The Impact of the COVID Pandemic on the Incidence of Presentations with Cancer-Related Symptoms in Primary Care
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Population
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Demographics
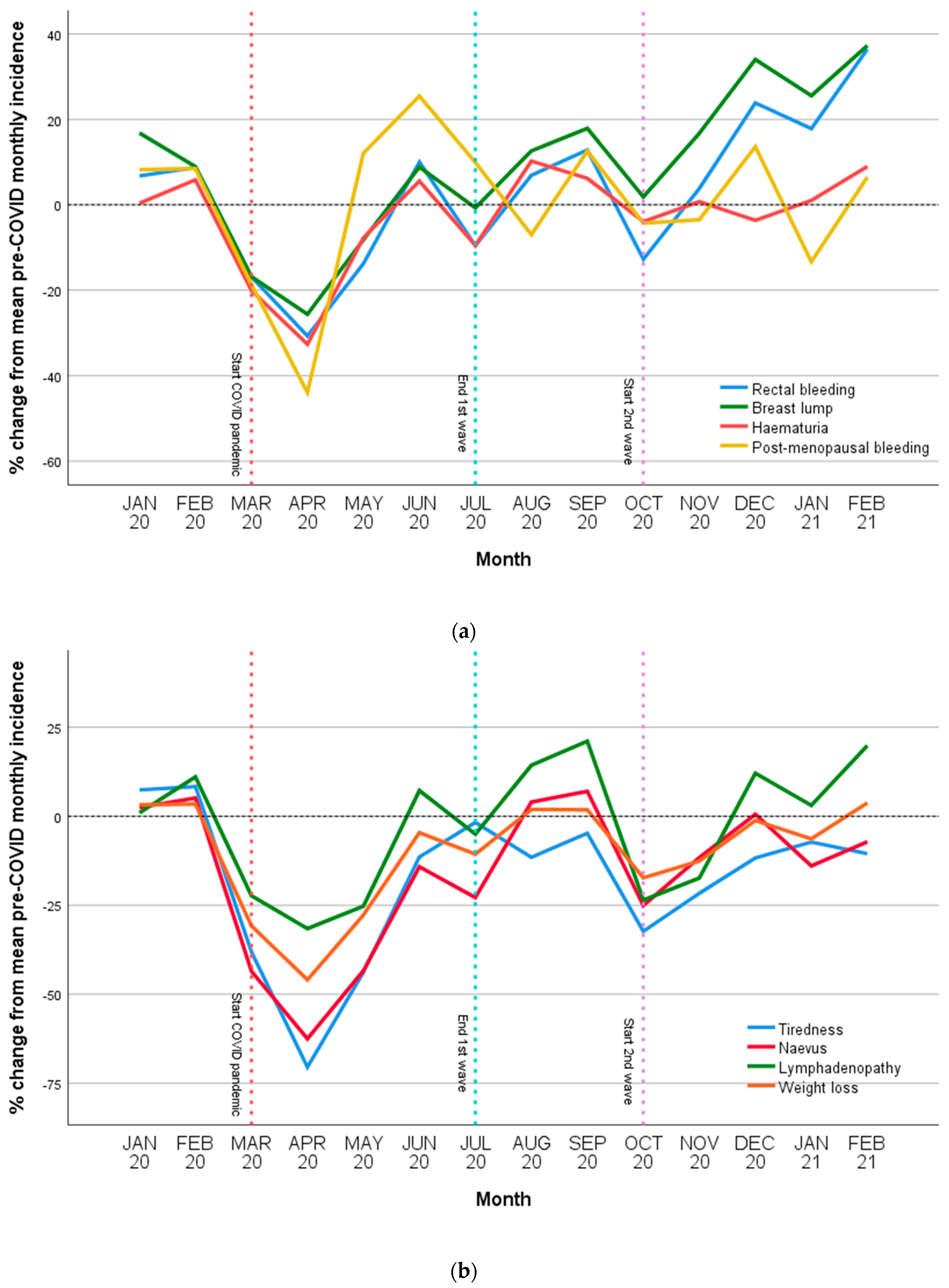
3.2. Incidence of Cancer-Related Symptoms
3.3. Group Differences
3.4. Differences in Incidence over First Year of COVID
4. Discussion
4.1. Main Findings/Results of the Study
4.2. What This Study Adds
4.3. Strengths and Weaknesses/Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Weert, H. After the First Wave: What Effects did the COVID-19 Measures Have on Regular Care and How can General Practitioners Respond to This? Eur. J. Gen. Pract. 2020, 26, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Papautsky, E.L.; Rice, D.R.; Ghoneima, H.; McKowen, A.L.W.; Anderson, N.; Wootton, A.R.; Veldhuis, C. Characterizing health care delays and interruptions in the United States during the COVID-19 pandemic: Internet-based, cross-sectional survey study. J. Med. Internet Res. 2021, 23, e25446. [Google Scholar] [CrossRef] [PubMed]
- Splinter, M.J.; Velek, P.; Ikram, M.K.; Kieboom, B.C.T.; Peeters, R.P.; Bindels, P.J.E.; Wolters, F.J.; Leening, M.J.G.; de Schepper, E.I.T.; Licher, S. Prevalence and determinants of healthcare avoidance during the COVID-19 pandemic: A population-based cross-sectional study. PLoS Med. 2021, 18, e1003854. [Google Scholar] [CrossRef] [PubMed]
- Van Ballegooijen, H.; Goossens, L.; Bruin, R.H.; Michels, R.; Krol, M. Concerns, quality of life, access to care and productivity of the general population during the first 8 weeks of the coronavirus lockdown in Belgium and The Netherlands. BMC Health Serv. Res. 2021, 21, 1–8. [Google Scholar] [CrossRef]
- Dinmohamed, A.G.; Visser, O.; Verhoeven, R.H.; Louwman, M.W.; van Nederveen, F.H.; Willems, S.M.; Merkx, M.A.; Lemmens, V.E.; Nagtegaal, I.D.; Siesling, S. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020, 21, 750–751. [Google Scholar] [CrossRef]
- Naughton, F.; Ward, E.; Khondoker, M.; Belderson, P.; Marie Minihane, A.; Dainty, J.; Hanson, S.; Holland, R.; Brown, T.; Notley, C. Health behaviour change during the UK COVID-19 lockdown: Findings from the first wave of the C-19 health behaviour and well-being daily tracker study. Br. J. Health Psychol. 2021, 26, 624–643. [Google Scholar] [CrossRef]
- Helsper, C.W.; Campbell, C.; Emery, J.; Neal, R.D.; Li, L.; Rubin, G.; Van Weert, H.; Vedsted, P.; Walter, F.M.; Weller, D.; et al. Cancer has not gone away: A primary care perspective to support a balanced approach for timely cancer diagnosis during COVID-19. Eur. J. Cancer Care 2020, 29, e13290. [Google Scholar] [CrossRef]
- Moynihan, R.; Sanders, S.; Michaleff, Z.A.; Scott, A.M.; Clark, J.; To, E.J.; Jones, M.; Kitchener, E.; Fox, M.; Johansson, M.; et al. Impact of COVID-19 pandemic on utilisation of healthcare services: A systematic review. BMJ Open 2021, 11, e045343. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.P.; Vedsted, P.; Sokolowski, I.; Søndergaard, J.; Olesen, F. Time intervals from first symptom to treatment of cancer: A cohort study of 2,212 newly diagnosed cancer patients. BMC Health Serv. Res. 2011, 11, 284. [Google Scholar] [CrossRef]
- Spicer, J.; Chamberlain, C.; Papa, S. Provision of cancer care during the COVID-19 pandemic. Nat. Rev. Clin. Oncol. 2020, 17, 329–331. [Google Scholar] [CrossRef]
- Sud, A.; Torr, B.; Jones, M.E.; Broggio, J.; Scott, S.; Loveday, C.; Garrett, A.; Gronthoud, F.; Nicol, D.L.; Jhanji, S.; et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: A modelling study. Lancet Oncol. 2020, 21, 1035–1044. [Google Scholar] [CrossRef]
- Quinn-Scoggins, H.D.; Cannings-John, R.; Moriarty, Y.; Whitelock, V.; Whitaker, K.L.; Grozeva, D.; Hughes, J.; Townson, J.; Osborne, K.; Goddard, M.; et al. Cancer symptom experience and help-seeking behaviour during the COVID-19 pandemic in the UK: A cross-sectional population survey. BMJ Open 2021, 11, e053095. [Google Scholar] [CrossRef] [PubMed]
- Coyer, L.; Boyd, A.; Schinkel, J.; Agyemang, C.; Galenkamp, H.; Koopman, A.D.; Leenstra, T.; van Duijnhoven, Y.T.; van Charante, E.P.M.; van den Born, B.J.H.; et al. Differences in SARS-CoV-2 infections during the first and second wave of SARS-CoV-2 between six ethnic groups in Amsterdam, the Netherlands: A population-based longitudinal serological study. Lancet Reg. Health-Eur. 2022, 13, 100284. [Google Scholar] [CrossRef] [PubMed]
- De Vincentiis, L.; Carr, R.A.; Mariani, M.P.; Ferrara, G. Cancer diagnostic rates during the 2020 ‘lockdown’, due to COVID-19 pandemic, compared with the 2018–2019: An audit study from cellular pathology. J. Clin. Pathol. 2021, 74, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Marques, N.P.; Silveira, D.M.M.; Marques, N.C.T.; Martelli, D.R.B.; Oliveira, E.A.; Martelli-Júnior, H. Cancer diagnosis in Brazil in the COVID-19 era. Semin. Oncol. 2021, 48, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, B.D.; Ordóñez-Mena, J.M.; Lay-Flurrie, S.; Sheppard, J.P.; Liyanage, H.; McGagh, D.; Sherlock, J.; Williams, J.; Smith, M.; Drakesmith, C.W.; et al. Consultations for clinical features of possible cancer and associated urgent referrals before and during the COVID-19 pandemic: An observational cohort study from English primary care. Br. J. Cancer 2021, 126, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Peacock, H.; Tambuyzer, T.; Verdoodt, F.; Calay, F.; Poirel, H.; De Schutter, H.; Francart, J.; Van Damme, N.; Van Eycken, L. Decline and incomplete recovery in cancer diagnoses during the COVID-19 pandemic in Belgium: A year-long, population-level analysis. ESMO Open 2021, 6, 100197. [Google Scholar] [CrossRef]
- Toes-Zoutendijk, E.; Vink, G.; Nagtegaal, I.D.; Spaander, M.C.; Dekker, E.; van Leerdam, M.E.; Siesling, S.; Lansdorp-Vogelaar, I.; Elferink, M.A. Impact of COVID-19 and suspension of colorectal cancer screening on incidence and stage distribution of colorectal cancers in the Netherlands. Eur. J. Cancer 2021, 161, 38–43. [Google Scholar] [CrossRef]
- Van Not, O.J.; van Breeschoten, J.; van den Eertwegh, A.J.; Hilarius, D.L.; De Meza, M.M.; Haanen, J.B.; Blank, C.U.; Aarts, M.J.; van den Berkmortel, F.W.; de Groot, J.W.B.; et al. The unfavorable effects of COVID-19 on Dutch advanced melanoma care. Int. J. Cancer 2022, 150, 816–824. [Google Scholar]
- Eijkelboom, A.H.; de Munck, L.; Peeters, M.-J.T.F.D.V.; Broeders, M.J.M.; Strobbe, L.J.A.; Bos, M.E.M.M.; Schmidt, M.K.; Paez, C.G.; Smidt, M.L.; Bessems, M.; et al. Impact of the COVID-19 pandemic on diagnosis, stage, and initial treatment of breast cancer in the Netherlands: A population-based study. J. Hematol. Oncol. 2021, 14, 1–12. [Google Scholar] [CrossRef]
- Weller, D.; Vedsted, P.; Rubin, G.; Walter, F.M.; Emery, J.; Scott, S.; Campbell, C.; Andersen, R.S.; Hamilton, W.; Olesen, F.; et al. The Aarhus statement: Improving design and reporting of studies on early cancer diagnosis. Br. J. Cancer 2012, 106, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.E.; Walter, F.M.; Webster, A.; Sutton, S.; Emery, J. The Model of Pathways to Treatment: Conceptualization and integration with existing theory. Br. J. Health Psychol. 2013, 18, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, W.; Kroneman, M.; Boerma, W.; Berg, M.V.D.; Westert, G.; Devillé, W.; Van Ginneken, E. The Netherlands: Health system review. Health Syst. Transit. 2010, 12. [Google Scholar]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. Faculty Opinions recommendation of The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Epidemiology 2008, 18, 867–872. [Google Scholar] [CrossRef]
- Environment NIoHat. Third Wave Shows Major Surge in Hospital Admissions in Younger Age Groups. 2021. Available online: https://www.rivm.nl/en/news/third-wave-shows-major-surge-in-hospital-admissions-in-younger-age-groups (accessed on 28 August 2022).
- Smeets, H.M.; Kortekaas, M.F.; Rutten, F.H.; Bots, M.L.; van der Kraan, W.; Daggelders, G.; Smits-Pelser, H.; Helsper, C.W.; Hoes, A.W.; de Wit, N.J. Routine primary care data for scientific research, quality of care programs and educational purposes: The Julius General Practitioners’ Network (JGPN). BMC Health Serv. Res. 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Dutch College of General Practitioners NTICoPC. Version 6. (Dutch translation derived from ICPC-1 by the WONCA International Classification Committee). 2018.
- Shapley, M.; Mansell, G.; Jordan, J.L.; Jordan, K.P. Positive predictive values of ≥5% in primary care for cancer: Systematic review. Br. J. Gen. Pract. 2010, 60, e366–e377. [Google Scholar] [CrossRef]
- Jones, R.; Latinovic, R.; Charlton, J.; Gulliford, M. Alarm symptoms in early diagnosis of cancer in primary care: Cohort study using General Practice Research Database. BMJ 2007, 334, 1040. [Google Scholar] [CrossRef]
- McLaughlin, J.M.; Anderson, R.T.; Ferketich, A.K.; Seiber, E.E.; Balkrishnan, R.; Paskett, E.D. Effect on Survival of Longer Intervals Between Confirmed Diagnosis and Treatment Initiation Among Low-Income Women With Breast Cancer. J. Clin. Oncol. 2012, 30, 4493–4500. [Google Scholar] [CrossRef]
- Degeling, K.; Baxter, N.N.; Emery, J.; Jenkins, M.A.; Franchini, F.; Gibbs, P.; Mann, G.B.; McArthur, G.; Solomon, B.J.; Ijzerman, M.J. An inverse stage-shift model to estimate the excess mortality and health economic impact of delayed access to cancer services due to the COVID-19 pandemic. Asia-Pacific J. Clin. Oncol. 2021, 17, 359–367. [Google Scholar] [CrossRef]
- Malagón, T.; Yong, J.H.; Tope, P.; Miller, W.H., Jr.; Franco, E.L.; McGill Task Force on the Impact of COVID-19 on Cancer Control and Care. Predicted long-term impact of COVID-19 pandemic-related care delays on cancer mortality in Canada. Int. J. Cancer 2022, 150, 1244–1254. [Google Scholar] [CrossRef]
- White, B.; Rafiq, M.; Gonzalez-Izquierdo, A.; Hamilton, W.; Price, S.; Lyratzopoulos, G. Risk of cancer following primary care presentation with fatigue: A population-based cohort study of a quarter of a million patients. Br. J. Cancer 2022, 126, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Schuster, N.A.; de Breij, S.; Schaap, L.A.; van Schoor, N.M.; Peters, M.J.; de Jongh, R.T.; Huisman, M.; Hoogendijk, E.O. Older adults report cancellation or avoidance of medical care during the COVID-19 pandemic: Results from the Longitudinal Aging Study Amsterdam. Eur. Geriatr. Med. 2021, 12, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
| n (%) | ||
|---|---|---|
| Number patients included | 1,233,035 | |
| Age Group | 18–49 | 681,447 (55%) |
| 50–75 | 440,017 (36%) | |
| 76 and older | 111,571 (9%) | |
| Sex | Female | 637,242 (52%) |
| Major comorbidity | Cardiovascular Disease | 128,796 (10%) |
| Diabetes | 71,665 (6%) | |
| Chronic obstructive airways disease (COPD) | 14,768 (1%) | |
| Psychiatric/psychological | 104,785 (9%) | |
| History of cancer | 46,779 (4%) |
| Mean Monthly pre-COVID Incidence | First Wave | Summer Period | Second Wave | ||||
|---|---|---|---|---|---|---|---|
| Per 100,000 Population | Incidence Rate Ratio | 95% CI | Incidence Rate Ratio | 95% CI | Incidence Rate Ratio | 95% CI | |
| Rectal bleeding | 163 | 0.87 | 0.76–1.00 | 1.04 | 0.90–1.21 | 1.14 | 1.00–1.30 |
| Breast lump | 190 | 0.83 | 0.73–0.94 | 1.02 | 0.90–1.16 | 1.14 | 1.02–1.27 |
| Postmenopausal bleeding | 69 | 0.87 | 0.73–1.03 | 0.99 | 0.82–1.19 | 0.93 | 0.80–1.09 |
| Haematuria | 120 | 0.85 | 0.76–0.94 | 1.01 | 0.89–1.13 | 0.99 | 0.90–1.10 |
| Dysphagia | 69 | 0.99 | 0.86–1.15 | 1.25 | 1.07–1.46 | 1.01 | 0.88–1.16 |
| Abdominal mass | 27 | 0.79 | 0.63–0.99 | 1.15 | 0.91–1.44 | 1.06 | 0.87–1.30 |
| Melaena | 23 | 0.78 | 0.61–0.99 | 1.27 | 0.99–1.63 | 1.18 | 0.96–1.46 |
| Change in bowel habit | 74 | 0.79 | 0.69–0.91 | 1.00 | 0.87–1.16 | 0.97 | 0.85–1.10 |
| Tiredness | 798 | 0.55 | 0.45–0.67 | 0.91 | 0.73–1.13 | 0.80 | 0.67–0.97 |
| Lymphadenopathy | 87 | 0.75 | 0.65–0.87 | 1.01 | 0.87–1.18 | 0.91 | 0.79–1.04 |
| Naevus | 379 | 0.63 | 0.53–0.75 | 1.04 | 0.86–1.25 | 0.96 | 0.81–1.13 |
| Weight loss | 124 | 0.78 | 0.67–0.90 | 1.04 | 0.90–1.21 | 0.99 | 0.87–1.13 |
| All cancer symptoms combined * | 582 | 0.66 | 0.57–0.77 | 0.98 | 0.84–1.15 | 0.92 | 0.80–1.06 |
| Mean Incidence per Month in The Netherlands Prior to COVID | Difference in Incidence 1st Wave | Difference in Incidence Summer Period | Difference in Incidence 2nd Wave | Annual Difference in Incidence March 2020 to Feb 2021 Compared to pre-COVID | |
|---|---|---|---|---|---|
| Rectal bleeding | 4578 | −2333 | +449 | +3393 | +1509 |
| Breast lump | 5354 | −2108 | +1514 | +6345 | +5751 |
| Haematuria | 3341 | −1770 | +195 | +176 | −1400 |
| Change in bowel habit | 2722 | −1847 | +351 | +123 | −1373 |
| Tiredness | 37,512 | −63,582 | −6600 | −30,702 | −100,883 |
| Lymphadenopathy | 3180 | −2332 | +875 | −210 | −1668 |
| Naevus | 17,239 | −28,145 | −3148 | −9044 | −40,337 |
| Weight loss | 4637 | −4712 | −359 | −1533 | −6605 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grant, M.P.; Helsper, C.W.; Stellato, R.; van Erp, N.; van Asselt, K.M.; Slottje, P.; Muris, J.; Brandenbarg, D.; de Wit, N.J.; van Gils, C.H. The Impact of the COVID Pandemic on the Incidence of Presentations with Cancer-Related Symptoms in Primary Care. Cancers 2022, 14, 5353. https://doi.org/10.3390/cancers14215353
Grant MP, Helsper CW, Stellato R, van Erp N, van Asselt KM, Slottje P, Muris J, Brandenbarg D, de Wit NJ, van Gils CH. The Impact of the COVID Pandemic on the Incidence of Presentations with Cancer-Related Symptoms in Primary Care. Cancers. 2022; 14(21):5353. https://doi.org/10.3390/cancers14215353
Chicago/Turabian StyleGrant, Matthew P., Charles W. Helsper, Rebecca Stellato, Nicole van Erp, Kristel M. van Asselt, Pauline Slottje, Jean Muris, Daan Brandenbarg, Niek J. de Wit, and Carla H. van Gils. 2022. "The Impact of the COVID Pandemic on the Incidence of Presentations with Cancer-Related Symptoms in Primary Care" Cancers 14, no. 21: 5353. https://doi.org/10.3390/cancers14215353
APA StyleGrant, M. P., Helsper, C. W., Stellato, R., van Erp, N., van Asselt, K. M., Slottje, P., Muris, J., Brandenbarg, D., de Wit, N. J., & van Gils, C. H. (2022). The Impact of the COVID Pandemic on the Incidence of Presentations with Cancer-Related Symptoms in Primary Care. Cancers, 14(21), 5353. https://doi.org/10.3390/cancers14215353





