A Novel Strategy to Model Age-Related Cancer for Elucidation of the Role of Th17 Inflammaging in Cancer Progression
Abstract
Simple Summary
Abstract
1. Introduction
2. The Importance of Examining the Effect of Aging in Cancer
3. Inflammaging: An Age-Related Driver of Cancer Progression
4. Th17 Inflammaging Plays a Pivotal Role in Age-Related Cancer Progression, Lessons from Aging Animal Models
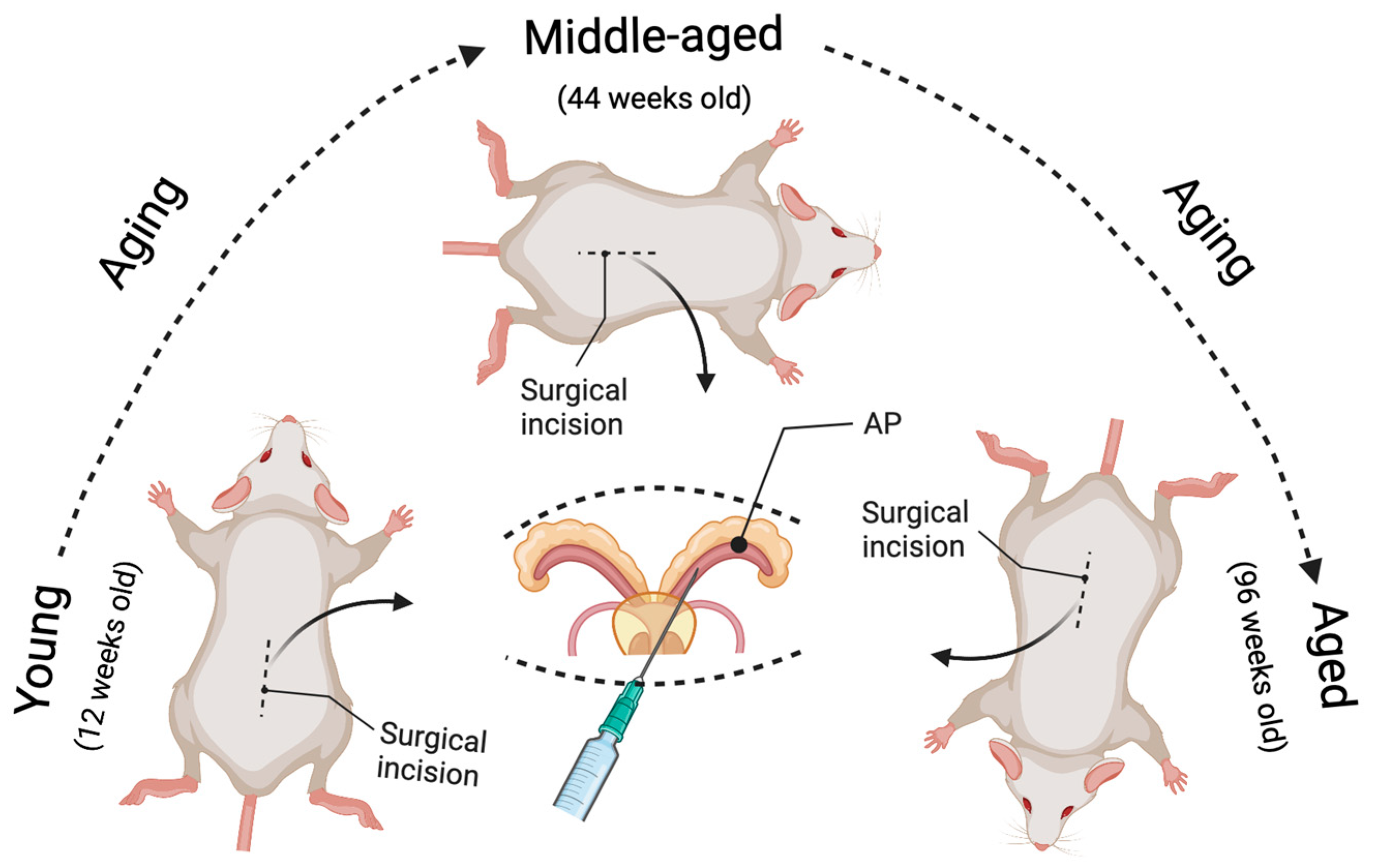
5. Novel Strategy for Generating Age-Related Cancer Models in Mice
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaul, E.; Barron, J. Age-Related Diseases and Clinical and Public Health Implications for the 85 Years Old and Over Population. Front Public Health 2017, 5, 335. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Fane, M.; Weeraratna, A.T. How the ageing microenvironment influences tumour progression. Nat. Rev. Cancer 2020, 20, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Schmauck-Medina, T.; Molière, A.; Lautrup, S.; Zhang, J.; Chlopicki, S.; Madsen, H.B.; Cao, S.; Soendenbroe, C.; Mansell, E.; Vestergaard, M.B.; et al. New hallmarks of ageing: A 2022 Copenhagen ageing meeting summary. Aging (Albany NY) 2022, 14, 6829–6839. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Gems, D.; de Magalhães, J.P. The hoverfly and the wasp: A critique of the hallmarks of aging as a paradigm. Ageing Res. Rev. 2021, 70, 101407. [Google Scholar] [CrossRef]
- Fraser, H.C.; Kuan, V.; Johnen, R.; Zwierzyna, M.; Hingorani, A.D.; Beyer, A.; Partridge, L. Biological mechanisms of aging predict age-related disease co-occurrence in patients. Aging Cell 2022, 21, e13524. [Google Scholar] [CrossRef]
- Leonardi, G.C.; Accardi, G.; Monastero, R.; Nicoletti, F.; Libra, M. Ageing: From inflammation to cancer. Immun. Ageing 2018, 15, 1. [Google Scholar] [CrossRef]
- Berben, L.; Floris, G.; Wildiers, H.; Hatse, S. Cancer and Aging: Two Tightly Interconnected Biological Processes. Cancers 2021, 13, 1400. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the global challenges of ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Editorial: The importance of aging in cancer research. Int. Nat. Aging 2022, 2, 365–366. [CrossRef]
- Haynes, L. Aging of the Immune System: Research Challenges to Enhance the Health Span of Older Adults. Front. Aging 2020, 1, 602108. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2017, 8, 1960. [Google Scholar] [CrossRef] [PubMed]
- Teissier, T.; Boulanger, E.; Cox, L.S. Interconnections between Inflammageing and Immunosenescence during Ageing. Cells 2022, 11, 359. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Lopez-Candales, A.; Hernández Burgos, P.M.; Hernandez-Suarez, D.F.; Harris, D. Linking Chronic Inflammation with Cardiovascular Disease: From Normal Aging to the Metabolic Syndrome. J. Nat. Sci. 2017, 3, e341. [Google Scholar]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Oishi, Y.; Manabe, I. Macrophages in age-related chronic inflammatory diseases. NPJ Aging Mech. Dis. 2016, 2, 16018. [Google Scholar] [CrossRef]
- Cakman, I.; Rohwer, J.; Schutz, R.M.; Kirchner, H.; Rink, L. Dysregulation between TH1 and TH2 T cell subpopulations in the elderly. Mech. Ageing Dev. 1996, 87, 197–209. [Google Scholar] [CrossRef]
- Karanfilov, C.I.; Liu, B.; Fox, C.C.; Lakshmanan, R.R.; Whisler, R.L. Age-related defects in Th1 and Th2 cytokine production by human T cells can be dissociated from altered frequencies of CD45RA+ and CD45RO+ T cell subsets. Mech. Ageing Dev. 1999, 109, 97–112. [Google Scholar] [CrossRef]
- Lewis, E.D.; Wu, D.; Meydani, S.N. Age-associated alterations in immune function and inflammation. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 118, 110576. [Google Scholar] [CrossRef] [PubMed]
- Uciechowski, P.; Kahmann, L.; Plümäkers, B.; Malavolta, M.; Mocchegiani, E.; Dedoussis, G.; Herbein, G.; Jajte, J.; Fulop, T.; Rink, L. TH1 and TH2 cell polarization increases with aging and is modulated by zinc supplementation. Exp. Gerontol. 2008, 43, 493–498. [Google Scholar] [CrossRef]
- Lustosa, L.P.; Xavier, D.R.; Ribeiro-Samora, G.A.; Pereira, D.S.; Parentoni, A.N.; Thomasini, R.L.; Pereira, L.S.M. Functional Capacity and Inflammatory Mediators in Elderly Residents of Counties with Different Human Development Index. J. Aging Res. 2020, 2020, 9250929. [Google Scholar] [CrossRef]
- Lim, M.A.; Lee, J.; Park, J.S.; Jhun, J.Y.; Moon, Y.M.; Cho, M.L.; Kim, H.Y. Increased Th17 differentiation in aged mice is significantly associated with high IL-1beta level and low IL-2 expression. Exp. Gerontol. 2014, 49, 55–62. [Google Scholar] [CrossRef]
- Schmitt, V.; Rink, L.; Uciechowski, P. The Th17/Treg balance is disturbed during aging. Exp. Gerontol. 2013, 48, 1379–1386. [Google Scholar] [CrossRef]
- van der Geest, K.S.; Abdulahad, W.H.; Tete, S.M.; Lorencetti, P.G.; Horst, G.; Bos, N.A.; Kroesen, B.J.; Brouwer, E.; Boots, A.M. Aging disturbs the balance between effector and regulatory CD4+ T cells. Exp. Gerontol. 2014, 60, 190–196. [Google Scholar] [CrossRef]
- Garg, S.K.; Delaney, C.; Toubai, T.; Ghosh, A.; Reddy, P.; Banerjee, R.; Yung, R. Aging is associated with increased regulatory T-cell function. Aging Cell 2014, 13, 441–448. [Google Scholar] [CrossRef]
- Tesar, B.M.; Du, W.; Shirali, A.C.; Walker, W.E.; Shen, H.; Goldstein, D.R. Aging augments IL-17 T-cell alloimmune responses. Am. J. Transplant. 2009, 9, 54–63. [Google Scholar] [CrossRef]
- De Angulo, A.; Faris, R.; Cavazos, D.; Jolly, C.; Daniel, B.; DeGraffenried, L. Age-related alterations in T-lymphocytes modulate key pathways in prostate tumorigenesis. Prostate 2013, 73, 855–864. [Google Scholar] [CrossRef] [PubMed]
- De Angulo, A.; Faris, R.; Daniel, B.; Jolly, C.; deGraffenried, L. Age-related increase in IL-17 activates pro-inflammatory signaling in prostate cells. Prostate 2015, 75, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Pieren, D.K.J.; Smits, N.A.M.; van de Garde, M.D.B.; Guichelaar, T. Response kinetics reveal novel features of ageing in murine T cells. Sci. Rep. 2019, 9, 5587. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, F.; Zhang, B.; Yan, P.; Rowan, B.G.; Abdel-Mageed, A.B.; Steele, C.; Jazwinski, S.M.; Moroz, K.; Norton, E.B.; et al. CD4+ T helper 17 cell response of aged mice promotes prostate cancer cell migration and invasion. Prostate 2020, 80, 764–776. [Google Scholar] [CrossRef]
- Bharath, L.P.; Agrawal, M.; McCambridge, G.; Nicholas, D.A.; Hasturk, H.; Liu, J.; Jiang, K.; Liu, R.; Guo, Z.; Deeney, J.; et al. Metformin Enhances Autophagy and Normalizes Mitochondrial Function to Alleviate Aging-Associated Inflammation. Cell Metab. 2020, 32, 44–55.e6. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, B.; Rowan, B.G.; Jazwinski, S.M.; Abdel-Mageed, A.B.; Steele, C.; Wang, A.R.; Sartor, O.; Niu, T.; Zhang, Q. A Novel Controlled PTEN-Knockout Mouse Model for Prostate Cancer Study. Front. Mol. Biosci. 2021, 8, 696537. [Google Scholar] [CrossRef]
- Li, H.; Wei, C.; Zhou, R.; Wang, B.; Zhang, Y.; Shao, C.; Luo, Y. Mouse models in modeling aging and cancer. Exp. Gerontol. 2019, 120, 88–94. [Google Scholar] [CrossRef]
- Cai, N.; Wu, Y.; Huang, Y. Induction of Accelerated Aging in a Mouse Model. Cells 2022, 11, 1418. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.; Im, S.K.; Fang, S. Mouse Cre-LoxP system: General principles to determine tissue-specific roles of target genes. Lab. Anim. Res. 2018, 34, 147–159. [Google Scholar] [CrossRef]
- Sternberg, N.; Hamilton, D. Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J. Mol. Biol. 1981, 150, 467–486. [Google Scholar] [CrossRef]
- Stachowski, K.; Norris, A.S.; Potter, D.; Wysocki, V.H.; Foster, M.P. Mechanisms of Cre recombinase synaptic complex assembly and activation illuminated by Cryo-EM. Nucleic Acids Res. 2022, 50, 1753–1769. [Google Scholar] [CrossRef] [PubMed]
- Indra, A.K.; Warot, X.; Brocard, J.; Bornert, J.M.; Xiao, J.H.; Chambon, P.; Metzger, D. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: Comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 1999, 27, 4324–4327. [Google Scholar] [CrossRef] [PubMed]
- Donocoff, R.S.; Teteloshvili, N.; Chung, H.; Shoulson, R.; Creusot, R.J. Optimization of tamoxifen-induced Cre activity and its effect on immune cell populations. Sci. Rep. 2020, 10, 15244. [Google Scholar] [CrossRef] [PubMed]
- Casanova, E.; Fehsenfeld, S.; Lemberger, T.; Shimshek, D.R.; Sprengel, R.; Mantamadiotis, T. ER-based double iCre fusion protein allows partial recombination in forebrain. Genesis 2002, 34, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Aznar, A.; Martínez-Corral, I.; Daubel, N.; Betsholtz, C.; Mäkinen, T.; Gaengel, K. Tamoxifen-independent recombination of reporter genes limits lineage tracing and mosaic analysis using CreER. Transgenic Res. 2020, 29, 53–68. [Google Scholar] [CrossRef]
- Chin, H.J.; Lee, S.Y.; Lee, D. Tamoxifen-inducible cardiac-specific Cre transgenic mouse using. Lab. Anim. Res. 2020, 36, 31. [Google Scholar] [CrossRef]
- Saranyutanon, S.; Deshmukh, S.K.; Dasgupta, S.; Pai, S.; Singh, S.; Singh, A.P. Cellular and Molecular Progression of Prostate Cancer: Models for Basic and Preclinical Research. Cancers 2020, 12, 2651. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Jazwinski, S.M. A Novel Strategy to Model Age-Related Cancer for Elucidation of the Role of Th17 Inflammaging in Cancer Progression. Cancers 2022, 14, 5185. https://doi.org/10.3390/cancers14215185
Zhang Q, Jazwinski SM. A Novel Strategy to Model Age-Related Cancer for Elucidation of the Role of Th17 Inflammaging in Cancer Progression. Cancers. 2022; 14(21):5185. https://doi.org/10.3390/cancers14215185
Chicago/Turabian StyleZhang, Qiuyang, and S. Michal Jazwinski. 2022. "A Novel Strategy to Model Age-Related Cancer for Elucidation of the Role of Th17 Inflammaging in Cancer Progression" Cancers 14, no. 21: 5185. https://doi.org/10.3390/cancers14215185
APA StyleZhang, Q., & Jazwinski, S. M. (2022). A Novel Strategy to Model Age-Related Cancer for Elucidation of the Role of Th17 Inflammaging in Cancer Progression. Cancers, 14(21), 5185. https://doi.org/10.3390/cancers14215185




