Minimising the Toxicities of First Line Hodgkin Lymphoma Treatment in the Modern Era
Abstract
Simple Summary
Abstract
1. Introduction
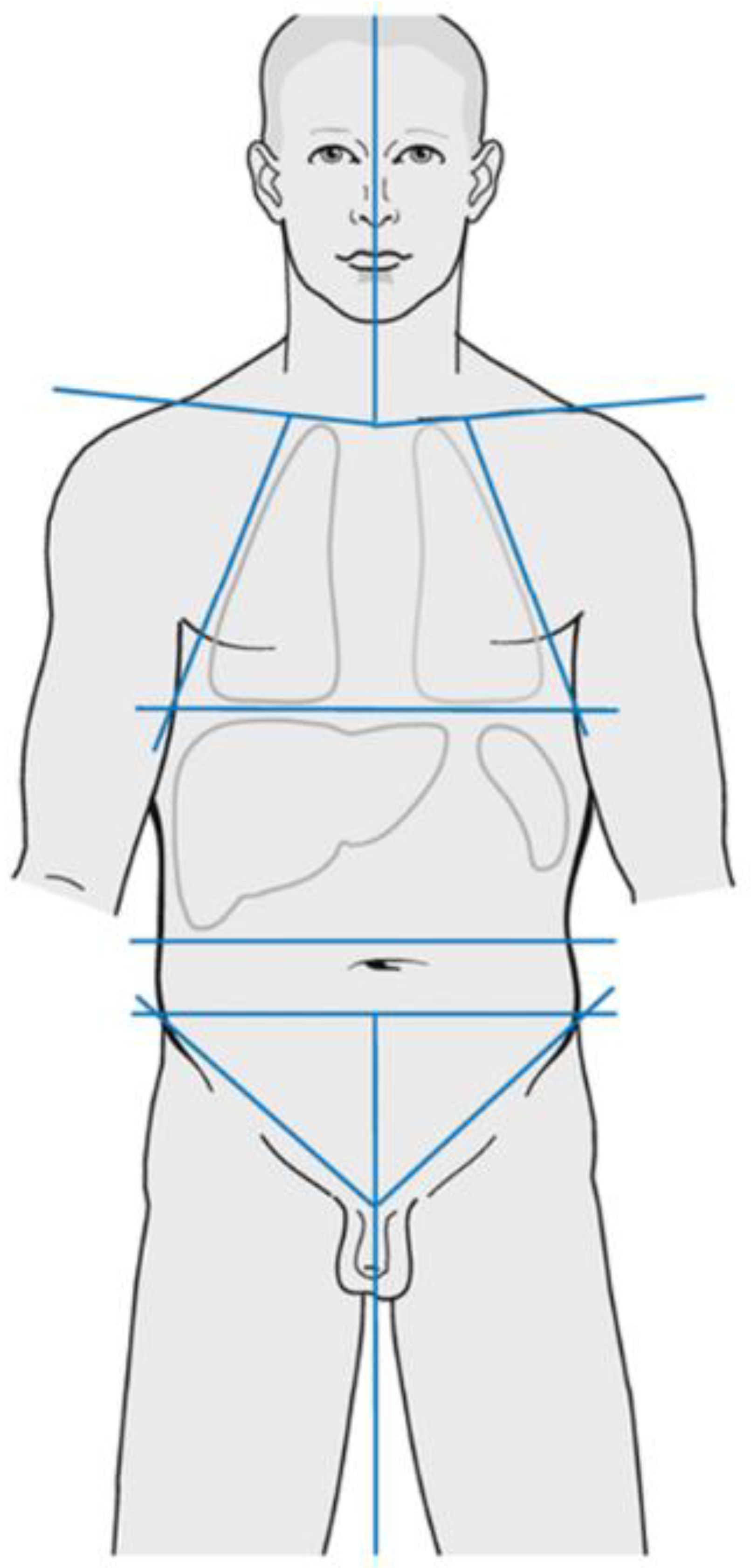
2. Early-Stage Favourable Hodgkin Lymphoma
3. Early-Stage Unfavourable Hodgkin Lymphoma
4. Advanced-Stage Hodgkin Lymphoma
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- ONS. Office for National Statistics. Cancer Survival in England: Adults Diagnosed in 2009 to 2013, Followed Up to 2014; Newport: Irvine, CA, USA, 2015. [Google Scholar]
- Link, B.K.; Vakkalanka, B. Neutropenia and neutropenic complications in ABVD chemotherapy for Hodgkin lymphoma. Adv. Hematol. 2011, 2011, 656013. [Google Scholar] [CrossRef][Green Version]
- Lang, N.; Crump, M. PET-adapted approaches to primary therapy for advanced Hodgkin lymphoma. Ther. Adv. Hematol. 2020, 11, 204062072091449. [Google Scholar] [CrossRef] [PubMed]
- Schaapveld, M.; Aleman, B.M.P.; van Eggermond, A.M.; Janus, C.P.M.; Krol, A.D.G.; van der Maazen, R.W.M.; Roesink, J.; Raemaekers, J.M.M.; de Boer, J.P.; Zijlstra, J.M.; et al. Second Cancer Risk Up to 40 Years after Treatment for Hodgkin’s Lymphoma. N. Engl. J. Med. 2015, 373, 2499–2511. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, E.; Mitchell, R.; Lane, S. Preservation of fertility in teenagers and young adults treated for haematological malignancies. Lancet Haematol. 2021, 8, e149–e160. [Google Scholar] [CrossRef]
- de Vries, S.; Schaapveld, M.; Janus, C.P.M.; Daniëls, L.A.; Petersen, E.J.; van der Maazen, R.W.M.; Zijlstra, J.M.; Beijert, M.; Nijziel, M.R.; Verschueren, K.M.S.; et al. Long-Term Cause-Specific Mortality in Hodgkin Lymphoma Patients. JNCI J. Natl. Cancer Inst. 2021, 113, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Mauz-Körholz, C.; Landman-Parker, J.; Balwierz, W.; Ammann, R.A.; Anderson, R.A.; Attarbaschi, A.; Bartelt, J.M.; Beishuizen, A.; Boudjemaa, S.; Cepelova, M.; et al. Response-adapted omission of radiotherapy and comparison of consolidation chemotherapy in children and adolescents with intermediate-stage and advanced-stage classical Hodgkin lymphoma (EuroNet-PHL-C1): A titration study with an open-label, embedded, multinational, non-inferiority, randomised controlled trial. Lancet Oncol. 2022, 23, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Geue, K.; Mehnert-Theuerkauf, A.; Stroske, I.; Brock, H.; Friedrich, M.; Leuteritz, K. Psychosocial Long-Term Effects of Young Adult Cancer Survivors: Study Protocol of the Longitudinal AYA-LE Long-Term Effects Study. Front. Psychol. 2021, 12, 688149. [Google Scholar] [CrossRef]
- Mahon, S.; Cella, D.; Donovan, M. Psychosocial adjustment to recurrent cancer. Oncol. Nurs. Forum. 1990, 17, 47–52. [Google Scholar]
- Engert, A.; Plütschow, A.; Eich, H.T.; Lohri, A.; Dörken, B.; Borchmann, P.; Berger, B.; Greil, R.; Willborn, K.C.; Wilhelm, M.; et al. Reduced Treatment Intensity in Patients with Early-Stage Hodgkin’s Lymphoma. N. Engl. J. Med. 2010, 363, 640–652. [Google Scholar] [CrossRef]
- Federico, M.; Luminari, S.; Iannitto, E.; Polimeno, G.; Marcheselli, L.; Montanini, A.; la Sala, A.; Merli, F.; Stelitano, C.; Pozzi, S.; et al. ABVD compared with BEACOPP compared with CEC for the initial treatment of patients with advanced hodgkin’s lymphoma: Results from the HD2000 gruppo italiano Per lo studio dei linfomi trial. J. Clin. Oncol. 2009, 27, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Duggan, D.B.; Petroni, G.R.; Johnson, J.L.; Glick, J.H.; Fisher, R.I.; Connors, J.M.; Canellos, G.P.; Peterson, B.A. Randomized Comparison of ABVD and MOPP/ABV Hybrid for the Treatment of Advanced Hodgkin’s Disease: Report of an Intergroup Trial. J. Clin. Oncol. 2003, 21, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Evens, A.M.; Hong, F.; Gordon, L.I.; Fisher, R.I.; Bartlett, N.L.; Connors, J.M.; Gascoyne, R.D.; Wagner, H.; Gospodarowicz, M.; Cheson, B.D.; et al. The efficacy and tolerability of adriamycin, bleomycin, vinblastine, dacarbazine and Stanford V in older Hodgkin lymphoma patients: A comprehensive analysis from the North American intergroup trial E. Br. J. Haematol. 2013, 161, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Behringer, K.; Goergen, H.; Hitz, F.; Zijlstra, J.M.; Greil, R.; Markova, J.; Sasse, S.; Fuchs, M.; Topp, M.S.; Soekler, M.; et al. Omission of dacarbazine or bleomycin, or both, from the ABVD regimen in treatment of early-stage favourable Hodgkin’s lymphoma (GHSG HD13): An open-label, randomised, non-inferiority trial. Lancet 2015, 385, 1418–1427. [Google Scholar] [CrossRef]
- O’Sullivan, J.M.; Huddart, R.A.; Norman, A.R.; Nicholls, J.; Dearnaley, D.P.; Horwich, A. Predicting the risk of bleomycin lung toxicity in patients with germ-cell tumours. Ann. Oncol. 2003, 14, 91–96. [Google Scholar] [CrossRef]
- Follows, G.A.; Barrington, S.F.; Bhuller, K.S.; Culligan, D.J.; Cutter, D.J.; Gallop-Evans, E.; Kassam, S.; Osborne, W.; Sadullah, S.; Townsend, W.; et al. Guideline for the first-line management of Classical Hodgkin Lymphoma—A British Society for Haematology guideline. Br. J. Haematol. 2022, 197, 558–572. [Google Scholar] [CrossRef]
- Johnson, P.W.M. Neutropenia and neutropenic complications in ABVD chemotherapy for Hodgkin lymphoma. Hematol. Am. Soc. Hematol. Educ. Program Book 2013, 2013, 400–405. [Google Scholar] [CrossRef][Green Version]
- Meyer, R.M.; Gospodarowicz, M.K.; Connors, J.M.; Pearcey, R.G.; Wells, W.A.; Winter, J.N.; Horning, S.J.; Dar, A.R.; Shustik, C.; Stewart, D.A.; et al. ABVD Alone versus Radiation-Based Therapy in Limited-Stage Hodgkin’s Lymphoma. N. Engl. J. Med. 2012, 366, 399–408. [Google Scholar] [CrossRef]
- Meyer, R.M.; Gospodarowicz, M.K.; Connors, J.M.; Pearcey, R.G.; Bezjak, A.; Wells, W.A.; Burns, B.F.; Winter, J.N.; Horning, S.J.; Dar, A.R.; et al. Randomized Comparison of ABVD Chemotherapy with a Strategy That Includes Radiation Therapy in Patients With Limited-Stage Hodgkin’s Lymphoma: National Cancer Institute of Canada Clinical Trials Group and the Eastern Cooperative Oncology Group. J. Clin. Oncol. 2005, 23, 4634–4642. [Google Scholar] [CrossRef]
- Radford, J.; Barrington, S.; Counsell, N.; Pettengell, R.; Johnson, P.; Wimperis, J.; Coltart, S.; Culligan, D.; Lister, A.; Bessell, E.; et al. Involved Field Radiotherapy Versus No Further Treatment in Patients with Clinical Stages IA and IIA Hodgkin Lymphoma and a ‘Negative’ PET Scan After 3 Cycles ABVD. Results of the UK NCRI RAPID Trial. Blood 2012, 120, 547. [Google Scholar] [CrossRef]
- André, M.P.; Girinsky, T.; Federico, M.; Reman, O.; Fortpied, C.; Gotti, M.; Casasnovas, O.; Brice, P.; van der Maazen, R.; Re, A.; et al. Early Positron Emission Tomography Response-Adapted Treatment in Stage I and II Hodgkin Lymphoma: Final Results of the Randomized EORTC/LYSA/FIL H10 Trial. J. Clin. Oncol. 2017, 35, 1786–1794. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, M.; Goergen, H.; Kobe, C.; Kuhnert, G.; Lohri, A.; Greil; Richard, S.S.; Topp, M.S.; Schäfer, E.; Schäfer, S.; et al. Positron Emission Tomography-Guided Treatment in Early-Stage Favorable Hodgkin Lymphoma: Final Results of the International, Randomized Phase III HD16 Trial by the German Hodgkin Study Group. J. Clin. Oncol. 2019, 37, 2835–2845. [Google Scholar] [CrossRef] [PubMed]
- Engert, A.; Haverkamp, H.; Kobe, C.; Markova, J.; Renner, C.; Ho, A.; Zijlstra, J.; Král, Z.; Fuchs, M.; Hallek, M.; et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): A randomised, open-label, phase 3 non-inferiority trial. Lancet 2012, 379, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Armenian, S.H.; Landier, W. How I monitor long-term and late effects after blood or marrow transplantation. Blood 2017, 130, 1302–1314. [Google Scholar] [CrossRef]
- Barrington, S.F.; Kirkwood, A.A.; Franceschetto, A.; Fulham, M.J.; Roberts, T.H.; En Almquist, H.; Brun, E.; Hjorthaug, K.; Viney, Z.N.; Pike, L.C.; et al. PET-CT for staging and early response: Results from the Response-Adapted Therapy in Advanced Hodgkin Lymphoma Study. Blood J. Am. Soc. Hematol. 2016, 127, 1531–1538. [Google Scholar] [CrossRef]
- Borchmann, P.; Goergen, H.; Kobe, C.; Lohri, A.; Greil, R.; Eichenauer, D.A.; Zijlstra, J.M.; Markova, J.; Meissner, J.; Feuring-Buske, M.; et al. PET-guided treatment in patients with advanced-stage Hodgkin’s lymphoma (HD18): Final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet 2017, 390, 2790–2802. [Google Scholar] [CrossRef]
- von Tresckow, B.; Plütschow, A.; Fuchs, M.; Klimm, B.; Markova, J.; Lohri, A.; Kral, Z.; Greil, R.; Topp, M.S.; Meissner, J.; et al. Dose-intensification in early unfavorable Hodgkin’s lymphoma: Final analysis of the German Hodgkin study group HD14 trial. J. Clin. Oncol. 2012, 30, 907–913. [Google Scholar] [CrossRef]
- Gillessen, S.; Plütschow, A.; Fuchs, M.; Markova, J.; Greil, R.; Topp, M.S.; Meissner, J.; Zijlstra, J.M.; Eichenauer, D.A.; Bröckelmann, P.J.; et al. Intensified treatment of patients with early stage, unfavourable Hodgkin lymphoma: Long-term follow-up of a randomised, international phase 3 trial of the German Hodgkin Study Group (GHSG HD14). Lancet Haematol. 2021, 8, e278–e288. [Google Scholar] [CrossRef]
- Borchmann, P.; Plütschow, A.; Kobe, C.; Greil, R.; Meissner, J.; Topp, M.S.; Ostermann, H.; Dierlamm, J.; Mohm, J.; Thiemer, J.; et al. PET-Guided Omission of Radiotherapy in Early-Stage Unfavourable Hodgkin Lymphoma (GHSG HD17): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2021, 22, 223–234. [Google Scholar]
- Bröckelmann, P.J.; McMullen, S.; Wilson, J.B.; Mueller, K.; Goring, S.; Stamatoullas, A.; Zagadailov, E.; Gautam, A.; Huebner, D.; Dalal, M.; et al. Patient and physician preferences for first-line treatment of classical Hodgkin lymphoma in Germany, France and the United Kingdom. Br. J. Haematol. 2019, 184, 202–214. [Google Scholar] [CrossRef]
- Uttenthal, B. (Department of Haematology, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK); Osborne, W. (Department of Haematology, Newcastle Hospitals NHS Foundation Trust, Tyne, UK). Personal communication, 2021.
- Fornecker, L.-M.; Lazarovici, J.; Aurer, I.; Casasnovas, R.-O.; Gac, A.-C.; Bonnet, C.; Bouabdallah, K.; Feugier, P.; Specht, L.; Molina, L.; et al. Brentuximab Vedotin Plus AVD for First-Line Treatment of Early-Stage Unfavorable Hodgkin Lymphoma (BREACH): A Multicenter, Open-Label, Randomized, Phase II Trial. J. Clin. Oncol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Skoetz, N.; Trelle, S.; Rancea, M.; Haverkamp, H.; Diehl, V.; Engert, A.; Borchmann, P. Effect of initial treatment strategy on survival of patients with advanced-stage Hodgkin’s lymphoma: A systematic review and network meta-analysis. Lancet Oncol. 2013, 14, 943–952. [Google Scholar] [CrossRef]
- Viviani, S.; Zinzani, P.L.; Rambaldi, A.; Brusamolino, E.; Levis, A.; Bonfante, V.; Vitolo, U.; Pulsoni, A.; Liberati, A.M.; Specchia, G.; et al. ABVD versus BEACOPP for Hodgkin’s Lymphoma When High-Dose Salvage Is Planned. N. Engl. J. Med. 2011, 365, 203–212. [Google Scholar] [CrossRef]
- Carde, P.; Karrasch, M.; Fortpied, C.; Brice, P.; Khaled, H.; Casasnovas, O.; Caillot, D.; Gaillard, I.; Bologna, S.; Ferme, C.; et al. Eight cycles of ABVD versus four cycles of BEACOPPescalated plus four cycles of BEACOPPbaseline in Stage III to IV, International Prognostic Score ≥ 3, high-risk Hodgkin lymphoma: First results of the phase III EORTC 20012 intergroup trial. J. Clin. Oncol. 2016, 34, 2028–2036. [Google Scholar] [CrossRef] [PubMed]
- Mounier, N.; Brice, P.; Bologna, S.; Briere, J.; Gaillard, I.; Heczko, M.; Gabarre, J.; Casasnovas, O.; Jaubert, J.; Colin, P.; et al. ABVD (8 cycles) versus BEACOPP (4 escalated cycles ≥4 baseline): Final results in stage III-IV low-risk Hodgkin lymphoma (IPS 0-2) of the LYSA H34 randomized trial. Ann. Oncol. 2014, 25, 1622–1628. [Google Scholar] [CrossRef]
- Johnson, P.; Federico, M.; Kirkwood, A.; Fosså, A.; Berkahn, L.; Carella, A.; d’Amore, F.; Enblad, G.; Franceschetto, A.; Fulham, M.; et al. Adapted Treatment Guided by Interim PET-CT Scan in Advanced Hodgkin’s Lymphoma. N. Engl. J. Med. 2016, 374, 2419–2429. [Google Scholar] [CrossRef]
- Stephens, D.M.; Li, H.; Schöder, H.; Straus, D.J.; Moskowitz, C.H.; LeBlanc, M.; Rimsza, L.M.; Bartlett, N.L.; Evens, A.M.; LaCasce, A.S.; et al. Five-year follow-up of SWOG S0816: Limitations and values of a PET-adapted approach with stage III/IV Hodgkin lymphoma. Blood 2019, 134, 1238–1246. [Google Scholar] [CrossRef]
- Gallamini, A.; Patti, C.; Viviani, S.; Rossi, A.; Fiore, F.; di Raimondo, F.; Cantonetti, M.; Stelitano, C.; Feldman, T.; Gavarotti, P.; et al. Early chemotherapy intensification with BEACOPP in advanced-stage Hodgkin lymphoma patients with a interim-PET positive after two ABVD courses. Br. J. Haematol. 2011, 152, 551–560. [Google Scholar] [CrossRef]
- Gallamini, A.; Hutchings, M.; Rigacci, L.; Specht, L.; Merli, F.; Hansen, M.; Patti, C.; Loft, A.; di Raimondo, F.; D’Amore, F.; et al. Early Interim 2-[ 18 F]Fluoro-2-Deoxy-D-Glucose Positron Emission Tomography Is Prognostically Superior to International Prognostic Score in Advanced-Stage Hodgkin’s Lymphoma: A Report From a Joint Italian-Danish Study. J. Clin. Oncol. 2007, 25, 3746–3752. [Google Scholar] [CrossRef]
- Russell, J.; Collins, A.; Fowler, A.; Karanth, M.; Saha, C.; Docherty, S.; Padayatty, J.; Maw, K.; Lentell, I.; Cooke, L.; et al. Advanced Hodgkin lymphoma in the East of England: A 10-year comparative analysis of outcomes for real-world patients treated with ABVD or escalated-BEACOPP, aged less than 60 years, compared with 5-year extended follow-up from the RATHL trial. Ann. Hematol. 2021, 100, 1049–1058. [Google Scholar] [CrossRef]
- Connors, J.M.; Jurczak, W.; Straus, D.J.; Ansell, S.M.; Kim, W.S.; Gallamini, A.; Younes, A.; Alekseev, S.; Illés, Á.; Picardi, M.; et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 378, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M.; Connors, J.M.; Radford, J.A.; Kim, W.S.; Gallamini, A.; Ramchandren, R.; Friedberg, J.W.; Advani, R.H.; Hutchings, M.; Evens, A.M.; et al. First-line brentuximab vedotin plus chemotherapy to improve overall survival in patients with stage III/IV classical Hodgkin lymphoma: An updated analysis of ECHELON-1. J. Clin. Oncol. 2022, 40, 7503. [Google Scholar] [CrossRef]
- Ansell, S.M.; Radford, J.; Connors, J.M.; Długosz-Danecka, M.; Kim, W.-S.; Gallamini, A.; Ramchandren, R.; Friedberg, J.W.; Advani, R.; Hutchings, M.; et al. Overall Survival with Brentuximab Vedotin in Stage III or IV Hodgkin’s Lymphoma. N. Engl. J. Med. 2022, 387, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Casasnovas, R.-O.; Bouabdallah, R.; Brice, P.; Lazarovici, J.; Ghesquieres, H.; Stamatoullas, A.; Dupuis, J.; Gac, A.-C.; Gastinne, T.; Joly, B.; et al. PET-adapted treatment for newly diagnosed advanced Hodgkin lymphoma (AHL2011): A randomised, multicentre, non-inferiority, phase 3 study. Lancet Oncol. 2019, 20, 202–215. [Google Scholar] [CrossRef]
- Santarsieri, A.; Sturgess, K.; Brice, P.; Menne, T.F.; Osborne, W.; Creasey, T.; Ardeshna, K.M.; Behan, S.; Bhuller, K.; Booth, S.; et al. Real World Escalated Beacopdac Delivers Similar Outcomes to Escalated Beacopp and Superior Outcomes to Response-Adapted (RATHL) ABVD, While Potentially Reducing Toxicity Compared with Escalated Beacopp. Blood 2021, 138, 877. [Google Scholar] [CrossRef]
- Santarsieri, A.; Mitchell, E.; Sturgess, K. Replacing procarbazine with dacarbazine in escalated BEACOPP dramatically reduces the post treatment haematopoietic stem and progenitor cell mutational burden in Hodgkin lymphoma patients with no apparent loss of clinical efficacy. Blood 2022. [Google Scholar]
- Follows, G.A.; Ardeshna, K.M.; Barrington, S.F.; Culligan, D.J.; Hoskin, P.J.; Linch, D.; Sadullah, S.; Williams, M.V.; Wimperis, J.Z. Guidelines for the first line management of classical Hodgkin lymphoma. Br. J. Haematol. 2014, 166, 34–49. [Google Scholar] [CrossRef]
- Johnson, P.W.M.; Sydes, M.R.; Hancock, B.W.; Cullen, M.; Radford, J.A.; Stenning, S.P. Consolidation Radiotherapy in Patients with Advanced Hodgkin’s Lymphoma: Survival Data from the UKLG LY09 Randomized Controlled Trial (ISRCTN97144519). J. Clin. Oncol. 2010, 28, 3352–3359. [Google Scholar] [CrossRef]
- Trotman, J.; Fosså, A.; Federico, M.; Stevens, L.; Kirkwood, A.; Clifton-Hadley, L.; Patrick, P.; Berkahn, L.; D’Amore, F.; Enblad, G.; et al. Response-adjusted therapy for advanced hodgkin lymphoma (RATHL) trial: Longer follow up confirms efficacy of de-escalation after a negative interim PET scan (CRUK/07/033). Hematol. Oncol. 2017, 35, 65–67. [Google Scholar] [CrossRef][Green Version]
- Gallamini, A.; Rossi, A.; Patti, C.; Picardi, M.; Romano, A.; Cantonetti, M.; Oppi, S.; Viviani, S.; Bolis, S.; Trentin, L.; et al. Consolidation Radiotherapy Could Be Safely Omitted in Advanced Hodgkin Lymphoma with Large Nodal Mass in Complete Metabolic Response After ABVD: Final Analysis of the Randomized GITIL/FIL HD0607 Trial. J. Clin. Oncol. 2020, 38, 3905–3913. [Google Scholar] [CrossRef]
- Allen, P.B.; Savas, H.; Evens, A.M.; Advani, R.H.; Palmer, B.; Pro, B.; Karmali, R.; Mou, E.; Bearden, J.; Dillehay, G.; et al. Pembrolizumab followed by AVD in untreated early unfavorable and advanced-stage classical Hodgkin lymphoma. Blood 2021, 137, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Bröckelmann, P.J.; Goergen, H.; Keller, U.; Meissner, J.; Ordemann, R.; Halbsguth, T.V.; Sasse, S.; Sökler, M.; Kerkhoff, A.; Mathas, S.; et al. Efficacy of Nivolumab and AVD in Early-Stage Unfavorable Classic Hodgkin Lymphoma. JAMA Oncol. 2020, 6, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef] [PubMed]
| Risk Factors | GHSG | EORTC | NCIC/ECOG |
|---|---|---|---|
| Large mediastinal mass | Yes, ratio ≥ 1/3 | Yes, ratio ≥ 0.35 | No |
| Extranodal disease | Yes | No | No |
| Nodal areas | Yes, ≥3 areas | Yes, ≥4 areas | Yes, ≥4 areas |
| ESR | Yes, ≥50 (A) or ≥30 (B) | Yes, ≥50 (A) or ≥30 (B) | Yes, ≥50 |
| Age | No | Yes, ≥50 years | Yes, ≥40 years |
| Histology other than LS/NS | No | No | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Follows, A.M.; Santarsieri, A. Minimising the Toxicities of First Line Hodgkin Lymphoma Treatment in the Modern Era. Cancers 2022, 14, 5390. https://doi.org/10.3390/cancers14215390
Follows AM, Santarsieri A. Minimising the Toxicities of First Line Hodgkin Lymphoma Treatment in the Modern Era. Cancers. 2022; 14(21):5390. https://doi.org/10.3390/cancers14215390
Chicago/Turabian StyleFollows, Annabel M., and Anna Santarsieri. 2022. "Minimising the Toxicities of First Line Hodgkin Lymphoma Treatment in the Modern Era" Cancers 14, no. 21: 5390. https://doi.org/10.3390/cancers14215390
APA StyleFollows, A. M., & Santarsieri, A. (2022). Minimising the Toxicities of First Line Hodgkin Lymphoma Treatment in the Modern Era. Cancers, 14(21), 5390. https://doi.org/10.3390/cancers14215390






