Role of Immunotherapy in the Treatment of Cancer: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Selection and Data Collection Process
2.4. Risk of Bias Assessment
2.5. Data Analysis
3. Results
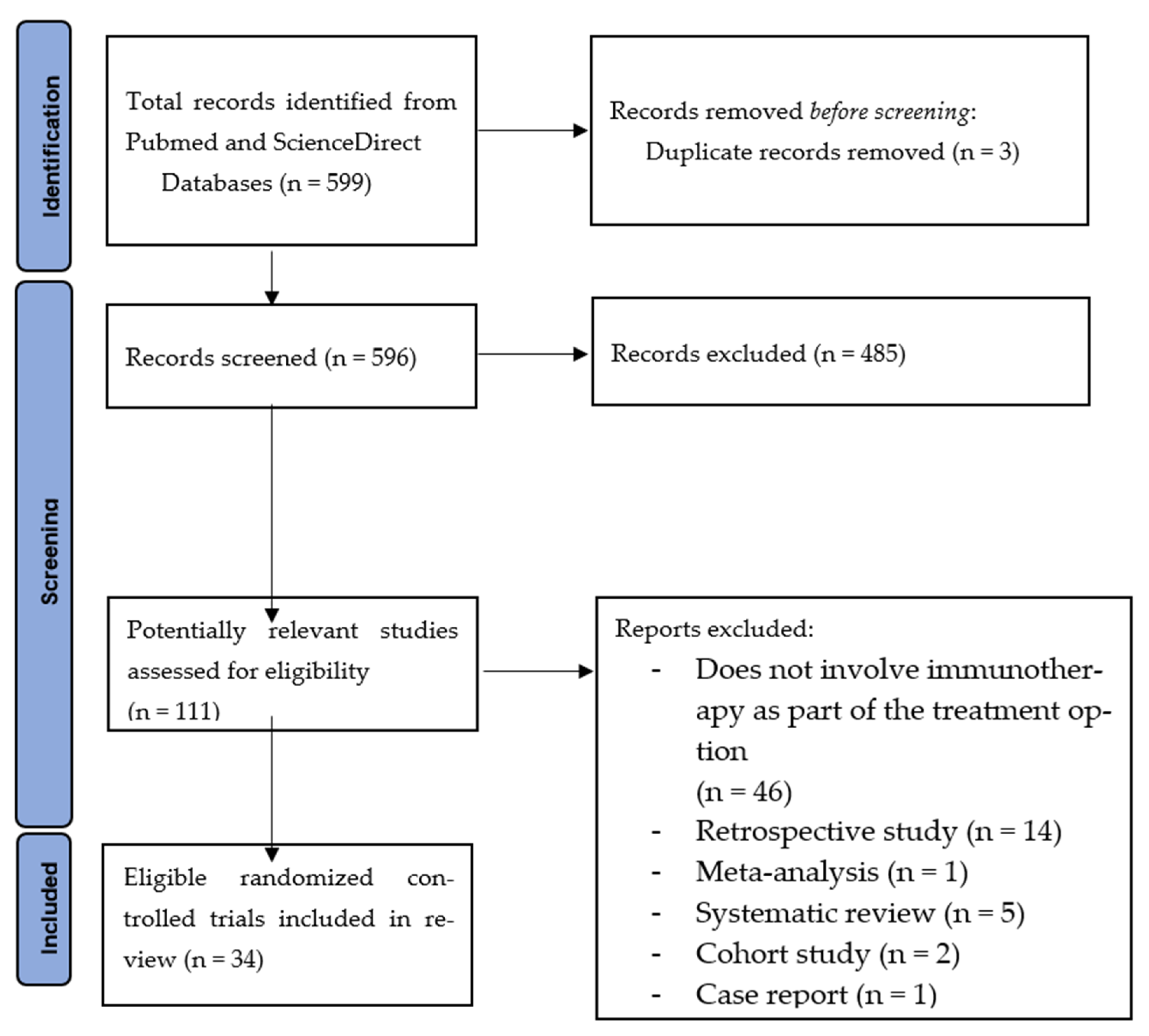
3.1. Study Selection
3.2. Reporting Biases
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Xiaomei, M.; Herbert, Y. Cancer Issue: Global Burden of Cancer. Yale J. Biol. Med. 2006, 79, 85. [Google Scholar]
- Arruebo, M.; Vilaboa, N.; Sáez-Gutierrez, B.; Lambea, J.; Tres, A.; Valladares, M.; González-Fernández, Á. Assessment of the Evolution of Cancer Treatment Therapies. Cancers 2011, 3, 3279–3330. [Google Scholar] [CrossRef]
- Whitaker, K. Earlier diagnosis: The importance of cancer symptoms. Lancet Oncol. 2019, 21, 6–8. [Google Scholar] [CrossRef]
- Borghaei, H.; Smith, M.R.; Campbell, K.S. Immunotherapy of cancer. Eur. J. Pharmacol. 2009, 625, 41–54. [Google Scholar] [CrossRef]
- Advances in Cancer Immunology and Cancer Immunotherapy—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/27011048/ (accessed on 20 August 2021).
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Cochrane Handbook for Systematic Reviews and Interventions. Available online: http://training.cochrane.org/handbook (accessed on 3 June 2020).
- Wang, F.H.; Wei, X.; Xu, N.; Shen, L.; Dai, G.; Yuan, X.; Chen, Y.; Yang, S.; Shi, J.; Hu, X.; et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann. Oncol. 2019, 30, 1479–1486. [Google Scholar] [CrossRef]
- Shore, N.D.; Boorjian, S.A.; Canter, D.J.; Ogan, K.; Karsh, L.I.; Downs, T.M.; Gomella, L.G.; Kamat, A.M.; Lotan, Y.; Svatek, R.S.; et al. Intravesical rAd–IFNα/Syn3 for Patients with High-Grade, Bacillus Calmette-Guerin–Refractory or Relapsed Non–Muscle-Invasive Bladder Cancer: A Phase II Randomized Study. J. Clin. Oncol. 2017, 35, 3410–3416. [Google Scholar] [CrossRef]
- Ding, X.; Cao, H.; Chen, X.; Zhao, Y.; Jin, H.; Niu, C.; Ma, K.; Liu, Z.; Chen, J.; Wang, X.; et al. Cellular immunotherapy as maintenance therapy prolongs the survival of the patients with small cell lung cancer in extensive stage. J. Cell. Immunother. 2016, 2, 36–43. [Google Scholar] [CrossRef][Green Version]
- Cho, B.C.; Yoh, K.; Perets, R.; Nagrial, A.; Spigel, D.R.; Gutierrez, M.; Kim, D.-W.; Kotasek, D.; Rasco, D.; Niu, J.; et al. Anti–cytotoxic T-lymphocyte–associated antigen-4 monoclonal antibody quavonlimab in combination with pembrolizumab: Safety and efficacy from a phase I study in previously treated extensive-stage small cell lung cancer. Lung Cancer 2021, 159, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Reinmuth, N.; Orlov, S.; Fischer, J.; Sugawara, S.; Mandziuk, S.; Marquez-Medina, D.; Novello, S.; Takeda, Y.; Soo, R.; et al. ARCTIC: Durvalumab with or without tremelimumab as third-line or later treatment of metastatic non-small-cell lung cancer. Ann. Oncol. 2020, 31, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Hui, R.; Garon, E.B.; Goldman, J.W.; Leighl, N.B.; Hellmann, M.D.; Patnaik, A.; Gandhi, L.; Eder, J.P.; Ahn, M.-J.; Horn, L.; et al. Pembrolizumab as first-line therapy for patients with PD-L1-positive advanced non-small cell lung cancer: A phase 1 trial. Ann. Oncol. 2017, 28, 874–881. [Google Scholar] [CrossRef]
- Spigel, D.R.; Chaft, J.E.; Gettinger, S.; Chao, B.H.; Dirix, L.; Schmid, P.; Chow, L.Q.; Hicks, R.J.; Leon, L.; Fredrickson, J.; et al. FIR: Efficacy, Safety, and Biomarker Analysis of a Phase II Open-Label Study of Atezolizumab in PD-L1–Selected Patients With NSCLC. J. Thorac. Oncol. 2018, 13, 1733–1742. [Google Scholar] [CrossRef]
- Mittendorf, E.; Clifton, G.; Holmes, J.; Schneble, E.; van Echo, D.; Ponniah, S.; Peoples, G. Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann. Oncol. 2014, 25, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Salgado, R.; Park, Y.; Muñoz-Couselo, E.; Kim, S.; Sohn, J.; Im, S.-A.; Foukakis, T.; Kuemmel, S.; Dent, R.; et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: Results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann. Oncol. 2020, 31, 569–581. [Google Scholar] [CrossRef]
- Chumsri, S.; Li, Z.; Serie, D.J.; Mashadi-Hossein, A.; Colon-Otero, G.; Song, N.; Pogue-Geile, K.L.; Gavin, P.; Paik, S.; Moreno-Aspitia, A.; et al. Incidence of Late Relapses in Patients with HER2-Positive Breast Cancer Receiving Adjuvant Trastuzumab: Combined Analysis of NCCTG N9831 (Alliance) and NRG Oncology/NSABP B-31. J. Clin. Oncol. 2019, 37, 3425–3435. [Google Scholar] [CrossRef]
- Antonilli, M.; Rahimi, H.; Visconti, V.; Napoletano, C.; Ruscito, I.; Zizzari, I.G.; Caponnetto, S.; Barchiesi, G.; Iadarola, R.; Pierelli, L.; et al. Triple peptide vaccination as consolidation treatment in women affected by ovarian and breast cancer: Clinical and immunological data of a phase I/II clinical trial. Int. J. Oncol. 2016, 48, 1369–1378. [Google Scholar] [CrossRef]
- Liau, L.M.; Ashkan, K.; Tran, D.D.; Campian, J.L.; Trusheim, J.E.; Cobbs, C.S.; Heth, J.A.; Salacz, M.; Taylor, S.; D’Andre, S.D.; et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J. Transl. Med. 2018, 16, 142. [Google Scholar] [CrossRef]
- Quispel-Janssen, J.; van der Noort, V.; de Vries, J.F.; Zimmerman, M.; Lalezari, F.; Thunnissen, E.; Monkhorst, K.; Schouten, R.; Schunselaar, L.; Disselhorst, M.; et al. Programmed Death 1 Blockade with Nivolumab in Patients With Recurrent Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2018, 13, 1569–1576. [Google Scholar] [CrossRef]
- Rischin, D.; Gil-Martin, M.; González-Martin, A.; Braña, I.; Hou, J.Y.; Cho, D.; Falchook, G.S.; Formenti, S.; Jabbour, S.; Moore, K.; et al. PD-1 blockade in recurrent or metastatic cervical cancer: Data from cemiplimab phase I expansion cohorts and characterization of PD-L1 expression in cervical cancer. Gynecol. Oncol. 2020, 159, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.M.; Nieminen, P.; Donders, G.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Stoler, M.H.; Glavini, K.; Attley, G.; Limacher, J.-M.; et al. The efficacy and safety of Tipapkinogen Sovacivec therapeutic HPV vaccine in cervical intraepithelial neoplasia grades 2 and 3: Randomized controlled phase II trial with 2.5 years of follow-up. Gynecol. Oncol. 2019, 153, 521–529. [Google Scholar] [CrossRef]
- Santin, A.D.; Deng, W.; Frumovitz, M.; Buza, N.; Bellone, S.; Huh, W.; Khleif, S.; Lankes, H.A.; Ratner, E.S.; O’Cearbhaill, R.E.; et al. Phase II evaluation of nivolumab in the treatment of persistent or recurrent cervical cancer (NCT02257528/NRG-GY002). Gynecol. Oncol. 2020, 157, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Brawley, V.S.; Hegde, M.; Robertson, C.; Ghazi, A.; Gerken, C.; Liu, E.; Dakhova, O.; Ashoori, A.; Corder, A.; et al. Human Epidermal Growth Factor Receptor 2 (HER2)–Specific Chimeric Antigen Receptor–Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J. Clin. Oncol. 2015, 33, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Miwa, S.; Nishida, H.; Tanzawa, Y.; Takeuchi, A.; Hayashi, K.; Yamamoto, N.; Mizukoshi, E.; Nakamoto, Y.; Kaneko, S.; Tsuchiya, H. Phase 1/2 study of immunotherapy with dendritic cells pulsed with autologous tumor lysate in patients with refractory bone and soft tissue sarcoma. Cancer 2017, 123, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.; Haddad, R.; Even, C.; Tahara, M.; Dvorkin, M.; Ciuleanu, T.; Clement, P.; Mesia, R.; Kutukova, S.; Zholudeva, L.; et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann. Oncol. 2020, 31, 942–950. [Google Scholar] [CrossRef]
- Saba, N.F.; Blumenschein, G., Jr.; Guigay, J.; Licitra, L.; Fayette, J.; Harrington, K.J.; Kiyota, N.; Gillison, M.L.; Ferris, R.L.; Jayaprakash, V.; et al. Nivolumab versus investigator’s choice in patients with recurrent or metastatic squamous cell carcinoma of the head and neck: Efficacy and safety in CheckMate 141 by age. Oral Oncol. 2019, 96, 7–14. [Google Scholar] [CrossRef]
- Zandberg, D.P.; Algazi, A.P.; Jimeno, A.; Good, J.S.; Fayette, J.; Bouganim, N.; Ready, N.E.; Clement, P.M.; Even, C.; Jang, R.W.; et al. Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: Results from a single-arm, phase II study in patients with ≥25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur. J. Cancer 2019, 107, 142–152. [Google Scholar] [CrossRef]
- Zhang, B.; Qi, L.; Wang, X.; Xu, J.; Liu, Y.; Mu, L.; Wang, X.; Bai, L.; Huang, J. Phase II clinical trial using camrelizumab combined with apatinib and chemotherapy as the first-line treatment of advanced esophageal squamous cell carcinoma. Cancer Commun. 2020, 40, 711–720. [Google Scholar] [CrossRef]
- Hansen, A.R.; Massard, C.; Ott, P.A.; Haas, N.B.; Lopez, J.S.; Ejadi, S.; Wallmark, J.M.; Keam, B.; Delord, J.-P.; Aggarwal, R.; et al. Pembrolizumab for advanced prostate adenocarcinoma: Findings of the KEYNOTE-028 study. Ann. Oncol. 2018, 29, 1807–1813. [Google Scholar] [CrossRef]
- Schuhmacher, J.; Heidu, S.; Balchen, T.; Richardson, J.R.; Schmeltz, C.; Sonne, J.; Schweiker, J.; Rammensee, H.-G.; Straten, P.T.; Røder, M.A.; et al. Vaccination against RhoC induces long-lasting immune responses in patients with prostate cancer: Results from a phase I/II clinical trial. J. Immunother. Cancer 2020, 8, e001157. [Google Scholar] [CrossRef] [PubMed]
- Garbe, C.; Radny, P.; Linse, R.; Dummer, R.; Gutzmer, R.; Ulrich, J.; Stadler, R.; Weichenthal, M.; Eigentler, T.; Ellwanger, U.; et al. Adjuvant low-dose interferon α2a with or without dacarbazine compared with surgery alone: A prospective-randomized phase III DeCOG trial in melanoma patients with regional lymph node metastasis. Ann. Oncol. 2008, 19, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Namikawa, K.; Kiyohara, Y.; Takenouchi, T.; Uhara, H.; Uchi, H.; Yoshikawa, S.; Takatsuka, S.; Koga, H.; Wada, N.; Minami, H.; et al. Efficacy and safety of nivolumab in combination with ipilimumab in Japanese patients with advanced melanoma: An open-label, single-arm, multicentre phase II study. Eur. J. Cancer 2018, 105, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Hemstock, M.; Amadi, A.; Kupas, K.; Roskell, N.; Kotapati, S.; Gooden, K.; Middleton, M.R.; Schadendorf, D. Indirect treatment comparison of nivolumab versus placebo for the adjuvant treatment of melanoma. Eur. J. Cancer 2020, 132, 176–186. [Google Scholar] [CrossRef]
- Anguille, S.; Van de Velde, A.L.; Smits, E.L.; Van Tendeloo, V.F.; Juliusson, G.; Cools, N.; Nijs, G.; Stein, B.; Lion, E.; Van Driessche, A.; et al. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia. Blood 2017, 130, 1713–1721. [Google Scholar] [CrossRef]
- Kreitman, R.J.; Dearden, C.; Zinzani, P.L.; Delgado, J.; Robak, T.; le Coutre, P.D.; Gjertsen, B.T.; Troussard, X.; Roboz, G.J.; Karlin, L.; et al. Moxetumomab pasudotox in heavily pre-treated patients with relapsed/refractory hairy cell leukemia (HCL): Long-term follow-up from the pivotal trial. J. Hematol. Oncol. 2021, 14, 1–11. [Google Scholar] [CrossRef]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef]
- Maruyama, D.; Hatake, K.; Kinoshita, T.; Fukuhara, N.; Choi, I.; Taniwaki, M.; Ando, K.; Terui, Y.; Higuchi, Y.; Onishi, Y.; et al. Multicenter phase II study of nivolumab in Japanese patients with relapsed or refractory classical Hodgkin lymphoma. Cancer Sci. 2017, 108, 1007–1012. [Google Scholar] [CrossRef]
- Fan, H.; Lu, X.; Wang, X.; Liu, Y.; Guo, B.; Zhang, Y.; Zhang, W.; Nie, J.; Feng, K.; Chen, M.; et al. Low-Dose Decitabine-Based Chemoimmunotherapy for Patients with Refractory Advanced Solid Tumors: A Phase I/II Report. J. Immunol. Res. 2014, 2014, 1–14. [Google Scholar] [CrossRef]
- Heiss, M.M.; Murawa, P.; Koralewski, P.; Kutarska, E.; Kolesnik, O.O.; Ivanchenko, V.V.; Dudnichenko, A.S.; Aleknaviciene, B.; Razbadauskas, A.; Gore, M.; et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial. Int. J. Cancer 2010, 127, 2209–2221. [Google Scholar] [CrossRef]
- Burges, A.; Wimberger, P.; Kümper, C.; Gorbounova, V.; Sommer, H.; Schmalfeldt, B.; Pfisterer, J.; Lichinitser, M.; Makhson, A.; Moiseyenko, V.; et al. Effective Relief of Malignant Ascites in Patients with Advanced Ovarian Cancer by a Trifunctional Anti-EpCAM × Anti-CD3 Antibody: A Phase I/II Study. Clin. Cancer Res. 2007, 13, 3899–3905. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.; McManus, J.M.; Sharifi, N. Hormonal Therapy for Prostate Cancer. Endocr. Rev. 2021, 42, 354–373. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.; Keefe, F.J.; Lyna, P.; Peterson, B.; Garst, J.; Kelley, M.; Bepler, G.; Bastian, L.A. Persistent Smoking After a Diagnosis of Lung Cancer Is Associated with Higher Reported Pain Levels. J. Pain 2009, 10, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Yanyan, H.; Dandan, L.; Lianhong, L. PD-1/PD-L1 Pathway: Current Researches in Cancer. Am. J. Cancer Res. 2020, 10, 727. [Google Scholar]
- Benjamin, D.J.; Xu, A.; Lythgoe, M.P.; Prasad, V. Cancer Drug Approvals That Displaced Existing Standard-of-Care Therapies, 2016-2021. JAMA Netw. Open 2022, 5, e222265. [Google Scholar] [CrossRef]
- Kichloo, A.; Albosta, M.; Dahiya, D.; Guidi, J.C.; Aljadah, M.; Singh, J.; Shaka, H.; Wani, F.; Kumar, A.; Lekkala, M. Systemic adverse effects and toxicities associated with immunotherapy: A review. World J. Clin. Oncol. 2021, 12, 150–163. [Google Scholar] [CrossRef]
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.F.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 341–351. [Google Scholar] [CrossRef]
- Ahmad, A.; Uddin, S.; Steinhoff, M. CAR-T Cell Therapies: An Overview of Clinical Studies Supporting Their Approved Use against Acute Lymphoblastic Leukemia and Large B-Cell Lymphomas. Int. J. Mol. Sci. 2020, 21, 3906. [Google Scholar] [CrossRef]
- Voelker, R. Immunotherapy Is Now First-line Therapy for Some Colorectal Cancers. JAMA 2020, 324, 433. [Google Scholar] [CrossRef]
- Xiong, A.; Wang, J.; Zhou, C. Immunotherapy in the First-Line Treatment of NSCLC: Current Status and Future Directions in China. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- Brown, T.J.; Mamtani, R.; Bange, E.M. Immunotherapy Adverse Effects. JAMA Oncol. 2021, 7, 1908. [Google Scholar] [CrossRef]
- Barber, F.D. Adverse Events of Oncologic Immunotherapy and Their Management. Asia-Pac. J. Oncol. Nurs. 2019, 6, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Geisler, A.N.; Phillips, G.S.; Barrios, D.M.; Wu, J.; Leung, D.Y.M.; Moy, A.P.; Kern, J.A.; Lacouture, M.E. Immune checkpoint inhibitor–related dermatologic adverse events. J. Am. Acad. Dermatol. 2020, 83, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; He, C.; Zhang, L.; Liu, X.; Li, Y.; Wang, H.; Guo, X.; Zhou, J.; Duan, L.; Wang, M.; et al. Management of immune checkpoint inhibitor-related dermatologic adverse events. Thorac. Cancer 2019, 11, 488–492. [Google Scholar] [CrossRef]
- Apalla, Z.; Rapoport, B.; Sibaud, V. Dermatologic immune-related adverse events: The toxicity spectrum and recommendations for management. Int. J. Women’s Dermatol. 2021, 7, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; Zang, X.-Y.; Wang, J.-C.; Huang, S.-S.; Xu, J.; Zhang, P. Diagnosis and Management of Immune Related Adverse Events (irAEs) in Cancer Immunotherapy. Biomed. Pharmacother. 2019, 120, 109437. [Google Scholar] [CrossRef]
- Rajha, E.; Chaftari, P.; Kamal, M.; Maamari, J.; Chaftari, C.; Yeung, S.-C.J. Gastrointestinal adverse events associated with immune checkpoint inhibitor therapy. Gastroenterol. Rep. 2019, 8, 25–30. [Google Scholar] [CrossRef]
- Ganatra, S.; Parikh, R.; Neilan, T.G. Cardiotoxicity of Immune Therapy. Cardiol. Clin. 2019, 37, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Ala, C.K.; Klein, A.L.; Moslehi, J.J. Cancer Treatment-Associated Pericardial Disease: Epidemiology, Clinical Presentation, Diagnosis, and Management. Curr. Cardiol. Rep. 2019, 21, 156. [Google Scholar] [CrossRef]
- Del Rivero, J.; Cordes, L.M.; Klubo-Gwiezdzinska, J.; Madan, R.A.; Nieman, L.K.; Gulley, J.L. Endocrine-Related Adverse Events Related to Immune Checkpoint Inhibitors: Proposed Algorithms for Management. Oncologist 2019, 25, 290–300. [Google Scholar] [CrossRef]
- Chalan, P.; Di Dalmazi, G.; Pani, F.; De Remigis, A.; Corsello, A.; Caturegli, P. Thyroid dysfunctions secondary to cancer immunotherapy. J. Endocrinol. Investig. 2017, 41, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Delaunay, M.; Prévot, G.; Collot, S.; Guilleminault, L.; Didier, A.; Mazières, J. Management of pulmonary toxicity associated with immune checkpoint inhibitors. Eur. Respir. Rev. 2019, 28, 190012. [Google Scholar] [CrossRef] [PubMed]
- Long, B.J.; Brem, E.; Koyfman, A. Oncologic Emergencies: Immune-Based Cancer Therapies and Complications. West. J. Emerg. Med. 2020, 21, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Chuzi, S.; Tavora, F.; Cruz, M.; Costa, R.; Chae, Y.K.; Carneiro, A.B.; Giles, F.J. Clinical features, diagnostic challenges, and management strategies in checkpoint inhibitor-related pneumonitis. Cancer Manag. Res. 2017, 9, 207–213. [Google Scholar] [CrossRef]
- Lipe, D.N.; Shafer, S. CAR-T and checkpoint inhibitors: Toxicities and antidotes in the emergency department. Clin. Toxicol. 2021, 59, 376–385. [Google Scholar] [CrossRef]
- Pallin, D.J.; Baugh, C.W.; Postow, M.A.; Caterino, J.M.; Erickson, T.B.; Lyman, G.H. Immune-related Adverse Events in Cancer Patients. Acad. Emerg. Med. 2018, 25, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Sibeoni, J.; Picard, C.; Orri, M.; Labey, M.; Bousquet, G.; Verneuil, L.; Revah-Levy, A. Patients’ quality of life during active cancer treatment: A qualitative study. BMC Cancer 2018, 18, 951. [Google Scholar] [CrossRef]
- Ramirez, R.A.; Lu, J.; Thomas, K.E.H. Quality of life for non-small cell lung cancer patients in the age of immunotherapy. Transl. Lung Cancer Res. 2018, 7, S149–S152. [Google Scholar] [CrossRef] [PubMed]
| Type of Cancer | Study Phase | Treatment Groups | Number of Patients | Mean Age, Years | Median Overall Survival, Months (95% CI); p-Value | Median Progression-Free Survival (95% CI); p-Value | |
|---|---|---|---|---|---|---|---|
| Gastric Cancer | Wang et al. (2019) [10] | 1b/2 | A: Toripalimab B: Toripalimab plus XELOX (oxaliplatin, capecitabine) | A: 58 B: 18 | A: 59.5 (52.0–66.0) B: 58.5 (48.0–69.0) | A: 4.8 months (N/A); p = N/A B: N/A | A: 1.9 months (N/A); p = N/A B: 5.8 months (N/A); p = N/A |
| Bladder Cancer | Shore et al. (2017) [11] | 2 | Low dose (LD) intravesical rAd-IFNα/Syn3 vs. high dose (HD) rAd-IFNα/Syn3 | LD: 22 HD: 21 | 70.5 (64.5–77.5) | 6.5 months (3.52–12.78) | LD: 3.52 months (3.02–12.78) HD: 11.73 months (5.88–N/A) |
| Non-Small Cell Lung Cancer | Ding et al. (2016) [12] | 1b/2 | Cytokine-induced killer (CIT group) vs. no treatment (control group) | 49 | CIT group: 63 (54–79) Control group: 57 (36–74) | CIT group: 13.3 months Control group: 8.2 months (N/A); p = 0.044 | CIT group: 5 months Control group: 3.1 months (N/A); p = 0.020 |
| Cho et al. (2021) [13] | 1 | Quavonlimab plus pembrolizumab | 40 | 66 (40–80) | 11.0 months (5.9, 15.5); p = N/A | 2.0 months (1.9, 3.9); p = N/A | |
| Planchard et al. (2020) [14] | 3 | A: Durvalumab vs. SoC B: Durvalumab plus tremelimumab (D + T) vs. SoC | A: 126 B: 469 | A: Durvalumab 63.5 (35–79), SoC 62.0 (41–81) B: D + T 62.5 (26–81), SoC 65.0 (42–83) | A: Durvalumab 11.7 months (8.2, 17.4); p = 0, SoC 6.8 months (4.9, 10.2); p = 0 B: D + T 11.5 months (8.7, 14.1); p = 0, SoC 8.7 months (6.5, 11.7); p = 0 | A: Durvalumab 3.8 months (1.9, 5.6); p = 0, SoC 2.2 months (1.9, 3.7); p = 0 B: D + T 9.1 months (6.6, 12.3); p = 0, SoC 3.5 months (1.9, 3.9); p = 0 | |
| Hui et al. (2017) [15] | 1 | Pembrolizumab monotherapy | 101 | 68.0 (N/A) | 22.1 months (17.1–27.2); p = N/A | 6.2 months (4.1, 8.6); p = N/A | |
| Spigel et al. (2018) [16] | 2 | Atezolizumab use in: Cohort 1: no previous treatment Cohort 2: prior platinum-based chemotherapy Cohort 3: prior platinum-based chemotherapy in brain metastases | Cohort 1: 31 Cohort 2: 93 Cohort 3: 13 | 1: 68 (42–85) 2: 65 (44–85) 3: 65 (52–74) | Cohort 1: 14.4 months (12.8, 22.1); p = N/A Cohort 2: 9.3 months (5.8, 17.6); p = N/A Cohort 3: 6.8 months (3.2, 19.4); p = N/A | Cohort 1: 4.5 months (3.3–8.3); p = N/A Cohort 2: 2.7 months (1.5–3.4); p = N/A Cohort 3: 2.5 months (1.2–4.2); p = N/A | |
| Breast Cancer | Mittendorf et al. (2014) [17] | 1/2 | Vaccinated group (VC) E75 plus granulocyte-macrophage colony-stimulating factor (GM-CSF) vs. control group (CG) no treatment | 187 | VC: 57 (28–78) CG: 53 (32–83) | N/A | VC: 89.7% CG: 80.2% (N/A); p = 0.8 |
| Schmid et al. (2020) [18] | 1b | Pembrolizumab plus chemotherapy | 60 | 48.5 (26–71) | 98% (90–100%); p = N/A | 98% (90–100%); p = N/A | |
| Chumsri et al. (2019) [19] | 3 | Adjuvant chemotherapy plus trastuzumab vs. chemotherapy | 3177 | 49.0 (23.0–80.0) | N/A | 81.39% (78.54%–84.34%); p = N/A | |
| Ovarian and Breast Cancer | Antonilli et al. (2016) [20] | 1/2 | Triple peptide vaccination | 14 | 53.0 (42–70) | N/A | N/A |
| Glioblastoma | Liau et al. (2018) [21] | 3 | Temozolomide plus autologous tumor lysate-pulsed dendritic cell vaccine or Temozolomide plus placebo | 331 | 56.0 (19–73) | 23.1 (21.2-25.4) | N/A |
| Mesothelioma | Janssen et al. (2018) [22] | 2 | Nivolumab monotherapy | 34 | 67.0 (50–81) | 11.8 months (9.7–15.7); p = N/A | 2.6 months (2.23–5.49); p = N/A |
| Cervical Cancer | Rischin et al. (2020) [23] | 1 | A: Cemiplimab monotherapy B: Cemiplimab plus hypofractionated radiation therapy (hfRT). | A: 10 B: 10 | A: 55.0 (31.0–76.0) B: 51.5 (29.0–65.0) | A: 10.3 months (2.1–N/A); p = N/A B: 8.0 months (1.7–N/A); p = N/A | A: 1.9 months (1.0–9.0); p = N/A B: 3.6 months (0.6–5.7); p = N/A |
| Harper et al. (2019) [24] | 2b | A: Tipapkinogen Sovacivec vaccine B: placebo | 206 | A: 30.1 (18–60) B: 29.8 (19–50) | N/A | N/A | |
| Santin et al. (2020) [25] | 2 | Nivolumab monotherapy | 26 | 45.0 (20–79) | 14.5 months (8.3–26.8); p = N/A | 3.5 months (1.9–5.1); p = N/A | |
| Sarcoma | Ahmed et al. (2015) [26] | 1/2 | Human Epidermal Growth Factor Receptor 2 (HER2)—Specific Chimeric Antigen Receptor-Modified T Cells | 19 | 17.0 (7.7–29.6) | 10.3 months (5.1, 29.1); p = N/A | N/A |
| Miwa et al. (2017) [27] | 1/2 | Dendritic cells pulsed with autologous tumor lysate | 37 | 37.8 (8–65) | 2.9% (N/A); p = N/A | 42.3% (N/A); p = N/A | |
| Head and Neck Squamous Cell Carcinoma | Ferris et al. (2020) [28] | 3 | A: Durvalumab vs. Soc B: Durvalumab plus tremelimumab vs. SoC | 736 | 60.0 (N/A) | A: 7.6 months (6.1–9.8); p = 0.20 B: 6.5 months (5.5–8.2); p = 0.76 | A: 2.1 months (1.9–3.0); p = N/A B: 2.0 months (1.9–2.3); p = N/A |
| Saba et al. (2019) [29] | 3 | A: Nivolumab vs. SoC in < 65 years old patients. B: Nivolumab vs. SoC in ≥ 65-year-old patients | 361 | 48.5 (26–71) | A: 8.2 months vs. 4.9 months (0.47–0.84); p = N/A B: 6.9 months vs. 6.0 months (0.51–1.12); p = N/A | A: 2.0 months vs. 2.7 months (0.71–1.30); p = N/A B: 2.1 months vs. 2.0 months (0.49–1.11); p = N/A | |
| Zandberg et al. (2019) [30] | 2 | Durvalumab monotherapy | 112 | 60.0 (24.0–84.0) | 7.1 months (1.9–5.6); p = N/A | 2.1 months (1.9–3.7); p = N/A | |
| Esophageal Squamous Cell Carcinoma | Zhang et al. (2020) [31] | 2 | Camrelizumab plus apatinib and chemotherapy | 30 | 61.5 (43–70) | 19.43 months (9.93–N/A); p = N/A | 6.85 months (4.46–14.20); p = N/A |
| Prostate Cancer | Hansen et al. (2018) [32] | 1b | Pembrolizumab monotherapy | 245 | 65.0 (46–83) | 7.9 months (6.5–N/A); p = N/A | 3.5 months (1.7–6.5); p = N/A |
| Schuhmacher et al. (2020) [33] | 1/2 | Ras homolog gene family member C vaccination | 22 | 66.0 (54–77) | N/A | N/A | |
| Melanoma | Garbe et al. (2008) [34] | 3 | Adjuvant interferon α2a with or without dacarbazine vs. surgery | 444 | N/A | 59.0% vs. 42.0% (N/A); p = 0.0045 | 39.0% vs. 27.0% (N/A); p = 0.018 |
| Namikawa et al. (2018) [35] | 2 | Nivolumab plus ipilimumab | 30 | 58.5 (31–81) | N/A | N/A | |
| Hemstock et al. (2020) [36] | 3 | Nivolumab vs. placebo | 928 | N/A | N/A | N/A | |
| Leukemia | Anguille et al. (2017) [37] | 2 | Adjuvant dendritic cell vaccination | 30 | 60.0 (30–79) | 41.8 months (N/A); p = N/A | N/A |
| Kreitman et al. (2021) [38] | 3 | Moxetummomab pasudotox | 80 | 60 | N/A | 41.5 months (29.5, N/A); p = N/A | |
| Lymphoma | Wang et al. (2020) [39] | 2 | KTE-X19 CAR T-Cell therapy | 60 | 65.0 (38–79) | N/A | N/A |
| Maruyama et al. (2017) [40] | 2 | Nivolumab | 17 | 63.0 (29–83) | N/A | N/A | |
| Fan et al. (2014) [41] | 1/2 | A: Decitabine B: Decitabine plus chemotherapy C: Decitabine plus cytokine induced killer cells | 32 | 58.8 (28–84) | N/A | A: 2.5 months (1–12); p = N/A B: 4 months (1–7); p = N/A C: 8 months (4–10); p = N/A | |
| Malignant Ascites | Heiss et al. (2010) [42] | 2/3 | A: Paracentesis plus catumaxomab B: Paracentesis alone | 258 | N/A | A: 72 days (61–96); p = N/A B: 68 days (49–81); p = N/A | A: 46 days (35–53); p = N/A B: 11 days (9–16); p = N/A |
| Burges et al. (2007) [43] | 1/2 | Catumaxomab | 23 | 61.7 (42–80) | N/A | N/A |
| (A) | |||||||||
| Study | Pre-Intervention | At Intervention | Post Intervention | Overall Risk of Bias | |||||
| First Author | Year | Bias Due to Confounding | Bias in Selection of Participants into the Study | Bias in Classification of Interventions | Bias Due to Deviations from Intended Interventions | Bias Due to Missing Data | Bias in Measurement of Outcomes | Bias in Selection of the Reported Result | Low, Moderate, Serious, Critical |
| Wang et al. [10] | 2019 | Low | Low | Low | Low | Low | Moderate | Low | Moderate |
| Ding et al. [12] | 2016 | Low | Low | Low | Low | Low | Moderate | Low | Moderate |
| Rischin et al. [23] | 2020 | Low | Low | Low | Low | Low | Moderate | Low | Moderate |
| Mittendorf et al. [17] | 2014 | Low | Low | Low | Moderate | Low | Moderate | Low | Moderate |
| Cho et al. [13] | 2021 | Low | Low | Low | Low | Low | Moderate | Low | Moderate |
| Janssen et al. [22] | 2018 | Low | Low | Low | Low | Low | Moderate | Low | Moderate |
| Hansen et al. [32] | 2018 | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate |
| Spigel et al. [16] | 2018 | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate |
| Zandberg et al. [30] | 2019 | Low | Low | Low | Low | Low | Moderate | Low | Moderate |
| Anguille et al. [37] | 2017 | Low | Low | Low | Low | Low | Moderate | Low | Moderate |
| Namikawa et al. [35] | 2018 | Low | Low | Low | Low | Low | Moderate | Low | Moderate |
| Wang et al. [39] | 2020 | Low | Low | Low | Low | Low | Moderate | Low | Moderate |
| Santin et al. [25] | 2020 | Low | Low | Low | Low | Low | Moderate | Low | Moderate |
| Zhang et al. [31] | 2020 | Low | Low | Low | Low | Low | Moderate | Low | Moderate |
| Ahmed et al. [26] | 2015 | Low | Low | Low | Low | Low | Moderate | Low | Moderate |
| Schuhmacher et al. [33] | 2020 | Low | Low | Low | Low | Low | Moderate | Low | Moderate |
| Antonilli et al. [20] | 2016 | Low | Low | Low | Low | Low | Moderate | Low | Moderate |
| Maruyama et al. [40] | 2017 | Low | Low | Low | Low | Low | Moderate | Low | Moderate |
| Fan et al. [41] | 2014 | Low | Low | Low | Low | Low | Moderate | Low | Moderate |
| Burges et al. [43] | 2007 | Low | Low | Low | Low | Low | Moderate | Low | Low |
| Miwa et al. [27] | 2017 | Low | Low | Low | Low | Low | Moderate | Low | Moderate |
| Kreitman et al. [38] | 2021 | Low | Low | Low | Low | Low | Moderate | Low | Moderate |
| (B) | |||||||||
| Study | Pre-Intervention | Post Intervention | Overall Risk of Bias | ||||||
| First Author | Year | Bias Arising from the Randomization Process | Bias Due to Deviations from Intended Interventions | Bias Due to Missing Outcome Data | Bias in Measurement of the Outcome | Bias in Selection of the Reported Result | Low, Some Concerns, High Risk of Bias | ||
| Hui et al. [15] | 2017 | Some concerns | Some concerns | Low | Low | Low | Some concerns | ||
| Schmid et al. [18] | 2020 | Low | Some concerns | Low | Low | Low | Some concerns | ||
| Harper et al. [24] | 2019 | Some concerns | Low | Low | Low | Low | Some concerns | ||
| Ferris et al. [28] | 2020 | Some concerns | Some concerns | Low | Low | Low | Some concerns | ||
| Saba et al. [29] | 2019 | Some concerns | Some concerns | Low | Low | Low | Some concerns | ||
| Garbe et al. [34] | 2008 | Some concerns | Some concerns | Low | Low | Low | Some concerns | ||
| Heiss et al. [42] | 2010 | Some concerns | Some concerns | Low | Low | Low | Some concerns | ||
| Chumsri et al. [19] | 2019 | Some concerns | Some concerns | Low | Low | Low | Some concerns | ||
| Shore et al. [11] | 2017 | Low | Some concerns | Low | Low | Low | Some concerns | ||
| Liau et al. [21] | 2018 | Low | Some concerns | Low | Low | Low | Some concerns | ||
| Planchard et al. [14] | 2020 | Some concerns | Some concerns | Low | Low | Low | Some concerns | ||
| Hemstock et al. [36] | 2020 | Some concerns | Low | Low | Low | Low | Some concerns | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, S.P.; Ming, L.C.; Dhaliwal, J.S.; Gupta, M.; Ardianto, C.; Goh, K.W.; Hussain, Z.; Shafqat, N. Role of Immunotherapy in the Treatment of Cancer: A Systematic Review. Cancers 2022, 14, 5205. https://doi.org/10.3390/cancers14215205
Ling SP, Ming LC, Dhaliwal JS, Gupta M, Ardianto C, Goh KW, Hussain Z, Shafqat N. Role of Immunotherapy in the Treatment of Cancer: A Systematic Review. Cancers. 2022; 14(21):5205. https://doi.org/10.3390/cancers14215205
Chicago/Turabian StyleLing, Sia Pei, Long Chiau Ming, Jagjit Singh Dhaliwal, Madhu Gupta, Chrismawan Ardianto, Khang Wen Goh, Zahid Hussain, and Naeem Shafqat. 2022. "Role of Immunotherapy in the Treatment of Cancer: A Systematic Review" Cancers 14, no. 21: 5205. https://doi.org/10.3390/cancers14215205
APA StyleLing, S. P., Ming, L. C., Dhaliwal, J. S., Gupta, M., Ardianto, C., Goh, K. W., Hussain, Z., & Shafqat, N. (2022). Role of Immunotherapy in the Treatment of Cancer: A Systematic Review. Cancers, 14(21), 5205. https://doi.org/10.3390/cancers14215205







