian 61 | CT-based segmentation at L3 Cutoff L3-SMI < 42 cm2/m2 for male: <38 cm2/m2 for women ΔL3-SMI > −5.73 cm2/m2/120 days ΔSFMI > −5.33 cm2/m2/120 days ∆VFMI > −3.95 cm2/m2/120 days | Rapid depletions of subcutaneous fat mass and skeletal muscle mass after the introduction of sorafenib indicate a poor prognosis |
| First Author and Year | Study Design | Cohort Characteristic | Method Used Cutoffs Used | Conclusion |
|---|---|---|---|---|
| Parikh et al., 2018 [89] | Retrospective | Asian 124 patients pre-LT | Multifrequency BIA | IMAT (HR = 3.898, 95% CI = 2.025–7.757, p < 0.001] and low PMI (HR = 3.635, 95% CI = 1.896–7.174, p < 0.001) were independent risk factors for death after LDLT |
| Montano-Loza et al., 2018 [83] | Retrospective | Canadian 678 patients (289 with HCC) pre-LT | CT-based segmentation at L3 | VATI ≥ 65 cm2/m2 independent risk factor for HCC in male patients with cirrhosis and for recurrence of HCC after LT |
| Hamaguchi et al., 2019 [51] | Retrospective | Asian 606 patients | Preoperative CT-based segmentation at L3 SMI cutoff: <40.31 cm2/m2 for men and <30.88 cm2/m2 for women | A high VRS (HR = 1.329, p = 0.020), low SMI, and high IMAT contributed to an increased risk of death (p < 0.001) and HCC recurrence (p < 0.001) in an additive manner |
| Ebadi et al., 2020 [87] | Retrospective | Canadian 89 HCC patients assess to SIRT | CT-based segmentation at L3 VAT cutoff: –85 HU | VAT ≥ –85 HU had a 2× higher risk of mortality (HR 2.01, 95% CI 1.14–3.54, p = 0.02) compared with their counterpart |
| Li Q et al., 2020 [88] | Retrospective | Asian 192 intermediate stage HCC patients assigned to TACE | CT-based segmentation at L3 VAT cutoff: −89.1 HU | VAT < −89.1 HU associated with better OS and PFS (25.1 mo, 95% CI: 18.1–32.1 vs. 17.6 mo, 95% CI: 16.3–18.8, p < 0.0001, 15.4 mo, 95% CI: 10.6–20.2 vs. 6.6 mo, 95% CI: 4.9–8.3, p < 0.0001, respectively) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radu, P.; Ebadi, M.; Montano-Loza, A.J.; Dufour, J.F. What Is the Role of Body Composition Assessment in HCC Management? Cancers 2022, 14, 5290. https://doi.org/10.3390/cancers14215290
Radu P, Ebadi M, Montano-Loza AJ, Dufour JF. What Is the Role of Body Composition Assessment in HCC Management? Cancers. 2022; 14(21):5290. https://doi.org/10.3390/cancers14215290
Chicago/Turabian StyleRadu, Pompilia, Maryam Ebadi, Aldo J. Montano-Loza, and Jean Francois Dufour. 2022. "What Is the Role of Body Composition Assessment in HCC Management?" Cancers 14, no. 21: 5290. https://doi.org/10.3390/cancers14215290
APA StyleRadu, P., Ebadi, M., Montano-Loza, A. J., & Dufour, J. F. (2022). What Is the Role of Body Composition Assessment in HCC Management? Cancers, 14(21), 5290. https://doi.org/10.3390/cancers14215290