Timely Leukapheresis May Interfere with the “Fitness” of Lymphocytes Collected for CAR-T Treatment in High Risk DLBCL Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. “Pre-Emptive” Ly-Apheresis Program
2.3. CAR-T Cell Treatment
2.4. Flow Cytometry Ly “Fitness” Analysis
2.5. Statistical Analyses
3. Results
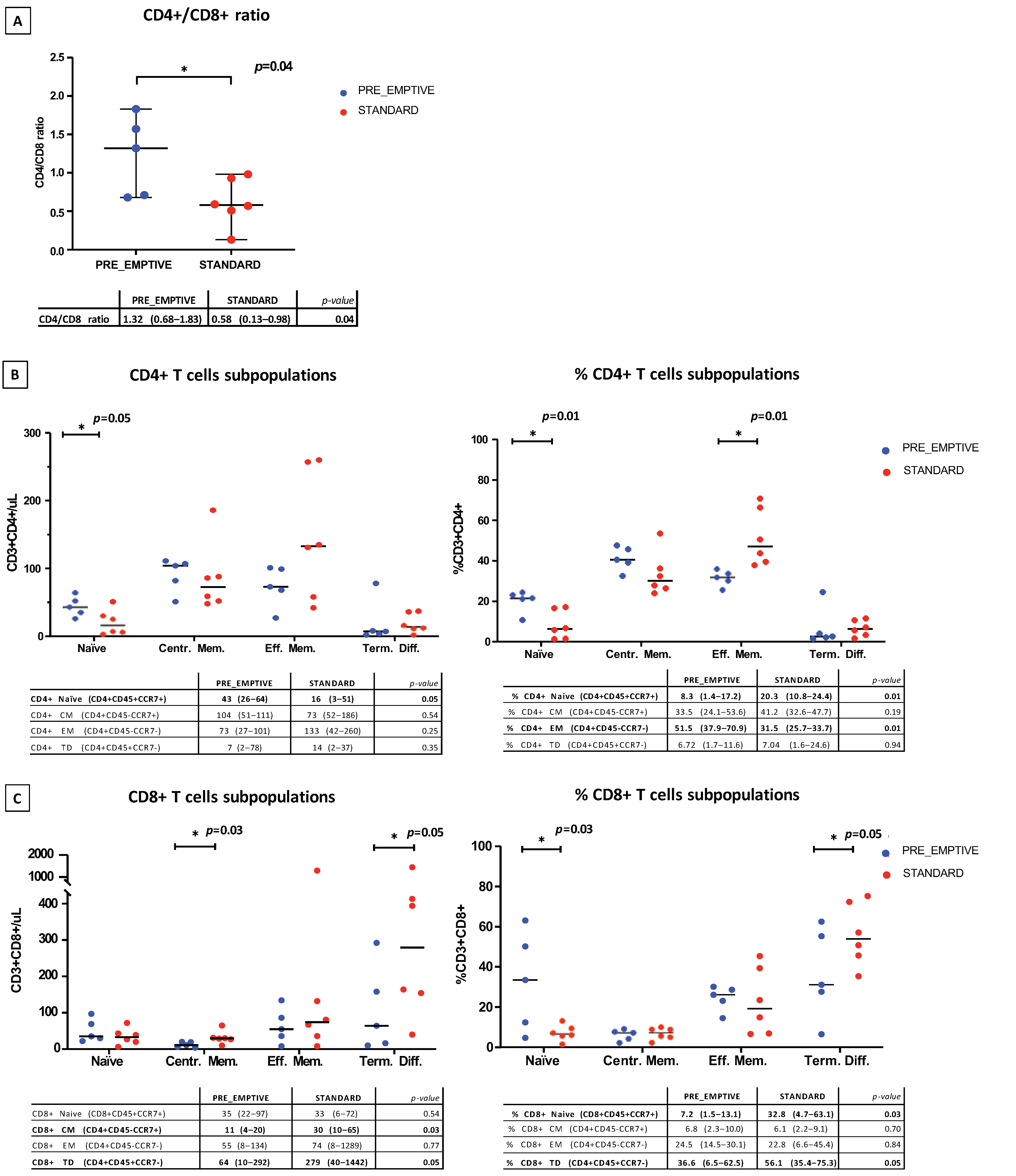
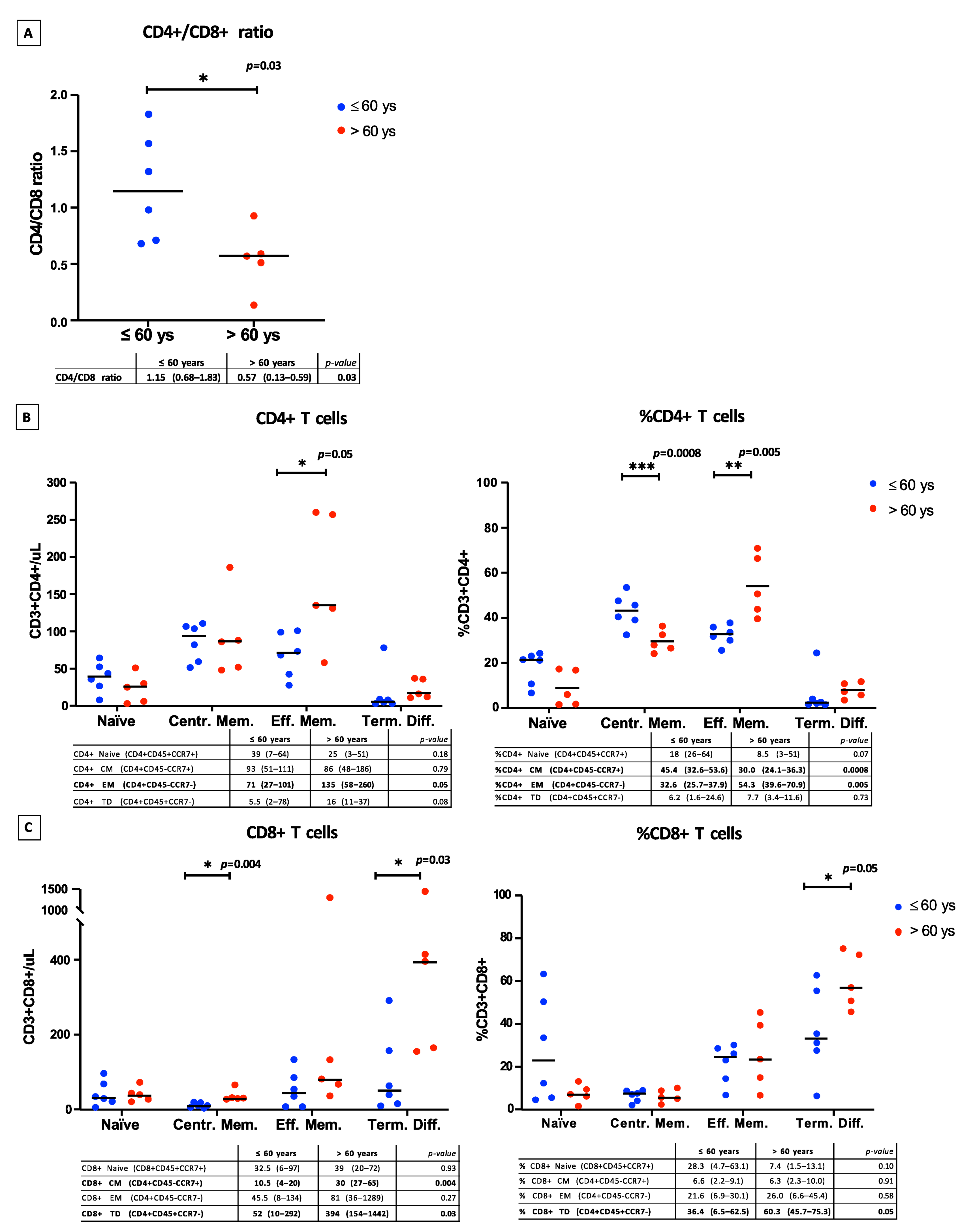
3.1. Lymphocyte Subpopulation Cell Analysis at Time of Ly-Apheresis
3.2. Follow-Up Post CAR-T Cell Infusion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larson, R.C.; Maus, M.V. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat. Rev. Cancer 2021, 21, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Hopfinger, G.; Jäger, U.; Worel, N. CAR-T Cell Therapy in Diffuse Large B Cell Lymphoma: Hype and Hope. HemaSphere 2019, 3, e185. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Locke, F.L.; Miklos, D.B.; Jacobson, C.A.; Perales, M.-A.; Kersten, M.-J.; Oluwole, O.O.; Ghobadi, A.; Rapoport, A.P.; McGuirk, J.; Pagel, J.M.; et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, C.A.; Hunter, B.D.; Redd, R.; Rodig, S.J.; Chen, P.H.; Wright, K.; Lipschitz, M.; Ritz, J.; Kamihara, Y.; Armand, P.; et al. Axicabtagene Ciloleucel in the Non-Trial Setting: Outcomes and Correlates of Response, Resistance, and Toxicity. J. Clin. Oncol. 2020, 38, 3095–3106. [Google Scholar] [CrossRef] [PubMed]
- Vercellino, L.; Di Blasi, R.; Kanoun, S.; Tessoulin, B.; Rossi, C.; D’Aveni-Piney, M.; Obéric, L.; Bodet-Milin, C.; Bories, P.; Olivier, P.; et al. Predictive factors of early progression after CAR T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2020, 4, 5607–5615. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Tam, C.S.; Borchmann, P.; Worel, N.; McGuirk, J.P.; Holte, H.; Waller, E.K.; Jaglowski, S.; Bishop, M.R.; Damon, L.E.; et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021, 22, 1403–1415. [Google Scholar] [CrossRef]
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef]
- Westin, J.R.; Kersten, M.J.; Salles, G.; Abramson, J.S.; Schuster, S.J.; Locke, F.L.; Andreadis, C. Efficacy and safety of CD19-directed CAR-T cell therapies in patients with relapsed/refractory aggressive B-cell lymphomas: Observations from the JULIET, ZUMA-1, and TRANSCEND trials. Am. J. Hematol. 2021, 96, 1295–1312. [Google Scholar] [CrossRef]
- Fraietta, J.A.; Lacey, S.F.; Orlando, E.J.; Pruteanu-Malinici, I.; Gohil, M.; Lundh, S.; Boesteanu, A.C.; Wang, Y.; O’connor, R.S.; Hwang, W.T.; et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 2018, 24, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Klaver, Y.; van Steenbergen, S.C.L.; Sleijfer, S.; Debets, R.; Lamers, C.H.J. T cell maturation stage prior to and during GMP processing informs on CAR T cell expansion in patients. Front. Immunol. 2016, 7, 648. [Google Scholar] [CrossRef] [PubMed]
- Garfall, A.L.; Dancy, E.K.; Cohen, A.D.; Hwang, W.T.; Fraietta, J.A.; Davis, M.M.; Levine, B.L.; Siegel, D.L.; Stadtmauer, E.A.; Vogl, D.T.; et al. T-cell phenotypes associated with effective CAR T-cell therapy in postinduction vs relapsed multiple myeloma. Blood Adv. 2019, 3, 2812–2815. [Google Scholar] [CrossRef]
- Shah, N.N.; Fry, T.J. Mechanisms of resistance to CAR T cell therapy. Nat. Rev. Clin. Oncol. 2019, 16, 372–385. [Google Scholar] [CrossRef]
- Nie, Y.; Lu, W.; Chen, D.; Tu, H.; Guo, Z.; Zhou, X.; Li, M.; Tu, S.; Li, Y. Mechanisms underlying CD19-positive ALL relapse after anti-CD19 CAR T cell therapy and associated strategies. Biomark. Res. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.H.; Fiorenza, S.; Koldej, R.M.; Jaworowski, A.; Ritchie, D.S.; Quinn, K.M. T Cell Fitness and Autologous CAR T Cell Therapy in Haematologic Malignancy. Front. Immunol. 2021, 12, 4971. [Google Scholar] [CrossRef] [PubMed]
- Gallimore, A.; Glithero, A.; Godkin, A.; Tissot, A.C.; Plückthun, A.; Elliott, T.; Hengartner, H.; Zinkernagel, R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 1998, 187, 1383–1393. [Google Scholar] [CrossRef]
- Singh, N.; Perazzelli, J.; Grupp, S.A.; Barrett, D.M. Early memory phenotypes drive T cell proliferation in patients with pediatric malignancies. Sci. Transl. Med. 2016, 8, 320ra3. [Google Scholar] [CrossRef]
- Skert, C.; Perucca, S.; Chiarini, M.; Giustini, V.; Sottini, A.; Ghidini, C.; Martellos, S.; Cattina, F.; Rambaldi, B.; Cancelli, V.; et al. Sequential monitoring of lymphocyte subsets and of T- and B-cell neogenesis indexes to identify time-varying immunologic profiles in relation to graft-versus-host disease and relapse after allogeneic stem cell transplantation. PLoS ONE 2017, 12, e0175337. [Google Scholar] [CrossRef]
- Chow, A.; Perica, K.; Klebanoff, C.A.; Wolchok, J.D. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Bianchetti, N.; Tucci, A.; Re, A.; Pagani, C.; Cattaneo, C.; Miscio, M.; Facchetti, F.; Fisogni, S.; Balzarini, P.; Albano, D.; et al. Very Poor Outcome of Patients with Relapsed/Refractory Aggressive B Cell Lymphoma after Autologous Stem Cell Transplantation (ASCT) or High Dose of Methotrexate and Cytarabine (HD-MTX/ARA-C) Regimens in the Clinical Care Setting. Blood 2018, 132, 4232. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Tummala, S.; Kebriaei, P.; Wierda, W.; Gutierrez, C.; Locke, F.L.; Komanduri, K.V.; Lin, Y.; Jain, N.; Daver, N.; et al. Chimeric antigen receptor T-cell therapy—Assessment and management of toxicities. Nat. Rev. Clin. Oncol. 2018, 15, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3067. [Google Scholar] [CrossRef] [PubMed]
- Kalos, M.; Levine, B.L.; Porter, D.L.; Katz, S.; Grupp, S.A.; Bagg, A.; June, C.H. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 2011, 3, 95ra73. [Google Scholar] [CrossRef] [PubMed]
- Savoldo, B.; Ramos, C.A.; Liu, E.; Mims, M.P.; Keating, M.J.; Carrum, G.; Kamble, R.T.; Bollard, C.M.; Gee, A.P.; Mei, Z.; et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J. Clin. Investig. 2011, 121, 1822–1826. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, M.; Ramos, C.A.; Durett, A.; Liu, E.; Dakhova, O.; Liu, H.; Creighton, C.J.; Gee, A.P.; Heslop, H.E.; et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood 2014, 123, 3750–3759. [Google Scholar] [CrossRef]
- Jaeger, U.; Bishop, M.R.; Salles, G.; Schuster, S.J.; Maziarz, R.T.; Han, X.; Savchenko, A.; Roscoe, N.; Orlando, E.; Knoblock, D.; et al. Myc Expression and Tumor-Infiltrating T Cells Are Associated with Response in Patients (Pts) with Relapsed/Refractory Diffuse Large B-Cell Lymphoma (r/r DLBCL) Treated with Tisagenlecleucel in the Juliet Trial. Blood 2020, 136, 48–49. [Google Scholar] [CrossRef]
- Finney, O.C.; Brakke, H.; Rawlings-Rhea, S.; Hicks, R.; Doolittle, D.; Lopez, M.; Futrell, B.; Orentas, R.J.; Li, D.; Gardner, R.; et al. CD19 CAR T cell product and disease attributes predict leukemia remission durability. J. Clin. Investig. 2019, 129, 2123–2132. [Google Scholar] [CrossRef]
- Sellmer, L.; Kovács, J.; Walter, J.; Kumbrink, J.; Neumann, J.; Kauffmann-Guerrero, D.; Kiefl, R.; Schneider, C.; Jung, A.; Behr, J.; et al. Markers of Immune Cell Exhaustion as Predictor of Survival in Surgically-Treated Early-Stage NSCLC. Front. Immunol. 2022, 13, 858212. [Google Scholar] [CrossRef]
- Gisselbrecht, C.; Glass, B.; Mounier, N.; Gill, D.S.; Linch, D.C.; Trneny, M.; Bosly, A.; Ketterer, N.; Shpilberg, O.; Hagberg, H.; et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J. Clin. Oncol. 2010, 28, 4184–4190. [Google Scholar] [CrossRef]
- Hamlin, P.A.; Zelenetz, A.D.; Kewalramani, T.; Qin, J.; Satagopan, J.M.; Verbel, D.; Noy, A.; Portlock, C.S.; Straus, D.J.; Yahalom, J.; et al. Age-adjusted International Prognostic Index predicts autologous stem cell transplantation outcome for patients with relapsed or primary refractory diffuse large B-cell lymphoma. Blood 2003, 102, 1989–1996. [Google Scholar] [CrossRef] [PubMed]
- Cooke, R.E.; Quinn, K.M.; Quach, H.; Harrison, S.; Prince, H.M.; Koldej, R.; Ritchie, D. Conventional Treatment for Multiple Myeloma Drives Premature Aging Phenotypes and Metabolic Dysfunction in T Cells. Front. Immunol. 2020, 11, 2153. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Kato, T.; Hasegawa, F.; Sunahara, M.; Tsurumaki, Y. The Past, Present, and Future of Clinically Applied Chimeric Antigen Receptor-T-Cell Therapy. Pharmaceuticals 2022, 15, 207. [Google Scholar] [CrossRef]
- Suen, H.; Brown, R.; Yang, S.; Weatherburn, C.; Ho, P.J.; Woodland, N.; Nassif, N.; Barbaro, P.; Bryant, C.; Hart, D.; et al. Multiple myeloma causes clonal T-cell immunosenescence: Identification of potential novel targets for promoting tumour immunity and implications for checkpoint blockade. Leukemia 2016, 30, 1716–1724. [Google Scholar] [CrossRef] [PubMed]

| Type of Selection | Criteria for “Pre-Emptive” Ly-Apheresis Selection | N° Cases/Year Expected | N° Cases Observed 1 | Ly-Apheresis Performed | CAR-T Program Activated | CAR-T Infusion Performed |
|---|---|---|---|---|---|---|
| A | Primary refractory DLBCL | 6 | 1 | 1 | 1 | 1 |
| B | Inter/High IPI score DLBCL or DE/DH DLBCL patients with MRD/PET positivity before ASCT | 6 | 4 | 4 | 2 | 2 |
| C | Low/Inter-Low IPI score DLBCL patients who had relapsed within 1-year of the first-line treatment | 4 | 0 | 0 | 0 | 0 |
| Total | Pre-Emptive Ly-Apheresis | Standard Ly-Apheresis | p-Value | |
|---|---|---|---|---|
| Number, n (%) | 11 | 5 (45%) | 6 (55%) | |
| Age, years, median (range) | 60 (29–70) | 51 (29–60) | 67 (56–70) | 0.02 |
| Male, n (%) | 3 (27%) | 2 (40%) | 1 (17%) | 0.55 |
| ECOG, n (%) | >0.99 | |||
| 0 | 5 (45%) | 2 (40%) | 3 (50%) | |
| 1 | 6 (55%) | 3 (60%) | 3 (50%) | |
| IPI, n (%) | 0.24 | |||
| 0–2 | 5 (45%) | 1 (20%) | 4 (67%) | |
| 3–5 | 6 (55%) | 4 (80%) | 2 (33%) | |
| Ann Arbor score, n (%) | >0.99 | |||
| 0–II | 1 (9%) | 0 (0%) | 1 (17%) | |
| III–IV | 10 (91%) | 5 (100%) | 5 (83%) | |
| Disease state at Ly-apheresis, n (%) | ||||
| Primary refractory (A) | 1 | 1 (20%) | n/a | |
| Partial response after 1 line (B) | 4 | 4 (80%) | n/a | |
| Relapse after I line (C) | 0 | 0 | n/a | |
| Non-responsive > II line | 3 | n/a | 3 (50%) | |
| Relapse after II line | 3 | n/a | 3 (50%) | |
| Previous ASCT, n (%) | 6 (43%) | 0 (0%) | 6 (100%) | 0.002 |
| CAR-T cell program activated, n (%) | 8 (73%) | 3 (60%) | 5 (83%) | 0.46 |
| Tisa-cel | 6 | 3 | 3 | |
| Axi-cel | 2 | n/a | 2 | |
| State of disease at CAR-T cell program activation, n (%) | 0.15 | |||
| Non-responsive > II line | 4 | 3 (100%) | 1 (20%) | |
| Relapse after II line | 4 | 0 (0%) | 4 (80%) | |
| Leukapheresis to CAR-T cell delivery, days, median (range) | 59 (27–98) | 89 (55–98) | 45.6 (27–64) | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farina, M.; Chiarini, M.; Almici, C.; Accorsi Buttini, E.; Zuccalà, F.; Piva, S.; Volonghi, I.; Poli, L.; Bernardi, S.; Colnaghi, F.; et al. Timely Leukapheresis May Interfere with the “Fitness” of Lymphocytes Collected for CAR-T Treatment in High Risk DLBCL Patients. Cancers 2022, 14, 5276. https://doi.org/10.3390/cancers14215276
Farina M, Chiarini M, Almici C, Accorsi Buttini E, Zuccalà F, Piva S, Volonghi I, Poli L, Bernardi S, Colnaghi F, et al. Timely Leukapheresis May Interfere with the “Fitness” of Lymphocytes Collected for CAR-T Treatment in High Risk DLBCL Patients. Cancers. 2022; 14(21):5276. https://doi.org/10.3390/cancers14215276
Chicago/Turabian StyleFarina, Mirko, Marco Chiarini, Camillo Almici, Eugenia Accorsi Buttini, Francesco Zuccalà, Simone Piva, Irene Volonghi, Loris Poli, Simona Bernardi, Federica Colnaghi, and et al. 2022. "Timely Leukapheresis May Interfere with the “Fitness” of Lymphocytes Collected for CAR-T Treatment in High Risk DLBCL Patients" Cancers 14, no. 21: 5276. https://doi.org/10.3390/cancers14215276
APA StyleFarina, M., Chiarini, M., Almici, C., Accorsi Buttini, E., Zuccalà, F., Piva, S., Volonghi, I., Poli, L., Bernardi, S., Colnaghi, F., Re, F., Leoni, A., Polverelli, N., Turra, A., Morello, E., Galvagni, A., Moratto, D., Brugnoni, D., Cattaneo, C., ... Russo, D. (2022). Timely Leukapheresis May Interfere with the “Fitness” of Lymphocytes Collected for CAR-T Treatment in High Risk DLBCL Patients. Cancers, 14(21), 5276. https://doi.org/10.3390/cancers14215276









