Emerging Role of ERBB2 in Targeted Therapy for Metastatic Colorectal Cancer: Signaling Pathways to Therapeutic Strategies
Abstract
Simple Summary
Abstract
1. Introduction
2. Molecular Biology of the ERBB2 Signaling Pathway
2.1. Structural Features of the ERBB2 Receptor
2.2. ERBB2 Downstream Signaling Pathways
2.3. ERBB2 Gene Alterations in mCRC
3. Detection of ERBB-Positivity in mCRC
4. ERBB2-Targeted Treatments in mCRC
4.1. Impact of ERBB2 Activation on Anti-EGFR Treatment Resistance
4.2. Role of ERBB2 Positivity in mCRC with Immunotherapy Tolerance
4.3. Novel Drugs and Clinical Trials for ERBB2-Positive mCRC
5. Perspectives
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Margonis, G.A.; Buettner, S.; Andreatos, N.; Kim, Y.; Wagner, D.; Sasaki, K.; Beer, A.; Schwarz, C.; Loes, I.M.; Smolle, M.; et al. Association of BRAF Mutations With Survival and Recurrence in Surgically Treated Patients With Metastatic Colorectal Liver Cancer. JAMA Surg. 2018, 153, e180996. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.; Lau, D.K.; Chau, I. HER2 targeted therapy in colorectal cancer: New horizons. Cancer Treat. Rev. 2022, 105, 102363. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef]
- Li, H.; Courtois, E.T.; Sengupta, D.; Tan, Y.; Chen, K.H.; Goh, J.J.L.; Kong, S.L.; Chua, C.; Hon, L.K.; Tan, W.S.; et al. Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat. Genet. 2017, 49, 708–718. [Google Scholar] [CrossRef]
- Lièvre, A.; Bachet, J.B.; Le Corre, D.; Boige, V.; Landi, B.; Emile, J.F.; Côté, J.F.; Tomasic, G.; Penna, C.; Ducreux, M.; et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006, 66, 3992–3995. [Google Scholar] [CrossRef]
- De Roock, W.; Claes, B.; Bernasconi, D.; De Schutter, J.; Biesmans, B.; Fountzilas, G.; Kalogeras, K.T.; Kotoula, V.; Papamichael, D.; Laurent-Puig, P.; et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: A retrospective consortium analysis. Lancet Oncol. 2010, 11, 753–762. [Google Scholar] [CrossRef]
- Bregni, G.; Sciallero, S.; Sobrero, A. HER2 Amplification and Anti-EGFR Sensitivity in Advanced Colorectal Cancer. JAMA Oncol. 2019, 5, 605–606. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Yuan, B.; Li, C.; Wang, Z.; Song, Y.; Liu, H. A narrative review of advances in treatment and survival prognosis of HER2-positive malignant lung cancers. J. Thorac. Dis. 2021, 13, 3708–3720. [Google Scholar] [CrossRef]
- Oh, D.Y.; Bang, Y.J. HER2-targeted therapies—A role beyond breast cancer. Nat. Rev. Clin. Oncol. 2020, 17, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Sartore-Bianchi, A.; Trusolino, L.; Martino, C.; Bencardino, K.; Lonardi, S.; Bergamo, F.; Zagonel, V.; Leone, F.; Depetris, I.; Martinelli, E.; et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): A proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 738–746. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Hurwitz, H.; Raghav, K.P.S.; McWilliams, R.R.; Fakih, M.; VanderWalde, A.; Swanton, C.; Kurzrock, R.; Burris, H.; Sweeney, C.; et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): An updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019, 20, 518–530. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Lonardi, S.; Martino, C.; Fenocchio, E.; Tosi, F.; Ghezzi, S.; Leone, F.; Bergamo, F.; Zagonel, V.; Ciardiello, F.; et al. Pertuzumab and trastuzumab emtansine in patients with HER2-amplified metastatic colorectal cancer: The phase II HERACLES-B trial. ESMO Open 2020, 5, e000911. [Google Scholar] [CrossRef] [PubMed]
- Siena, S.; Di Bartolomeo, M.; Raghav, K.; Masuishi, T.; Loupakis, F.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Fakih, M.; Elez, E.; et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2021, 22, 779–789. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Marsoni, S.; Siena, S. Human Epidermal Growth Factor Receptor 2 as a Molecular Biomarker for Metastatic Colorectal Cancer. JAMA Oncol. 2018, 4, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Swain, S.M. Novel anticancer targets: Revisiting ERBB2 and discovering ERBB3. Nat. Rev. Cancer 2009, 9, 463–475. [Google Scholar] [CrossRef]
- Hynes, N.E.; Lane, H.A. ERBB receptors and cancer: The complexity of targeted inhibitors. Nat. Rev. Cancer 2005, 5, 341–354. [Google Scholar] [CrossRef]
- Kovacs, E.; Zorn, J.A.; Huang, Y.; Barros, T.; Kuriyan, J. A structural perspective on the regulation of the epidermal growth factor receptor. Annu. Rev. Rev. Rev. Biochem. 2015, 84, 739–764. [Google Scholar] [CrossRef]
- Pawson, T.; Gish, G.D.; Nash, P. SH2 domains, interaction modules and cellular wiring. Trends Cell Biol. 2001, 11, 504–511. [Google Scholar] [CrossRef]
- Olayioye, M.A.; Graus-Porta, D.; Beerli, R.R.; Rohrer, J.; Gay, B.; Hynes, N.E. ErbB-1 and ErbB-2 acquire distinct signaling properties dependent upon their dimerization partner. Mol. Cell. Biol. 1998, 18, 5042–5051. [Google Scholar] [CrossRef]
- Cohen, S. The epidermal growth factor (EGF). Cancer 1983, 51, 1787–1791. [Google Scholar] [CrossRef]
- Alonso, A.; Sasin, J.; Bottini, N.; Friedberg, I.; Friedberg, I.; Osterman, A.; Godzik, A.; Hunter, T.; Dixon, J.; Mustelin, T. Protein tyrosine phosphatases in the human genome. Cell 2004, 117, 699–711. [Google Scholar] [CrossRef]
- Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2000, 103, 211–225. [Google Scholar] [CrossRef]
- Garrett, T.P.; McKern, N.M.; Lou, M.; Elleman, T.C.; Adams, T.E.; Lovrecz, G.O.; Kofler, M.; Jorissen, R.N.; Nice, E.C.; Burgess, A.W.; et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol. Cell 2003, 11, 495–505. [Google Scholar] [CrossRef]
- Cho, H.S.; Mason, K.; Ramyar, K.X.; Stanley, A.M.; Gabelli, S.B.; Denney, D.W., Jr.; Leahy, D.J. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 2003, 421, 756–760. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol. Res. 2014, 79, 34–74. [Google Scholar] [CrossRef]
- Tzahar, E.; Waterman, H.; Chen, X.; Levkowitz, G.; Karunagaran, D.; Lavi, S.; Ratzkin, B.J.; Yarden, Y. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol. Cell. Biol. 1996, 16, 5276–5287. [Google Scholar] [CrossRef]
- Graus-Porta, D.; Beerli, R.R.; Daly, J.M.; Hynes, N.E. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997, 16, 1647–1655. [Google Scholar] [CrossRef]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef]
- Pinkas-Kramarski, R.; Soussan, L.; Waterman, H.; Levkowitz, G.; Alroy, I.; Klapper, L.; Lavi, S.; Seger, R.; Ratzkin, B.J.; Sela, M.; et al. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J. 1996, 15, 2452–2467. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, A.C.; Moasser, M.M. Targeting HER proteins in cancer therapy and the role of the non-target HER3. Br. J. Cancer 2007, 97, 453–457. [Google Scholar] [CrossRef]
- Lee-Hoeflich, S.T.; Crocker, L.; Yao, E.; Pham, T.; Munroe, X.; Hoeflich, K.P.; Sliwkowski, M.X.; Stern, H.M. A central role for HER3 in HER2-amplified breast cancer: Implications for targeted therapy. Cancer Res. 2008, 68, 5878–5887. [Google Scholar] [CrossRef] [PubMed]
- Liberelle, M.; Jonckheere, N.; Melnyk, P.; Van Seuningen, I.; Lebègue, N. EGF-Containing Membrane-Bound Mucins: A Hidden ErbB2 Targeting Pathway? J. Med. Chem. 2020, 63, 5074–5088. [Google Scholar] [CrossRef] [PubMed]
- Salomon, D.S.; Brandt, R.; Ciardiello, F.; Normanno, N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit. Rev. Oncol. Hematol. 1995, 19, 183–232. [Google Scholar] [CrossRef]
- Fang, J.Y.; Richardson, B.C. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 2005, 6, 322–327. [Google Scholar] [CrossRef]
- Hommes, D.W.; Peppelenbosch, M.P.; van Deventer, S.J. Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut 2003, 52, 144–151. [Google Scholar] [CrossRef]
- Cantley, L.C. The phosphoinositide 3-kinase pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A.; Luo, J.; Cantley, L.C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006, 7, 606–619. [Google Scholar] [CrossRef]
- DiNitto, J.P.; Cronin, T.C.; Lambright, D.G. Membrane recognition and targeting by lipid-binding domains. Sci. STKE Signal Transduct. Knowl. Environ. 2003, 2003, re16. [Google Scholar] [CrossRef]
- Franke, T.F.; Kaplan, D.R.; Cantley, L.C.; Toker, A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science 1997, 275, 665–668. [Google Scholar] [CrossRef]
- Klippel, A.; Kavanaugh, W.M.; Pot, D.; Williams, L.T. A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain. Mol. Cell. Biol. 1997, 17, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Mora, A.; Komander, D.; van Aalten, D.M.; Alessi, D.R. PDK1, the master regulator of AGC kinase signal transduction. Semin. Cell Dev. Biol. 2004, 15, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas, N. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef]
- Richman, S.D.; Southward, K.; Chambers, P.; Cross, D.; Barrett, J.; Hemmings, G.; Taylor, M.; Wood, H.; Hutchins, G.; Foster, J.M.; et al. HER2 overexpression and amplification as a potential therapeutic target in colorectal cancer: Analysis of 3256 patients enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials. J. Pathol. 2016, 238, 562–570. [Google Scholar] [CrossRef]
- Loree, J.M.; Kopetz, S.; Raghav, K.P. Current companion diagnostics in advanced colorectal cancer; getting a bigger and better piece of the pie. J. Gastrointest. Oncol. 2017, 8, 199–212. [Google Scholar] [CrossRef]
- Ingold Heppner, B.; Behrens, H.M.; Balschun, K.; Haag, J.; Krüger, S.; Becker, T.; Röcken, C. HER2/neu testing in primary colorectal carcinoma. Br. J. Cancer 2014, 111, 1977–1984. [Google Scholar] [CrossRef]
- Lee, W.S.; Park, Y.H.; Lee, J.N.; Baek, J.H.; Lee, T.H.; Ha, S.Y. Comparison of HER2 expression between primary colorectal cancer and their corresponding metastases. Cancer Med. 2014, 3, 674–680. [Google Scholar] [CrossRef]
- Shan, L.; Lv, Y.; Bai, B.; Huang, X.; Zhu, H. Variability in HER2 expression between primary colorectal cancer and corresponding metastases. J. Cancer Res. Clin. Oncol. 2018, 144, 2275–2281. [Google Scholar] [CrossRef]
- Salem, M.E.; Weinberg, B.A.; Xiu, J.; El-Deiry, W.S.; Hwang, J.J.; Gatalica, Z.; Philip, P.A.; Shields, A.F.; Lenz, H.J.; Marshall, J.L. Comparative molecular analyses of left-sided colon, right-sided colon, and rectal cancers. Oncotarget 2017, 8, 86356–86368. [Google Scholar] [CrossRef] [PubMed]
- Loree, J.M.; Pereira, A.A.L.; Lam, M.; Willauer, A.N.; Raghav, K.; Dasari, A.; Morris, V.K.; Advani, S.; Menter, D.G.; Eng, C.; et al. Classifying Colorectal Cancer by Tumor Location Rather than Sidedness Highlights a Continuum in Mutation Profiles and Consensus Molecular Subtypes. Clin. Cancer Res. 2018, 24, 1062–1072. [Google Scholar] [CrossRef]
- Stintzing, S.; Tejpar, S.; Gibbs, P.; Thiebach, L.; Lenz, H.J. Understanding the role of primary tumour localisation in colorectal cancer treatment and outcomes. Eur. J. Cancer 2017, 84, 69–80. [Google Scholar] [CrossRef] [PubMed]
- La Salvia, A.; Lopez-Gomez, V.; Garcia-Carbonero, R. HER2-targeted therapy: An emerging strategy in advanced colorectal cancer. Expert Opin. Investig. Drugs 2019, 28, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Robichaux, J.P.; Elamin, Y.Y.; Vijayan, R.S.K.; Nilsson, M.B.; Hu, L.; He, J.; Zhang, F.; Pisegna, M.; Poteete, A.; Sun, H.; et al. Pan-Cancer Landscape and Analysis of ERBB2 Mutations Identifies Poziotinib as a Clinically Active Inhibitor and Enhancer of T-DM1 Activity. Cancer Cell 2019, 36, 444–457.e447. [Google Scholar] [CrossRef]
- Kavuri, S.M.; Jain, N.; Galimi, F.; Cottino, F.; Leto, S.M.; Migliardi, G.; Searleman, A.C.; Shen, W.; Monsey, J.; Trusolino, L.; et al. HER2 activating mutations are targets for colorectal cancer treatment. Cancer Discov. 2015, 5, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Zabransky, D.J.; Yankaskas, C.L.; Cochran, R.L.; Wong, H.Y.; Croessmann, S.; Chu, D.; Kavuri, S.M.; Red Brewer, M.; Rosen, D.M.; Dalton, W.B.; et al. HER2 missense mutations have distinct effects on oncogenic signaling and migration. Proc. Natl. Acad. Sci. USA 2015, 112, E6205–E6214. [Google Scholar] [CrossRef]
- Pahuja, K.B.; Nguyen, T.T.; Jaiswal, B.S.; Prabhash, K.; Thaker, T.M.; Senger, K.; Chaudhuri, S.; Kljavin, N.M.; Antony, A.; Phalke, S.; et al. Actionable Activating Oncogenic ERBB2/HER2 Transmembrane and Juxtamembrane Domain Mutations. Cancer Cell 2018, 34, 792–806.e795. [Google Scholar] [CrossRef]
- Greulich, H.; Kaplan, B.; Mertins, P.; Chen, T.H.; Tanaka, K.E.; Yun, C.H.; Zhang, X.; Lee, S.H.; Cho, J.; Ambrogio, L.; et al. Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2. Proc. Natl. Acad. Sci. USA 2012, 109, 14476–14481. [Google Scholar] [CrossRef]
- Wang, S.E.; Narasanna, A.; Perez-Torres, M.; Xiang, B.; Wu, F.Y.; Yang, S.; Carpenter, G.; Gazdar, A.F.; Muthuswamy, S.K.; Arteaga, C.L. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell 2006, 10, 25–38. [Google Scholar] [CrossRef]
- Bose, R.; Kavuri, S.M.; Searleman, A.C.; Shen, W.; Shen, D.; Koboldt, D.C.; Monsey, J.; Goel, N.; Aronson, A.B.; Li, S.; et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013, 3, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Slodkowska, E.A.; Symmans, W.F.; Pusztai, L.; Ravdin, P.M.; Hortobagyi, G.N. The HER-2 receptor and breast cancer: Ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist 2009, 14, 320–368. [Google Scholar] [CrossRef] [PubMed]
- Valtorta, E.; Martino, C.; Sartore-Bianchi, A.; Penaullt-Llorca, F.; Viale, G.; Risio, M.; Rugge, M.; Grigioni, W.; Bencardino, K.; Lonardi, S.; et al. Assessment of a HER2 scoring system for colorectal cancer: Results from a validation study. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc. 2015, 28, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Siravegna, G.; Sartore-Bianchi, A.; Nagy, R.J.; Raghav, K.; Odegaard, J.I.; Lanman, R.B.; Trusolino, L.; Marsoni, S.; Siena, S.; Bardelli, A. Plasma HER2 (ERBB2) Copy Number Predicts Response to HER2-targeted Therapy in Metastatic Colorectal Cancer. Clin. Cancer Res. 2019, 25, 3046–3053. [Google Scholar] [CrossRef]
- Dabbs, D.J.; Klein, M.E.; Mohsin, S.K.; Tubbs, R.R.; Shuai, Y.; Bhargava, R. High false-negative rate of HER2 quantitative reverse transcription polymerase chain reaction of the Oncotype DX test: An independent quality assurance study. J. Clin. Oncol. 2011, 29, 4279–4285. [Google Scholar] [CrossRef]
- Bai, J.; Gao, J.; Mao, Z.; Wang, J.; Li, J.; Li, W.; Lei, Y.; Li, S.; Wu, Z.; Tang, C.; et al. Genetic mutations in human rectal cancers detected by targeted sequencing. J. Hum. Genet. 2015, 60, 589–596. [Google Scholar] [CrossRef]
- El-Deiry, W.S.; Vijayvergia, N.; Xiu, J.; Scicchitano, A.; Lim, B.; Yee, N.S.; Harvey, H.A.; Gatalica, Z.; Reddy, S. Molecular profiling of 6892 colorectal cancer samples suggests different possible treatment options specific to metastatic sites. Cancer Biol. Ther. 2015, 16, 1726–1737. [Google Scholar] [CrossRef]
- D’Haene, N.; Le Mercier, M.; De Nève, N.; Blanchard, O.; Delaunoy, M.; El Housni, H.; Dessars, B.; Heimann, P.; Remmelink, M.; Demetter, P.; et al. Clinical Validation of Targeted Next Generation Sequencing for Colon and Lung Cancers. PLoS ONE 2015, 10, e0138245. [Google Scholar] [CrossRef]
- Shanmugam, V.; Ramanathan, R.K.; Lavender, N.A.; Sinari, S.; Chadha, M.; Liang, W.S.; Kurdoglu, A.; Izatt, T.; Christoforides, A.; Benson, H.; et al. Whole genome sequencing reveals potential targets for therapy in patients with refractory KRAS mutated metastatic colorectal cancer. BMC Med. Genom. 2014, 7, 36. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Chen, L.; Sah, S.; Latham, G.J.; Patel, R.; Song, Q.; Koeppen, H.; Tam, R.; Schleifman, E.; Mashhedi, H.; et al. Profiling cancer gene mutations in clinical formalin-fixed, paraffin-embedded colorectal tumor specimens using targeted next-generation sequencing. Oncologist 2014, 19, 336–343. [Google Scholar] [CrossRef]
- Rankin, A.; Klempner, S.J.; Erlich, R.; Sun, J.X.; Grothey, A.; Fakih, M.; George, T.J., Jr.; Lee, J.; Ross, J.S.; Stephens, P.J.; et al. Broad Detection of Alterations Predicted to Confer Lack of Benefit From EGFR Antibodies or Sensitivity to Targeted Therapy in Advanced Colorectal Cancer. Oncologist 2016, 21, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.H.; Dintzis, S.M.; Schmidt, R.A. Frequency of HER2 Heterogeneity by Fluorescence In Situ Hybridization According to CAP Expert Panel Recommendations: Time for a New Look at How to Report Heterogeneity. Am. J. Clin. Pathol. 2011, 136, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Öhlschlegel, C.; Zahel, K.; Kradolfer, D.; Hell, M.; Jochum, W. HER2 genetic heterogeneity in breast carcinoma. J. Clin. Pathol. 2011, 64, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Seol, H.; Lee, H.J.; Choi, Y.; Lee, H.E.; Kim, Y.J.; Kim, J.H.; Kang, E.; Kim, S.-W.; Park, S.Y. Intratumoral heterogeneity of HER2 gene amplification in breast cancer: Its clinicopathological significance. Mod. Pathol. 2012, 25, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Seo, A.N.; Kim, E.J.; Jang, M.H.; Suh, K.J.; Ryu, H.S.; Kim, Y.J.; Kim, J.H.; Im, S.A.; Gong, G.; et al. HER2 heterogeneity affects trastuzumab responses and survival in patients with HER2-positive metastatic breast cancer. Am. J. Clin. Pathol. 2014, 142, 755–766. [Google Scholar] [CrossRef]
- Grob, T.J.; Kannengiesser, I.; Tsourlakis, M.C.; Atanackovic, D.; Koenig, A.M.; Vashist, Y.K.; Klose, H.; Marx, A.H.; Koops, S.; Simon, R.; et al. Heterogeneity of ERBB2 amplification in adenocarcinoma, squamous cell carcinoma and large cell undifferentiated carcinoma of the lung. Mod. Pathol.: Off. J. United States Can. Acad. Pathol. Inc. 2012, 25, 1566–1573. [Google Scholar] [CrossRef]
- Hamilton, E.; Shastry, M.; Shiller, S.M.; Ren, R. Targeting HER2 heterogeneity in breast cancer. Cancer Treat. Rev. 2021, 100, 102286. [Google Scholar] [CrossRef]
- Kijima, T.; Arigami, T.; Uenosono, Y.; Hiraki, T.; Yanagita, S.; Matsushita, D.; Okubo, K.; Shimonosono, M.; Ishigami, S.; Maemura, K.; et al. Comparison of HER2 Status Before and After Trastuzumab-based Chemotherapy in Patients With Advanced Gastric Cancer. Anticancer Res. 2020, 40, 75–80. [Google Scholar] [CrossRef]
- Regitnig, P.; Schippinger, W.; Lindbauer, M.; Samonigg, H.; Lax, S.F. Change of HER-2/neu status in a subset of distant metastases from breast carcinomas. J. Pathol. 2004, 203, 918–926. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Modest, D.P.; Stintzing, S.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.E.; Heintges, T.; Lerchenmüller, C.; Kahl, C.; et al. Impact of Subsequent Therapies on Outcome of the FIRE-3/AIO KRK0306 Trial: First-Line Therapy With FOLFIRI Plus Cetuximab or Bevacizumab in Patients With KRAS Wild-Type Tumors in Metastatic Colorectal Cancer. J. Clin. Oncol. 2015, 33, 3718–3726. [Google Scholar] [CrossRef] [PubMed]
- Bertotti, A.; Migliardi, G.; Galimi, F.; Sassi, F.; Torti, D.; Isella, C.; Cora, D.; Di Nicolantonio, F.; Buscarino, M.; Petti, C.; et al. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 2011, 1, 508–523. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Yu, Y.; Liu, W.; Chen, S. Significance of human epidermal growth factor receptor 2 expression in colorectal cancer. Exp. Ther. Med. 2015, 9, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Belli, V.; Matrone, N.; Napolitano, S.; Migliardi, G.; Cottino, F.; Bertotti, A.; Trusolino, L.; Martinelli, E.; Morgillo, F.; Ciardiello, D.; et al. Combined blockade of MEK and PI3KCA as an effective antitumor strategy in HER2 gene amplified human colorectal cancer models. J. Exp. Clin. Cancer Res. 2019, 38, 236. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Amatu, A.; Porcu, L.; Ghezzi, S.; Lonardi, S.; Leone, F.; Bergamo, F.; Fenocchio, E.; Martinelli, E.; Borelli, B.; et al. HER2 Positivity Predicts Unresponsiveness to EGFR-Targeted Treatment in Metastatic Colorectal Cancer. Oncologist 2019, 24, 1395–1402. [Google Scholar] [CrossRef]
- Raghav, K.P.S.; Overman, M.J.; Yu, R.; Meric-Bernstam, F.; Menter, D.; Kee, B.K.; Muranyi, A.; Singh, S.; Routbort, M.; Chen, K.; et al. HER2 amplification as a negative predictive biomarker for anti-epidermal growth factor receptor antibody therapy in metastatic colorectal cancer. J. Clin. Oncol. 2016, 34, 3517. [Google Scholar] [CrossRef]
- Mangiapane, L.R.; Nicotra, A.; Turdo, A.; Gaggianesi, M.; Bianca, P.; Di Franco, S.; Sardina, D.S.; Veschi, V.; Signore, M.; Beyes, S.; et al. PI3K-driven HER2 expression is a potential therapeutic target in colorectal cancer stem cells. Gut 2022, 71, 119–128. [Google Scholar] [CrossRef]
- Rau, A.; Janssen, N.; Kühl, L.; Sell, T.; Kalmykova, S.; Mürdter, T.E.; Dahlke, M.H.; Sers, C.; Morkel, M.; Schwab, M.; et al. Triple Targeting of HER Receptors Overcomes Heregulin-mediated Resistance to EGFR Blockade in Colorectal Cancer. Mol. Cancer Ther. 2022, 21, 799–809. [Google Scholar] [CrossRef]
- Lupo, B.; Sassi, F.; Pinnelli, M.; Galimi, F.; Zanella, E.R.; Vurchio, V.; Migliardi, G.; Gagliardi, P.A.; Puliafito, A.; Manganaro, D.; et al. Colorectal cancer residual disease at maximal response to EGFR blockade displays a druggable Paneth cell-like phenotype. Sci. Transl. Med. 2020, 12, eaax8313. [Google Scholar] [CrossRef]
- Yonesaka, K.; Zejnullahu, K.; Okamoto, I.; Satoh, T.; Cappuzzo, F.; Souglakos, J.; Ercan, D.; Rogers, A.; Roncalli, M.; Takeda, M.; et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci. Transl. Med. 2011, 3, 99ra86. [Google Scholar] [CrossRef]
- Punt, C.J.; Koopman, M.; Vermeulen, L. From tumour heterogeneity to advances in precision treatment of colorectal cancer. Nat. Rev. Clin. Oncol. 2017, 14, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Andre, T.; Amonkar, M.; Norquist, J.M.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.J.A.; Smith, D.; Garcia-Carbonero, R.; et al. Health-related quality of life in patients with microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer treated with first-line pembrolizumab versus chemotherapy (KEYNOTE-177): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 665–677. [Google Scholar] [CrossRef]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A., Jr. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Reviews. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, S.; Koyama, S.; Nishikawa, H. Antitumour immunity regulated by aberrant ERBB family signalling. Nat. Rev. Cancer 2021, 21, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e814. [Google Scholar] [CrossRef]
- Augustin, J.E.; Soussan, P.; Bass, A.J. Targeting the complexity of ERBB2 biology in gastroesophageal carcinoma. Ann. Oncol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, Q.; Zhang, F.; Meng, F.; Liu, S.; Zhou, R.; Wu, Q.; Li, X.; Shen, L.; Huang, J.; et al. HER2 recruits AKT1 to disrupt STING signalling and suppress antiviral defence and antitumour immunity. Nat. Cell Biol. 2019, 21, 1027–1040. [Google Scholar] [CrossRef]
- Xu, J.; Meng, Q.; Sun, H.; Zhang, X.; Yun, J.; Li, B.; Wu, S.; Li, X.; Yang, H.; Zhu, H.; et al. HER2-specific chimeric antigen receptor-T cells for targeted therapy of metastatic colorectal cancer. Cell Death Dis. 2021, 12, 1109. [Google Scholar] [CrossRef]
- Hernandez-Lopez, R.A.; Yu, W.; Cabral, K.A.; Creasey, O.A.; Lopez Pazmino, M.D.P.; Tonai, Y.; De Guzman, A.; Mäkelä, A.; Saksela, K.; Gartner, Z.J.; et al. T cell circuits that sense antigen density with an ultrasensitive threshold. Science 2021, 371, 1166–1171. [Google Scholar] [CrossRef]
- Lynch, K.T.; Squeo, G.C.; Kane, W.J.; Meneveau, M.O.; Petroni, G.; Olson, W.C.; Chianese-Bullock, K.A.; Slingluff, C.L., Jr.; Foley, E.F.; Friel, C.M. A pilot trial of vaccination with Carcinoembryonic antigen and Her2/neu peptides in advanced colorectal cancer. Int. J. Cancer 2022, 150, 164–173. [Google Scholar] [CrossRef]
- Kubota, Y.; Kawazoe, A.; Sasaki, A.; Mishima, S.; Sawada, K.; Nakamura, Y.; Kotani, D.; Kuboki, Y.; Taniguchi, H.; Kojima, T.; et al. The Impact of Molecular Subtype on Efficacy of Chemotherapy and Checkpoint Inhibition in Advanced Gastric Cancer. Clin. Cancer Res. 2020, 26, 3784–3790. [Google Scholar] [CrossRef] [PubMed]
- Dirix, L.Y.; Takacs, I.; Jerusalem, G.; Nikolinakos, P.; Arkenau, H.T.; Forero-Torres, A.; Boccia, R.; Lippman, M.E.; Somer, R.; Smakal, M.; et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase 1b JAVELIN Solid Tumor study. Breast Cancer Res. Treat. 2018, 167, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Nazemalhosseini Mojarad, E.; Kuppen, P.J. HER2 and immunotherapy using monoclonal antibodies in colorectal cancer. Immunotherapy 2013, 5, 1267–1269. [Google Scholar] [CrossRef]
- Burstein, H.J. The distinctive nature of HER2-positive breast cancers. N. Engl. J. Med. 2005, 353, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Schuell, B.; Gruenberger, T.; Scheithauer, W.; Zielinski, C.; Wrba, F. HER 2/neu protein expression in colorectal cancer. BMC Cancer 2006, 6, 123. [Google Scholar] [CrossRef]
- Blok, E.J.; Kuppen, P.J.; van Leeuwen, J.E.; Sier, C.F. Cytoplasmic Overexpression of HER2: A Key Factor in Colorectal Cancer. Clin. Med. Insights Oncol. 2013, 7, 41–51. [Google Scholar] [CrossRef]
- Lewis Phillips, G.D.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.; Lutz, R.J.; et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef]
- Iwata, T.N.; Ishii, C.; Ishida, S.; Ogitani, Y.; Wada, T.; Agatsuma, T. A HER2-Targeting Antibody-Drug Conjugate, Trastuzumab Deruxtecan (DS-8201a), Enhances Antitumor Immunity in a Mouse Model. Mol. Cancer Ther. 2018, 17, 1494–1503. [Google Scholar] [CrossRef]
- Tsurutani, J.; Iwata, H.; Krop, I.; Jänne, P.A.; Doi, T.; Takahashi, S.; Park, H.; Redfern, C.; Tamura, K.; Wise-Draper, T.M.; et al. Targeting HER2 with Trastuzumab Deruxtecan: A Dose-Expansion, Phase I Study in Multiple Advanced Solid Tumors. Cancer Discov. 2020, 10, 688–701. [Google Scholar] [CrossRef]
- Abuhelwa, Z.; Alloghbi, A.; Alqahtani, A.; Nagasaka, M. Trastuzumab Deruxtecan-Induced Interstitial Lung Disease/Pneumonitis in ERBB2-Positive Advanced Solid Malignancies: A Systematic Review. Drugs 2022, 82, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, J.; Wang, C.; Fakih, M. Clinical Response to T-DM1 in HER2-Amplified, KRAS-Mutated Metastatic Colorectal Cancer. J. Natl. Compr. Cancer Netw. 2020, 18, 116–119. [Google Scholar] [CrossRef]
- Ringhieri, P.; Mannucci, S.; Conti, G.; Nicolato, E.; Fracasso, G.; Marzola, P.; Morelli, G.; Accardo, A. Liposomes derivatized with multimeric copies of KCCYSL peptide as targeting agents for HER-2-overexpressing tumor cells. Int. J. Nanomed. 2017, 12, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Laha, D.; Dash, S.K.; Chattopadhyay, S.; Roy, S.; Das, D.K.; Pramanik, P.; Karmakar, P. An in-vivo study for targeted delivery of copper-organic complex to breast cancer using chitosan polymer nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Wu, F.G.; Chen, X. Antibody-Incorporated Nanomedicines for Cancer Therapy. Adv. Mater. 2022, 34, e2109210. [Google Scholar] [CrossRef] [PubMed]
- Vivek, R.; Thangam, R.; NipunBabu, V.; Rejeeth, C.; Sivasubramanian, S.; Gunasekaran, P.; Muthuchelian, K.; Kannan, S. Multifunctional HER2-antibody conjugated polymeric nanocarrier-based drug delivery system for multi-drug-resistant breast cancer therapy. ACS Appl. Mater. Interfaces 2014, 6, 6469–6480. [Google Scholar] [CrossRef]
- ZW25 Effective in HER2-Positive Cancers. Cancer Discov. 2019, 9, 8. [CrossRef]
- Ye, P.; Wang, Y.; Li, R.; Chen, W.; Wan, L.; Cai, P. The HER family as therapeutic targets in colorectal cancer. Crit Rev. Oncol. Hematol. 2022, 174, 103681. [Google Scholar] [CrossRef]
- Deeks, E.D. Neratinib: First Global Approval. Drugs 2017, 77, 1695–1704. [Google Scholar] [CrossRef]
- Ito, Y.; Suenaga, M.; Hatake, K.; Takahashi, S.; Yokoyama, M.; Onozawa, Y.; Yamazaki, K.; Hironaka, S.; Hashigami, K.; Hasegawa, H.; et al. Safety, efficacy and pharmacokinetics of neratinib (HKI-272) in Japanese patients with advanced solid tumors: A Phase 1 dose-escalation study. Jpn. J. Clin. Oncol. 2012, 42, 278–286. [Google Scholar] [CrossRef][Green Version]
- Jacobs, S.A.; Lee, J.J.; George, T.J.; Wade, J.L., 3rd; Stella, P.J.; Wang, D.; Sama, A.R.; Piette, F.; Pogue-Geile, K.L.; Kim, R.S.; et al. Neratinib-Plus-Cetuximab in Quadruple-WT (KRAS, NRAS, BRAF, PIK3CA) Metastatic Colorectal Cancer Resistant to Cetuximab or Panitumumab: NSABP FC-7, A Phase Ib Study. Clin. Cancer Res. 2021, 27, 1612–1622. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Pyrotinib: First Global Approval. Drugs 2018, 78, 1751–1755. [Google Scholar] [CrossRef]
- Kulukian, A.; Lee, P.; Taylor, J.; Rosler, R.; de Vries, P.; Watson, D.; Forero-Torres, A.; Peterson, S. Preclinical Activity of HER2-Selective Tyrosine Kinase Inhibitor Tucatinib as a Single Agent or in Combination with Trastuzumab or Docetaxel in Solid Tumor Models. Mol. Cancer Ther. 2020, 19, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Wedam, S.; Cheng, J.; Fiero, M.H.; Xia, H.; Li, F.; Fan, J.; Zhang, X.; Yu, J.; Song, P.; et al. FDA Approval Summary: Tucatinib for the Treatment of Patients with Advanced or Metastatic HER2-positive Breast Cancer. Clin. Cancer Res. 2021, 27, 1220–1226. [Google Scholar] [CrossRef]
- Lee, A. Tucatinib: First Approval. Drugs 2020, 80, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Budi, H.S.; Ahmad, F.N.; Achmad, H.; Ansari, M.J.; Mikhailova, M.V.; Suksatan, W.; Chupradit, S.; Shomali, N.; Marofi, F. Human epidermal growth factor receptor 2 (HER2)-specific chimeric antigen receptor (CAR) for tumor immunotherapy; recent progress. Stem Cell Res. Ther. 2022, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, Z.; Tan, X.; Jiang, H.; Xu, Z.; Fang, Y.; Han, D.; Hong, W.; Wei, W.; Tu, J. CAR-macrophage: A new immunotherapy candidate against solid tumors. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 139, 111605. [Google Scholar] [CrossRef]
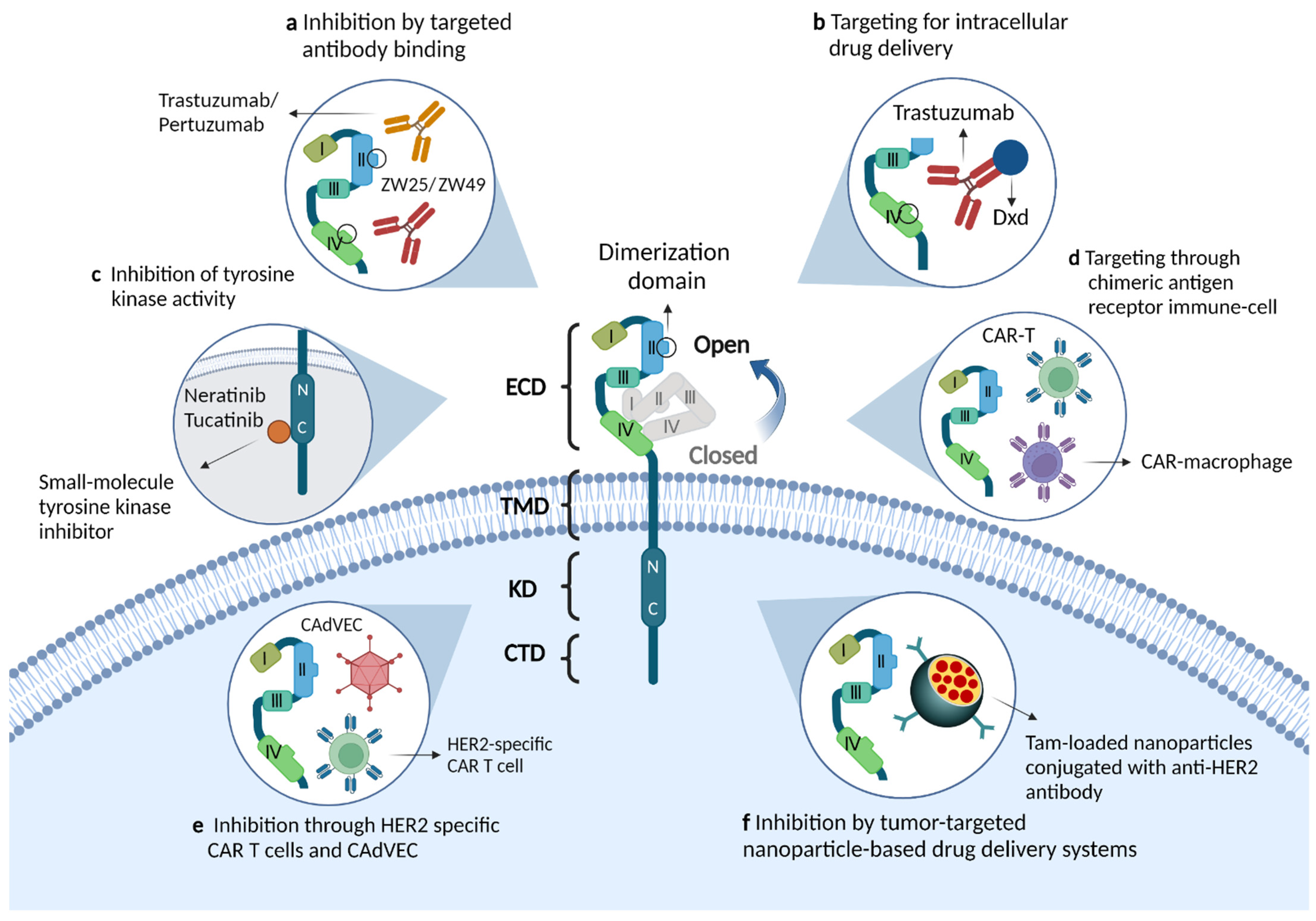

| Types of Drugs | Trial/Phase | Interventions | Description | Outcomes/Status |
|---|---|---|---|---|
| Anti-HER2 mAbs | My Pathway (phase II) | Trastuzumab plus pertuzumab | KRAS-unselected, chemorefractory, HER2 amplified mCRC (n = 56); HER2 positivity assigned based on IHC (3+ staining), FISH (ERBB2:CEP17 > 2.0) and/or NGS (ERBB2 copy number gain) | mOS 11.5 months, mPFS 2.9 months, ORR 32% |
| TRIUMPH (phase II) | Pertuzumab plus trastuzumab | HER2 amplification mCRC (n = 30); ERBB2 amplifications determined using tissue and/or ctDNA analysis | mOS 10.1 months (tissue+) and 8.8 months (ctDNA+), mPFS 4.0 months (tissue+) and 3.1 months (ctDNA+), ORR 30% (tissue+) and 28% (ctDNA+) | |
| Anti-HER2 mAbs+TKIs | HERACLES-A (phase II) | Trastuzumab plus lapatinib | KRAS-WT, chemorefractory, HER2-positive mCRC (n = 27); HER2 positivity (HERACLES pathological criteria) | mOS 10 months, mPFS 4.7 months, ORR 28% |
| Anti-HER2 mAbs+TKIs | MOUNTAINEER (phase II) | Tucatinib plus trastuzumab | RAS-WT, chemorefractory, HER2 amplification/overexpression mCRC (n = 26); HER2 positivity determined using IHC (3+ or 2+ staining and FISH-positive), FISH and/or NGS | mOS 17.3 months, mPFS 6.2 months, ORR 55%, DCR NR |
| NCT04579380 (phase II) | Tucatinib plus trastuzumab | HER2 overexpression/alterations, unresectable, or metastatic solid timors (n = 270, including CRC); HER2 status detected in fresh or archival tumor tissue or blood | Recruiting | |
| NCT04380012 (phase II) | Pyrotinib plus trastuzumab | HER2-positive advanced CRC(n = 40); HER2 positivity confirmed by IHC (3+ or 2+ in more than 50% of cells) and SISH/FISH (HER2:CEP17 > 2.0) | Recruiting | |
| Anti-HER2 ADCs | HERACLES-B (phase II) | T-DM1 plus pertuzumab | RAS-/BRAF-WT, chemorefractory and HER2+mCRC (n = 31); HER2 positivity (HERACLES pathological criteria) | ORR 80%, mOS 4.1 months, DCR 9.7% |
| DESTINY-CRC01 (phase II) | T-DXd (DS-8201a) | RAS-/BRAF V600E-WT, disease progression on two or more prior regimens, HER2 expressing mCRC (n = 78); HER2 positivityCohort A: HER2 IHC 3+ or IHC 2+ staining and ISH-positive (n = 53) Cohort B: IHC 2+ staining and ISH-negative (n = 7) Cohort C: IHC 1+ staining (n = 18) | ORR 83%, mPFS NR, mOS NR, DCR 45.3% | |
| TKIs+anti-EGFR therapy | NSABP FC-7 (phase Ib) | Neratinib plus cetuximab | Resistant to Cetuximab or Panitumumab and quadruple-WT (KRAS, NRAS, BRAF, PI3KCA) disease (n = 21); HER2 amplification assessed by CISH (ERBB2:CEP17 > 2.0) or NGS (ERBB2 copy number > 2.0) | RP2D of neratinib: 240 mg/day; no OS; SD was seen at all neratinib doses |
| Dual-targeted antibodies | NCT03929666 (phase II) | ZW25 plus standard QT | Unresectable, locally advanced, recurrent, or metastatic HER2-expressing cancers (n = 362, including CRC); HER2 expressing (IHC 3+ with or without gene amplification; or IHC 0, 1+ or 2+ with gene amplification) | Recruiting |
| NCT03821233 (phase I) | ZW49 | Locally advanced (unresectable) or metastatic HER2-expressing cancers (n = 174) | Recruiting | |
| NCT03602079 (phase I/II) | A166 | Relapsed/refractory, HER2 expressing/amplified cancers (n = 49, including CRC); Low HER2 expression (IHC 1+ and IHC 2+ without FISH confirmation) and HER2 positive (IHC2+ with FISH confirmation and IHC 3+) | Active, not recruiting | |
| Anti-HER2 CAR- macrophages | NCT04660929 (phase I) | CT-0508 | HER2 overexpressing solid tumors (n = 18, including CRC) | Recruiting |
| CAR-T+Oncolytic adenovirus | NCT03740256 (phase I) | CAdVEC | Advanced HER2 positive solid tumors (n = 45, including CRC); HER2 positivity defined as IHC 3+ or IHC 2+ staining | Recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Cao, Y.; Si, C.; Shao, P.; Su, G.; Wang, K.; Bao, J.; Yang, L. Emerging Role of ERBB2 in Targeted Therapy for Metastatic Colorectal Cancer: Signaling Pathways to Therapeutic Strategies. Cancers 2022, 14, 5160. https://doi.org/10.3390/cancers14205160
Wang N, Cao Y, Si C, Shao P, Su G, Wang K, Bao J, Yang L. Emerging Role of ERBB2 in Targeted Therapy for Metastatic Colorectal Cancer: Signaling Pathways to Therapeutic Strategies. Cancers. 2022; 14(20):5160. https://doi.org/10.3390/cancers14205160
Chicago/Turabian StyleWang, Nannan, Yuepeng Cao, Chengshuai Si, Peng Shao, Guoqing Su, Ke Wang, Jun Bao, and Liu Yang. 2022. "Emerging Role of ERBB2 in Targeted Therapy for Metastatic Colorectal Cancer: Signaling Pathways to Therapeutic Strategies" Cancers 14, no. 20: 5160. https://doi.org/10.3390/cancers14205160
APA StyleWang, N., Cao, Y., Si, C., Shao, P., Su, G., Wang, K., Bao, J., & Yang, L. (2022). Emerging Role of ERBB2 in Targeted Therapy for Metastatic Colorectal Cancer: Signaling Pathways to Therapeutic Strategies. Cancers, 14(20), 5160. https://doi.org/10.3390/cancers14205160






