Simple Summary
The recommended treatment for locally advanced cervical cancer (LACC) consists of chemoradiotherapy (CRT) followed by brachytherapy (BT). Although BT is considered a minimally invasive procedure, patients still suffer severe discomfort from it and risk uterine perforation. Dosimetric uncertainties are often inevitable due to anatomical variations and inconsistencies in applicator loadings. These issues prompted us to explore the use of stereotactic body radiotherapy (SBRT) as a viable alternative. It has been well described that the CyberKnife (CK), a robotic image-guided SBRT delivery system, is capable of producing rapidly fall-off dose gradients with submillimeter accuracy. The aim of this study was to compare the dose distributions and radiobiological effects of a CK-based SBRT boost and a BT boost. We found a tumor volume threshold target, below which the CK-based SBRT plan could result in significantly better target coverage, OAR sparing and radiobiological effects compared to the BT plan. With improved precision of target localization, a reduced PTV margin might increase the eligibility of patients to receive a CK-based SBRT boost after CRT, rather than BT. CK-based SBRT could be an alternative option for patients who are not candidate for BT.
Abstract
(1) Aim: To compare the treatment plans of stereotactic body radiotherapy (SBRT) with CyberKnife (CK) and high-dose-rate (HDR) intracavitary/interstitial brachytherapy (IC/ISBT) and examine the feasibility of CK-SBRT as a viable alternative to BT in patients with locally advanced cervical cancer (LACC). (2) Methods: A BT plan of 28 Gy in four fractions delivered previously to 20 patients with LACC was compared with a CK plan based on the same CT images with structures delineation for BT. The SBRT treatment plan was further divided according to two different approaches, with the high-risk planning target volume (HR-PTV) defined by the high-risk clinical target volume (HR-CTV) without and with a 5 mm margin, which were named CK-CTV plan and CK-PTV plan, respectively. The dose distributions and dosimetric parameters of the target volumes and organs at risk (OARs) were recorded and compared for the three boost plans. Radiobiological metrics were calculated based on the EUD for the hybrid plans. Additionally, the relationship between tumor volume and tolerance doses for the OARs in the BT plan and CK-PTV plan was investigated. (3) Results: Target coverage was better with the CK plan than with the BT plan, as the D95%, D98%, HI and CI of the CK-CTV plan and CK-PTV plan were higher than those of the BT plan; an exception was the D50%. Similarly, the TCP of the target was also significantly in favor of the CK hybrid plans (p < 0.01). For the OARs, the CK-CTV plan was superior to the BT plan as regards the rectum D2cc, bladder D2cc and bladder Dmax. The CK-PTV plan could achieve dosimetric parameters comparable to those of the BT plan for OARs concerning the small residual tumor volume. The NTCP of the rectum for the WPI+CK-CTV plans was significantly lower than that of the WPI+BT plans (p < 0.01). (4) Conclusions: CK-based SBRT can achieve better target coverage, dose sparing for the OARs and radiobiological effects compared with the BT plan for tumors that are not excessively large. CK-based SBRT could be an alternative option to administer a radiation boost for patients with LACC.
1. Introduction
Cervical cancer is the fourth frequent malignancy in women globally and remains a leading cause of cancer death in developing countries. Approximately 604,000 new cases of cervical carcinoma were diagnosed worldwide and 342,000 people died of the disease in 2020 [1]. External beam radiotherapy (EBRT) with concomitant chemotherapy followed by brachytherapy (BT) is the recommended treatment of choice for locally advanced cervical cancer (LACC) [2]. With the demonstrated improvement in clinical outcomes, BT is a crucial component in the management of LACC [3]. However, the use of BT has progressively declined since the early 2000s due to the rapid development of high-precision EBRT [4,5,6]. In certain circumstances, the practicability of BT is compromised because of unfavorable anatomy, medical comorbidities and patient refusal to receive the procedure. Furthermore, BT is operator-dependent, and radiation centers with a low treatment volume tend to pursue alternative EBRT boost modalities [5,6]. Previously published studies concluded that the clinical outcomes were adequately admissible to consider an EBRT boost if patients could not undergo brachytherapy [7,8].
With the advent of radiation delivery and image-guiding techniques, stereotactic body radiotherapy (SBRT) can now deliver a substantially higher dose to defined target volumes and effectively protect organs at risk (OARs). In addition, compared with other EBRT boost techniques, a higher biologically equivalent dose can be achieved by SBRT [9]. Earlier studies indicated that SBRT could be similar to BT in clinical outcomes and minimal toxicities for patients with cervical cancer [10,11,12,13]. In a propensity-matched analysis based on the National Cancer Database, those who underwent the SBRT boost had equal overall survival rates when compared with patients who received the BT one [14], which indicates that SBRT is a promising alternative for patients who are not candidates for BT.
The CyberKnife (CK), a robotic-based SBRT delivery system, enables the target volumes to be irradiated preeminently and produces rapid fall-off dose gradients, with submillimeter accuracy. The prescribed doses for linac-based SBRT in LACC treatment mostly remained below the recommended biologically equivalent doses of 2 Gy per fraction (EQD2) of at least 85 Gy [7], whereas the CK-based therapy could closely mimic the dose distribution of BT [11,15]. Moreover, radiobiological metrics such as tumor control probability (TCP) and normal tissue complication probability (NTCP) can be assessed and provide a more robust comparison of the efficacy of different radiotherapy modalities. However, there is very little information in the current literature regarding plan quality and the radiobiological effects of a CK-based SBRT boost in LACC patients compared with BT.
Given the challenges of administering BT and the benefits of CK-based SBRT, this study aimed to further investigate the dosimetric and radiobiological feasibility of SBRT with CK as an alternative to BT for patients with LACC.
2. Materials and Methods
2.1. Patient Characteristics
Twenty patients with pathologically confirmed LACC and FIGO stage IIA to IVA diseases examined from May to September 2021 were retrospectively reviewed in this study. All protocols were reviewed and approved by the Research Ethics Committee of Fujian Medical University Union Hospital (No. 2022ky175). The median age of the patients was 54.5 years (range 39 to 72 years). The median values of high-risk clinical target volume (HR-CTV) was 78.8 cm3 (range 37.78–128.44) in the BT plans. The median values of high-risk planning target volume (HR-PTV) was 137.58 cm3 (range 80.05–206.54) in the CK plans. The patient characteristics are summarized in Table 1. The prescribed dose of whole pelvic irradiation (WPI) was 48.6 Gy in 27 fractions, using the volumetric-modulated arc therapy technique. A BT boost to the HR-CTV was given with the prescribed dose of 28 Gy in 4 fractions. All cases did not receive EBRT on the day of BT irradiation. The Nucletro microSelectron-HDR remote afterloading unit (Elekta Inc., Stockholm, Sweden) was used for BT delivery.

Table 1.
Patients characteristics.
2.2. CT Simulation
The patients were asked to prepare for the simulation, which included having a full bladder and an empty rectum. All patients underwent local anesthesia before the implantation of the applicator. Nucletron standard tandem/ovoid (T/O) applicators and interstitial needles were used to deliver the IC/ISBT treatment. The bladder and rectum were kept away from the applicator by a radio-opaque gauze. After the insertion, patients with the tandem and ovoid applicators in place were immobilized in the supine position. Computed tomography (CT) images with a 2.5 mm slice thickness were acquired on a CT simulator (Brilliance Big Bore, Philips, The Netherlands). The scanning range was set from the third lumbar vertebra to 5 cm below the ischial tuberosity.
2.3. Brachytherapy Planning
The BT plans of 20 patients were confirmed and delivered. The simulation CT images were transferred to an Oncentra treatment planning system V4.3 (Elekta Inc., Stockholm, Sweden). The HR-CTV was contoured in accordance with the Gynaecological Groupe Europeen de Curitherapie and the European Society for Therapeutic Radiology and Oncology (GYN GEC-ESTRO) recommendations. The OARs, including rectum, bladder, sigmoid, were outlined according to the Radiation Therapy Oncology Group (RTOG) consensus atlas. Briefly, the bladder wall, sigmoid wall and rectal wall were delineated as the outer wall of the organs minus 3 mm for the inner wall, so that a shell structure was created. A total of 28 Gy in 7 Gy per fraction was prescribed to the HR-CTV. The dose volume constraints in this study followed the National Comprehensive Cancer Network clinical practice guidelines [16]. Although a new plan was generated for each individual fraction of BT for every patient, for comparison purpose, the total sum of the 4-fraction BT was created from each patient’s first plan.
2.4. CyberKnife Planning
For the 20 patients, CT images with structures delineation for BT were transferred to the CK MultiPlan planning system V4.6.1. (Accuray Inc., Sunnyvale, CA, USA). The prescribed dose of target volume was 28 Gy in 4 fractions. Two different SBRT treatment plans were generated by MultiPlan and were named CK-CTV plan and CK-PTV plan, respectively. In the CK-CTV plan, the HR-PTV was equal to the HR-CTV of the BT plan. Considering the setup and systematic error in EBRT and tumor motion, previous studies indicated that the margin of planning target volume (PTV) from the HR-CTV was 5 mm in LACC [9,13,17]. Hence, the HR-PTV was built from the HR-CTV of the BT plan with a margin of 5 mm in the CK-PTV plan. The DVH data from the BT plan were used as input parameters in the CK plan’s optimization process for the corresponding patient. Dose volume constraints for the different OARs were according to the report of the American Association of Physicists in Medicine (AAPM) Task Group 101 during CK plan optimization [18]. The maximum monitor unit restrictions per beam and per node were set to 800 and 1200, respectively. The dose was calculated by the Monte Carlo algorithm (high resolution: 512 × 512 × number of slices, with 1% uncertainty). The grid of calculation was 0.2 cm. The Iris variable aperture collimator was used. Inverse planning was performed to acquire the optimal dose distribution. Overall, three plans were generated for each patient, i.e., a BT plan, a CK-CTV plan and a CK-PTV plan.
2.5. Evaluation of the Treatment Plans
The target volume coverage was assessed by calculating the D50%, D95% and D98% (dose delivered to 50%, 95% and 98% of the target volume). The dose homogeneity and conformity of the target volume were assessed by the homogeneity index (HI) and the conformity index (CI). The HI is an objective tool to analyze the uniformity of dose distribution in the target volume. The HI is defined as
where D2%, D98% and D50% are the doses to the 2%, 98% and 50% of the target volume, respectively [19].
The CI helps to assess the degree of congruence between prescription isodose and planning target volume. CI is defined as
where Vt,ref is the volume of the target covered by the reference isodose, Vt is the target volume, and Vref is the volume covered by the reference isodose [20]. Regarding the OARs, the following dosimetric parameters were recorded. D2cc (dose to a volume of 2cc), Dmax (maximum point dose to the organ), V15Gy and V24.5Gy (volume of rectum receiving 15 Gy and 24.5 Gy), V17.55Gy (volume of bladder receiving 17.55 Gy).
2.6. Calculation of EUD, TCP and NTCP
The dose–volume histograms (DVHs) of the tumor CTV and OARs were derived from the WPI and three different boost plans. The equivalent uniform dose (EUD) is used as an evaluation tool under the assumption that two plans with the same value of EUD are equivalent in their therapeutic effectiveness. The dose was converted to EQD2 based on the LQ model to calculate the EUD [21,22]. Because 2 patients received WPI in other hospital, the DVHs for the WPI plans of only 18 patients were used. The EUD for three boost plans were separately summed with the EUD for the WPI plans to generate the total EUD for the hybrid plans (WPI+BT, WPI+CK-CTV and WPI+CK-PTV plans).
where is the fraction of the target volume irradiated by a dose , and is a unitless model parameter that is specific to the normal structure or the tumor of interest.
The tumor control probability (TCP) and normal tissue complication probability (NTCP)were calculated based on the total EUD [22]:
where is the dose leading to a 50% chance of controlling the tumor, is the tolerance dose for a 50% complication rate of the normal structure at a specific time interval, is a specific parameter that describes the slope of the dose–response curve. The following parameter values were used. For the tumor: , , . For the rectum: , , . For the bladder: , , .
2.7. Statistical Analysis
Statistical analyses were performed with the SPSS Statistics 25.0 (IBM Corp, Armonk, NY, USA). The dosimetric parameters of the three plans (BT, CK-CTV and CT-PTV) were compared in a two-way analysis of variance (ANOVA), followed by Bonferroni post hoc testing. The radiobiological model results were compared in a paired t-test. To investigate the relationship between CTV and tolerance doses of the OARs, a paired t-test was used to test for the differences between the BT plans and the CK-PTV plans. The graphs were implemented in Origin 2021b (Origin software Inc., Northampton, MA, USA). p < 0.05 was considered statistically significant.
3. Results
3.1. Target Volume Coverage
Target volume coverage in the CK-CTV plan and CK-PTV plan was superior to that in the BT plan. It was shown that the 120%, 100% and 80% isodose lines matched the tumor volume shape in the three plans (Figure 1). Except for D50%, the D95% and D98% of the CK-CTV plan and CK-PTV plan were higher than those of the BT plan (p < 0.001). Nevertheless, there were no statistically significant differences in all the dosimetric parameters between the CK-CTV plan and the CK-PTV plan (p > 0.05). The HI (p < 0.001) and CI (p < 0.001) were significantly better for the CK-CTV plan and the CK-PTV plan than for the BT plan, as the CK-CTV plan and CK-PTV plan showed significantly lower HI and CI than the BT plan, respectively. The results of the target volume parameters are presented in Table 2 and Figure 2.
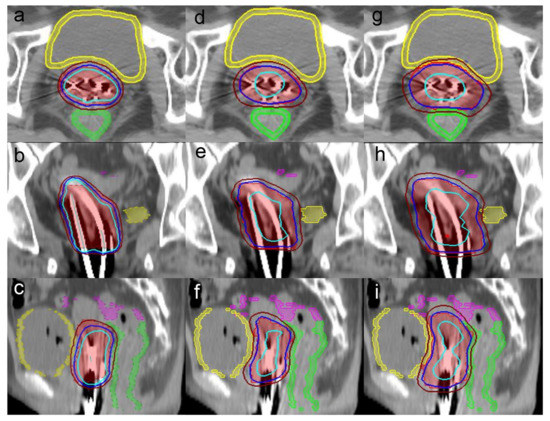
Figure 1.
Axial, coronal and sagittal views of the dose distribution among the (a–c) BT plan, (d–f) CK-CTV plan and (g–i) CK-PTV plan for a representative patient. The 120% (33.6 Gy), 100% (28 Gy) and 80% (22.4 Gy) isodose lines are in light blue, dark blue, and brown, respectively. The images also illustrate the target volume in red, the rectum in green, the bladder in yellow and the sigmoid in pink.

Table 2.
Dose parameters comparison in relation to the target volume in the BT, CK-CTV and CK-PTV plans.
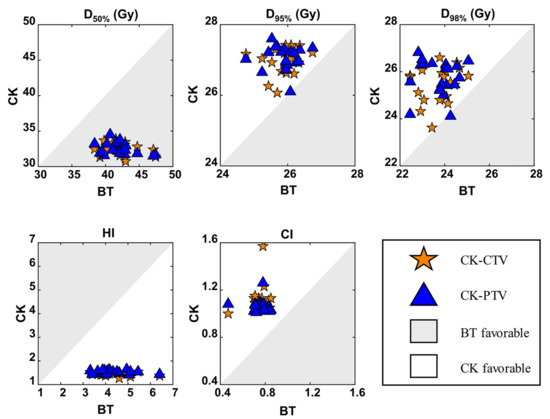
Figure 2.
Comparisons of the BT, CK-CTV and CK-PTV plans by target volume dosimetric parameters. For every patient, each parameter is associated with three values (from the BT plan, CK-CTV plan and CK-PTV plan). In each graph, for example that of CI, each patient will contribute a pair of symbols, i.e., a star and a triangle; the star is plotted by the coordinates CIBT, CICK-CTV, while the triangle is plotted by CIBT, CICK-PTV. A symbol falling in the gray area implies the parameter is favorable in the BT plan. A symbol falling in the white area implies the parameter is favorable in the CK plan.
3.2. Dose to Organs at Risk
A comparison of the dose parameters in relation to the OARs in the BT, CK-CTV and CK-PTV plans is presented in Table 3 and Figure 3.

Table 3.
Dose parameters comparison in relation to the OARs in the BT, CK-CTV and CK-PTV plans.
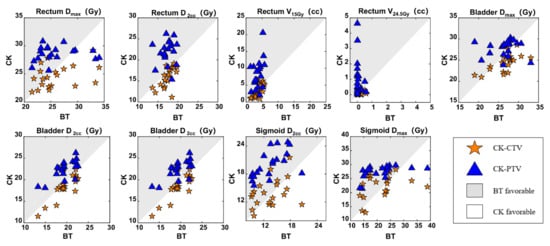
Figure 3.
Comparisons of the BT, CK-CTV, and CK-PTV plans by the dosimetric parameters in relation to the OARs. For every patient, each parameter is associated with three values (from the BT plan, CK-CTV plan and CK-PTV plan). In each graph, for the parameter X, each patient will contribute a pair of symbols, i.e., a star and a triangle; the star is plotted by the coordinates XBT, XCK-CTV, while the triangle is plotted by XBT, XCK-PTV. A symbol falling in the gray area implies the parameter is favorable in the BT plan. A symbol falling in the white area implies the parameter is favorable in the CK plan.
3.2.1. Rectum
The D2cc was better with the CK-CTV plan (p = 0.019), whereas the Dmax, V15Gy and V24.5Gy showed no statistically significant differences between the CK-CTV plan and the BT plan (p > 0.05). All rectum dosimetric parameters were inferior in the CK-PTV plan compared with the CK-CTV plan and BT plan (p < 0.05).
3.2.2. Bladder
For the bladder, the D2cc (p = 0.007) and Dmax (p = 0.003) were significantly lower in the CK-CTV plan than in the BT plan; an exception was V17.55Gy. In addition, there were also statistically significant differences between the CK-CTV plan and the CK-PTV plan (p < 0.001), as the CK-CTV plan was superior to the CK-PTV plan in all dosimetric parameters. Except for Dmax (p = 0.159), the D2cc and V17.55Gy were significantly lower in the BT plan than in the CK-PTV plan (p < 0.001).
3.2.3. Sigmoid
The D2cc and Dmax showed no statistically significant differences between the CK-CTV plan and the BT plan (p > 0.05). Compared to the CK-PTV plan, the D2cc (p < 0.001) and Dmax (p = 0.002) were statistically significantly in favor of the CK-CTV plan. On the other hand, the D2cc and Dmax were lower with the BT plan compared to the CK-PTV plan (p < 0.001).
3.3. TCP and NTCP Analysis
The EUD and TCP of the target volume for the WPI+CK-CTV and WPI+CK-PTV plans were higher than those of the WPI+ BT plans (p < 0.001). The WPI+CK-CTV plans exhibited lower EUD and NTCP for the rectum, compared with the WPI+ BT plans (p < 0.001). For the bladder, the EUD and NTCP were comparable between the WPI+CK-CTV and WPI+BT plans. However, the EUD and NTCP of the OARs for the WPI+CK-PTV plan were higher than for the WPI+BT plans (p < 0.05). Table 4 shows the results of the comparison of the radiobiological metrics.

Table 4.
Radiobiological comparison of CTV and OARs in the WPI+BT, WPI+CK-CTV and WPI+CK-PTV plans.
3.4. Relationship between CTV and Tolerance Doses for the OARs
We investigated the correlation between the size of the CTV and the quality of the CK-PTV. A threshold value of CTV was determined by using the paired t-test to screen the volumes of CTV from the largest to the smallest until no statistically significant difference in the OAR dosimetric indicators was shown between the BT and CK-PTV plans. For a CTV smaller than this threshold value, one could expect the CK-PTV plan to be comparable or better than the BT plan.
3.4.1. Rectum
When the CTV was less than 65.55 cm3, no significant differences between rectum D2cc and rectum V24.5Gy were found between the BT plan and the CK-PTV plan, (p = 0.068 and p = 0.099). The maximum dose to the rectum was similar in the two plans when the CTV was less than 93.99 cm3 (p = 0.062). In addition, the rectum V15Gy was comparable between the BT plan and the CK-PTV plan when the CTV was less than 75.52 cm3 (p = 0.081).
3.4.2. Bladder
When the CTV was less than 56.50 cm3, there was no significant difference in the bladder D2cc between the BT plan and the CK-PTV plan (p = 0.107). Moreover, the bladder Dmax was similar for the BT plan and the CK-PTV plan when the CTV was less than 128.44 cm3 (p = 0.149). The bladder V17.55Gy was comparable between the BT plan and the CK-PTV plan when the CTV was less than 57.99 cm3 (p = 0.051).
3.4.3. Sigmoid
The sigmoid D2cc was comparable between the BT plan and the CK-PTV plan when the CTV was less than 56.50 cm3 (p = 0.088). When the CTV was less than 75.52 cm3, there was no difference in the sigmoid Dmax between the BT plan and the CK-PTV plan (p = 0.083).
When the CTV was less than 56.50 cm3, all of the OAR dosimetric parameters showed no statistically significant differences between the BT plan and the CK-PTV plan (p = 0.531 for rectum D2cc, p = 0.708 for rectum Dmax, p = 0.631 for rectum V15Gy, p = 0.635 for rectum V24.5Gy, p = 0.107 for bladder D2cc, p = 0.540 for bladder Dmax, p = 0.143 for bladder V17.55Gy, p = 0.088 for sigmoid D2cc, and p = 0.468 for sigmoid Dmax).
In summary, for patients with a CTV less than 56.50 cm3, the toxicity for all OAR was comparable between the BT plan and the CK-PTV plan, indicating that the CK SBRT is a viable alternative. The results are detailed in Table 5.

Table 5.
Relationship between CTV and tolerance doses for OARs in the BT and CK-PTV plans.
4. Discussion
Cervical cancer is one of the most prevalent cancers among females worldwide [23]. A combination of chemoradiotherapy and a BT boost is the standard of care for patients with LACC [24]. Although BT is considered a minimally invasive procedure, patients may suffer from vaginal pain, uterine perforation and anesthesia-associated risks [25]. Conversely, if patients are treated with SBRT, these risks could in principle be eliminated, with the added benefits of comfort and a shorter treatment time. Meanwhile, the SBRT allows a higher degree of dose control and a precise dose delivery to the cervical tumor volume, sparing the normal organs, compared to conventional radiation delivery methods. Therefore, many studies attempted to use SBRT to achieve a boost dose distribution similar to that of BT plans. Previously published results showed that SBRT could provide a potential alternative to boost cervical carcinomas when BT is not performed [15,26,27]. Moreover, Guerrero et al. suggested that SIB-IMRT was radiobiologically feasible for LACC patients who cannot undergo BT [28]. Unlike their study, our study not only presents a dosimetric comparison but also shows the achievement of the expected clinical outcome based on TCP and NTCP.
Earlier research demonstrated that a poor target coverage during radiation therapy is closely related to an increased risk of local and probably distant recurrence [29,30]. Our study indicates a consistent superiority of CK-based SBRT as a boost compared with BT in regard to target volume coverage and OAR sparing, and these results are similar to those previously published [11,26,31]. D90% is considered the essential parameter for HR-CTV [32], but D98% seems to be a better predictor of local control rates [33]. In our study, the CK-CTV plan showed statistically significant differences in the D98% and D95% when compared to the BT plan. D98% and D95% in the CK-CTV plan were higher than in the BT plan, while D50% was on average 22.8% less than in the BT plan. That is to say that the results regarding the higher isodose volume outperformed those for the lower isodose volume. These findings are in line with the results of Malhotra et al. [34]. A study by Morgenthaler et al. [15] reported that the D90% and V100 in the target volume were almost optimal when the boost was delivered by robotic radiosurgery. We also found that the EUD and TCP of the target volume for the WPI+CK-CTV plans were higher than those of the WPI+BT plans (p < 0.001). Additionally, the target volume coverage in the CK-PTV plan was superior compares to that of the BT plan in our study. With regard to plan quality, CK improved the dose homogeneity in the target volume. Our study showed that the HI and CI were also better with the CK-CTV plan and CK-PTV plan compared to the BT plan. The reason might be the geometrical variation and uncertainty of implantation in BT and, especially, the nature of the radiation from the BT source, resulting in a hot spot, a heterogeneous distribution and a suboptimal conformation of the prescribed dose.
The position of the cervix changes appreciably during the course of radiotherapy for cervical cancer. Previous studies examined the mean maximal inter-fractional movement of the cervix in the superior–inferior, anterior–posterior and right–left lateral dimensions and reported values of 2.1, 1.6 and 0.82 cm, respectively [35]. Moreover, cervix displacements greater than 1.5 cm occurred in intra-fractional radiation treatments [36]. Given organ motion and the uncertainties of the setup, HR-PTV was obtained from HR-CTV by a 5 mm margin in the CK-PTV plan in our study. It is notable that the CK-CTV plan was superior to the BT plan as regards rectum D2cc (p = 0.019), bladder D2cc (p = 0.007) and bladder Dmax (p = 0.003) in our study. In addition, the EUD and NTCP values for the rectum were significantly lower in the WPI+CK-CTV plans (p < 0.001). For the bladder, the EUD and NTCP were comparable between the WPI+CK-CTV and WPI+BT plans. Cengiz et al. [11] compared the dose distribution characteristics in SBRT plans generated by a CK and in HDR BT plans for 11 patients with cervical cancer. No margin was added around the CTV to construct the PTV in the SBRT plans. Their study revealed distinct advantages in terms of target coverage and dose distributions to the surrounding normal tissues in SBRT plans, except for the bone marrow. The results are similar to ours with the CK-CTV plans. When the range of tumor motion is large, the internal target volume may be large, and this could cause increased the treatment toxicity [37]. However, the CK can achieve a submillimeter dose delivery with high precision, as tumor motion is tracked in real time using image guidance with different tracking system [38,39]. Hadi et al. assessed the feasibility of an MR-guided SBRT boost modality in patients who were ineligible for BT. Online-adaptive treatment planning was conducted to adjust the tumor volumes derived from daily anatomy [17]. If cervix motion is monitored or modified, it means that the margin of HR-PTV can be reduced. Hence, in order to minimize the margin of HR-PTV, it is suggested that adaptive radiotherapy planning be generated for each SBRT fraction, and real-time tracking and correction of cervix and tumor displacement be implemented. Such approach may result in a decrease in the volume of normal tissues exposed to the low-dose region, thereby reducing the late toxicity. Accordingly, dose distributions to the adjacent organs in a CK plan may be better than in a BT plan.
As has been previously revealed, the cervical tumor size is a critical independent prognostic factor for local control and pelvic recurrences after irradiation [40,41]. Promising clinical outcomes were reported by Mantz et al. [42], using a stereotactic ablative radiotherapy (SABR) boost in gross tumor volume (GTV) in 55 patients with FIGO stage IB–IIB cervical cancer. No grade 3 or greater late toxicities were observed, but this GTV-only modality needs to be further investigated. Albuquerque et al. [43] carried out a phase II trial of SABR as a boost for LACC, but the trial was closed due to severe toxicity concerns. The high rate of toxicity may be associated with the fact that large tumors were treated in this study. What accounts for late high-grade toxicity is the increased radiation exposure to OARs in the presence of bulky tumors. If the OARs are close to the PTV, the doses to the OARs may increase with a rise in the PTV [44]. Gultekin et al. retrospectively evaluated the oncological outcomes of an SBRT boost in 21 patients with cervical cancer. They found that while the 2-year LC rate was 75% in patients with residual tumor size < 4 cm, it was 50% when there was ≥4 cm residual tumor after definitive chemoradiotherapy (p < 0.001) [45]. It is suggested that a certain threshold of tumor size in patients with cervical cancer should be considered for eligibility to receive an SBRT boost. According to the size of the patient’s tumor, we arranged all dosimetric parameters in regard to the OARs and investigated the relationship between the tumor volume and OARs tolerance in the BT and CK-PTV plans. For the cohort of our study, when the tumor volume was less than 56.50 cm3, there was no statistical difference between the BT and the CK-PTV plans in all dosimetric parameters in relation to the OARs. This indicated that for patients with a target volume less than 56.50 cm3, lower OARs toxicity might be achieved with a CK boost. Systemic clinical trials are necessary for to provide a rigorous proof.
The main limitation of this study is that CT images with applicators were used to generate the CK plans, which is not in line with the actual treatment situation by CK. The applicators are not expected to be used in clinical treatments using the CK. The shape and location of the tumor will be affected during the implantation of the applicators. In a proposed future prospective study, a CT simulation without applicators will be performed after patients have received the last EBRT fraction. However, by the principle of physics, it is anticipated that the results of this study should hold true when no implanted applicators are in use.
5. Conclusions
Consistent with previous studies, CK-CTV plans can produce significantly better target coverage, OAR sparing, and radiobiological effects compared to BT plans. When the target volume is less than 56.50 cm3, CK-PTV plans with a 5 mm PTV margin can achieve a dose distribution comparable to that of BT plans. CK-based SBRT could be an effective alternative to BT for patients with LACC. With improved precision of target localization, a reduced PTV margin might increase the eligibility of patients with large tumors. Further clinical investigation to provide a higher level of evidence of the efficacy of a CK-based SBRT boost is needed.
Author Contributions
Conceptualization, X.W. and Y.C.; methodology, J.G. and Y.C.; software, M.H. and X.L.; formal analysis, J.G.; investigation, J.G. and Z.X.; resources, B.X. and Y.L.; data curation, J.G. and Y.L.; writing—original draft preparation, J.G. and Y.C.; writing—review and editing, X.W. and Y.C.; visualization, J.G. and Z.X.; supervision, B.X.; project administration, X.W. and Y.C.; funding acquisition, Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Joint Funds for the Innovation of Science and Technology, Fujian Province (2018Y9001) and Natural Science Foundation of Fujian Province (2021J01740).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Research Ethics Committee of Fujian Medical University Union Hospital.
Informed Consent Statement
Patient consent was waived by the Research Ethics Committee of Fujian Medical University Union Hospital due to the retrospective nature of the study.
Data Availability Statement
The data and clinical information will be shared upon reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Pötter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Meder, C.H.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2018, 127, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, A.N.; Creutzberg, C.L.; Craighead, P.; McCormack, M.; Toita, T.; Narayan, K.; Reed, N.; Long, H.; Kim, H.-J.; Marth, C.; et al. International brachytherapy practice patterns: A survey of the Gynecologic Cancer Intergroup (GCIG). Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 250–255. [Google Scholar] [CrossRef]
- Tagliaferri, L.; Vavassori, A.; Lancellotta, V.; De Sanctis, V.; Barbera, F.; Fusco, V.; Vidali, C.; Fionda, B.; Colloca, G.; Gambacorta, M.A.; et al. Can brachytherapy be properly considered in the clinical practice? Trilogy project: The vision of the AIRO (Italian Association of Radiotherapy and Clinical Oncology) Interventional Radiotherapy study group. J. Contemp. Brachytherapy 2020, 12, 84–89. [Google Scholar] [CrossRef]
- Han, K.; Milosevic, M.; Fyles, A.; Pintilie, M.; Viswanathan, A. Trends in the utilization of brachytherapy in cervical cancer in the United States. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Gill, B.S.; Lin, J.F.; Krivak, T.C.; Sukumvanich, P.; Laskey, R.A.; Ross, M.S.; Lesnock, J.L.; Beriwal, S. National Cancer Data Base analysis of radiation therapy consolidation modality for cervical cancer: The impact of new technological advancements. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Barraclough, L.H.; Swindell, R.; Livsey, J.E.; Hunter, R.D.; Davidson, S.E. External beam boost for cancer of the cervix uteri when intracavitary therapy cannot be performed. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, Y.S.; Joo, J.H.; Eom, K.Y.; Park, W.; Kim, J.H.; Lee, J.H.; Kim, Y.S.; Lee, S.H.; Ahn, K.; et al. Tumor Boost Using External Beam Radiation in Cervical Cancer Patients Unable to Receive Intracavitary Brachytherapy: Outcome From a Multicenter Retrospective Study (Korean Radiation Oncology Group 1419). Int. J. Gynecol. Cancer 2018, 28, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Song, C.; Kim, I.A.; Kim, J.S.; Kim, Y.B.; Kim, K.; No, J.H.; Suh, D.H.; Chung, J.B.; Eom, K.Y. Stereotactic ablative body radiotherapy boost for cervical cancer when brachytherapy boost is not feasible. Radiat. Oncol. 2021, 16, 148. [Google Scholar] [CrossRef]
- Mollà, M.; Escude, L.; Nouet, P.; Popowski, Y.; Hidalgo, A.; Rouzaud, M.; Linero, D.; Miralbell, R. Fractionated stereotactic radiotherapy boost for gynecologic tumors: An alternative to brachytherapy? Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, M.; Dogan, A.; Ozyigit, G.; Erturk, E.; Yildiz, F.; Selek, U.; Ulger, S.; Colak, F.; Zorlu, F. Comparison of intracavitary brachytherapy and stereotactic body radiotherapy dose distribution for cervical cancer. Brachytherapy 2012, 11, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Kubicek, G.J.; Xue, J.; Xu, Q.; Asbell, S.O.; Hughes, L.; Kramer, N.; Youssef, A.; Chen, Y.; Aikens, J.; Saul, H.; et al. Stereotactic body radiotherapy as an alternative to brachytherapy in gynecologic cancer. BioMed Res. Int. 2013, 2013, 898953. [Google Scholar] [CrossRef]
- Haas, J.A.; Witten, M.R.; Clancey, O.; Episcopia, K.; Accordino, D.; Chalas, E. CyberKnife Boost for Patients with Cervical Cancer Unable to Undergo Brachytherapy. Front. Oncol. 2012, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, B.; Shiao, J.C.; Pezzi, T.A.; Waheed, N.; Sharma, S.; Bonnen, M.D.; Ludwig, M.S. Stereotactic Body Radiation Therapy, Intensity-Modulated Radiation Therapy, and Brachytherapy Boost Modalities in Invasive Cervical Cancer: A Study of the National Cancer Data Base. Int. J. Gynecol. Cancer 2018, 28, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Morgenthaler, J.; Köhler, C.; Budach, V.; Sehouli, J.; Stromberger, C.; Besserer, A.; Trommer, M.; Baues, C.; Marnitz, S. Long-term results of robotic radiosurgery for non brachytherapy patients with cervical cancer. Strahlenther. Onkol. 2021, 197, 474–486. [Google Scholar] [CrossRef]
- Koh, W.J.; Abu-Rustum, N.R.; Bean, S.; Bradley, K.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Chu, C.; Clark, R.; Cohn, D.; et al. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 64–84. [Google Scholar] [CrossRef]
- Hadi, I.; Eze, C.; Schönecker, S.; von Bestenbostel, R.; Rogowski, P.; Nierer, L.; Bodensohn, R.; Reiner, M.; Landry, G.; Belka, C.; et al. MR-guided SBRT boost for patients with locally advanced or recurrent gynecological cancers ineligible for brachytherapy: Feasibility and early clinical experience. Radiat. Oncol. 2022, 17, 8. [Google Scholar] [CrossRef]
- Benedict, S.H.; Yenice, K.M.; Followill, D.; Galvin, J.M.; Hinson, W.; Kavanagh, B.; Keall, P.; Lovelock, M.; Meeks, S.; Papiez, L.; et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med. Phys. 2010, 37, 4078–4101. [Google Scholar] [CrossRef]
- Grégoire, V.; Mackie, T.R. State of the art on dose prescription, reporting and recording in Intensity-Modulated Radiation Therapy (ICRU report No. 83). Cancer Radiother. 2011, 15, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Baltas, D.; Kolotas, C.; Geramani, K.; Mould, R.F.; Ioannidis, G.; Kekchidi, M.; Zamboglou, N. A conformal index (COIN) to evaluate implant quality and dose specification in brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 515–524. [Google Scholar] [CrossRef]
- Niemierko, A. Reporting and analyzing dose distributions: A concept of equivalent uniform dose. Med. Phys. 1997, 24, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Gay, H.A.; Niemierko, A. A free program for calculating EUD-based NTCP and TCP in external beam radiotherapy. Phys. Med. 2007, 23, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Mahantshetty, U.; Engineer, R.; Chopra, S.; Hawaldar, R.; Hande, V.; Kerkar, R.A.; Maheshwari, A.; Shylasree, T.S.; Ghosh, J.; et al. Cisplatin Chemoradiotherapy vs Radiotherapy in FIGO Stage IIIB Squamous Cell Carcinoma of the Uterine Cervix: A Randomized Clinical Trial. JAMA Oncol. 2018, 4, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Pötter, R.; Georg, P.; Dimopoulos, J.C.A.; Grimm, M.; Berger, D.; Nesvacil, N.; Georg, D.; Schmid, M.P.; Reinthaller, A.; Sturdza, A.; et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother. Oncol. 2011, 100, 116–123. [Google Scholar] [CrossRef]
- Mahmoud, O.; Kilic, S.; Khan, A.J.; Beriwal, S.; Small, W. External beam techniques to boost cervical cancer when brachytherapy is not an option-theories and applications. Ann. Transl. Med. 2017, 5, 207. [Google Scholar] [CrossRef]
- Neumann, O.; Kluge, A.; Lyubina, O.; Wlodarczyk, W.; Jahn, U.; Köhler, C.; Budach, V.; Kufeld, M.; Marnitz, S. Robotic radiosurgery as an alternative to brachytherapy for cervical cancer patients. Strahlenther. Onkol. 2014, 190, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Merrow, C.; deBoer, S.; Podgorsak, M.B. VMAT for the treatment of gynecologic malignancies for patients unable to receive HDR brachytherapy. J. Appl. Clin. Med. Phys. 2014, 15, 66–73. [Google Scholar] [CrossRef]
- Guerrero, M.; Li, X.A.; Ma, L.; Linder, J.; Deyoung, C.; Erickson, B. Simultaneous integrated intensity-modulated radiotherapy boost for locally advanced gynecological cancer: Radiobiological and dosimetric considerations. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Fuks, Z.; Leibel, S.A.; Wallner, K.E.; Begg, C.B.; Fair, W.R.; Anderson, L.L.; Hilaris, B.S.; Whitmore, W.F. The effect of local control on metastatic dissemination in carcinoma of the prostate: Long-term results in patients treated with 125I implantation. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 537–547. [Google Scholar] [CrossRef]
- Yorke, E.D.; Fuks, Z.; Norton, L.; Whitmore, W.; Ling, C.C. Modeling the development of metastases from primary and locally recurrent tumors: Comparison with a clinical data base for prostatic cancer. Cancer Res. 1993, 53, 2987–2993. [Google Scholar] [PubMed]
- Marnitz, S.; Köhler, C.; Budach, V.; Neumann, O.; Kluge, A.; Wlodarczyk, W.; Jahn, U.; Gebauer, B.; Kufeld, M. Brachytherapy-emulating robotic radiosurgery in patients with cervical carcinoma. Radiat. Oncol. 2013, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Pötter, R.; Haie-Meder, C.; Van Limbergen, E.; Barillot, I.; De Brabandere, M.; Dimopoulos, J.; Dumas, I.; Erickson, B.; Lang, S.; Nulens, A.; et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother. Oncol. 2006, 78, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, J.C.; Lang, S.; Kirisits, C.; Fidarova, E.F.; Berger, D.; Georg, P.; Dörr, W.; Pötter, R. Dose-volume histogram parameters and local tumor control in magnetic resonance image-guided cervical cancer brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, H.K.; Avadhani, J.S.; de Boer, S.F.; Jaggernauth, W.; Kuettel, M.R.; Podgorsak, M.B. Duplicating a tandem and ovoids distribution with intensity-modulated radiotherapy: A feasibility study. J. Appl. Clin. Med. Phys. 2007, 8, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Beadle, B.; Jhingran, A.; Salehpour, M.; Sam, M.; Iyer, R.; Eifel, P. Cervix regression and motion during the course of external beam chemoradiation for cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 235–241. [Google Scholar] [CrossRef]
- Haripotepornkul, N.H.; Nath, S.K.; Scanderbeg, D.; Saenz, C.; Yashar, C.M. Evaluation of intra- and inter-fraction movement of the cervix during intensity modulated radiation therapy. Radiother. Oncol. 2011, 98, 347–351. [Google Scholar] [CrossRef]
- Lin, H.; Zou, W.; Li, T.; Feigenberg, S.J.; Teo, B.K.; Dong, L. A Super-Learner Model for Tumor Motion Prediction and Management in Radiation Therapy: Development and Feasibility Evaluation. Sci. Rep. 2019, 9, 14868. [Google Scholar] [CrossRef]
- Biasi, G.; Petasecca, M.; Guatelli, S.; Martin, E.A.; Grogan, G.; Hug, B.; Lane, J.; Perevertaylo, V.; Kron, T.; Rosenfeld, A.B. CyberKnife® fixed cone and Iris™ defined small radiation fields: Assessment with a high-resolution solid-state detector array. J. Appl. Clin. Med. Phys. 2018, 19, 547–557. [Google Scholar] [CrossRef]
- Chang, S.D.; Main, W.; Martin, D.P.; Gibbs, I.C.; Heilbrun, M.P. An analysis of the accuracy of the CyberKnife: A robotic frameless stereotactic radiosurgical system. Neurosurgery 2003, 52, 140–146; discussion 146–147. [Google Scholar] [CrossRef]
- Eifel, P.J.; Morris, M.; Wharton, J.T.; Oswald, M.J. The influence of tumor size and morphology on the outcome of patients with FIGO stage IB squamous cell carcinoma of the uterine cervix. Int. J. Radiat. Oncol. Biol. Phys. 1994, 29, 9–16. [Google Scholar] [CrossRef]
- Perez, C.A.; Grigsby, P.W.; Nene, S.M.; Camel, H.M.; Galakatos, A.; Kao, M.S.; Lockett, M.A. Effect of tumor size on the prognosis of carcinoma of the uterine cervix treated with irradiation alone. Cancer 1992, 69, 2796–2806. [Google Scholar] [CrossRef]
- Mantz, C.A. Definitive External Beam Radiation Therapy with Stereotactic Body Radiation Therapy Boost for FIGO Stage IB-IIB Cancer of the Cervix: Minimum 5-Year Disease Control, Toxicity, and Quality of Life Outcomes From a Phase 2 Trial. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, S225. [Google Scholar] [CrossRef]
- Albuquerque, K.; Tumati, V.; Lea, J.; Ahn, C.; Richardson, D.; Miller, D.; Timmerman, R. A Phase II Trial of Stereotactic Ablative Radiation Therapy as a Boost for Locally Advanced Cervical Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Uto, M.; Mizowaki, T.; Ogura, K.; Hiraoka, M. Non-coplanar volumetric-modulated arc therapy (VMAT) for craniopharyngiomas reduces radiation doses to the bilateral hippocampus: A planning study comparing dynamic conformal arc therapy, coplanar VMAT, and non-coplanar VMAT. Radiat. Oncol. 2016, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Gultekin, M.; Yilmaz, M.T.; Sari, S.Y.; Yildiz, D.; Ozyigit, G.; Yildiz, F. Stereotactic body radiotherapy boost in patients with cervical cancer. J. Obstet. Gynaecol. 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).