Simple Summary
Proteoglycans are important structural and functional components of extracellular matrices that govern phenotype and functions of resident cells. They are often deregulated in cancer cells and their stroma, providing a favorable microenvironment for cancer cell growth and spread. Proteoglycans, by influencing cell–cell and cell–matrix interactions and oncogenic signaling, affect cancer cell phenotype and properties. In this article, we discussed the implication of proteoglycans with cancer cell stemness and epithelial-to-mesenchymal transition.
Abstract
Proteoglycans (PGs) are pivotal components of extracellular matrices, involved in a variety of processes such as migration, invasion, morphogenesis, differentiation, drug resistance, and epithelial-to-mesenchymal transition (EMT). Cellular plasticity is a crucial intermediate phenotypic state acquired by cancer cells, which can modulate EMT and the generation of cancer stem cells (CSCs). PGs affect cell plasticity, stemness, and EMT, altering the cellular shape and functions. PGs control these functions, either by direct activation of signaling cascades, acting as co-receptors, or through regulation of the availability of biological compounds such as growth factors and cytokines. Differential expression of microRNAs is also associated with the expression of PGs and their interplay is implicated in the fine tuning of cancer cell phenotype and potential. This review summarizes the involvement of PGs in the regulation of EMT and stemness of cancer cells and highlights the molecular mechanisms.
1. Introduction
Extracellular matrices (ECMs) are dynamic three-dimensional macromolecular networks which together with the embedded cells form complex structures called tissues, which are further assembled to create organs [1]. Besides acting as physical scaffolds, ECMs support all different cell tissue functions and constitute the main regulators of numerous cell properties, such as survival, proliferation, adhesion, migrations, differentiation, and apoptosis. The main macromolecules of ECMs are collagens, proteoglycans (PGs), laminins, fibronectin, elastin, other glycoproteins, and ECM-degrading enzymes such as proteinases [2]. PGs are crucial ECMs constituents, as they localize throughout the ECMs and are key mediators of cell behavior both in physiological and pathological conditions [3,4]. All PGs are composed of a core protein, which is decorated with variable number and type(s) of five different glycosaminoglycans (GAGs), named chondroitin sulfate (CS), dermatan sulfate (DS), keratan sulfate (KS), heparan sulfate (HS), and heparin (HP). As a result, a variety of PG editing can be achieved, with diverse interaction motifs and roles in the orchestration of cell signaling and phenotype [5,6]. Several members of the PG family have been shown to be deregulated during tumorigenesis and cell differentiation, leading to changes in epithelial-to-mesenchymal transition (EMT) (Figure 1) [5,7]. EMT is a cellular process during which epithelial cells repress their epithelial characteristics and acquire mesenchymal phenotypes and behavior [8,9]. The process has drawn much attention due to its association with cancer metastasis and resistance (Figure 1) [10,11,12].
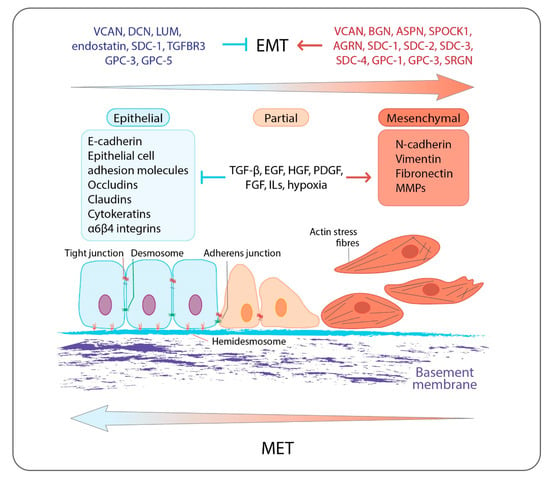
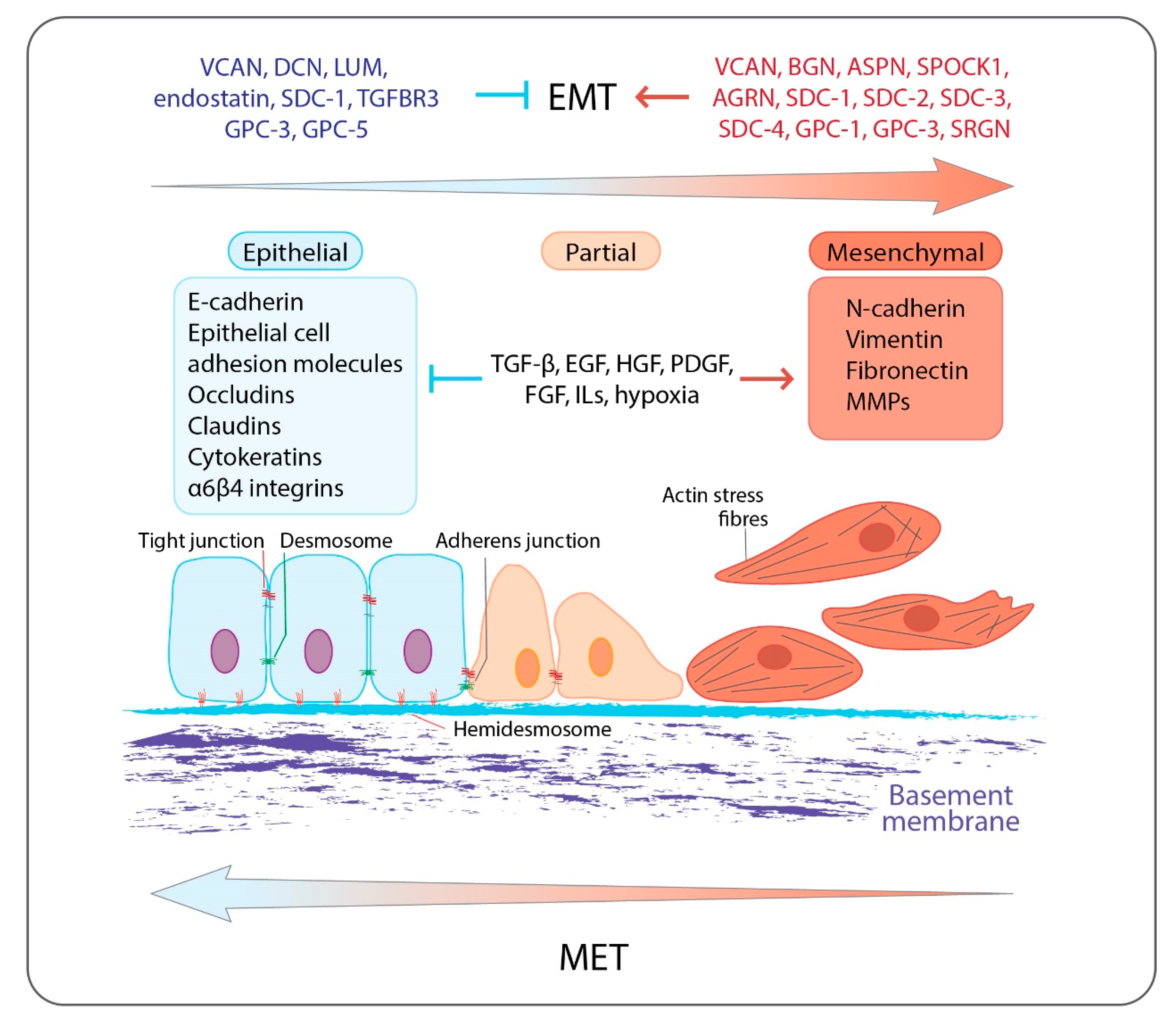
Figure 1.
Overview of EMT: epithelial cells are anchored to the underlying basement membrane by hemidesmosomes and are interconnected by tight junctions, adherens junctions, and desmosomes. They express specific cell surface and cytoskeletal proteins associated with epithelial phenotype such as E-cadherin, epithelial cell adhesion molecules, occludins and claudins, epithelial cell-associated integrins α6β4, and cytokeratins. EMT is triggered by numerous growth factors, cytokines, and hypoxia, which activate a transcription program, which is associated with the inhibition of the expression of epithelial-related genes and the induction of the expression of mesenchymal-related genes including N-cadherin, vimentin, ECM molecules, fibronectin, and MMPs. Mesenchymal cells display increased motility and invasiveness. Epithelial cells during their progressive transition to a mesenchymal phenotype acquire an intermediate state (partial EMT), into which cells exhibit a mixed cell phenotype expressing both epithelial and mesenchymal markers. PGs are involved in the EMT program affecting, among others, EMT-related signaling pathways acting either as inducers or as inhibitors. EMT can be reversed where the mesenchymal cells can revert to the epithelial phenotype by undergoing MET. Both EMT and MET occur during cancer progression and the development of distant metastases.
EMT is an essential biological process under normal physiology, as it is involved in embryonic development and tissue regeneration and repair. In embryonic development, it takes part in gastrulation and renal, heart valve, and neural crest formation, while in wound healing EMT is mediated by inflammatory cells and fibroblasts, and provides the epithelial cells lining the wound with migratory properties so that they can repopulate the wound [13,14,15]. EMT is induced during normal and pathological conditions by a range of cytokines and growth factor-signaling pathways including transforming growth factor β (TGFβ), bone morphogenetic protein (BMP), interleukins (ILs), Wnt–β-catenin, Notch, Hedgehog, receptor tyrosine kinases, and hypoxia (Figure 1) [16]. The major reprogramming of the gene expression during EMT is conducted by a group of transcription factors (TFs) commonly referred to as EMT-TFs, including the Snail family (Snail, encoded by SNAI1, and Slug, encoded by SNAI2), the zinc finger E-box-binding homeobox (ZEB) family (ZEB1 and ZEB2), and the Twist family BHLH transcription factors (TWIST1 and TWIST2) [17,18], yet other transcription factors, such as paired-related homeobox 1 (PRRX1) and forkhead box C2 (FOXC2), can also induce EMT [19,20].
One of the main features of EMT is the acquisition of resistance to anoikis-apoptosis upon loss of attachment to the ECM and neighboring cells [21]. Anoikis comes from the Greek word meaning homelessness, and is a mechanism to prevent inappropriate translocation and attachment of cells in tissues. In the process of EMT, mesenchymal cells lose epithelial adherens junctions, rearrange their actin cytoskeleton, and alter their ECM interactions [8,9]. These changes allow the cells to become motile, and, in addition, the cells induce expression of proteins with properties that can modulate the ECM, such as matrix metalloproteinases (MMPs) (Figure 1) [8,22]. Epithelial cells, on the contrary, have a tight connection with the ECM. Integrins, cell adhesion molecules, and various adaptor proteins take part in these interactions, and bridge the ECM molecules with the cell cytoskeleton (Figure 1) [8,9]. The interconnections established between various ECM components as well as between matrix components with cells allow mechanical force and regulatory signals to be transmitted from the ECM to the cells [23]. For this reason, the ECM is of major importance for tissue homeostasis, and the composition, or changes in composition, of ECM molecules have a direct impact on the signaling networks of the cells. Cancer cells that have undergone EMT and other cells within the tumor stroma, such as tumor-associated macrophages (TAMs) and cancer-associated fibroblasts (CAFs), contribute to ECM degradation and remodeling to facilitate tumor cell plasticity [24]. Further, specific ECM–integrin interactions have been associated with changes in the EMT phenotypes of cells, an example being hyaluronan (HA), which has been shown to induce EMT via it its receptor, CD44 [25]. CD44 has been tightly linked to EMT, as it is upregulated by TGFβ, is directly involved with STAT3, β-catenin, and AKT signaling, and promotes tumor invasion and metastasis by contributing to the adhesion of cancer cells to the endothelium and fibronectin-enriched matrices [26]. CD44 is both considered to be a mesenchymal marker gene and also a marker gene for cancer stem cells.
In vivo experiments show that in breast cancer, but also other cancer types, only a subpopulation of the cancer cells have self-renewing capabilities and stem-like characteristics [27]. These cells have been termed cancer stem cells (CSCs), or also known as tumor-initiating cells. Several cell-surface markers have so far been proposed as makers of CSCs, including EMA, CALLA, CD49f, EpCAM, CD44, and CD24 [28], and many of these markers overlap with cells that have undergone EMT [27]. In addition, CSC express EMT transcription factors, suggesting that mechanisms regulating EMT and stemness are closely integrated [27]. It has been suggested that CSCs have a combined epithelial and mesenchymal phenotype, defined as epithelial mesenchymal plasticity (EMP), permitting these cells to move between an epithelial and a mesenchymal state [29]. EMT, EMP, and the acquisition of stem-like characteristics have all been linked to chemotherapy resistance, dormancy, and relapse [30,31,32]. ECM components, such as several collagens, glycoproteins, PGs, and HA are produced in high quantities by CSCs and take part in regulating their properties. Furthermore, the physical and mechanical properties of the ECM affect CSCs functions, and it has been suggested that a deeper understanding of the involvement of ECM components on cancer stemness could provide novel opportunities for therapies against CSCs [33].
2. Proteoglycans in Brief
PGs are classified into four categories by taking into consideration their localization, the extracellular, pericellular, cell surface, and intracellular states, and further subdivided based on their structural and functional characteristics [7,34,35].
The extracellular PGs are classified into three main subfamilies: the hyalectans, the small leucine-rich proteoglycans (SLRPs), and the Testican/SPARC/Osteonectin CWCV and Kazal-like domain (SPOCK).
Aggrecan, versican (VCAN), brevican, and neurocan are the four members that comprise the hyalectan family and are characterized by C-terminus and N-terminus globular domains that interact with a plethora of ECM molecules and a central part where GAGs, especially CS and KS, bind. Their distribution and functions are exceptionally diverse. For example, aggrecan is mainly found in cartilage and brain tissues, bridging different ECM molecules. The four isoforms of VCAN, V0, V1, V2, and V3 are located in the blood vessels, heart, breast, and brain tissues in diverse proportions, while brevican and neurocan are mainly expressed in the central nervous system. VCAN is a multifunctional molecule that affects cell adhesion, proliferation, and migration via binding to CD44, integrin-β1, and toll-like receptors (TLRs) as well as activating epidermal growth factor receptor (EGFR) signaling pathway by direct binding through its EGF-like domain present at the C-terminus globular domain [7,34,35].
SLRPs constitute a wide PGs family, with repeating leucine residues in their protein core, and are expressed in various tissues. SLRPs can be subdivided based on their structure and organization characteristics into five different classes: three canonical SLRP classes, types I–III, and two non-canonical classes, types IV and V. Decorin (DCN) and biglycan (BGN) belong to SLRP class I, and contain one and two CS/DS chains, respectively, whereas lumican (LUM) is a member of SLRP class II, and carries two to four KS chains. All three of them contain domains that interact with various growth factors (GFs) and GF receptors, such as vascular endothelial growth factor A (VEGFA), TGFβ, platelet-derived growth factor (PDGF), connective tissue growth factor (CTGF), EGFR, insulin-like growth factor receptor I (IGFR-I), VEGFR-II, as well as TLRs, modulating processes including cell growth, development, and inflammation [5,7,34,35].
The pericellular PGs family contains the following four members: Agrin (AGRN), perlecan/HSPG2, and collagen types XV and XVIII. All four members bear a combination of CS and HS chains attached to their diverse protein cores. CS and HS chains mediate the interactions of pericellular PGs with GFs as well as with inflammation and matrix remodeling-related molecules. Interestingly, the C-terminal domain of perlecan/HSPG2 and collagen type XVIII can be proteolytically cleaved, thereby generating bioactive fragments named endorepellin and endostatin, respectively [5,7,34,35].
The cell surface PG superfamily is mainly divided in two subclasses, syndecans and glypicans, whereas three more proteoglycans, CSPG4, phosphacan, and betaglycan, are enlisted in this superfamily. The family of syndecans contains four members of transmembrane PGs (syndecan 1–4), expressed in numerous cell types. Syndecans mainly carry HS chains [5]. They play crucial roles both in physiological processes, such as development, and in certain pathological conditions [5,7,36]. For example, altered expression levels of syndecans can lead to cancer progression, stemness, EMT, and metastasis. Glypicans consist of six members (glypican 1–6) of glycosylphosphatidylinositol (GPI)-anchored cell membrane PGs. They are mainly substituted with HS chains. Their main role is in the regulation of several signaling pathways such as Wnt, Hedgehog, fibroblast growth factor (FGF), TGFβ, and BMPs [5,7,37]. Lastly, betaglycan, also known as TGFβ receptor III (TGFBR3), is a co-receptor of the TGFβ family, regulating its ligands’ bioavailability. The role of TGFBR3 in EMT is highly relevant, as the TGFβ signaling pathway is a crucial modulator, both in physiological and pathological conditions [38].
Serglycin (SRGN) is the only PG that has been characterized as an intracellular PG. The core of SRGN contains a domain with repeating Ser–Gly residues, where eight CS, DS, HS, or HP chains can attach in a cell- and condition-dependent manner. SRGN is expressed by various cells such as hematopoietic, endothelial, smooth muscle cells, fibroblasts, and tumor cells. It can regulate the formation and maturation of secretory granules, and the secretion and the bioavailability of various growth factors, cytokines, and functional ECM molecules involved in tumorigenesis and inflammation. SRGN also directly stimulates oncogenic signaling in several tumor cell types [39,40].
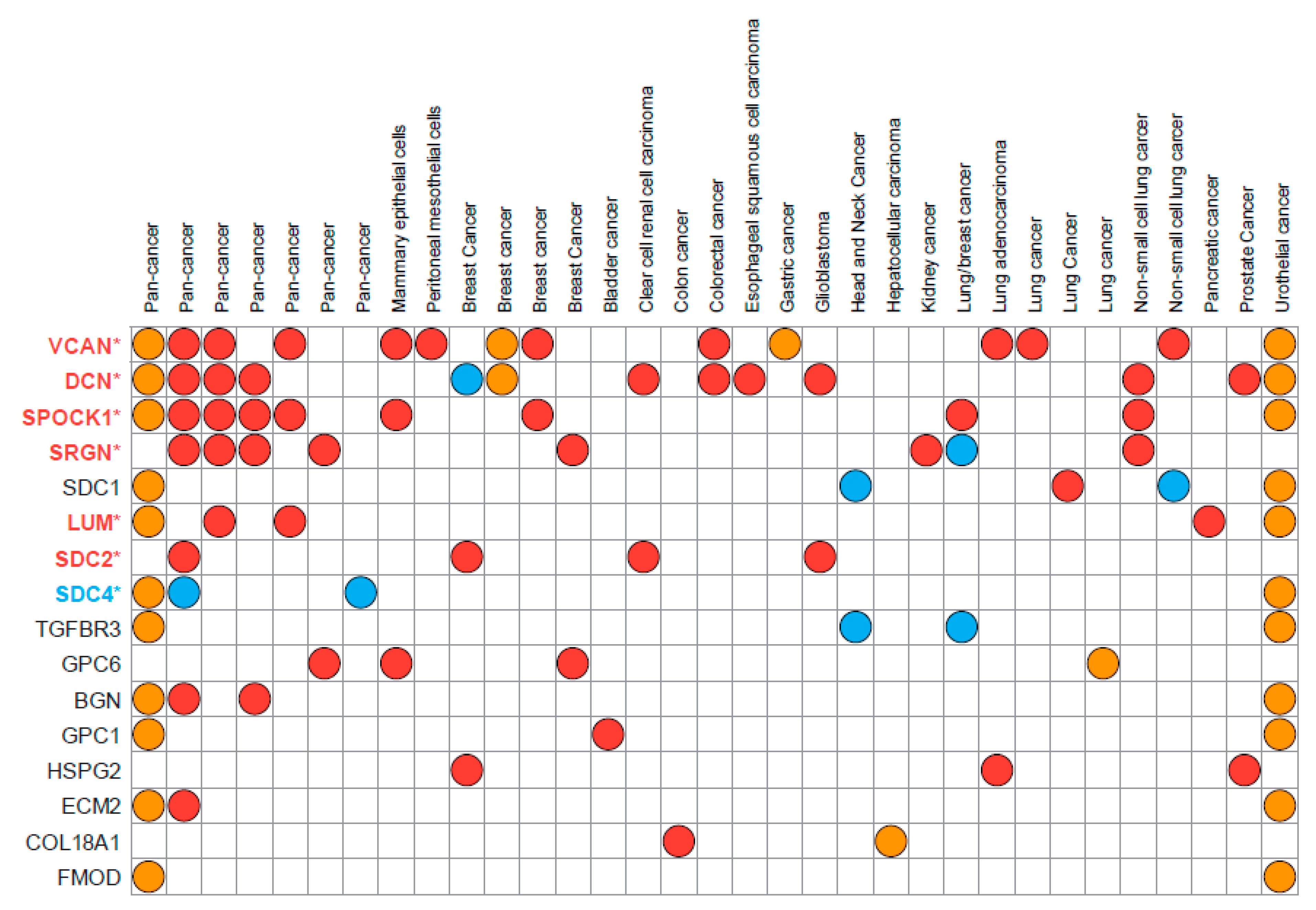
PGs participate in cell–cell and cell–matrix interactions and oncogenic signaling affecting cancer cell phenotype and functional properties. Differential expression of PGs is associated with EMT in various cancer types, and multiple PGs have been identified in proposed EMT signatures deposited in the EMTome database (http://www.emtome.org/ (accessed on 19 August 2022)) [41] (Figure 2).
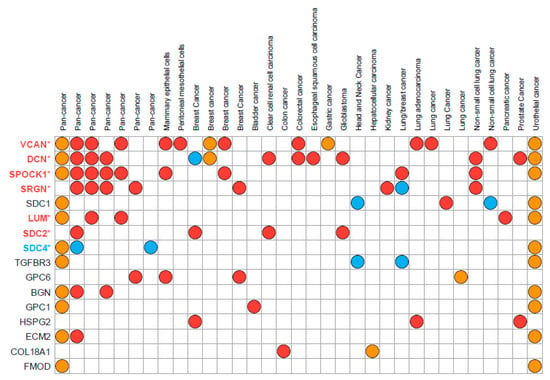
Figure 2.
Proteoglycans found in the proposed EMT signatures deposited in the EMTome database. Eighty-four EMT signatures were downloaded from the EMTome database (http://www.emtome.org/ (accessed on 19 August 2022)) and searched for overlap with 42 proteoglycans. Only proteoglycans identified in at least two signatures are displayed in the figure. Red color indicates association towards the mesenchymal phenotype, blue towards the epithelial phenotype, and yellow indicates that no association towards either the mesenchymal or epithelial phenotype was found in the proposed dataset. Proteoglycans with stars after their name were also discovered as TGFβ-responsive genes in our own publication (PMID: 35494005) Here, colors of the name represent their association to either epithelial (blue) or mesenchymal (red) phenotypes.
3. Proteoglycans as Regulators of EMT and Cell Stemness
3.1. Association of Versican with EMT
VCAN is an extracellular EMT mediator that affects cell phenotypes and functions. A crucial step for embryogenesis is the differentiation of pluripotent embryonic stem cells (ESCs) via embryoid bodies (EBs), a phenomenon driven by induction of EMT in ESCs, in which the expression and specific distribution of VCAN and HA are of great importance. VCAN is highly expressed by CSCs [33,42,43]. Suppressed VCAN expression blocks breast cancer cell self-renewal, while overexpression of the G3-domain of VCAN promotes cancer cell self-renewal via the EGFR/AKT/GSK3β pathway [42] (Table 1). In CD133+/CD44+ prostate CSCs, VCAN causes the suppression of cancer cell attachment to fibronectin and thereby increases cell motility [43]. VCAN is positively regulated by the EMT transcription factor Snail. In addition to VCAN, Snail induces transcription of the sulfate donor PAPSS2, and thus leads to elevated sulfation levels of VCAN, which in turn is associated with increased migration and metastasis [44]. Moreover, the role of VCAN in EMT during cancer seems to be isoform specific. For instance, in breast cancer cells, VCAN V2 expression suppressed Snail and induced E-cadherin protein levels through inhibition of the EGFR/ERK/GSK3β axis. On the contrary, VCAN V1 is proposed to enhance the EGFR pathway [45] (Table 1).

Table 1.
Main roles of PGs in cancer cell EMT and stemness.
VCAN is also reported to be associated with mesenchymal-to-epithelial transition (MET), the reversed process of EMT [46,47,48]. In human metastatic breast model, VCAN derived from bone marrow myeloid progenitor cells induces MET of tumor cells in the premetastatic niche via inhibition of the TGFβ/SMAD2/3 pathway [46] (Table 1).
3.2. SLRPs: Diverse Regulatory Roles in Cancer Cell Signaling Related to Stemness and EMT
BGN has been associated with poor prognosis in several cancer types and seems to contribute to the EMT process [49,50,51,52,53,54]. In bladder cancer, BGN overexpression is associated with the EMT markers such as Snail, Slug, ZEB1-2, TWIST1-2, vimentin, and fibronectin, and negatively correlates with E-cadherin. BGN correlates with overall survival and the expression of the EMT markers N-cadherin and vimentin in head and neck squamous cell carcinoma patients [50]. BGN is further highly expressed in gastric cancer and has been associated with poor prognosis. Here, BGN expression is directly correlated with a mesenchymal gene signature. By knocking out BGN in gastric cancer cells, decreased levels of mesenchymal markers N-cadherin, ZEB1, ZEB2, Snail, NANOG, DPP4, and 3/4 October (POU5F1), and increased expression of the epithelial marker E-cadherin are observed. Treatment of KO cells with exogenous BGN leads to recovery of NANOG, but no differences in E-cadherin and N-cadherin expression levels are observed. Furthermore, the loss of BGN suppresses the colony formation capability of the cells [51]. BGN is a factor that participates in the integrated pathway of TGFβ/Snail with TNFα/NF-κB, which may facilitate tumor–stroma cross-talk during EMT in colorectal cancer [52] (Table 1). Furthermore, BGN enhances cancer cell stemness and EMT by affecting the interaction between cancer cells and mesenchymal stem cells during liver metastasis of colon cancer cells [53]. The expression of BGN in colon cancer cells is induced by high sugar concentration, fatty acids, and insulin, and its contact co-culture with mesenchymal stem cells. BGN overexpression in cancer cells is associated with phosphorylation of AKT, expression of mesenchymal markers, and liver metastasis [53].
Additionally, BGN appears to be highly expressed in cancer stem cells, such as colon and MCF-7-derived breast CSCs [33,54]. In the latter, depletion of BGN leads to decreased CSCs populations as shown by the reduction in the number of CD29 high, CD61+, and ALDH+ CSCs. BGN expression is required for the activation of NF-κB signaling pathway in breast CSCs as well as for increased tumorsphere forming ability, invasion, and metastasis (Table 1). Thus, BGN seems to contribute to the maintenance of breast CSC properties and is associated with worse prognosis in breast cancer [54]. On the contrary to the above, BGN is reported to have a tumor-suppressive function in pancreatic ductal adenocarcinoma, likely via trapping TGFβ within the ECM, hence decreasing the activation of the TGFβ/SMAD-independent pathway [55].
DCN exhibits a tumor-suppressive role as a pan-receptor tyrosine kinases inhibitor [56,57]. In normal gastric tissue, elevated levels of DCN have been reported, while in silico data and co-immunoprecipitation assays reveal its interaction with TGFβ. The interplay of asporin (ASPN), DCN, and TGFβ has been shown in a gastric adenocarcinoma dataset. Both ASPN and DCN interact with TGFβ and differentially regulate the activation of this pathway. DCN interacts with TGFβ in normal gastric epithelium, inhibiting the TGFβ canonical pathway [58]. Additionally, DCN inhibits the EMT-like phenotype in glioma through the inhibition of the c-Met/AKT/mTOR pathway and by inducing autophagy [59] (Table 1). In inflammatory breast cancer (IBC), DCN is significantly downregulated, while its overexpression suppresses cancer cell migration, invasion, and cancer cell stemness in vitro as well as cancer cell growth and metastasis in vivo. DCN physically interacts with E-cadherin and mediates its autophagic degradation and elimination, which in turn inhibits the E-cadherin/EGFR/ERK signaling axis [60]. Several treatment approaches based on DCN-mediated suppression of TGFβ and EMT have been proposed [61,62,63] (Table 1). For instance, the delivery of an oncolytic adenovirus expressing DCN blocks breast cancer cell growth and development of lung metastasis in a breast cancer mice model through multiple mechanisms involve inhibition of EMT, regulation of Wnt/β-catenin, VEGF and Met signaling as well as modulation of inflammatory and immune responses [61]. Furthermore, an oncolytic adenovirus co-expressing DCN and a soluble Wnt decoy receptor markedly prevents EMT and cancer cell metastasis in an orthotopic pancreatic xenograft tumor model [62]. DCN deficiency significantly promotes EMT in a DCN−/− mice model of colitis-associated cancer. The intestinal epithelium of the DCN−/− mice exhibits a marked increase in the protein levels of EMT markers including N-cadherin, vimentin, Snail, Slug, Twist, and MMP-2 and reduced levels of E-cadherin under unchallenged basal conditions. Induction of colitis-associated cancer further increases the expression of EMT-associated markers in DCN−/− mice. Moreover, treatment with a combination of Celecoxib and DCN markedly inhibits EMT and proliferation of colorectal cancer cells in DCN−/− mice and it is proposed as a potential adjuvant protein therapy [63].
ASPN is highly expressed by CAFs and plays important roles in cancer progression [64,65,66]. In pancreatic cancer, ASPN induces EMT in a paracrine manner via expression by pancreatic stellate cells. ASPN binds to CD44 in pancreatic cancer cells and activates the CD44/AKT/ERK-NF-kB signaling axis, which lead to EMT induction and increased migration and invasion [65] (Table 1). As mentioned above, in normal gastric tissue, DCN interacts with TGFβ, leading to tumor suppression. On the other hand, ASPN is increased in gastric cancer and can also interact with TGFβ. ASPN is thought to activate the SMAD2 pathway to induce EMT, as higher levels of phosphorylated SMAD2 have been observed in gastric cancer tissue compared with adjacent normal tissue [58] (Table 1). ASPN exhibits significantly increased expression levels in CAFs compared with normal fibroblasts. Scirrhous gastric cancer cells stimulate CAFs to express ASPN, which in turn causes increased invasion of both cell types in vitro and in vivo. The underlying mechanism includes the ASPN-mediated activation of Rac1 in both CAFs and cancer cells through its interaction with CD44 [67]. ASPN is also overexpressed in colorectal cancer tissues compared with adjacent normal tissues and its expression is associated with lymph node metastasis and TNM stage. In vitro experiments show that ASPN enhances migration and invasion of colorectal cancer cells. In colorectal cancer tissues, elevated co-expression of ASPN and p-cortactin (Tyr 241), an essential component for invadopodia formation and cellular migration, are found. When ASPN is overexpressed in HT-29 and LoVo colorectal cancer cells, the EGFR/Src axis is activated, leading to increased phosphorylation (Tyr 241) of cortactin. These data support that ASPN could be a biomarker and a potential therapeutic target for colorectal cancer [68]. Of interest, ASPN can be located intracellularly, acting as an EMT promoter. Cytoplasmic ASPN can recruit the TGFβ-induced p-SMAD2/3 on the nuclear membrane, facilitating their transportation to the nucleus inducing the expression of EMT-related genes, such as MMP-2, MMP-9, AHR, GLI2, ZEB1, TCF4, and TNC in colorectal cancer cells [69] (Table 1). Nevertheless, ASPN is expressed in the Hs578T breast cancer cell line, a cell line associated with a mesenchymal phenotype, and its expression can be regulated by BMP4, serum starvation, and 3D cultivation. Invasion of breast cancer cells is dependent on ASPN and it seems to be facilitated by CAFs [64]. Further studies verify that ASPN expression is low in non-tumor tissues, including breast tissue, on the contrary to DCN and BGN, which present high expression levels in normal tissues. In tumor tissues, ASPN is mainly detectable in the stroma secreted by CAFs and not by cancer cells, while the majority of breast cancer cells independently of their receptor status do not express ASPN. Normal breast fibroblasts can be stimulated to secrete ASPN by noninvasive luminal-like hormone receptor-positive cell lines such as T47D and MCF7 and not by highly metastatic triple-negative breast cancer cells such as MDA-MB-231 and MDA-MB-468 cells. This interesting observation is due to the secretion of IL-1β from triple-negative breast cancer cells, which blocks ASPN expression. Contrary to the previous studies, ASPN has also been found to be able to sequester TGFβ1, and thereby inhibiting the activation of SMAD2 and the subsequent promotion of EMT [70].
LUM is involved in multiple cancer-related processes, including EMT, and the impact of LUM in cancer aggressiveness is cancer-type specific [71,72]. In breast cancer cells, the expression of estrogen receptors ERα and ERβ is associated with EMT. Treatment of breast cancer cells with different expression of estrogen receptors with LUM induces the epithelial phenotype, especially in the highly invasive mesenchymal cells. These data indicate that LUM is a negative regulator of EMT and could be potentially used for breast cancer therapy [71,73,74]. In melanoma, the TF Snail triggers EMT, increases migration, invasion, and MMP-14 activity, while the inhibition of Snail-mediated EMT suppresses metastasis. LUM, through the suppression of elevated activity of MMP-14, decreases Snail-induced cell proliferation, migration, and invasion, and suppresses EMT (Table 1). Therefore, LUM could be applied for melanoma treatment [71,72,75,76]. LUM is transcriptionally suppressed by HMGA2, which is an oncogene which induces tumorigenesis and regulates specific genes and microRNAs associated with EMT, in ovarian cancer [77]. Glioblastoma and neuroblastoma cells increase the mRNA and protein levels of DCN and LUM when cultured under CSC enrichment conditions. The DCN+/LUM+ CSC-like glioblastoma and neuroblastoma cells form secondary neurospheres when cultured in anchorage-independent conditions, and present a quiescent, less proliferative phenotype and temozolomide resistance. It is possible that LUM and DCN play a dual role in the maintenance of CSCs and cell fate which is dependent on the tumor microenvironment (TME) [78].
3.3. SPOCK1 Is a Potent Inducer of EMT
The Testican/SPARC/Osteonectin, CWCV and Kazal-like domain (SPOCK) family of extracellular PGs is comprised of SPOCK1, SPOCK2, and SPOCK3. SPOCK1 has been shown to contribute to cancer cell invasion and metastasis through the promotion of EMT in multiple cancer types. Specifically, SPOCK1 is a downstream regulator of TGFβ and regulates Snail and Slug via the PI3K/AKT and Wnt/β-catenin signaling pathways, which lead to the regulation of the epithelial markers E-cadherin and ZO-1, and the mesenchymal markers vimentin and N-cadherin [79,80,81] (Table 1). SPOCK1 is upregulated in gastric [82], colorectal [83], lung [81], pancreatic [84], breast [85,86], esophageal squamous cell [87], and prostate cancer [88], as well as in glioblastoma [89], and it is associated with poor prognosis and EMT. In colorectal cancer, knockdown of SPOCK1 through shRNA results in increased levels of E-cadherin and decreased N-cadherin through the suppression of the PI3K/AKT pathway [83] (Table 1). Ectopic expression of SPOCK1 in epithelial cancer cell lines, including lung [81], pancreatic [84], breast [85,86], and esophageal squamous cell cancer [87], leads to the induction of EMT. In pancreatic cancer, SPOCK1 directly interacts with IκBα, thereby regulating NF-κB and ZEB2 [84] (Table 1). In breast cancer, the AKT/mTOR pathway is activated by SPOCK1 regulation of SIX1, and also induces cell proliferation and cell cycle progression [85]. In glioblastoma cells, SPOCK1 expression is positively correlated with the mRNA and protein levels of vimentin and N-cadherin as shown by gain or loss expression of SPOCK1, while SPOCK1 can induce temozolomide resistance [89]. HER2-positive gastric cancer cells acquire resistance to lapatinib via activation of c-Met and HER3 signaling. These cells exhibit an EMT phenotype with upregulated levels of SPOCK1. Inhibition of SPOCK1 restores sensitivity to lapatinib by suppressing c-Met and HER3 activation and the expression of β-catenin and promotes MET (Table 1). WNT/β-catenin signaling inhibition using XAV939, a small-molecule inhibitor, restores lapatinib sensitivity abrogated by SPOCK1 overexpression [82]. SPOCK1, due to its important roles in cancer, could serve as a potential therapeutic target. For instance, the flavonoid apigenin exerts an anti-metastatic effect in metastatic prostate cancer cell lines. Here, apigenin targets SPOCK1, which causes a reduction of Snail and Slug expression, further causing changes in vimentin, N-cadherin, and E-cadherin expression [88].
3.4. Versatile Functions of Pericellular PGs
AGRN has been shown to contribute to EMT in liver and pancreatic cancer [90,91,92]. In hepatocellular carcinoma (HCC) cells, AGRN is overexpressed and secreted in high levels compared with non-tumor liver cells. Furthermore, AGRN depletion leads to increased E-cadherin and reduced N-cadherin, vimentin, and Snail levels, while E-cadherin and vimentin levels are reversed after treatment with soluble AGRN, indicating that secreted AGRN can control the expression of EMT markers. AGRN through the LRP4/Musk axis activates FAK signaling and the Arp2/3 complex, which are critical for ECM degradation, cancer cell invasion, induction of EMT, and tumorigenesis [90] (Table 1). AGRN has also been associated with poor prognosis in patients with pancreatic ductal adenocarcinoma. Communication between cancer stem cells and non-stem cancer cells is mainly through extracellular vesicles that flow from CSCs to non-stem cancer cells. Extracellular vesicles intratumor communication network, which is formed through this procedure, contributes to the adaptation of cancer cells to microenvironmental changes and can lead to therapy resistance. Additionally, extracellular vesicles derived from CSCs have a distinct protein cargo, enriched in AGRN. AGRN-positive CSCs EVs enhance the activation of YAP through LRP4, promoting tumor growth (Table 1). Downregulation of AGRN in CSCs and in CSCs extracellular vesicles reduces tumor growth, and the use of a neutralizing anti-human AGRN antibody also reduces cell growth, as well as the activation of YAP. These results suggest that AGRN could be a therapeutic target in pancreatic cancer and, simultaneously, AGRN-positive CSCs extracellular vesicles can serve as prognostic biomarkers associated with increased risk for disease progression [92].
Collagen XVIII is a HSPG, associated with EMT of endothelial cells during endocardial cushions formation [93]. Endostatin is a fragment created by proteolytic cleavage of the carboxy-terminal non-collagenous domain of collagen XVIII and exerts anti-cancer properties [7]. In esophageal squamous cell carcinoma cells, recombinant human endostatin treatment combined with irradiation leads to the upregulation of PTEN and E-cadherin and inhibition of AKT/GSK-3β/Snail signaling axis and EMT, suggesting that this combination could be a potential approach for metastasis suppression [94] (Table 1). After treatment of basal carcinoma cells with endostatin, protein levels of the epithelial marker E-cadherin are increased, while N-cadherin, fibronectin, and vimentin are decreased, indicating that endostatin could induce MET [95]. In lung cancer, endostatin is used as an anti-angiogenic drug, but resistance to it has been observed. Endostatin therapy seems to increase the CSC population through the induction of TGFβ1 secretion and hypoxia, leading to endostatin resistance [96]. Endostar, a recombinant human endostatin, also has anti-tumor activity and suppresses EMT in ovarian and lung cancer [97,98]. Treatment of ovarian cancer cells with Endostar results in increased protein levels of E-cadherin and decreased N-cadherin, vimentin, Snail, MMP-2, and MMP-3. It is indicated that EMT suppression succeeds through blocking the expression of PD-L1 and the activation of STAT3, which are associated with tumor metastasis [97] (Table 1). Additionally, when A549 and H1975 lung cancer cells are cultured with supernatant of lung cancer-related polarized macrophages, EMT is induced. After co-treatment with Endostar and polarized macrophages, these alterations of EMT-related genes are restored, and it is proposed that Endostar-related inhibition of EMT occurs through the HGF-c-Met pathway [98]. Furthermore, in vitro experiments have shown that treatment of HCC cells with HYD-PEP06, an endostatin-derived synthetic polypeptide, inhibits metastasis by suppressing EMT through blocking of PI3K/AKT signaling pathway (Table 1). Simultaneously, HYD-PEP06 diminishes HCC CSCs metastasis via inhibition of the canonical Wnt/β-catenin pathway. These data suggest that HYD-PEP06 could be potentially used as a short peptide-agent for HCC treatment [99].
3.5. Syndecans: Dual Roles in EMT and Stemness
Syndecan-1 (SDC1) is a molecular biomarker for EMT during carcinogenesis. SDC1 mediates migration and adhesion through the interaction with GFs, and has a regulatory role on CSCs. Abnormal expression of SDC1 acts as a disease indicator in triple-negative breast [100,101], pancreatic [102], gastric, endometrial, and ovarian cancer [103]. SDC1 has emerged as a regulator of CSCs phenotype in triple-negative breast cancer [101], where SDC1 gene silencing in MDA-MB-231 cells [100] causes a downregulation in several components of the IL-6/STAT3, Notch, EGFR, and LRP6 pathways [100,101] (Table 1). Moreover, SDC1 holds a vital role in HCC sphere formation. The suppression of SDC1 induces the downregulation of the CSC markers CD133, CD44, and CD90, while the hepatoma spheres size is drastically decreased [104]. Numerous studies on different cancer types have characterized SDC1 as a key factor in EMT, as its repression in most carcinomas favors EMT [105]. In ductal breast cancers, the loss of SDC1 induces EMT, which can be utilized as a prognostic tool of survival [106]. Activation of the FGF-2/PI3K/AKT signaling axis promotes EMT in renal proximal tubular cell line HK2 via the overexpression of MMP-9 and heparanase (HPSE). These enzymes degrade SDC1 leading to sustained FGF-2 signaling [107] (Table 1). The FGF-2/SDC1/HPSE axis is also important for the regulation of EMT in pancreatic cancer [108]. This multifunctional role of SDC1 as a regulator of EMT was further investigated in prostate cancer, where the concurrent upregulation of ZEB1, a repressor of SDC1 expression [109], and Snail, and the downregulation of SDC1 induce a mesenchymal phenotype [110]. SDC1 knockdown also leads to increased proliferation, invasion, and migration of gallbladder cancer cells by regulating ERK/Snail signaling and induction of EMT [111] (Table 1). The suppression of SDC1 also induces EMT in oral cancer cells by activating the ERK/Snail signaling axis [112]. An association between EMT markers’ expression, such as SDC1, E-cadherin, and β-catenin, with reduced risk of poor outcome in colorectal cancer has been identified [113]. Finally, the addition of sphingosine-1-phospate in HCC cells induces EMT via an SDC1/MMP-7/TGFβ/HPSE mechanism. In more detail, S1P binds to its receptor and activates the PI3K/AKT and ERK1/2 signaling pathways. The PI3K/AKT pathway triggers HPSE, which further activates ERK1/2 and MMP-7. MMP-7 sheds SDC1, which leads to TGFβ1 production and EMT [114]. A correlation between EMT and nuclear translocated SDC1 has been observed in epithelial origin A549 adenocarcinoma cells. In more detail, during TGFβ-induced EMT in A549 adenocarcinoma cells, a loss of nuclear SDC1 is observed that is associated with EMT. Moreover, silencing of SDC1 leads to increased N-cadherin, nuclear localization of ZEB1, and invasion. The observed role of SDC1 strengthens the view about its involvement in modulation of EMP [115]. Finally, in invasive ductal carcinoma in situ cells, SDC1 gene silencing leads to the upregulation of several markers of EMT, such as the epithelial markers claudin and E-cadherin, the mesenchymal marker fibronectin, as well as the invasive markers MMP-3, MMP-9, and HPSE. These results may suggest a protective role of SDC1 in early stages of ductal carcinoma in situ, as its silencing leads to partial EMT accompanied by deregulation of ECM degradation enzymes [116].
Overexpression of syndecan-2 (SDC2) in HT29 colorectal cancer cells activates PKCγ and the FAK/ERK signaling pathway that induces the expression of MMP-7 involved in the shedding of E-cadherin [117,118] (Table 1). The overexpression of SDC2 in colorectal cancer results in induction of EMT and the upregulation of N-cadherin, Slug, Twist, vimentin, and MAPK signaling pathway activation, while SDC2 knockdown reduces cancer cell proliferation, migration, and invasion, and induces cell apoptosis [119]. Finally, circNFATC3, a circular RNA that is overexpressed in cervical cancer, works as a molecular sponge for miR-9-5p, which is a suppressor of SDC2 expression. As a result, the overexpression of circNFATC3 in cervical cancer leads to the overexpression of SDC2 and its downstream targets syntenin-1, NF-κB, and MMP-9 [120].
The data on the contribution of syndecan 3 (SDC3) in the induction of a mesenchymal phenotype are limited. Nevertheless, the receptor protein tyrosine phosphatase β/ζ seems to induce EMT in prostate cancer via cooperation with SDC3. In the early stages of prostate cancer, both molecules are expressed and the growth factor pleiotrophin binds to receptor protein tyrosine phosphatase β/ζ. As the cancer progresses, receptor protein tyrosine phosphatase β/ζ is downregulated and pleiotrophin binds to its second receptor, SDC3. This event triggers the induction of a mesenchymal phenotype via MAPK signaling pathway activation [121] (Table 1). SDC3 is associated with an EMT-stemness phenotype in ovarian cancer [122]. In particular, silencing of SDC3 reduces colony formation and 3D spheroid growth and stemness in ovarian cancer cells. Bioinformatic tools revealed numerous direct and indirect functional interactions of SDC3 with several stemness- and EMT-associated markers, such as E-cadherin, Snail, SDC1, and its involvement in multiple pathways such as Notch, Wnt, and Hedgehog [122].
Syndecan 4 (SDC4) has a universal expression in almost every cell type under physiological situations as well as in development and pathological conditions. SDC4 is localized at the focal adhesion sites interacting with a plethora of actin modulating molecules, such as PKCα, Rho GTPases, and FAK [123,124]. SDC4 is associated with aggressiveness in testicular germ tumors. The aggressive non-seminomatous testicular germ tumor cells present decreased levels of SDC4, which are further correlated with disease stage, lymph node metastasis, and vascular and lymphatic invasion. [125]. SDC4 levels are increased at the invasion front of colorectal cancer cells. SDC4 expression is significantly correlated with the pathological characteristics that indicate disease progression and inversely associated with disease-free and overall survival, meaning SDC4 emerges as a molecular marker of colorectal cancer cell aggressiveness [126]. Moreover, SDC4 is upregulated in TGFβ1-induced EMT in A549 lung adenocarcinoma cells. SDC4 knockdown results in recovered E-cadherin expression and decreased expression of mesenchymal markers vimentin and Snail; however, Slug protein expression is not affected by the SDC4 knockdown. To achieve a full epithelial morphology, both SDC4 and Slug need to be silenced, suggesting that Snail is a transcription factor downstream of SDC4, and that SDC4 regulates TGFβ1-induced EMT by cooperating with Slug [127]. In papillary thyroid cancer cells, the knockdown of SDC4 ameliorates their aggressive mesenchymal phenotype via the downregulation of Wnt/β-catenin signaling (Table 1). Additionally, the expression of SCD4 is positively associated with clinicopathological characteristics of papillary thyroid cancer patients, including tumor and lymph node metastasis, differentiation, and disease stage [128]. The mechanism behind the relation of SDC4 and Wnt/β-catenin is partially understood in Xenopus, where the molecule R-spondin 3, a modulator of Wnt, interacts with SDC4 and induces Wnt signaling via receptor complex endocytosis, resulting in the morphological differentiation of the cells [129]. To continue, chondroitin polymerizing factor was demonstrated to promote breast cancer aggressiveness via the structural alteration of SDC4 and especially addition of CS chains [130]. SDC4 further acts as a receptor for tenascin-C, an ECM molecule secreted from various bladder cancer cells. This interaction activates NF-κΒ and induces EMT, as shown by the expression of mesenchymal markers, such as Snail, N-cadherin, and vimentin [131] (Table 1).
3.6. Loss of Betaglycan (TGFBR3) Evokes EMT in Cancer Cells
The first extensive study in cancer about the role of TGFBR3 in EMT revealed that the deprivation of TGFBR3 expression can lead to increased invasion and migration of the pancreatic cancer cells PANC-1 without affecting Snail or E-cadherin levels. This is not mediated by its cytoplasmic domain or its co-receptor function, but is partially mediated by ectodomain shedding of TGFBR3, generating soluble TGFBR3 (sTGFBR3). TGFBR3 is downregulated during EMT and precedes downregulation of E-cadherin and cytoskeletal reorganization during the early stages of EMT. Surprisingly, the maintenance of TGFBR3 expression does not inhibit EMT, but instead suppresses the increased motility and invasiveness associated with the mesenchymal phenotype [38]. Both stimulations with TGFβ1 or BMPs repress expression of TGFBR3 in PANC-1 cells. In particular, BMPs cause the suppression of protein and mRNA levels of TGFBR3, which in basal conditions inhibits the phosphorylation of SMAD1. In EMT conditions, the BMP type I receptor ALK3 unhindered regulates p-SMAD1, which in turns activates the expression of MMP-2. The active form of MMP-2 seems to control the increased invasiveness observed in PANC-1 cells after the treatment with BMPs [132]. Moreover, TGFβ1 can also repress the expression of TGFBR3 in HCC cell lines, resulting in subsequent activation of the SMAD2 and AKT pathways and enhanced migratory and invasive cellular capacity [133].
Additionally, in oral squamous cancer, the simultaneous loss of TGFBR3 and growth differentiation factor 10/BMP3b induces EMT and chemotherapy resistance via the SMAD2 and ERK1/2 signaling pathways (Table 1). TGFBR3 is an upstream activator of growth differentiation factor 10 expression and they negatively regulate EMT, migration, and invasion via the same signaling pathways [134]. TGFBR3 can cooperate with a number of important miRNAs, including miR-19a, miR-424 [135], and miR-323b-3p [136]. In particular, in tongue squamous cancer miR-19a and miR-424 inhibit TGFBR3. TGFBR3 overexpression alone exerts a strong inhibitory effect on migration and EMT of CAL-27 tongue squamous cancer cells, while overexpression of miR-19a and miR-424 downregulates TGFBR3 and triggers EMT and cancer cell migration. Mechanistically, TGFBR3 directly interacts with β-arrestin 2, which in turns interacts with ΙκΒα, resulting in the inhibition of NF-κΒ signaling and migratory ability of CAL-27 cells [135] (Table 1). The implication of TGFBR3 in the inhibition of the NF-κB pathway and cellular migration via the interaction with β-arrestin 2 has also been demonstrated in MCF10A breast epithelial and MDA-MB-231 breast cancer cells [137]. MiR-323b-3p expression associates with the expression of TGFBR3 in an inverse manner. MiR-323b-3p is upregulated and TGFBR3 is downregulated in osteosarcoma to evoke tumorigenesis, with the opposite expression pattern being true in pulmonary metastasis where TGFBR3 acts as a tumor promoter for the development of pulmonary metastasis [136]. In murine mammary epithelial cells, TGFBR3 presents a basolateral localization which is directed by specific cytosolic residues to sustain an epithelial phenotype. Mutations of the proline 826 residue of TGFBR3 lead to apical localization of TGFBR3 that occurs before, and perhaps leads to the subsequent loss of epithelial cell polarity and promotes EMT independently of TGFβ ligand and ΤGFβR1 kinase activity. Apical localization of TGFBR3 can further lead to enhanced proliferation, migration, and invasion in vitro and enhanced tumorigenesis in an in vivo mouse model of breast carcinoma. The proper localization of TGFBR3 to the basolateral membrane domain is essential for the maintenance of epithelial cell polarity and suppression of breast cancer progression [138].
3.7. Glypicans: Contradictory Roles in EMT and cell Stemness
Data regarding the role of glypican 1 (GPC1) in the induction of EMT have revealed that GPC1 mainly interacts with TGFβ, FGF, and Wnt signaling pathways, all crucial regulators of EMT [37]. The pivotal role of GPC1 in EMT induction has been confirmed in several pathological conditions, such as esophageal carcinoma and colon adenocarcinoma. In particular, the overexpression of GPC1 in HT29 and HCT-116 colon cancer cells downregulates several epithelial markers, such as E-cadherin and upregulates vimentin, Snail, and Slug, leading to EMT. Moreover, GPC1 is negatively regulated via miR-96-5p and miR-149 [139]. Overexpression of Snail in MC38 colon adenocarcinoma cells induces EMT, and the Snail-overexpressing cells produce extracellular vesicles containing elevated quantities of GPC1 [140]. Interestingly, increased expression levels of GPC1 induce a mesenchymal phenotype in esophageal squamous cell carcinoma through the PTEN/AKT/β-catenin signaling pathway (Table 1). In particular, the overexpression of GPC1 reduces the levels and the activation of PTEN and increases the activation of AKT and the levels of β-catenin. GPC1 overexpression induces EMT as it eliminates the levels of E-cadherin and upregulates the expression of N-cadherin [141].
Glypican-3 (GPC3) displays diverse biological roles regarding tumor progression in a cell- and tissue-specific manner [7]. Several studies have outlined the role of GPC3 in the regulation of EMT, in a diverse number of cancer types, including breast [142] and HCC [143]. GPC3 is highly expressed in normal breast tissue and is markedly decreased in tumors. In breast cancer, GPC3 silencing in low metastatic MCF7 breast cancer cells stimulates an aggressive phenotype with EMT features; while, on the other hand, induction of its expression attenuates the aggressiveness of MDA-MB-231 cells, leading to MET. Part of the regulation of EMT characteristics from GPC3 is mediated by the inhibition of the Wnt/β-catenin signaling pathway and downregulation of ZEB1 [142] (Table 1). In another study, GPC3 re-expression in aggressive breast cancer cells returned mesenchymal-like breast cancer cells to an epithelial phenotype as shown by the suppression of Snail, ZEB1, vimentin, and increased expression of E-cadherin. It also impaired in vivo metastasis and induced tumor in vivo dormancy at the secondary tumor site through p38 MAPK signaling activation [144].
On the other hand, GPC3 evokes EMT in hepatocellular carcinoma, thereby displaying a tissue-specific dual role [7]. GPC3 knockdown in HCC HepG2 cells decreases proliferation, invasion, migration, and the expression of EMT-related molecules including MMP-2, MMP-9, Snail, Slug, and β-catenin, while it promotes the expression of E-cadherin as well as apoptosis [143]. GPC3-induced ERK activation promotes EMT and enhances cell migration and invasion in hepatocellular carcinoma [145] (Table 1). GPC3 and the transmembrane protein FAT1 directly interact and co-operate to evoke cell migration and EMT in HCC cell lines. Double silencing of those molecules regulates the expression of important EMT markers such as Snail, vimentin, and E-cadherin [146].
Several studies have shown the crucial role of glypican-4 (GPC4) as a mediator of stem cell maintenance, differentiation, self-renewal, and pluripotency. GPC4 is essential in ESCs and neural stem cells for the maintenance of progenitors and the response of ESCs to Wnt ligands [147,148]. In particular, the loss of GPC4 function leads to the acceleration of differentiation of mouse ESCs, as early markers of ectoderm, mesoderm, and endoderm differentiation are increased and SOX2 expression is decreased. Additionally, stem cells with GPC4 mutations are able to integrate into all three germ layers [147,148]. GPC4 is vital for the cell fate determination, pluripotency, tumorigenic potential, and Wnt pathway induction by downregulating GSK3 protein levels, as well as a number of other signal transduction pathways [147]. In neural SCs, GPC4 plays a key role in the astrocyte differentiation [149]. An important association is observed between GPC4 and 5-FU resistance in pancreatic cancer. GPC4 silencing results in the decrease of several stem markers, such as SOX2 and OCT4, and better response to 5-FU treatment, through the suppression of the Wnt/β-catenin signaling pathway (Table 1). GPC4 functions by facilitating the localization of Wnts on the cell membrane, thus activating Wnt/β-catenin signaling, which causes the increased chemoresistance and stem cell-like properties [150].
The suppressive role of glypican-5 (GPC5) in EMT has been described in lung adenocarcinoma [151] and prostate cancer [152]. Here, overexpression of GPC5 leads to the downregulation of EMT markers, such as vimentin and Snail, upregulation of E-cadherin, and inhibition of the Wnt/β-catenin signaling pathway, via the direct binding of GPC5 to Wnt3a (Table 1).
3.8. Serglycin Triggers Oncogenic Signaling and EMT
Serlgycin (SRGN) is the only intracellularly classified PG that can also be secreted by a number of different cell types, exerting crucial roles in the ECM and TME [40]. The role of SRGN in EMT and cancer cell stemness has been confirmed in a plethora of cancer types, such as HCC [153], breast cancer [154,155,156], colorectal [157], lung [158], and nasopharyngeal cancer [159]. SRGN also has an effect on tumor cell phenotype and stemness in glioblastoma [160]. SRGN is positively correlated with vimentin and negatively with E-cadherin, in HCC and nasopharyngeal carcinoma samples as well as in the nasopharyngeal carcinoma cell line CNE-2 and its highly metastatic clones [153,159]. Concerning breast cancer, knockdown of SRGN in mesenchymal-like MDA-MB-231 and the induction of the SRGN expression in epithelial MCF-7 cancer cells confirmed the vital role of SRGN in the induction of EMT [154,155]. High expression levels of SRGN facilitate EMT by upregulation of several ECM-degrading enzymes, activation of IL-8/CXCR2, and modulation of downstream signaling cascades including PI3K, SRC, and Rac [154] as well as the CD44/CREB1/TGFβ2 signaling axis, [155] (Table 1). SRGN is markedly upregulated during TGFβ-induced EMT [161]. SRGN/TGFβ2 interlay establishes a positive feedback loop that promotes EMT in breast cancer cells. TGFβ2 binds to TGFβR, activating SMAD2/3 that promotes the expression and secretion of SRGN. Then, secreted SRGN binds to CD44 on the breast cancer cell membrane, triggering intracellular signals activating CREB1 that increase the expression of TGFβ2 [155]. Importantly, epithelial breast cells are more responsive to TGFβ-induced EMT when expressing SRGN, as serglycin CRISPR/Cas9 knockout delays the onset of EMT upon TGFβ treatment. Nevertheless, after the induction of EMT, the suppression of SRGN by siRNA knockdown could not revert the acquired mesenchymal phenotype. Moreover, during the EMT process, the expression of SRGN is regulated by ZEB1 in mammary epithelial cells [161]. Knockout of SRGN has no impact on the growth of primary tumor or the number of mammary tumors formed in the MMTV-PyMT-driven mouse breast cancer model. In contrast, the ablation of SRGN completely inhibits lung metastasis. E-cadherin expression is significantly higher in the SRGN-deficient primary tumors, indicating a more epithelial cancer cells phenotype [162]. SRGN further plays an important role in chemoresistance and stemness of breast cancer cells. Specifically, SRGN via the activation of the ITGA5/FAK/CREB/YAP signaling axis establishes a positive feedback loop that upregulates SRGN and HDAC2 and prompts cell stemness [156] (Table 1). The SRGN/CD44 interaction was thoroughly investigated in A549 and H460 lung cancer cell lines [158]. SRGN interacts with CD44, which serves as a stem-cell marker, via its CS residues, activating NF-κΒ and upregulating claudin-1 expression, which disturbs tight cell junctions, leading to EMT and increased cell stemness [158] (Table 1). Furthermore, in colorectal cancer, SRGN transcriptional overexpression is associated with a mesenchymal phenotype, as N-cadherin and vimentin are upregulated and E-cadherin is downregulated through induction by the hypoxia-inducible factor 1a [157]. SRGN is also a mediator of cell stemness and glioblastoma to astrocytic differentiation. Suppression of SRGN in LN-18 glioblastoma cells triggers the downregulation of several pluripotency and self-renewal markers, such as SOX2, OCT4, LIF, MSI1, NES, KLF4, Nanog, and ALDH-1, while it simultaneously upregulates the expression of astrocyte differentiation factors including GFAP, SPARCL-1, and Snail. Moreover, LN-18 glioblastoma cells with suppressed levels of SRGN exhibit a low ability for colony formation and a reduction of tumorigenesis in vivo [160].
4. Conclusions
PGs are important structural and functional constituents of ECMs that participate in matrix organization and cell–cell and cell–matrix interactions. Their expression is often deregulated in tumor cells and the surrounding stroma, providing a provisional matrix for tumor cell growth and spread. Recent studies show PGs as potent players in the landscape of cancer cell signaling and plasticity, as PGs are implicated in the regulation of EMT and cancer cell stemness by intervening in a variety of signaling pathways. PGs exhibit complicated roles as they often can control cancer cell stemness and EMT in a cell- and context-dependent manner. The elucidation of the underlying molecular mechanisms may provide us with new therapeutic tools for cancer treatment.
Author Contributions
Conceptualization, A.D.T. and E.K.; literature survey: Z.K., P.N.F. and D.M.; writing—original draft preparation, Z.K., P.N.F. and D.M.; writing—review and editing, A.D.T. and E.K.; supervision, A.D.T. All authors have read and agreed to the published version of the manuscript.
Funding
E.K. is funded by Helse Nord (HNF1585-21).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AGRN | Agrin |
| ASPN | asporin |
| BGN | biglycan |
| BMP | bone morphogenetic protein |
| CAFs | cancer-associated fibroblasts |
| CSCs | cancer stem cells |
| CS | chondroitin sulfate |
| CTGF | connective tissue growth factor |
| DCN | decorin |
| DS | dermatan sulfate |
| EBs | embryoid bodies |
| ESCs | embryonic stem cells |
| EGFR | epidermal growth factor receptor |
| EMP | epithelial mesenchymal plasticity |
| EMT | epithelial-to-mesenchymal transition |
| ECMs | extracellular matrices |
| FGF | fibroblast growth factor |
| FOXC2 | forkhead box C2 |
| GAGs | glycosaminoglycans |
| GPC1 | glypican 1 |
| GPC3 | glypican-3 |
| GPC4 | glypican-4 |
| GPC5 | glypican-5 |
| GFs | growth factors |
| HCC | hepatocellular carcinoma |
| HPSE | heparanase |
| HS | heparan sulfate |
| HP | heparin |
| HA | hyaluronan |
| IBC | inflammatory breast cancer |
| IGFR-I | insulin-like growth factor receptor I |
| ILs | interleukins |
| KS | keratan sulfate |
| LUM | lumican |
| MMPs | matrix metalloproteinases |
| MET | mesenchymal-to-epithelial transition |
| PRRX1 | paired-related homeobox 1 |
| PDGF | platelet-derived growth factor |
| PGs | proteoglycans |
| SRGN | serglycin |
| SLRPs | small leucine-rich proteoglycans |
| SDC1 | syndecan-1 |
| SDC2 | syndecan-2 |
| SDC3 | syndecan 3 |
| SDC4 | syndecan 4 |
| SPOCK | Testican/SPARC/Osteonectin CWCV and Kazal-like domain |
| TGFBR3 | TGFβ receptor III |
| TLRs | toll like receptors |
| TFs | transcription factors |
| TGFβ | transforming growth factor β |
| TAMs | tumor-associated macrophages |
| TME | tumor microenvironment |
| VEGFA | vascular endothelial growth factor A |
| VCAN | versican |
| ZEB | zinc finger E-box binding homeobox |
References
- Theocharis, A.D.; Manou, D.; Karamanos, N.K. The extracellular matrix as a multitasking player in disease. FEBS J. 2019, 286, 2830–2869. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- Manou, D.; Caon, I.; Bouris, P.; Triantaphyllidou, I.E.; Giaroni, C.; Passi, A.; Karamanos, N.K.; Vigetti, D.; Theocharis, A.D. The Complex Interplay between Extracellular Matrix and Cells in Tissues. Methods Mol. Biol. 2019, 1952, 1–20. [Google Scholar] [PubMed]
- Karamanos, N.K.; Piperigkou, Z.; Theocharis, A.D.; Watanabe, H.; Franchi, M.; Baud, S.; Brezillon, S.; Gotte, M.; Passi, A.; Vigetti, D.; et al. Proteoglycan Chemical Diversity Drives Multifunctional Cell Regulation and Therapeutics. Chem. Rev. 2018, 118, 9152–9232. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Skandalis, S.S.; Tzanakakis, G.N.; Karamanos, N.K. Proteoglycans in health and disease: Novel roles for proteoglycans in malignancy and their pharmacological targeting. FEBS J. 2010, 277, 3904–3923. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Karamanos, N.K. Proteoglycans remodeling in cancer: Underlying molecular mechanisms. Matrix Biol. 2019, 75–76, 220–259. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.E.; Weinberg, S.H.; Lemmon, C.A. Mechanochemical Signaling of the Extracellular Matrix in Epithelial-Mesenchymal Transition. Front. Cell Dev. Biol. 2019, 7, 135. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Zhang, Y.; Weinberg, R.A. Epithelial-to-mesenchymal transition in cancer: Complexity and opportunities. Front. Med. 2018, 12, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Solnica-Krezel, L. Conserved Patterns of Cell Movements during Vertebrate Gastrulation. Curr. Biol. 2005, 15, R213–R228. [Google Scholar] [CrossRef] [PubMed]
- Tucker, R.P. Neural crest cells: A model for invasive behavior. Int. J. Biochem. Cell Biol. 2004, 36, 173–177. [Google Scholar] [CrossRef]
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells 2021, 10, 1587. [Google Scholar] [CrossRef]
- Gonzalez, D.M.; Medici, D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci. Signal. 2014, 7, re8. [Google Scholar] [CrossRef]
- Stemmler, M.P.; Eccles, R.L.; Brabletz, S.; Brabletz, T. Non-redundant functions of EMT transcription factors. Nat. Cell Biol. 2019, 21, 102–112. [Google Scholar] [CrossRef]
- Lambert, A.W.; Weinberg, R.A. Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat. Rev. Cancer 2021, 21, 325–338. [Google Scholar] [CrossRef]
- Ocana, O.H.; Corcoles, R.; Fabra, A.; Moreno-Bueno, G.; Acloque, H.; Vega, S.; Barrallo-Gimeno, A.; Cano, A.; Nieto, M.A. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell 2012, 22, 709–724. [Google Scholar] [CrossRef]
- Mani, S.A.; Yang, J.; Brooks, M.; Schwaninger, G.; Zhou, A.; Miura, N.; Kutok, J.L.; Hartwell, K.; Richardson, A.L.; Weinberg, R.A. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc. Natl. Acad. Sci. USA 2007, 104, 10069–10074. [Google Scholar] [CrossRef]
- Cao, Z.; Livas, T.; Kyprianou, N. Anoikis and EMT: Lethal “Liaisons” during Cancer Progression. Crit. Rev. Oncog. 2016, 21, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Gialeli, C.; Theocharis, A.D.; Karamanos, N.K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011, 278, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Riveline, D.; Zamir, E.; Balaban, N.Q.; Schwarz, U.S.; Ishizaki, T.; Narumiya, S.; Kam, Z.; Geiger, B.; Bershadsky, A.D. Focal contacts as mechanosensors: Externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol. 2001, 153, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Poltavets, V.; Kochetkova, M.; Pitson, S.M.; Samuel, M.S. The Role of the Extracellular Matrix and Its Molecular and Cellular Regulators in Cancer Cell Plasticity. Front. Oncol. 2018, 8, 431. [Google Scholar] [CrossRef]
- Xu, H.; Tian, Y.; Yuan, X.; Wu, H.; Liu, Q.; Pestell, R.G.; Wu, K. The role of CD44 in epithelial-mesenchymal transition and cancer development. OncoTargets Ther. 2015, 8, 3783–3792. [Google Scholar]
- McFarlane, S.; McFarlane, C.; Montgomery, N.; Hill, A.; Waugh, D.J. CD44-mediated activation of alpha5beta1-integrin. Cortactin and paxillin signaling underpins adhesion of basal-like breast cancer cells to endothelium and fibronectin-enriched matrices. Oncotarget 2015, 6, 36762–36773. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]
- Domenici, G.; Aurrekoetxea-Rodriguez, I.; Simoes, B.M.; Rabano, M.; Lee, S.Y.; Millan, J.S.; Comaills, V.; Oliemuller, E.; Lopez-Ruiz, J.A.; Zabalza, I.; et al. A Sox2-Sox9 signalling axis maintains human breast luminal progenitor and breast cancer stem cells. Oncogene 2019, 38, 3151–3169. [Google Scholar] [CrossRef]
- Ye, X.; Weinberg, R.A. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol. 2015, 25, 675–686. [Google Scholar] [CrossRef]
- De Angelis, M.L.; Francescangeli, F.; Zeuner, A. Breast Cancer Stem Cells as Drivers of Tumor Chemoresistance, Dormancy and Relapse: New Challenges and Therapeutic Opportunities. Cancers 2019, 11, 1569. [Google Scholar] [CrossRef]
- De Angelis, M.L.; Francescangeli, F.; La Torre, F.; Zeuner, A. Stem Cell Plasticity and Dormancy in the Development of Cancer Therapy Resistance. Front. Oncol. 2019, 9, 626. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells: Current status and evolving complexities. Cell Stem Cell 2012, 10, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Nallanthighal, S.; Heiserman, J.P.; Cheon, D.J. The Role of the Extracellular Matrix in Cancer Stemness. Front. Cell Dev. Biol. 2019, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Skandalis, S.S.; Neill, T.; Multhaupt, H.A.; Hubo, M.; Frey, H.; Gopal, S.; Gomes, A.; Afratis, N.; Lim, H.C.; et al. Insights into the key roles of proteoglycans in breast cancer biology and translational medicine. Biochim. Biophys. Acta 2015, 1855, 276–300. [Google Scholar] [CrossRef]
- Afratis, N.A.; Nikitovic, D.; Multhaupt, H.A.; Theocharis, A.D.; Couchman, J.R.; Karamanos, N.K. Syndecans—Key regulators of cell signaling and biological functions. FEBS J. 2017, 284, 27–41. [Google Scholar] [CrossRef]
- Lund, M.E.; Campbell, D.H.; Walsh, B.J. The Role of Glypican-1 in the Tumour Microenvironment. Adv. Exp. Med. Biol. 2020, 1245, 163–176. [Google Scholar]
- Gordon, K.J.; Dong, M.; Chislock, E.M.; Fields, T.A.; Blobe, G.C. Loss of type III transforming growth factor beta receptor expression increases motility and invasiveness associated with epithelial to mesenchymal transition during pancreatic cancer progression. Carcinogenesis 2008, 29, 252–262. [Google Scholar] [CrossRef]
- Korpetinou, A.; Skandalis, S.S.; Labropoulou, V.T.; Smirlaki, G.; Noulas, A.; Karamanos, N.K.; Theocharis, A.D. Serglycin: At the crossroad of inflammation and malignancy. Front. Oncol. 2014, 3, 327. [Google Scholar] [CrossRef]
- Manou, D.; Karamanos, N.K.; Theocharis, A.D. Tumorigenic functions of serglycin: Regulatory roles in epithelial to mesenchymal transition and oncogenic signaling. Semin. Cancer Biol. 2020, 62, 108–115. [Google Scholar] [CrossRef]
- Vasaikar, S.V.; Deshmukh, A.P.; den Hollander, P.; Addanki, S.; Kuburich, N.A.; Kudaravalli, S.; Joseph, R.; Chang, J.T.; Soundararajan, R.; Mani, S.A. EMTome: A resource for pan-cancer analysis of epithelial-mesenchymal transition genes and signatures. Br. J. Cancer 2021, 124, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Du, W.W.; Fang, L.; Yang, X.; Sheng, W.; Yang, B.L.; Seth, A.; Zhang, Y.; Yang, B.B.; Yee, A.J. The role of versican in modulating breast cancer cell self-renewal. Mol. Cancer Res. 2013, 11, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Oktem, G.; Sercan, O.; Guven, U.; Uslu, R.; Uysal, A.; Goksel, G.; Ayla, S.; Bilir, A. Cancer stem cell differentiation: TGFβ1 and versican may trigger molecules for the organization of tumor spheroids. Oncol. Rep. 2014, 32, 641–649. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, X.; Qian, W.; Weng, X.; Zhang, L.; Zhang, L.; Wang, S.; Cao, X.; Ma, L.; Wei, G.; et al. Enhanced PAPSS2/VCAN sulfation axis is essential for Snail-mediated breast cancer cell migration and metastasis. Cell Death Differ. 2019, 26, 565–579. [Google Scholar] [CrossRef]
- Lee, H.C.; Su, M.Y.; Lo, H.C.; Wu, C.C.; Hu, J.R.; Lo, D.M.; Chao, T.Y.; Tsai, H.J.; Dai, M.S. Cancer metastasis and EGFR signaling is suppressed by amiodarone-induced versican V2. Oncotarget 2015, 6, 42976–42987. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Vahdat, L.T.; Wong, S.; Chang, J.C.; Mittal, V. Microenvironmental regulation of epithelial-mesenchymal transitions in cancer. Cancer Res. 2012, 72, 4883–4889. [Google Scholar] [CrossRef]
- Sheng, W.; Wang, G.; La Pierre, D.P.; Wen, J.; Deng, Z.; Wong, C.-K.A.; Lee, D.Y.; Yang, B.B. Versican mediates mesenchymal-epithelial transition. Mol. Biol. Cell 2006, 17, 2009–2020. [Google Scholar] [CrossRef][Green Version]
- Soltermann, A.; Tischler, V.; Arbogast, S.; Braun, J.; Probst-Hensch, N.; Weder, W.; Moch, H.; Kristiansen, G. Prognostic significance of epithelial-mesenchymal and mesenchymal-epithelial transition protein expression in non-small cell lung cancer. Clin. Cancer Res. 2008, 14, 7430–7437. [Google Scholar] [CrossRef]
- Schulz, G.B.; Grimm, T.; Sers, C.; Riemer, P.; Elmasry, M.; Kirchner, T.; Stief, C.G.; Karl, A.; Horst, D. Prognostic value and association with epithelial-mesenchymal transition and molecular subtypes of the proteoglycan biglycan in advanced bladder cancer. Urol. Oncol. 2019, 37, 530.e9–530.e18. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, R.; Feng, L.; Ma, H.; Fang, J. LINC00460 Promotes Cell Proliferation, Migration, Invasion, and Epithelial-Mesenchymal Transition of Head and Neck Squamous Cell Carcinoma via miR-320a/BGN Axis. OncoTargets Ther. 2021, 14, 2279–2291. [Google Scholar] [CrossRef]
- Pinto, F.; Santos-Ferreira, L.; Pinto, M.T.; Gomes, C.; Reis, C.A. The Extracellular Small Leucine-Rich Proteoglycan Biglycan Is a Key Player in Gastric Cancer Aggressiveness. Cancers 2021, 13, 1330. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhong, A.; Li, S.; Meng, X.; Wang, X.; Xu, F.; Lai, M. The integrated pathway of TGFβ/Snail with TNFα/NFκB may facilitate the tumor-stroma interaction in the EMT process and colorectal cancer prognosis. Sci. Rep. 2017, 7, 4915. [Google Scholar] [CrossRef]
- Fujiwara-Tani, R.; Sasaki, T.; Fujii, K.; Luo, Y.; Mori, T.; Kishi, S.; Mori, S.; Matsushima-Otsuka, S.; Nishiguchi, Y.; Goto, K.; et al. Diabetes mellitus is associated with liver metastasis of colorectal cancer through production of biglycan-rich cancer stroma. Oncotarget 2020, 11, 2982–2994. [Google Scholar] [CrossRef] [PubMed]
- Manupati, K.; Paul, R.; Hao, M.; Haas, M.; Bian, Z.C.; Holm, T.M.; Guan, J.L.; Yeo, S.K. Biglycan Promotes Cancer Stem Cell Properties, NFκB Signaling and Metastatic Potential in Breast Cancer Cells. Cancers 2022, 14, 455. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.K.; Nigri, J.; Lac, S.; Leca, J.; Bressy, C.; Berthezene, P.; Bartholin, L.; Chan, P.; Calvo, E.; Iovanna, J.L.; et al. TAp73 loss favors Smad-independent TGF-β signaling that drives EMT in pancreatic ductal adenocarcinoma. Cell Death Differ. 2016, 23, 1358–1370. [Google Scholar] [CrossRef]
- Xie, C.; Mondal, D.K.; Ulas, M.; Neill, T.; Iozzo, R.V. Oncosuppressive roles of decorin through regulation of multiple receptors and diverse signaling pathways. Am. J. Physiol. Cell Physiol. 2022, 322, C554–C566. [Google Scholar] [CrossRef]
- Neill, T.; Iozzo, R.V. The Role of Decorin Proteoglycan in Mitophagy. Cancers 2022, 14, 804. [Google Scholar] [CrossRef]
- Basak, D.; Jamal, Z.; Ghosh, A.; Mondal, P.K.; Dey Talukdar, P.; Ghosh, S.; Ghosh Roy, B.; Ghosh, R.; Halder, A.; Chowdhury, A.; et al. Reciprocal interplay between asporin and decorin: Implications in gastric cancer prognosis. PLoS ONE 2021, 16, e0255915. [Google Scholar] [CrossRef]
- Jia, Y.; Feng, Q.; Tang, B.; Luo, X.; Yang, Q.; Yang, H.; Li, Q. Decorin Suppresses Invasion and EMT Phenotype of Glioma by Inducing Autophagy via c-Met/Akt/mTOR Axis. Front. Oncol. 2021, 11, 659353. [Google Scholar] [CrossRef]
- Hu, X.; Villodre, E.S.; Larson, R.; Rahal, O.M.; Wang, X.; Gong, Y.; Song, J.; Krishnamurthy, S.; Ueno, N.T.; Tripathy, D.; et al. Decorin-mediated suppression of tumorigenesis, invasion, and metastasis in inflammatory breast cancer. Commun. Biol. 2021, 4, 72. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, H.; Kong, F.; Xu, W.; Wang, T.; Xiao, F.; Wang, L.; Huang, D.; Seth, P.; Yang, Y.; et al. Oncolytic Adenovirus rAd.DCN Inhibits Breast Tumor Growth and Lung Metastasis in an Immune-Competent Orthotopic Xenograft Model. Hum. Gene Ther. 2019, 30, 197–210. [Google Scholar] [CrossRef]
- Li, Y.; Hong, J.; Jung, B.K.; Oh, E.; Yun, C.O. Oncolytic Ad co-expressing decorin and Wnt decoy receptor overcomes chemoresistance of desmoplastic tumor through degradation of ECM and inhibition of EMT. Cancer Lett. 2019, 459, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Yang, J.; Yue, J.; Chen, Y.; Zhou, H.; Fan, D.; Zhang, Q.; Buraschi, S.; Iozzo, R.V.; Bi, X. Decorin deficiency promotes epithelial-mesenchymal transition and colon cancer metastasis. Matrix Biol. 2021, 95, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Simkova, D.; Kharaishvili, G.; Korinkova, G.; Ozdian, T.; Suchankova-Kleplova, T.; Soukup, T.; Krupka, M.; Galandakova, A.; Dzubak, P.; Janikova, M.; et al. The dual role of asporin in breast cancer progression. Oncotarget 2016, 7, 52045–52060. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, H.; Wang, L.; Zhang, H.; Lu, J.; Liang, Z.; Liu, T. Asporin promotes pancreatic cancer cell invasion and migration by regulating the epithelial-to-mesenchymal transition (EMT) through both autocrine and paracrine mechanisms. Cancer Lett. 2017, 398, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Simkova, D.; Kharaishvili, G.; Slabakova, E.; Murray, P.G.; Bouchal, J. Glycoprotein asporin as a novel player in tumour microenvironment and cancer progression. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov. 2016, 160, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Satoyoshi, R.; Kuriyama, S.; Aiba, N.; Yashiro, M.; Tanaka, M. Asporin activates coordinated invasion of scirrhous gastric cancer and cancer-associated fibroblasts. Oncogene 2015, 34, 650–660. [Google Scholar] [CrossRef]
- Wu, H.; Jing, X.; Cheng, X.; He, Y.; Hu, L.; Wu, H.; Ye, F.; Zhao, R. Asporin enhances colorectal cancer metastasis through activating the EGFR/src/cortactin signaling pathway. Oncotarget 2016, 7, 73402–73413. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Chen, L.; Sun, X.; Zhao, Y.; Guo, Q.; Zhu, S.; Li, P.; Min, L.; Zhang, S. Cytoplasmic Asporin promotes cell migration by regulating TGF-β/Smad2/3 pathway and indicates a poor prognosis in colorectal cancer. Cell Death Dis. 2019, 10, 109. [Google Scholar] [CrossRef]
- Maris, P.; Blomme, A.; Palacios, A.P.; Costanza, B.; Bellahcène, A.; Bianchi, E.; Gofflot, S.; Drion, P.; Trombino, G.E.; Di Valentin, E.; et al. Asporin Is a Fibroblast-Derived TGF-β1 Inhibitor and a Tumor Suppressor Associated with Good Prognosis in Breast Cancer. PLoS Med. 2015, 12, e1001871. [Google Scholar] [CrossRef]
- Karamanou, K.; Franchi, M.; Vynios, D.; Brezillon, S. Epithelial-to-mesenchymal transition and invadopodia markers in breast cancer: Lumican a key regulator. Semin. Cancer Biol. 2020, 62, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Giatagana, E.M.; Berdiaki, A.; Tsatsakis, A.; Tzanakakis, G.N.; Nikitovic, D. Lumican in Carcinogenesis-Revisited. Biomolecules 2021, 11, 1319. [Google Scholar] [CrossRef] [PubMed]
- Karamanou, K.; Franchi, M.; Piperigkou, Z.; Perreau, C.; Maquart, F.X.; Vynios, D.H.; Brezillon, S. Lumican effectively regulates the estrogen receptors-associated functional properties of breast cancer cells, expression of matrix effectors and epithelial-to-mesenchymal transition. Sci. Rep. 2017, 7, 45138. [Google Scholar] [CrossRef] [PubMed]
- Karamanou, K.; Franchi, M.; Onisto, M.; Passi, A.; Vynios, D.H.; Brezillon, S. Evaluation of lumican effects on morphology of invading breast cancer cells, expression of integrins and downstream signaling. FEBS J. 2020, 287, 4862–4880. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, M.; Boncela, J.; Perreau, C.; Karamanou, K.; Chatron-Colliet, A.; Proult, I.; Przygodzka, P.; Chakravarti, S.; Maquart, F.-X.; Kowalska, M.A.; et al. Lumican Inhibits SNAIL-Induced Melanoma Cell Migration Specifically by Blocking MMP-14 Activity. PLoS ONE 2016, 11, e0150226. [Google Scholar] [CrossRef]
- Karamanou, K.; Franchi, M.; Proult, I.; Rivet, R.; Vynios, D.; Brezillon, S. Lumican Inhibits In Vivo Melanoma Metastasis by Altering Matrix-Effectors and Invadopodia Markers. Cells 2021, 10, 841. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Z.; Shao, C.; Gong, Y.; Hernando, E.; Lee, P.; Narita, M.; Muller, W.; Liu, J.; Wei, J.J. HMGA2 overexpression-induced ovarian surface epithelial transformation is mediated through regulation of EMT genes. Cancer Res. 2011, 71, 349–359. [Google Scholar] [CrossRef]
- Farace, C.; Oliver, J.A.; Melguizo, C.; Alvarez, P.; Bandiera, P.; Rama, A.R.; Malaguarnera, G.; Ortiz, R.; Madeddu, R.; Prados, J. Microenvironmental Modulation of Decorin and Lumican in Temozolomide-Resistant Glioblastoma and Neuroblastoma Cancer Stem-Like Cells. PLoS ONE 2015, 10, e0134111. [Google Scholar]
- Sun, L.R.; Li, S.Y.; Guo, Q.S.; Zhou, W.; Zhang, H.M. SPOCK1 Involvement in Epithelial-to-Mesenchymal Transition: A New Target in Cancer Therapy? Cancer Manag. Res. 2020, 12, 3561–3569. [Google Scholar] [CrossRef]
- Ye, Z.; Chen, J.; Hu, X.; Yang, S.; Xuan, Z.; Lu, X.; Zhao, Q. SPOCK1: A multi-domain proteoglycan at the crossroads of extracellular matrix remodeling and cancer development. Am. J. Cancer Res. 2020, 10, 3127–3137. [Google Scholar]
- Miao, L.; Wang, Y.; Xia, H.; Yao, C.; Cai, H.; Song, Y. SPOCK1 is a novel transforming growth factor-β target gene that regulates lung cancer cell epithelial-mesenchymal transition. Biochem. Biophys. Res. Commun. 2013, 440, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Han, S.W.; Song, S.H.; Jeong, E.G.; Lee, M.Y.; Hwang, D.; Im, S.A.; Bang, Y.J.; Kim, T.Y. Testican-1-mediated epithelial-mesenchymal transition signaling confers acquired resistance to lapatinib in HER2-positive gastric cancer. Oncogene 2014, 33, 3334–3341. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Guan, H.T.; Dai, Z.J.; Ma, Y.G.; Liu, X.X.; Wang, X.J. Knockdown of SPOCK1 Inhibits the Proliferation and Invasion in Colorectal Cancer Cells by Suppressing the PI3K/Akt Pathway. Oncol. Res. 2016, 24, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wang, Y.; Lan, W.; Wang, S.; Cui, Y.; Zhang, X.; Lin, Z.; Piao, J. SPOCK1 promotes metastasis in pancreatic cancer via NF-κB-dependent epithelial-mesenchymal transition by interacting with IκB-α. Cell. Oncol. 2022, 45, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, X.; Zhang, S.; Piao, J.; Yang, Y.; Wang, X.; Lin, Z. SPOCK1/SIX1axis promotes breast cancer progression by activating AKT/mTOR signaling. Aging 2020, 13, 1032–1050. [Google Scholar] [CrossRef]
- Fan, L.C.; Jeng, Y.M.; Lu, Y.T.; Lien, H.C. SPOCK1 Is a Novel Transforming Growth Factor-β-Induced Myoepithelial Marker That Enhances Invasion and Correlates with Poor Prognosis in Breast Cancer. PLoS ONE 2016, 11, e0162933. [Google Scholar] [CrossRef]
- Song, X.; Han, P.; Liu, J.; Wang, Y.; Li, D.; He, J.; Gong, J.; Li, M.; Tu, W.; Yan, W.; et al. Up-regulation of SPOCK1 induces epithelial-mesenchymal transition and promotes migration and invasion in esophageal squamous cell carcinoma. J. Mol. Histol. 2015, 46, 347–356. [Google Scholar] [CrossRef]
- Chien, M.H.; Lin, Y.W.; Wen, Y.C.; Yang, Y.C.; Hsiao, M.; Chang, J.L.; Huang, H.C.; Lee, W.J. Targeting the SPOCK1-snail/slug axis-mediated epithelial-to-mesenchymal transition by apigenin contributes to repression of prostate cancer metastasis. J. Exp. Clin. Cancer Res. 2019, 38, 246. [Google Scholar] [CrossRef]
- Yu, F.; Li, G.; Gao, J.; Sun, Y.; Liu, P.; Gao, H.; Li, P.; Lei, T.; Chen, Y.; Cheng, Y.; et al. SPOCK1 is upregulated in recurrent glioblastoma and contributes to metastasis and Temozolomide resistance. Cell Prolif. 2016, 49, 195–206. [Google Scholar] [CrossRef]
- Chakraborty, S.; Lakshmanan, M.; Swa, H.L.; Chen, J.; Zhang, X.; Ong, Y.S.; Loo, L.S.; Akincilar, S.C.; Gunaratne, J.; Tergaonkar, V.; et al. An oncogenic role of Agrin in regulating focal adhesion integrity in hepatocellular carcinoma. Nat. Commun. 2015, 6, 6184. [Google Scholar] [CrossRef]
- Lv, X.; Fang, C.; Yin, R.; Qiao, B.; Shang, R.; Wang, J.; Song, W.; He, Y.; Chen, Y. Agrin para-secreted by PDGF-activated human hepatic stellate cells promotes hepatocarcinogenesis in vitro and in vivo. Oncotarget 2017, 8, 105340–105355. [Google Scholar] [CrossRef] [PubMed]
- Ruivo, C.F.; Bastos, N.; Adem, B.; Batista, I.; Duraes, C.; Melo, C.A.; Castaldo, S.A.; Campos-Laborie, F.; Moutinho-Ribeiro, P.; Morao, B.; et al. Extracellular Vesicles from Pancreatic Cancer Stem Cells Lead an Intratumor Communication Network (EVNet) to fuel tumour progression. Gut 2022, 71, 2043–2068. [Google Scholar] [CrossRef] [PubMed]
- Carvalhaes, L.S.; Gervasio, O.L.; Guatimosim, C.; Heljasvaara, R.; Sormunen, R.; Pihlajaniemi, T.; Kitten, G.T. Collagen XVIII/endostatin is associated with the epithelial-mesenchymal transformation in the atrioventricular valves during cardiac development. Dev. Dyn. 2006, 235, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, H.; Zhu, H.; Yang, X.; Yang, Y.; Yang, Y.; Min, H.; Chen, G.; Liu, J.; Lu, J.; et al. Endostatin combined with radiotherapy suppresses vasculogenic mimicry formation through inhibition of epithelial-mesenchymal transition in esophageal cancer. Tumour Biol. 2016, 37, 4679–4688. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Ma, L.; Wu, Z.Q.; Zheng, G.Y.; Li, J.T. Effect of endostatin on proliferation. invasion and epithelial-mesenchymal transition of basal cell carcinoma cell A431. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 877–884. [Google Scholar]
- Wang, Y.; Jiang, M.; Li, Z.; Wang, J.; Du, C.; Yanyang, L.; Yu, Y.; Wang, X.; Zhang, N.; Zhao, M.; et al. Hypoxia and TGF-β1 lead to endostatin resistance by cooperatively increasing cancer stem cells in A549 transplantation tumors. Cell Biosci. 2015, 5, 72. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Cui, J.; Si, T. Endostar blocks the metastasis. invasion and angiogenesis of ovarian cancer cells. Neoplasma 2020, 67, 595–603. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, Q.; Li, L. Endostar regulates EMT. migration and invasion of lung cancer cells through the HGF-Met pathway. Mol. Cell. Probes 2019, 45, 57–64. [Google Scholar] [CrossRef]
- Tian, W.; Li, J.; Wang, Z.; Zhang, T.; Han, Y.; Liu, Y.; Chu, W.; Liu, Y.; Yang, B. HYD-PEP06 suppresses hepatocellular carcinoma metastasis, epithelial-mesenchymal transition and cancer stem cell-like properties by inhibiting PI3K/AKT and WNT/β-catenin signaling activation. Acta Pharm. Sin. B 2021, 11, 1592–1606. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Hassan, H.; Vilardo, L.; Kumar, S.K.; Kumar, A.V.; Kelsch, R.; Schneider, C.; Kiesel, L.; Eich, H.T.; Zucchi, I.; et al. Syndecan-1 (CD138) modulates triple-negative breast cancer stem cell properties via regulation of LRP-6 and IL-6-mediated STAT3 signaling. PLoS ONE 2013, 8, e85737. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Gadalla, R.; El-Ghonaimy, E.A.; Samir, O.; Mohamed, H.T.; Hassan, H.; Greve, B.; El-Shinawi, M.; Mohamed, M.M.; Götte, M. Syndecan-1 Is a Novel Molecular Marker for Triple Negative Inflammatory Breast Cancer and Modulates the Cancer Stem Cell Phenotype via the IL-6/STAT3, Notch and EGFR Signaling Pathways; BioMed Central Ltd.: London, UK, 2017. [Google Scholar]
- Juuti, A.; Nordling, S.; Lundin, J.; Louhimo, J.; Haglund, C. Syndecan-1 expression—A novel prognostic marker in pancreatic cancer. Oncology 2005, 68, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Gharbaran, R. Advances in the molecular functions of syndecan-1 (SDC1/CD138) in the pathogenesis of malignancies. Crit. Rev. Oncol. Hematol. 2015, 94, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Wu, C.P.; Tseng, T.; Jhang, Y.; Lee, S.C. Role of syndecan-1 and exogenous heparin in hepatoma sphere formation. Biochem. Cell Biol. 2020, 98, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Couchman, J.R. Syndecan-1 (CD138), Carcinomas and EMT. Int. J. Mol. Sci. 2021, 22, 4227. [Google Scholar] [CrossRef]
- Loussouarn, D.; Campion, L.; Sagan, C.; Frenel, J.S.; Dravet, F.; Classe, J.M.; Pioud-Martigny, R.; Berton-Rigaud, D.; Bourbouloux, E.; Mosnier, J.F.; et al. Prognostic impact of syndecan-1 expression in invasive ductal breast carcinomas. Br. J. Cancer 2008, 98, 1993–1998. [Google Scholar] [CrossRef]
- Masola, V.; Gambaro, G.; Tibaldi, E.; Brunati, A.M.; Gastaldello, A.; D’Angelo, A.; Onisto, M.; Lupo, A. Heparanase and syndecan-1 interplay orchestrates fibroblast growth factor-2-induced epithelial-mesenchymal transition in renal tubular cells. J. Biol. Chem. 2012, 287, 1478–1488. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, H.; Chen, C.; Li, J.; He, J.; Fu, X.; Zhao, H. The HPA/SDC1 axis promotes invasion and metastasis of pancreatic cancer cells by activating EMT via FGF2 upregulation. Oncol. Lett. 2020, 19, 211–220. [Google Scholar] [CrossRef]
- Farfan, N.; Ocarez, N.; Castellon, E.A.; Mejia, N.; de Herreros, A.G.; Contreras, H.R. The transcriptional factor ZEB1 represses Syndecan 1 expression in prostate cancer. Sci. Rep. 2018, 8, 11467. [Google Scholar] [CrossRef]
- Poblete, C.E.; Fulla, J.; Gallardo, M.; Munoz, V.; Castellon, E.A.; Gallegos, I.; Contreras, H.R. Increased SNAIL expression and low syndecan levels are associated with high Gleason grade in prostate cancer. Int. J. Oncol. 2014, 44, 647–654. [Google Scholar] [CrossRef]
- Liu, Z.X.; Jin, H.; Yang, S.; Cao, H.M.; Zhang, Z.Y.; Wen, B.; Zhou, S.B. SDC1 knockdown induces epithelial-mesenchymal transition and invasion of gallbladder cancer cells via the ERK/Snail pathway. J. Int. Med. Res. 2020, 48. [Google Scholar] [CrossRef]
- Wang, X.; He, J.; Zhao, X.; Qi, T.; Zhang, T.; Kong, C. Syndecan-1 suppresses epithelial-mesenchymal transition and migration in human oral cancer cells. Oncol. Rep. 2018, 39, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Mitselou, A.; Galani, V.; Skoufi, U.; Arvanitis, D.L.; Lampri, E.; Ioachim, E. Syndecan-1, Epithelial-Mesenchymal Transition Markers (E-cadherin/beta-catenin) and Neoangiogenesis-related Proteins (PCAM-1 and Endoglin) in Colorectal Cancer. Anticancer Res. 2016, 36, 2271–2280. [Google Scholar] [PubMed]
- Zeng, Y.; Yao, X.; Chen, L.; Yan, Z.; Liu, J.; Zhang, Y.; Feng, T.; Wu, J.; Liu, X. Sphingosine-1-phosphate induced epithelial-mesenchymal transition of hepatocellular carcinoma via an MMP-7/syndecan-1/TGF-beta autocrine loop. Oncotarget 2016, 7, 63324–63337. [Google Scholar] [CrossRef] [PubMed]
- Kumar-Singh, A.; Parniewska, M.M.; Giotopoulou, N.; Javadi, J.; Sun, W.; Szatmari, T.; Dobra, K.; Hjerpe, A.; Fuxe, J. Nuclear Syndecan-1 Regulates Epithelial-Mesenchymal Plasticity in Tumor Cells. Biology 2021, 10, 521. [Google Scholar] [CrossRef]
- D’Arcy, C.; Zimmermann, C.C.; Espinoza-Sanchez, N.A.; Greve, B.; Schmidt, A.; Kiesel, L.; von Wahlde, M.-K.; Götte, M. The heparan sulphate proteoglycan Syndecan-1 (CD138) regulates tumour progression in a 3D model of ductal carcinoma in situ of the breast. IUBMB Life 2022, 74, 955–968. [Google Scholar] [CrossRef]
- Jang, B.; Jung, H.; Chung, H.; Moon, B.I.; Oh, E.S. Syndecan-2 enhances E-cadherin shedding and fibroblast-like morphological changes by inducing MMP-7 expression in colon cancer cells. Biochem. Biophys. Res. Commun. 2016, 477, 47–53. [Google Scholar] [CrossRef]
- Jang, B.; Jung, H.; Choi, S.; Lee, Y.H.; Lee, S.T.; Oh, E.S. Syndecan-2 cytoplasmic domain up-regulates matrix metalloproteinase-7 expression via the protein kinase Cgamma-mediated FAK/ERK signaling pathway in colon cancer. J. Biol. Chem. 2017, 292, 16321–16332. [Google Scholar] [CrossRef]
- Hua, R.; Yu, J.; Yan, X.; Ni, Q.; Zhi, X.; Li, X.; Jiang, B.; Zhu, J. Syndecan-2 in colorectal cancer plays oncogenic role via epithelial-mesenchymal transition and MAPK pathway. Biomed. Pharmacother. 2020, 121, 109630. [Google Scholar] [CrossRef]
- Ma, N.; Li, X.; Wei, H.; Zhang, H.; Zhang, S. Circular RNA circNFATC3 acts as a miR-9-5p sponge to promote cervical cancer development by upregulating SDC2. Cell. Oncol. 2021, 44, 93–107. [Google Scholar] [CrossRef]
- Diamantopoulou, Z.; Kitsou, P.; Menashi, S.; Courty, J.; Katsoris, P. Loss of receptor protein tyrosine phosphatase beta/zeta (RPTPbeta/zeta) promotes prostate cancer metastasis. J. Biol. Chem. 2012, 287, 40339–40349. [Google Scholar] [CrossRef]
- Hillemeyer, L.; Espinoza-Sanchez, N.A.; Greve, B.; Hassan, N.; Chelariu-Raicu, A.; Kiesel, L.; Gotte, M. The Cell Surface Heparan Sulfate Proteoglycan Syndecan-3 Promotes Ovarian Cancer Pathogenesis. Int. J. Mol. Sci. 2022, 23, 5793. [Google Scholar] [CrossRef] [PubMed]
- Keller-Pinter, A.; Gyulai-Nagy, S.; Becsky, D.; Dux, L.; Rovo, L. Syndecan-4 in Tumor Cell Motility. Cancers 2021, 13, 3322. [Google Scholar] [CrossRef] [PubMed]
- Onyeisi, J.O.S.; Lopes, C.C.; Gotte, M. Syndecan-4 as a Pathogenesis Factor and Therapeutic Target in Cancer. Biomolecules 2021, 11, 503. [Google Scholar] [CrossRef] [PubMed]
- Labropoulou, V.T.; Skandalis, S.S.; Ravazoula, P.; Perimenis, P.; Karamanos, N.K.; Kalofonos, H.P.; Theocharis, A.D. Expression of syndecan-4 and correlation with metastatic potential in testicular germ cell tumours. BioMed Res. Int. 2013, 2013, 214864. [Google Scholar] [CrossRef]
- Jechorek, D.; Haeusler-Pliske, I.; Meyer, F.; Roessner, A. Diagnostic value of syndecan-4 protein expression in colorectal cancer. Pathol. Res. Pract. 2021, 222, 153431. [Google Scholar] [CrossRef]
- Toba-Ichihashi, Y.; Yamaoka, T.; Ohmori, T.; Ohba, M. Up-regulation of Syndecan-4 contributes to TGF-beta1-induced epithelial to mesenchymal transition in lung adenocarcinoma A549 cells. Biochem. Biophys. Rep. 2016, 5, 1–7. [Google Scholar]
- Chen, L.L.; Gao, G.X.; Shen, F.X.; Chen, X.; Gong, X.H.; Wu, W.J. SDC4 Gene Silencing Favors Human Papillary Thyroid Carcinoma Cell Apoptosis and Inhibits Epithelial Mesenchymal Transition via Wnt/beta-Catenin Pathway. Mol. Cells 2018, 41, 853–867. [Google Scholar]
- Ohkawara, B.; Glinka, A.; Niehrs, C. Rspo3 binds syndecan 4 and induces Wnt/PCP signaling via clathrin-mediated endocytosis to promote morphogenesis. Dev. Cell 2011, 20, 303–314. [Google Scholar] [CrossRef]
- Liao, W.C.; Yen, H.R.; Chen, C.H.; Chu, Y.H.; Song, Y.C.; Tseng, T.J.; Liu, C.H. CHPF promotes malignancy of breast cancer cells by modifying syndecan-4 and the tumor microenvironment. Am. J. Cancer Res. 2021, 11, 812–826. [Google Scholar]
- Guan, Z.; Sun, Y.; Mu, L.; Jiang, Y.; Fan, J. Tenascin-C promotes bladder cancer progression and its action depends on syndecan-4 and involves NF-kappaB signaling activation. BMC Cancer 2022, 22, 240. [Google Scholar] [CrossRef]
- Gordon, K.J.; Kirkbride, K.C.; How, T.; Blobe, G.C. Bone morphogenetic proteins induce pancreatic cancer cell invasiveness through a Smad1-dependent mechanism that involves matrix metalloproteinase-2. Carcinogenesis 2009, 30, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sun, W.Y.; Wu, J.J.; Gu, Y.J.; Wei, W. Decreased expression of the type III TGF-beta receptor enhances metastasis and invasion in hepatocellullar carcinoma progression. Oncol. Rep. 2016, 35, 2373–2381. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.W.; Hsiao, J.R.; Fan, C.C.; Lo, Y.K.; Tzen, C.Y.; Wu, L.W.; Fang, W.Y.; Cheng, A.J.; Chen, C.H.; Chang, I.S.; et al. Loss of GDF10/BMP3b as a prognostic marker collaborates with TGFBR3 to enhance chemotherapy resistance and epithelial-mesenchymal transition in oral squamous cell carcinoma. Mol. Carcinogen. 2016, 55, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, K.; Li, Z.; Wang, J.; Wang, X. miR-19a and miR-424 target TGFBR3 to promote epithelial-to-mesenchymal transition and migration of tongue squamous cell carcinoma cells. Cell Adhes. Migr. 2018, 12, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Yao, Z.; Zhang, Y.; Li, D.; Hu, F.; Liao, Y.; Zhou, L.; Zhou, Y.; Huang, Z.; He, Z.; et al. Deep RNA sequencing reveals the dynamic regulation of miRNA, lncRNAs, and mRNAs in osteosarcoma tumorigenesis and pulmonary metastasis. Cell Death Dis. 2018, 9, 772. [Google Scholar] [CrossRef]
- You, H.J.; How, T.; Blobe, G.C. The type III transforming growth factor-β receptor negatively regulates nuclear factor kappa B signaling through its interaction with β-arrestin2. Carcinogenesis 2009, 30, 1281–1287. [Google Scholar] [CrossRef]
- Meyer, A.E.; Gatza, C.E.; How, T.; Starr, M.; Nixon, A.B.; Blobe, G.C. Role of TGF-beta receptor III localization in polarity and breast cancer progression. Mol. Biol. Cell 2014, 25, 2291–2304. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Ren, C.; Chen, Y.; Guo, X.; Zhou, L.; Peng, Z.; Tang, Y.; Chen, Y.; Liu, W.; et al. The clinical significance of circulating GPC1 positive exosomes and its regulative miRNAs in colon cancer patients. Oncotarget 2017, 8, 101189–101202. [Google Scholar] [CrossRef]
- Papiewska-Pajak, I.; Krzyzanowski, D.; Katela, M.; Rivet, R.; Michlewska, S.; Przygodzka, P.; Kowalska, M.A.; Brezillon, S. Glypican-1 Level Is Elevated in Extracellular Vesicles Released from MC38 Colon Adenocarcinoma Cells Overexpressing Snail. Cells 2020, 9, 1585. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Zhan, C.; Zhu, J.; Weng, S.; Dong, L.; Liu, T.; Shen, X. Glypican-1 Promotes Tumorigenesis by Regulating the PTEN/Akt/beta-Catenin Signaling Pathway in Esophageal Squamous Cell Carcinoma. Dig. Dis. Sci. 2019, 64, 1493–1502. [Google Scholar] [CrossRef]
- Castillo, L.F.; Tascon, R.; Lago Huvelle, M.R.; Novack, G.; Llorens, M.C.; Dos Santos, A.F.; Shortrede, J.; Cabanillas, A.M.; Bal de Kier Joffe, E.; Labriola, L.; et al. Glypican-3 induces a mesenchymal to epithelial transition in human breast cancer cells. Oncotarget 2016, 7, 60133–60154. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.H.; Wu, D.; Cui, H.X.; Ma, N.; Su, J.; Wang, Y.T.; Jiang, Y.H. Silencing of the glypican-3 gene affects the biological behavior of human hepatocellular carcinoma cells. Mol. Med. Rep. 2014, 10, 3177–3184. [Google Scholar] [CrossRef] [PubMed]
- Guereno, M.; Delgado Pastore, M.; Lugones, A.C.; Cercato, M.; Todaro, L.; Urtreger, A.; Peters, M.G. Glypican-3 (GPC3) inhibits metastasis development promoting dormancy in breast cancer cells by p38 MAPK pathway activation. Eur. J. Cell Biol. 2020, 99, 151096. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, H.; Weng, H.; Zhang, X.; Li, P.; Fan, C.L.; Li, B.; Dong, P.L.; Li, L.; Dooley, S.; et al. Glypican-3 promotes epithelial-mesenchymal transition of hepatocellular carcinoma cells through ERK signaling pathway. Int. J. Oncol. 2015, 46, 1275–1285. [Google Scholar] [CrossRef]
- Meng, P.; Zhang, Y.F.; Zhang, W.; Chen, X.; Xu, T.; Hu, S.; Liang, X.; Feng, M.; Yang, X.; Ho, M. Identification of the atypical cadherin FAT1 as a novel glypican-3 interacting protein in liver cancer cells. Sci. Rep. 2021, 11, 40. [Google Scholar] [CrossRef]
- Fico, A.; De Chevigny, A.; Egea, J.; Bosl, M.R.; Cremer, H.; Maina, F.; Dono, R. Modulating Glypican4 suppresses tumorigenicity of embryonic stem cells while preserving self-renewal and pluripotency. Stem Cells 2012, 30, 1863–1874. [Google Scholar] [CrossRef]
- Vitale, D.; Kumar Katakam, S.; Greve, B.; Jang, B.; Oh, E.S.; Alaniz, L.; Gotte, M. Proteoglycans and glycosaminoglycans as regulators of cancer stem cell function and therapeutic resistance. FEBS J. 2019, 286, 2870–2882. [Google Scholar] [CrossRef]
- Oikari, L.E.; Okolicsanyi, R.K.; Qin, A.; Yu, C.; Griffiths, L.R.; Haupt, L.M. Cell surface heparan sulfate proteoglycans as novel markers of human neural stem cell fate determination. Stem Cell Res. 2016, 16, 92–104. [Google Scholar] [CrossRef]
- Cao, J.; Ma, J.; Sun, L.; Li, J.; Qin, T.; Zhou, C.; Cheng, L.; Chen, K.; Qian, W.; Duan, W.; et al. Targeting glypican-4 overcomes 5-FU resistance and attenuates stem cell-like properties via suppression of Wnt/beta-catenin pathway in pancreatic cancer cells. J. Cell. Biochem. 2018, 119, 9498–9512. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, M.; Xia, W.; Xu, Y.; Mao, Q.; Wang, J.; Dong, G.; Xu, L.; Yang, X.; Yin, R. Glypican-5 suppresses Epithelial-Mesenchymal Transition of the lung adenocarcinoma by competitively binding to Wnt3a. Oncotarget 2016, 7, 79736–79746. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, K.; He, M.; Fan, G.; Lu, H. Overexpression of Glypican 5 (GPC5) Inhibits Prostate Cancer Cell Proliferation and Invasion via Suppressing Sp1-Mediated EMT and Activation of Wnt/beta-Catenin Signaling. Oncol. Res. 2018, 26, 565–572. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhou, X.; Qu, C.; Tang, Y.; Zhang, Q.; Hong, J. Serglycin (SRGN) overexpression predicts poor prognosis in hepatocellular carcinoma patients. Med. Oncol. 2013, 30, 707. [Google Scholar] [CrossRef] [PubMed]
- Bouris, P.; Manou, D.; Sopaki-Valalaki, A.; Kolokotroni, A.; Moustakas, A.; Kapoor, A.; Iozzo, R.V.; Karamanos, N.K.; Theocharis, A.D. Serglycin promotes breast cancer cell aggressiveness: Induction of epithelial to mesenchymal transition. Proteolytic activity and IL-8 signaling. Matrix Biol. 2018, 74, 35–51. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, Y.; Zheng, G.; Jia, X.; Xiong, Y.; Luo, K.; Qiu, Q.; Qiu, N.; Yin, J.; Lu, M.; et al. SRGN-TGFbeta2 regulatory loop confers invasion and metastasis in triple-negative breast cancer. Oncogenesis 2017, 6, e360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qiu, N.; Yin, J.; Zhang, J.; Liu, H.; Guo, W.; Liu, M.; Liu, T.; Chen, D.; Luo, K.; et al. SRGN crosstalks with YAP to maintain chemoresistance and stemness in breast cancer cells by modulating HDAC2 expression. Theranostics 2020, 10, 4290–4307. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, J.; Yang, Y.; Zhu, L.; Li, X.; Zhao, W. SRGN Promotes Colorectal Cancer Metastasis as a Critical Downstream Target of HIF-1alpha. Cell. Physiol. Biochem. 2018, 48, 2429–2440. [Google Scholar] [CrossRef]
- Guo, J.Y.; Hsu, H.S.; Tyan, S.W.; Li, F.Y.; Shew, J.Y.; Lee, W.H.; Chen, J.Y. Serglycin in tumor microenvironment promotes non-small cell lung cancer aggressiveness in a CD44-dependent manner. Oncogene 2017, 36, 2457–2471. [Google Scholar] [CrossRef]
- Li, X.J.; Ong, C.K.; Cao, Y.; Xiang, Y.Q.; Shao, J.Y.; Ooi, A.; Peng, L.X.; Lu, W.H.; Zhang, Z.; Petillo, D.; et al. Serglycin is a theranostic target in nasopharyngeal carcinoma that promotes metastasis. Cancer Res. 2011, 71, 3162–3172. [Google Scholar] [CrossRef]
- Manou, D.; Bouris, P.; Kletsas, D.; Gotte, M.; Greve, B.; Moustakas, A.; Karamanos, N.K.; Theocharis, A.D. Serglycin activates pro-tumorigenic signaling and controls glioblastoma cell stemness, differentiation and invasive potential. Matrix Biol. Plus 2020, 6–7, 100033. [Google Scholar] [CrossRef]
- Tellez-Gabriel, M.; Tekpli, X.; Reine, T.M.; Hegge, B.; Nielsen, S.R.; Chen, M.; Moi, L.; Normann, L.S.; Busund, L.-T.R.; Calin, G.A.; et al. Serglycin Is Involved in TGF-β Induced Epithelial-Mesenchymal Transition and Is Highly Expressed by Immune Cells in Breast Cancer Tissue. Front. Oncol. 2022, 12, 868868. [Google Scholar] [CrossRef]
- Roy, A.; Femel, J.; Huijbers, E.J.; Spillmann, D.; Larsson, E.; Ringvall, M.; Olsson, A.K.; Abrink, M. Targeting Serglycin Prevents Metastasis in Murine Mammary Carcinoma. PLoS ONE 2016, 11, e0156151. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).