Quantifying Liver Heterogeneity via R2*-MRI with Super-Paramagnetic Iron Oxide Nanoparticles (SPION) to Characterize Liver Function and Tumor
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Two Pre- and Post-SPION MRI Sessions
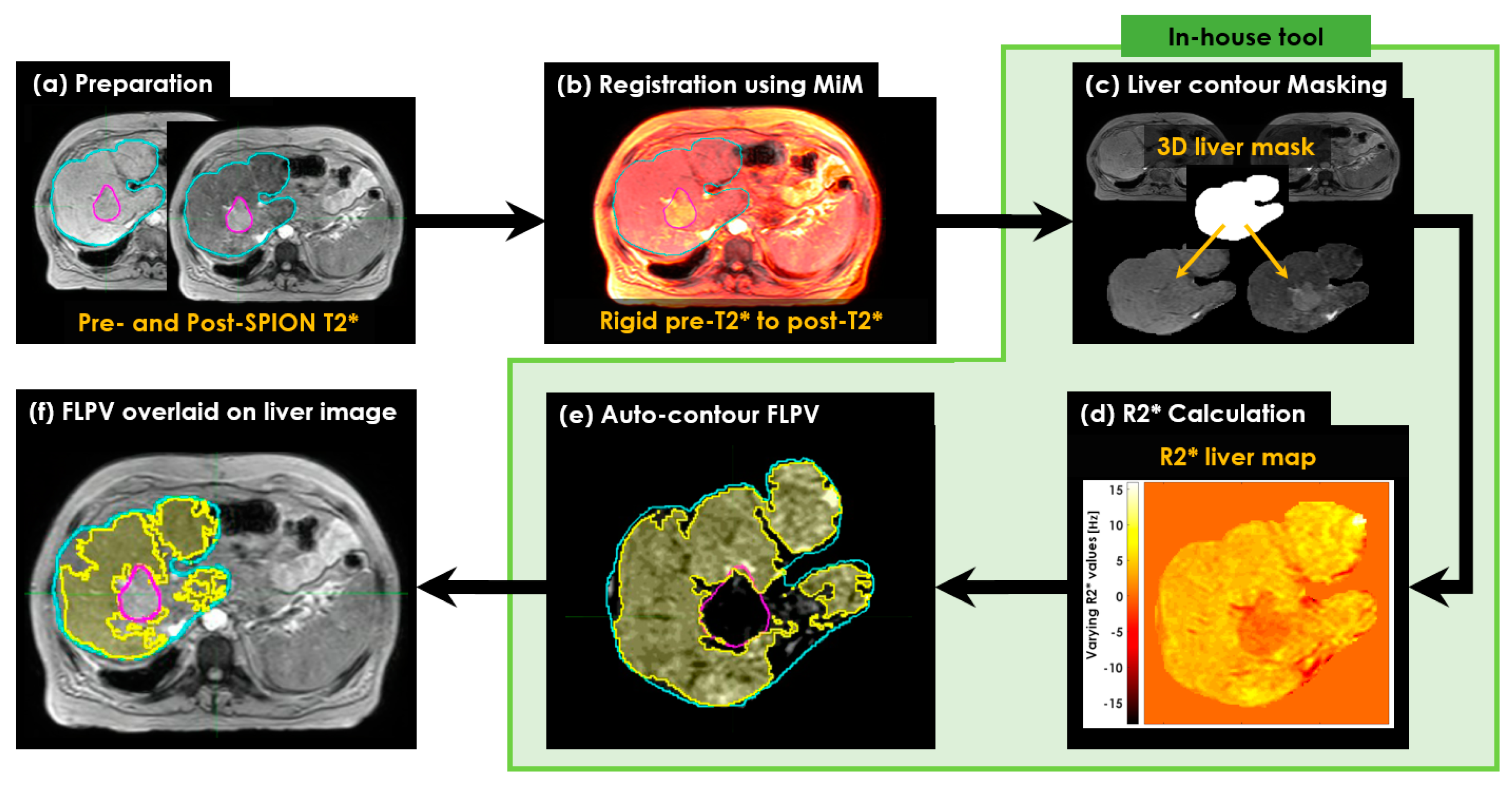
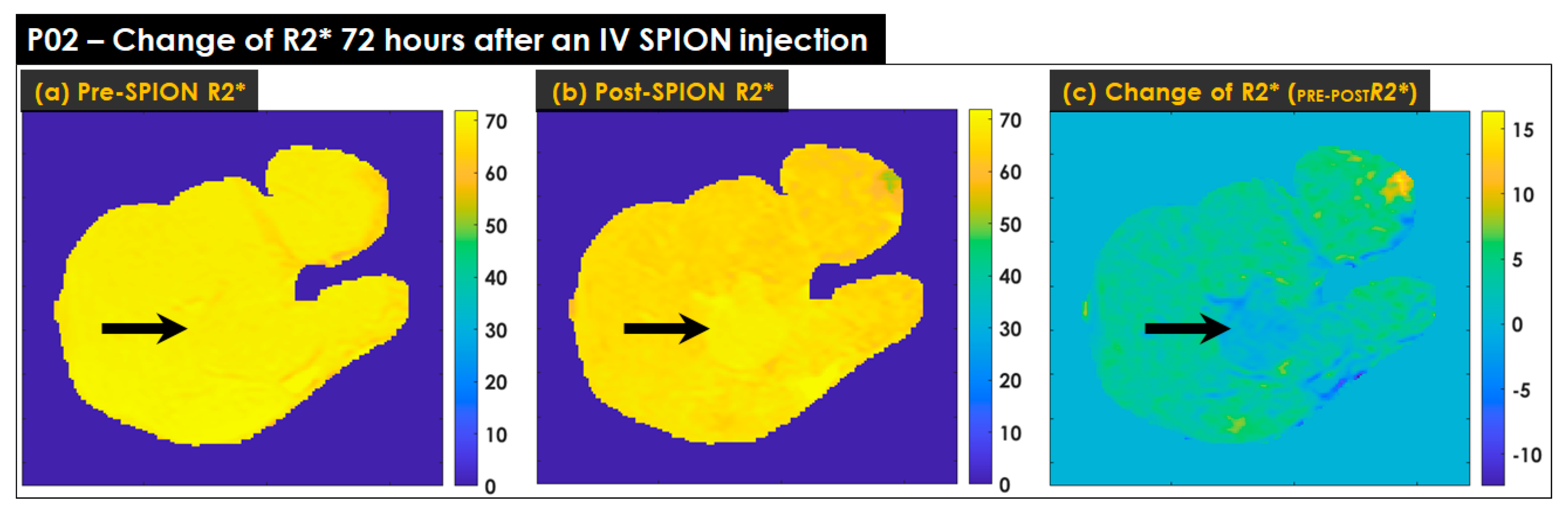
2.4. Calculating R2* Relaxation Rates of Liver
2.5. Characterizing Liver Heterogeneity
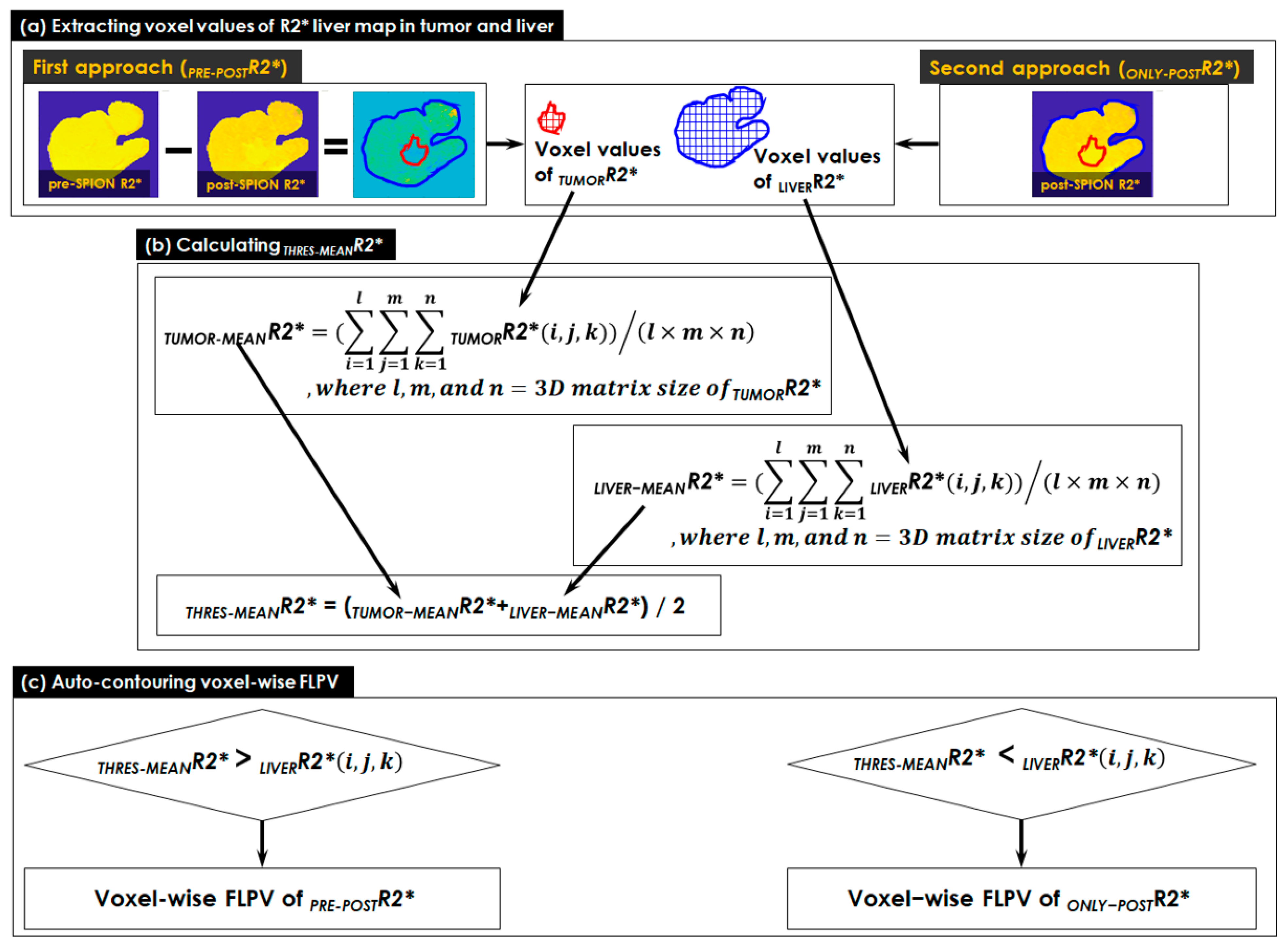
- The voxel values of the R2* liver map in tumor and liver contours were extracted in two approaches (see Figure 2a). Both pre- and post-SPION R2* liver maps were used in the first approach (PRE-POSTR2*), but only a single post-SPION R2* liver map was used in the second approach (ONLY-POSTR2*).
- A threshold (THRES-MEANR2*) was calculated using the middle value of TUMOR-MEANR2* (an average of all voxel values in TUMORR2*) and LIVER-MEANR2* (an average of all voxel values in LIVERR2*). All voxel values in TUMORR2* and LIVERR2* were totaled and the sum was divided by the number of voxels in the tumor and liver contours, respectively (see Figure 2b).
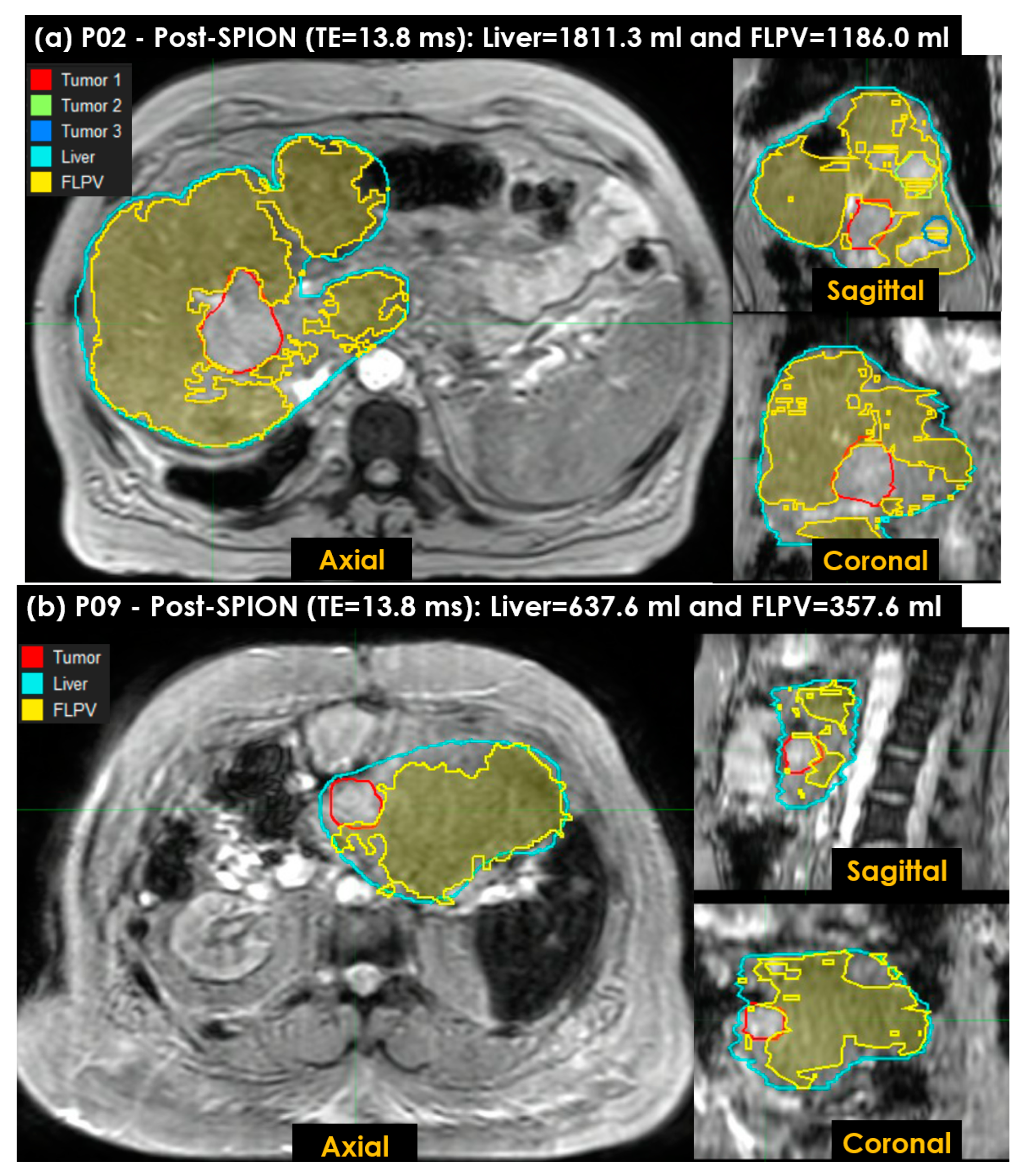
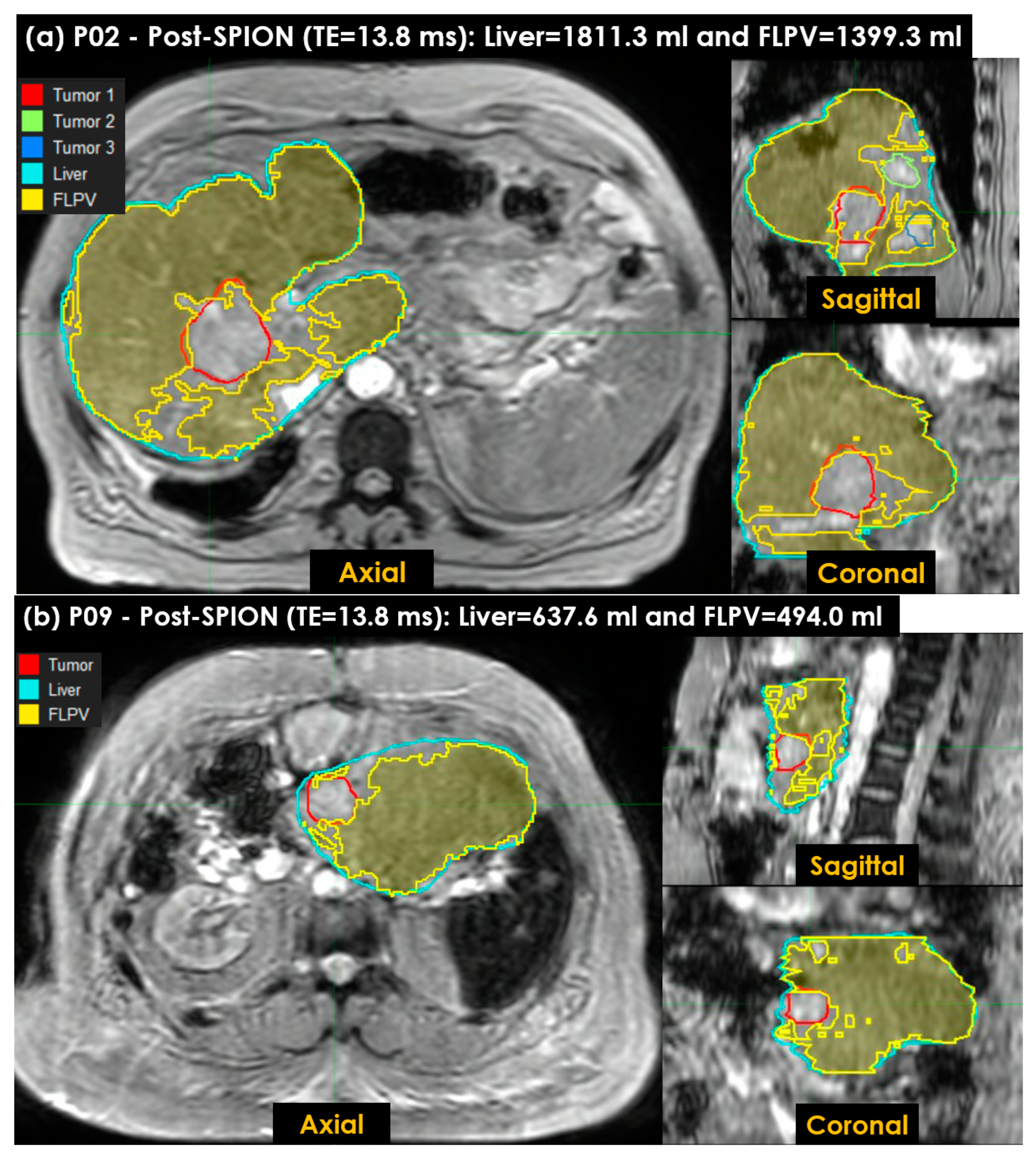
- A voxel-wise FLPV was automatically determined by comparing each voxel value of an R2* liver map to the THRES-MEANR2* (see Figure 2c). In the PRE-POSTR2* approach, if each voxel was greater than THRES-MEANR2*, it became a voxel of FLPV. However, the opposite worked in the ONLY-POSTR2* approach. The voxel-wise FLPV was saved as a new contour together with the physician-identified tumor and liver contours and transferred to MiM. This study developed the in-house auto-contouring tool in Matlab version 9.10 (The MathWorks, Natick, MA, USA). A long TE (13.8 ms) negatively enhanced more R2* in the liver map and led to an improvement in heterogeneity in the R2* liver map, so this study utilized the R2* liver map of a long TE (13.8 ms) for auto-contouring FLPV.
3. Results
3.1. Patients
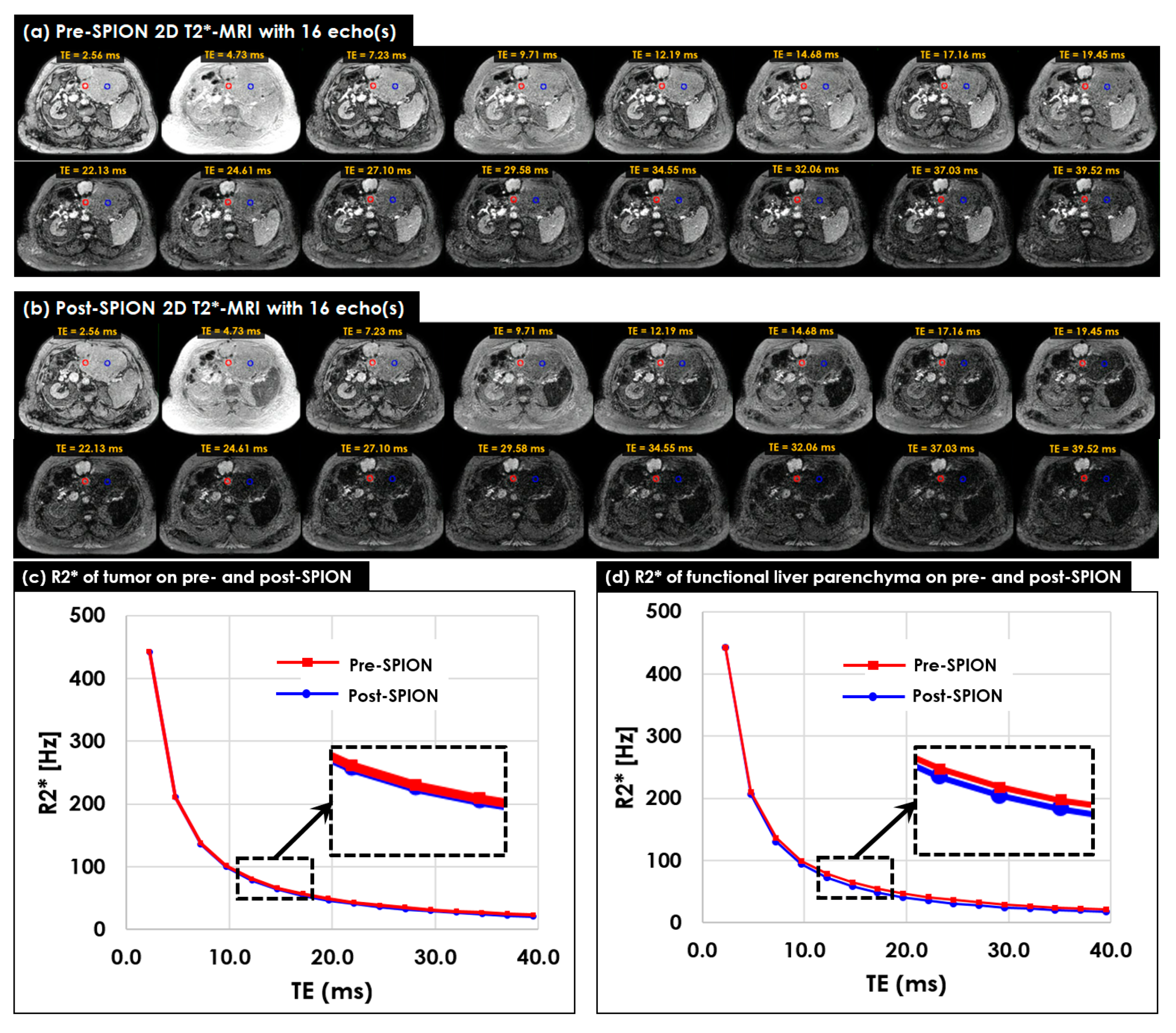
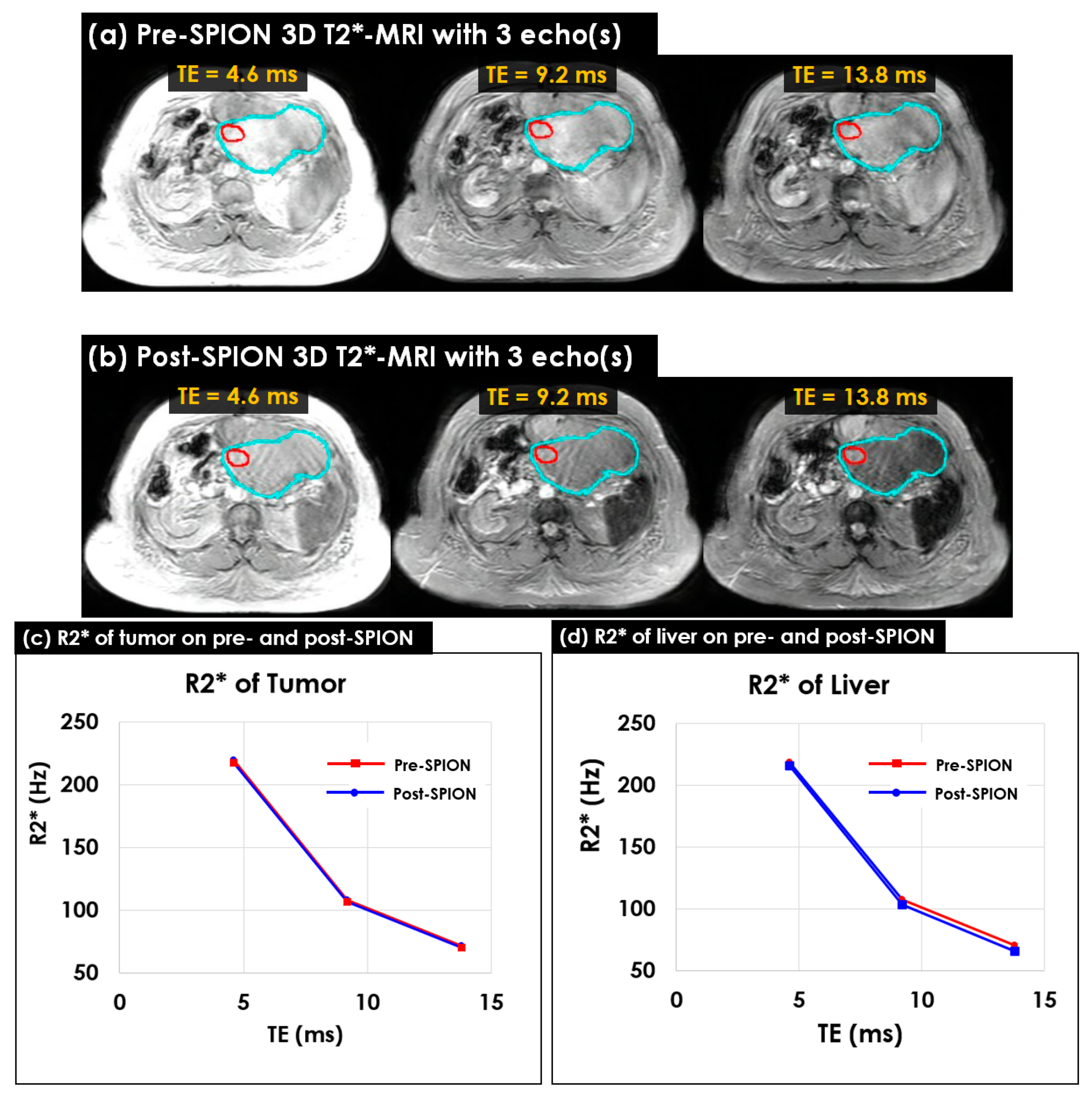
3.2. Multi-Echo 2D/3D T2*- and R2*-MRI
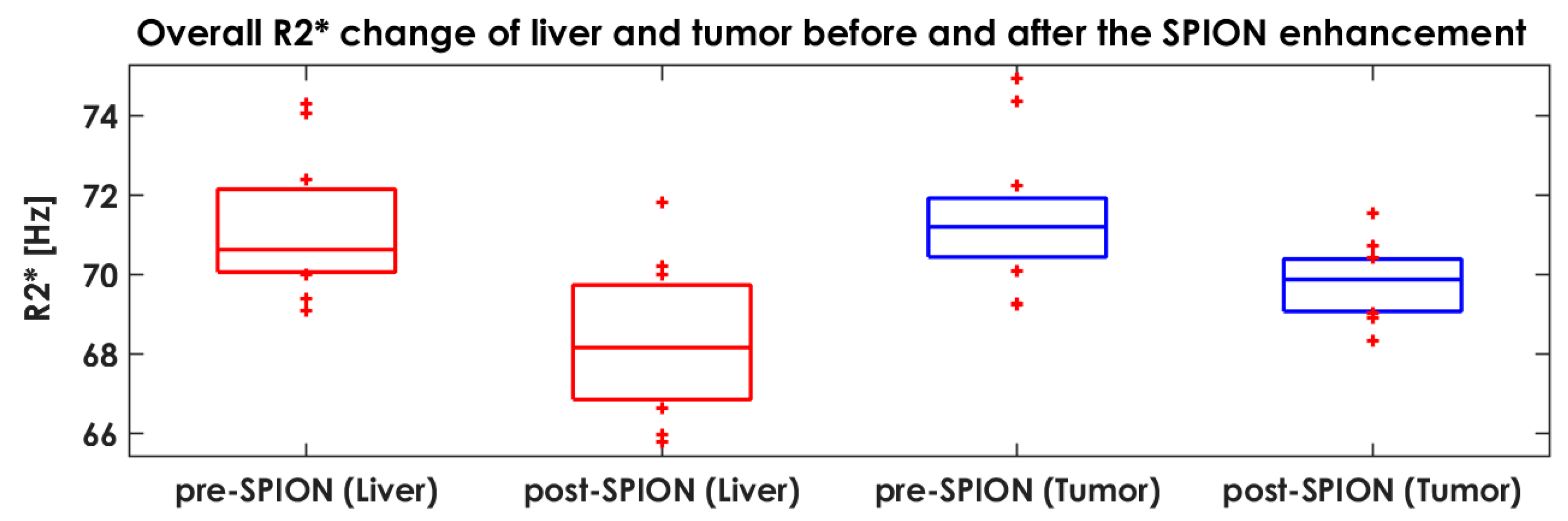
3.3. Characterizing Liver Heterogeneity in R2*
3.4. Characterizing FLP Using an in-House Tool
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen-Lefebvre, A.T.; Horuzsko, A. Kupffer Cell Metabolism and Function. J. Enzymol. Metab. 2015, 1, 101. [Google Scholar]
- Guillot, A.; Tacke, F. Liver Macrophages: Old Dogmas and New Insights. Hepatol. Commun. 2019, 3, 730–743. [Google Scholar] [CrossRef]
- Arvanitakis, K.; Koletsa, T.; Mitroulis, I.; Germanidis, G. Tumor-Associated Macrophages in Hepatocellular Carcinoma Pathogenesis, Prognosis and Therapy. Cancers 2022, 14, 226. [Google Scholar] [CrossRef]
- Bilzer, M.; Roggel, F.; Gerbes, A.L. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006, 26, 1175–1186. [Google Scholar] [CrossRef]
- Mahfouz, A.E.; Hamm, B.; Taupitz, M. Contrast agents for MR imaging of the liver: A clinical overview. Eur. Radiol. 1997, 7, 507–513. [Google Scholar] [CrossRef]
- Choi, J.; Kim, H.Y.; Ju, E.J.; Jung, J.; Park, J.; Chung, H.-K.; Lee, J.S.; Lee, J.S.; Park, H.J.; Song, S.Y.; et al. Use of macrophages to deliver therapeutic and imaging contrast agents to tumors. Biomaterials 2012, 33, 4195–4203. [Google Scholar] [CrossRef]
- Toth, G.B.; Varallyay, C.G.; Horvath, A.; Bashir, M.R.; Choyke, P.L.; Daldrup-Link, H.E.; Dosa, E.; Finn, J.P.; Gahramanov, S.; Harisinghani, M.; et al. Current and potential imaging applications of ferumoxytol for magnetic resonance imaging. Kidney Int. 2017, 92, 47–66. [Google Scholar] [CrossRef]
- Zhou, Q.; Wei, Y. For Better or Worse, Iron Overload by Superparamagnetic Iron Oxide Nanoparticles as a MRI Contrast Agent for Chronic Liver Diseases. Chem. Res. Toxicol. 2017, 30, 73–80. [Google Scholar] [CrossRef]
- Oh, K.Y.; Roberts, V.H.J.; Schabel, M.C.; Grove, K.L.; Woods, M.; Frias, A.E. Gadolinium Chelate Contrast Material in Pregnancy: Fetal Biodistribution in the Nonhuman Primate. Radiology 2015, 276, 110–118. [Google Scholar] [CrossRef]
- Ahmad, F.; Treanor, L.; McGrath, T.A.; Walker, D.; McInnes, M.D.F.; Schieda, N. Safety of Off-Label Use of Ferumoxtyol as a Contrast Agent for MRI: A Systematic Review and Meta-Analysis of Adverse Events. J. Magn. Reson. Imaging 2021, 53, 840–858. [Google Scholar] [CrossRef]
- Kashani, R.; Olsen, J.R. Magnetic Resonance Imaging for Target Delineation and Daily Treatment Modification. Semin. Radiat. Oncol. 2018, 28, 178–184. [Google Scholar] [CrossRef]
- Boldrini, L.; Corradini, S.; Gani, C.; Henke, L.; Hosni, A.; Romano, A.; Dawson, L. MR-Guided Radiotherapy for Liver Malignancies. Front. Oncol. 2021, 11, 616027. [Google Scholar] [CrossRef]
- Hama, Y.; Tate, E. SPIO-enhanced 0.35T MRI-guided radiotherapy for liver malignancies: Usefulness in tumor visualization. Br. J. Radiol. 2022, 95, 20211131. [Google Scholar] [CrossRef]
- Chavhan, G.B.; Babyn, P.S.; Thomas, B.; Shroff, M.M.; Haacke, E.M. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics 2009, 29, 1433–1449. [Google Scholar] [CrossRef]
- Henninger, B.; Alustiza, J.; Garbowski, M.; Gandon, Y. Practical guide to quantification of hepatic iron with MRI. Eur. Radiol. 2020, 30, 383–393. [Google Scholar] [CrossRef]
- Obrzut, M.; Atamaniuk, V.; Glaser, K.J.; Chen, J.; Ehman, R.L.; Obrzut, B.; Cholewa, M.; Gutkowski, K. Value of liver iron concentration in healthy volunteers assessed by MRI. Sci. Rep. 2020, 10, 17887. [Google Scholar] [CrossRef]
- Muhi, A.; Ichikawa, T.; Motosugi, U.; Sou, H.; Nakajima, H.; Sano, K.; Kitamura, T.; Faima, Z.; Fukushima, K.; Araki, T.; et al. Diagnosis of colorectal hepatic metastases: Contrast-enhanced ultrasonography versus contrast-enhanced computed tomography versus superparamagnetic iron oxide-enhanced magnetic resonance imaging with diffusion-weighted imaging. J. Magn. Reson. Imaging 2010, 32, 1132–1140. [Google Scholar] [CrossRef]
- Bashir, M.R.; Bhatti, L.; Marin, D.; Nelson, R.C. Emerging applications for ferumoxytol as a contrast agent in MRI: Emerging Applications of Ferumoxytol. J. Magn. Reson. Imaging 2015, 41, 884–898. [Google Scholar] [CrossRef]
- Sharkey, J.; Lewis, P.J.S.; Barrow, M.; Alwahsh, S.M.; Noble, J.; Livingstone, E.; Lennen, R.J.; Jansen, M.A.; Carrion, J.G.; Liptrott, N.; et al. Functionalized superparamagnetic iron oxide nanoparticles provide highly efficient iron-labeling in macrophages for magnetic resonance—Based detection in vivo. Cytotherapy 2017, 19, 555–569. [Google Scholar] [CrossRef]
- Hankins, J.S.; McCarville, M.B.; Loeffler, R.B.; Smeltzer, M.P.; Onciu, M.; Hoffer, F.A.; Li, C.-S.; Wang, W.C.; Ware, R.E.; Hillenbrand, C.M. R2* magnetic resonance imaging of the liver in patients with iron overload. Blood 2009, 113, 4853–4855. [Google Scholar] [CrossRef]
- Muehler, M.R.; Vigen, K.; Hernando, D.; Zhu, A.; Colgan, T.J.; Reeder, S.B. Reproducibility of liver R2* quantification for liver iron quantification from cardiac R2* acquisitions. Abdom. Radiol. 2021, 46, 4200–4209. [Google Scholar] [CrossRef] [PubMed]
- Kirichenko, A.; Gayou, O.; Parda, D.; Kudithipudi, V.; Tom, K.; Khan, A.; Abrams, P.; Szramowski, M.; Oliva, J.; Monga, D.; et al. Stereotactic body radiotherapy (SBRT) with or without surgery for primary and metastatic liver tumors. HPB 2016, 18, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Kudithipudi, V.; Day, E.; Thai, N.; Kirichenko, A. Liver stereotactic radiotherapy (SRT) with functional treatment planning for patients with intermediate stage hepatocellular carcinoma (HCC). J. Radiat. Oncol. 2017, 6, 371–377. [Google Scholar] [CrossRef]
- Kirichenko, A.; Koay, E.J.; Hasan, S.; Crane, C. Stereotactic Body Radiotherapy with Functional Treatment Planning in Hepatocellular Carcinoma. In Alternate Fractionation in Radiotherapy; Medical Radiology; Trombetta, M., Pignol, J.P., Montemaggi, P., Brady, L.W., Eds.; Springer International Publishing: Chem, Switzerland, 2017; pp. 203–210. [Google Scholar] [CrossRef]
- Gresswell, S.; Tobillo, R.; Hasan, S.; Uemura, T.; Machado, L.; Thai, N.; Kirichenko, A. Stereotactic body radiotherapy used as a bridge to liver transplant in patients with hepatocellular carcinoma and Child-Pugh score ≥8 cirrhosis. J. Radiosurgery SBRT 2018, 5, 261–267. [Google Scholar]
- Uemura, T.; Kirichenko, A.; Bunker, M.; Vincent, M.; Machado, L.; Thai, N. Stereotactic Body Radiation Therapy: A New Strategy for Loco-Regional Treatment for Hepatocellular Carcinoma While Awaiting Liver Transplantation. World J. Surg. 2019, 43, 886–893. [Google Scholar] [CrossRef]
- Péran, P.; Hagberg, G.; Luccichenti, G.; Cherubini, A.; Brainovich, V.; Celsis, P.; Caltagirone, C.; Sabatini, U. Voxel-based analysis of R2* maps in the healthy human brain. J. Magn. Reson. Imaging 2007, 26, 1413–1420. [Google Scholar] [CrossRef]
- Liu, K.; He, X.; Lei, X.Z.; Zhao, L.-S.; Tang, H.; Liu, L.; Lei, B.-J. Pathomorphological study on location and distribution of Kupffer cells in hepatocellular carcinoma. World J. Gastroenterol. 2003, 9, 1946–1949. [Google Scholar] [CrossRef]
- Matsumura, H.; Kondo, T.; Ogawa, K.; Tamura, T.; Fukunaga, K.; Murata, S.; Ohkohchi, N. Kupffer cells decrease metastasis of colon cancer cells to the liver in the early stage. Int. J. Oncol. 2014, 45, 2303–2310. [Google Scholar] [CrossRef]
- Kubota, K. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology 1997, 26, 1176–1181. [Google Scholar] [CrossRef]
- Khan, A.S.; Garcia-Aroz, S.; Ansari, M.A.; Atiq, S.M.; Senter-Zapata, M.; Fowler, K.; Doyle, M.B.; Chapman, W.C. Assessment and optimization of liver volume before major hepatic resection: Current guidelines and a narrative review. Int. J. Surg. 2018, 52, 74–81. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, H.; Wang, Z.; Cong, W.; Wang, J.; Zeng, M.; Zhou, W.; Bie, P.; Liu, L.; Wen, T.; et al. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer 2020, 9, 682–720. [Google Scholar] [CrossRef] [PubMed]
- Maolood, I.Y.; Al-Salhi, Y.E.A.; Lu, S. Thresholding for Medical Image Segmentation for Cancer using Fuzzy Entropy with Level Set Algorithm. OpenMed 2018, 13, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Tamal, M. Intensity threshold based solid tumour segmentation method for Positron Emission Tomography (PET) images: A review. Heliyon 2020, 6, e05267. [Google Scholar] [CrossRef] [PubMed]
- Turkbey, B.; Agarwal, H.K.; Shih, J.; Bernardo, M.; McKinney, Y.L.; Daar, D.; Griffiths, G.L.; Sankineni, S.; Johnson, L.; Grant, K.B.; et al. A Phase I Dosing Study of Ferumoxytol for MR Lymphography at 3 T in Patients With Prostate Cancer. AJR Am. J. Roentgenol. 2015, 205, 64–69. [Google Scholar] [CrossRef]
- Christen, T.; Ni, W.; Qiu, D.; Schmiedeskamp, H.; Bammer, R.; Moseley, M.; Zaharchuk, G. High-resolution cerebral blood volume imaging in humans using the blood pool contrast agent ferumoxytol. Magn. Reson. Med. 2013, 70, 705–710. [Google Scholar] [CrossRef]
- Finn, J.P.; Nguyen, K.L.; Han, F.; Zhou, Z.; Salusky, I.; Ayad, I.; Hu, P. Cardiovascular MRI with ferumoxytol. Clin. Radiol. 2016, 71, 796–806. [Google Scholar] [CrossRef]
- Varallyay, P.; Nesbit, G.; Muldoon, L.L.; Nixon, R.R.; Delashaw, J.; Cohen, J.I.; Petrillo, A.; Rink, D.; Neuwelt, E.A. Comparison of two superparamagnetic viral-sized iron oxide particles ferumoxides and ferumoxtran-10 with a gadolinium chelate in imaging intracranial tumors. AJNR Am. J. Neuroradiol. 2002, 23, 510–519. [Google Scholar]
- Hama, Y.; Tate, E. Superparamagnetic iron oxide-enhanced MRI-guided stereotactic ablative radiation therapy for liver metastasis. Rep. Pract. Oncol. Radiother. 2021, 26, 470–474. [Google Scholar] [CrossRef]
- Lee, D.; Greer, P.B.; Lapuz, C.; Ludbrook, J.; Hunter, P.; Arm, J.; Pollock, S.; Makhija, K.; O’Brien, R.T.; Kim, T.; et al. Audiovisual biofeedback guided breath-hold improves lung tumor position reproducibility and volume consistency. Adv. Radiat. Oncol. 2017, 2, 354–362. [Google Scholar] [CrossRef]
- Kanal, E.; Barkovich, A.J.; Bell, C.; Borgstede, J.P.; Bradley, W.G.; Froelich, J.W., Jr.; Gimbel, J.R.; Gosbee, J.W.; Kuhni-Kaminski, E.; Larson, P.A.; et al. ACR guidance document on MR safe practices: 2013. J. Magn. Reson. Imaging 2013, 37, 501–530. [Google Scholar] [CrossRef]
- Greenberg, T.D.; Hoff, M.N.; Gilk, T.B.; Jackson, E.F.; Kanal, E.; McKinney, A.M.; Och, J.G.; Pedrosa, I.; Rampulla, T.L.; Reeder, S.B.; et al. ACR guidance document on MR safe practices: Updates and critical information 2019. J. Magn. Reson. Imaging 2020, 51, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Calusi, S.; Labanca, G.; Zani, M.; Casati, M.; Marrazzo, L.; Noferini, L.; Talamonti, C.; Fusi, F.; Desideri, I.; Bonomo, P.; et al. A multiparametric method to assess the MIM deformable image registration algorithm. J. Appl. Clin. Med. Phys. 2019, 20, 75–82. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, W.; Bennink, R.J.; Veteläinen, R.; van Gulik, T.M. Nuclear Imaging Techniques for the Assessment of Hepatic Function in Liver Surgery and Transplantation. J. Nucl. Med. 2010, 51, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Bártulos, C.R.; Senk, K.; Schumacher, M.; Plath, J.; Kaiser, N.; Bade, R.; Woetzel, J.; Wiggermann, P. Assessment of Liver Function with MRI: Where DO We Stand? Front. Med. 2022, 9, 839919. [Google Scholar] [CrossRef] [PubMed]
- Kellman, P.; Xue, H.; Spottiswoode, B.S.; Sandino, C.M.; Hansen, M.S.; Abdel-Gadir, A.; Treibel, T.A.; Rosmini, S.; Mancini, C.; Bandettini, W.P.; et al. Free-breathing T2* mapping using respiratory motion corrected averaging. J. Cardiovasc. Magn. Reson. 2015, 17, 3. [Google Scholar] [CrossRef] [PubMed]
| Patient # | Gender | Age | Child–Pugh Score | Diagnosis | Tumor Location | Liver Volume (mL) |
|---|---|---|---|---|---|---|
| P01 | M | 80 | - | HCC | Liver Seg 4 | 1635.6 |
| P02 | M | 55 | - | Metastases | Liver Right lobe | 1811.3 |
| P03 | F | 79 | Child–Pugh B Nash Cirrhosis | HCC | Liver Seg 6 and Seg 2 | 1439.4 |
| P04 | M | 53 | - | Metastases | Liver Seg 8 | 1267.2 |
| P05 | M | 62 | Child–Pugh A Nash Cirrhosis | HCC | Liver Seg 7 | 1791.8 |
| P06 | M | 53 | - | Metastases | Liver Seg 8 | 1858.5 |
| P07 | F | 81 | Child–Pugh B Cirrhosis | HCC | Liver Seg 7 | 901.1 |
| P08 | M | 60 | Child–Pugh B Cirrhosis | HCC | Liver Seg 4A/8 | 1985.7 |
| P09 | F | 56 | - | Metastases | Right Hepatic Lobe | 637.6 |
| P10 | M | 74 | Child–Pugh A Cirrhosis Nash | HCC | Liver Seg 6 and Seg 2 | 2637.8 |
| P11 | M | 73 | Child–Pugh A Cirrhosis Nash | HCC | Liver Seg 7 Hilum | 1187.1 |
| P12 | F | 86 | Child–Pugh A Cirrhosis Nash | HCC | Liver Seg 5/6 | 1971.5 |
| Volume | Patients | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P01 | P02 | P03 | P04 | P05 | P06 | P07 | P08 | P09 | P10 | P11 | P12 | |
| Tumor (mL) | 51.2 | 77.3 | 6.1 | 1.8 | 7.8 | 42.3 | 6.9 | 13.5 | 12.0 | 227.6 | 45.9 | 6.1 |
| Liver (mL) | 1635.6 | 1811.3 | 1439.4 | 1267.2 | 1791.8 | 1858.5 | 901.1 | 1985.7 | 637.6 | 2637.8 | 1187.1 | 1971.5 |
| PRE-POST FLPV (mL) | 1256.3 | 1186.0 | 957.3 | 729.9 | 713.7 | 1289.7 | 734.5 | 1207.1 | 357.6 | 1681.0 | 505.0 | 830.4 |
| PRE-POST FLPV (%) | 76.8 | 65.5 | 66.5 | 57.6 | 39.8 | 69.4 | 81.5 | 60.8 | 56.1 | 63.0 | 42.5 | 42.1 |
| ONLY-POST FLPV (mL) | 1060.0 | 1399.3 | 766.7 | 590.0 | 1166.1 | 1212.0 | 691.8 | 1049.4 | 494.0 | 1610.1 | 636.6 | 1253.8 |
| ONLY-POST FLPV (%) | 64.8 | 77.3 | 53.3 | 46.6 | 65.1 | 65.2 | 76.8 | 52.8 | 77.5 | 61.0 | 53.6 | 63.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Sohn, J.; Kirichenko, A. Quantifying Liver Heterogeneity via R2*-MRI with Super-Paramagnetic Iron Oxide Nanoparticles (SPION) to Characterize Liver Function and Tumor. Cancers 2022, 14, 5269. https://doi.org/10.3390/cancers14215269
Lee D, Sohn J, Kirichenko A. Quantifying Liver Heterogeneity via R2*-MRI with Super-Paramagnetic Iron Oxide Nanoparticles (SPION) to Characterize Liver Function and Tumor. Cancers. 2022; 14(21):5269. https://doi.org/10.3390/cancers14215269
Chicago/Turabian StyleLee, Danny, Jason Sohn, and Alexander Kirichenko. 2022. "Quantifying Liver Heterogeneity via R2*-MRI with Super-Paramagnetic Iron Oxide Nanoparticles (SPION) to Characterize Liver Function and Tumor" Cancers 14, no. 21: 5269. https://doi.org/10.3390/cancers14215269
APA StyleLee, D., Sohn, J., & Kirichenko, A. (2022). Quantifying Liver Heterogeneity via R2*-MRI with Super-Paramagnetic Iron Oxide Nanoparticles (SPION) to Characterize Liver Function and Tumor. Cancers, 14(21), 5269. https://doi.org/10.3390/cancers14215269





