Use of Cannabis and Cannabinoids for Treatment of Cancer
Simple Summary
Abstract
1. Introduction
2. Role of Endocannabinoids in the Human Body
2.1. Mechanism of Action—Ligand/Receptor
2.2. Role in the Control of Cell Division and Cell Proliferation
2.3. Changes in the ECS with Age
3. ECS and Cancer
3.1. Changes in ECS in Cancer
3.1.1. Changes in Expression Pattern of Cannabinoid Receptors in Cancer
CB1R and CB2R
GPR55
3.1.2. Changes in Cannabinoid Receptor Endogenous Ligands in Cancer
3.1.3. Changes in the Expression Pattern of Endocannabinoid Hydrolytic Enzymes in Cancer
3.2. Changes of ECS Signaling Pathways in Cancer—Potential Molecular Targets
3.2.1. Receptor-Dependent Signaling and Changes in Response to Cannabinoids
3.2.2. Receptor-Independent Signaling and Changes in Response to Cannabinoids
3.2.3. Signaling When the Receptor Status Is Unknown
4. Effect of Cannabinoids on Various Hallmarks of Cancer
4.1. Induction of Autophagy and Apoptosis
4.2. Reduction of Inflammation and Inhibition of Proliferation
4.3. Inhibition of Angiogenesis, Tumor Invasiveness, and Metastasis
5. Effect of Terpenes and Flavonoids
6. Preclinical and Clinical Use of Cannabinoids
6.1. Cannabis and Cannabinoids for Primary Care—Tumor Shrinkage
6.1.1. Data on Humans Are Limited
6.1.2. Combination of Cannabinoids with Other Drugs—There Is Potential Benefit, but Caution Is to Be Exercised
6.2. Cannabis for Palliative Care
6.2.1. Cannabis for Pain
6.2.2. Reducing Nausea and Vomiting
6.2.3. Improving Appetite
6.2.4. Reducing Anxiety and Improving Sleep
7. Adverse, Unexpected, and Unintended Effects of Cannabinoids
8. Sex-Specific Differences in ECS, Ethical Considerations of Cannabis Use and Equal Access to Cannabis
8.1. Sex-Specific Difference in Cancer and Use of Cannabis
8.2. Effect of Cannabis as a Function of Age
8.3. Equal Access to Cannabis for Everyone
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- McPartland, J.M.; Matias, I.; Di Marzo, V.; Glass, M. Evolutionary origins of the endocannabinoid system. Gene 2006, 370, 64–74. [Google Scholar] [CrossRef]
- Cristino, L.; Becker, T.; Di Marzo, V. Endocannabinoids and energy homeostasis: An update. BioFactors 2014, 40, 389–397. [Google Scholar] [CrossRef]
- Rodríguez-Valentín, R.; Torres-Mejía, G.; Martínez-Matsushita, L.; Angeles-Llerenas, A.; Gómez-Flores-Ramos, L.; Wolff, R.K.; Baumgartner, K.B.; Hines, L.M.; Ziv, E.; Flores-Luna, L.; et al. Energy homeostasis genes modify the association between serum concentrations of IGF-1 and IGFBP-3 and breast cancer risk. Sci. Rep. 2022, 12, 1837. [Google Scholar] [CrossRef]
- Khandekar, M.J.; Cohen, P.; Spiegelman, B.M. Molecular mechanisms of cancer development in obesity. Nat. Cancer 2011, 11, 886–895. [Google Scholar] [CrossRef]
- Kunos, G.; Osei-Hyiaman, D.; Liu, J.; Godlewski, G.; Bátkai, S. Endocannabinoids and the control of energy homeostasis. J. Biol. Chem. 2008, 283, 33021–33025. [Google Scholar] [CrossRef]
- Loeb, L.A.; Loeb, K.R.; Anderson, J.P. Multiple mutations and cancer. Proc. Natl. Acad. Sci. USA 2003, 100, 776–781. [Google Scholar] [CrossRef]
- Battista, N.; Bari, M.; Maccarrone, M. Endocannabinoids and Reproductive Events in Health and Disease. Endocannabinoids 2015, 231, 341–365. [Google Scholar] [CrossRef]
- Kozakiewicz, M.L.; Grotegut, C.A.; Howlett, A.C. Endocannabinoid System in Pregnancy Maintenance and Labor: A Mini-Review. Front. Endocrinol. 2021, 12, 699951. [Google Scholar] [CrossRef]
- Nghdawagsb, E.F. The endocannabinoid system during development: Emphasis on perinatal events and delayed effects. In Vitamins and Hormones; Elsevier: Amsterdam, The Netherlands, 2009; pp. 139–158. [Google Scholar]
- Skaper, S.D.; di Marzo, V. Endocannabinoids in nervous system health and disease: The big picture in a nutshell. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3193–3200. [Google Scholar] [CrossRef]
- Pagotto, U.; Marsicano, G.; Cota, D.; Lutz, B.; Pasquali, R. The Emerging Role of the Endocannabinoid System in Endocrine Regulation and Energy Balance. Endocr. Rev. 2005, 27, 73–100. [Google Scholar] [CrossRef]
- Almogi-Hazan, O.; Or, R. Cannabis, the Endocannabinoid System and Immunity—The Journey from the Bedside to the Bench and Back. Int. J. Mol. Sci. 2020, 21, 4448. [Google Scholar] [CrossRef]
- Bilkei-Gorzo, A. The endocannabinoid system in normal and pathological brain ageing. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3326–3341. [Google Scholar] [CrossRef]
- Michela, G.; Giuseppe, C.; Ornella, P.; Patrizia, P.; Daniela, C.; Renza, V.; Romano, L.L. Anandamide-induced apoptosis in Chang liver cells involves ceramide and JNK/AP-1 pathway. Int. J. Mol. Med. 2006, 17, 811–819. [Google Scholar]
- Noonan, J.; Tanveer, R.; Klompas, A.; Gowran, A.; McKiernan, J.; Campbell, V.A. Endocannabinoids Prevent β-Amyloid-mediated Lysosomal Destabilization in Cultured Neurons. J. Biol. Chem. 2010, 285, 38543–38554. [Google Scholar] [CrossRef]
- Velez-Pardo, C.; Jimenez-Del-Rio, M.; Lores-Arnaiz, S.; Bustamante, J. Protective Effects of the Synthetic Cannabinoids CP55,940 and JWH-015 on Rat Brain Mitochondria upon Paraquat Exposure. Neurochem. Res. 2010, 35, 1323–1332. [Google Scholar] [CrossRef]
- Zaccagnino, P.; D’Oria, S.; Romano, L.L.; Di Venere, A.; Sardanelli, A.M.; Lorusso, M. The endocannabinoid 2-arachidonoylglicerol decreases calcium induced cytochrome c release from liver mitochondria. J. Bioenerg. Biomembr. 2012, 44, 273–280. [Google Scholar] [CrossRef]
- Mackie, K.; Mackie, K. Cannabinoid Receptors: Where They are and What They do. J. Neuroendocr. 2008, 20, 10–14. [Google Scholar] [CrossRef]
- Tóth, K.F.; Ádám, D.; Bíró, T.; Oláh, A. Cannabinoid signaling in the skin: Therapeutic potential of the “c(ut)annabinoid” system. Molecules 2019, 24, 918. [Google Scholar] [CrossRef]
- Baron, E.P. Medicinal Properties of Cannabinoids, Terpenes, and Flavonoids in Cannabis, and Benefits in Migraine, Headache, and Pain: An Update on Current Evidence and Cannabis Science. Headache J. Head Face Pain 2018, 58, 1139–1186. [Google Scholar] [CrossRef]
- del, R.C.; Millán, E.; García, V.; Appendino, G.; DeMesa, J.; Muñoz, E. The endocannabinoid system of the skin. A potential approach for the treatment of skin disorders. Biochem. Pharmacol. 2018, 157, 122–133. [Google Scholar]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. J. Cereb. Blood Flow Metab. 2015, 172, 4790–4805. [Google Scholar] [CrossRef]
- Burstein, S. Cannabidiol (CBD) and its analogs: A review of their effects on inflammation. Bioorganic Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef]
- Martínez, V.; Iriondo De-Hond, A.; Borrelli, F.; Capasso, R.; del Castillo, M.D.; Abalo, R. Cannabidiol and other non-psychoactive cannabinoids for prevention and treatment of gastrointestinal disorders: Useful nutraceuticals? Int. J. Mol. Sci. 2020, 9, 3067. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.; Romero, J.; Ramos, J.A. Endocannabinoids and neurodegenerative disorders: Parkinson’s disease, Huntington’s chorea, Alzheimer’s disease, and others. In Endocannabinoids; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 233–259. [Google Scholar]
- Elphick, M.R. The evolution and comparative neurobiology of endocannabinoid signalling. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3201–3215. [Google Scholar] [CrossRef]
- Gomez, O.; Arevalo-Martin, A.; Garcia-Ovejero, D.; Ortega-Gutierrez, S.; Cisneros, J.A.; Almazan, G.; Sánchez-Rodriguez, M.A.; Molina-Holgado, F.; Molina-Holgado, E. The constitutive production of the endocannabinoid 2-arachidonoylglycerol participates in oligodendrocyte differentiation. Glia 2010, 58, 1913–1927. [Google Scholar] [CrossRef]
- Bíró, T.; Tóth, B.I.; Haskó, G.; Paus, R.; Pacher, P. The endocannabinoid system of the skin in health and disease: Novel perspectives and therapeutic opportunities. Trends Pharmacol. Sci. 2009, 30, 411–420. [Google Scholar] [CrossRef]
- Ständer, S.; Schmelz, M.; Metze, D.; Luger, T.; Rukwied, R. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J. Dermatol. Sci. 2005, 38, 177–188. [Google Scholar] [CrossRef]
- Telek, A.; Bíró, T.; Bodó, E.; Tóth, B.I.; Borbíró, I.; Kunos, G.; Sardanelli, A.M. Inhibition of human hair follicle growth by endo-and exocannabinoids. FASEB J. 2007, 21, 3534–3541. [Google Scholar] [CrossRef]
- Dobrosi, N.; Tóth, B.I.; Nagy, G.; Dózsa, A.; Géczy, T.; Nagy, L.; Harvey-White, J.; Loureiro, A.I. Endocannabinoids enhance lipid synthesis and apoptosis of human sebocytes via cannabinoid receptor-2-mediated signaling. FASEB J. 2008, 22, 3685–3695. [Google Scholar] [CrossRef]
- Ibsen, M.S.; Connor, M.; Glass, M. Cannabinoid CB1 and CB2 Receptor Signaling and Bias. Cannabis Cannabinoid Res. NLM (Medlin.) 2017, 2, 48–60. [Google Scholar] [CrossRef]
- Schwarz, H.; Blanco, F.J.; Lotz, M. Anadamide, an endogenous cannabinoid receptor agonist inhibits lymphocyte proliferation and induces apoptosis. J. Neuroimmunol. 1994, 55, 107–115. [Google Scholar] [CrossRef]
- Joseph, J.; Niggemann, B.; Zaenker, K.S.; Entschladen, F. Anandamide is an endogenous inhibitor for the migration of tumor cells and T lymphocytes. Cancer Immunol. Immunother. 2004, 53, 723–728. [Google Scholar] [CrossRef]
- Kishimoto, S.; Muramatsu, M.; Gokoh, M.; Oka, S.; Waku, K.; Sugiura, T. Endogenous Cannabinoid Receptor Ligand Induces the Migration of Human Natural Killer Cells. J. Biochem. 2005, 137, 217–223. [Google Scholar] [CrossRef]
- Rahaman, O.; Ganguly, D. Endocannabinoids in immune regulation and immunopathologies. Immunology 2021, 164, 242–252. [Google Scholar] [CrossRef]
- Do, Y.; McKallip, R.J.; Nagarkatti, M.; Nagarkatti, P.S. Activation through Cannabinoid Receptors 1 and 2 on Dendritic Cells Triggers NF-κB-Dependent Apoptosis: Novel Role for Endogenous and Exogenous Cannabinoids in Immunoregulation. J. Immunology. 2004, 173, 2373–2382. [Google Scholar] [CrossRef]
- Berrendero, F.; Romero, J.; García-Gil, L.; Suarez, I.; De la Cruz, P.; Ramos, J.; Fernández-Ruiz, J. Changes in cannabinoid receptor binding and mRNA levels in several brain regions of aged rats. Biochim. et Biophys. Acta (BBA)-Mol. Basis Dis. 1998, 1407, 205–214. [Google Scholar] [CrossRef]
- Liu, P.; Bilkey, D.K.; Darlington, C.L.; Smith, P.F. Cannabinoid CB1 receptor protein expression in the rat hippocampus and entorhinal, perirhinal, postrhinal and temporal cortices: Regional variations and age-related changes. Brain Res. 2003, 979, 235–239. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Harvey-White, J.; Zimmer, A.; Kunos, G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc. Natl. Acad. Sci. USA 2003, 100, 1393–1398. [Google Scholar] [CrossRef]
- Van Laere, K.; Goffin, K.; Casteels, C.; Dupont, P.; Mortelmans, L.; de Hoon, J.; Bormans, G. Gender-dependent increases with healthy aging of the human cerebral cannabinoid-type 1 receptor binding using [18F]MK-9470 PET. Neuroimage 2008, 39, 1533–1541. [Google Scholar] [CrossRef]
- Bátkai, S.; Rajesh, M.; Mukhopadhyay, P.; Haskó, G.; Liaudet, L.; Cravatt, B.F.; Csiszár, A.; Ungvári, Z.; Pacher, P. Decreased age-related cardiac dysfunction, myocardial nitrative stress, inflammatory gene expression, and apoptosis in mice lacking fatty acid amide hydrolase-1) expression and monocyte-endothelial adhesion in Downloaded from. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, 909–918. [Google Scholar] [CrossRef]
- Correia-Sá, I.B.; Carvalho, C.M.; Serrão, P.V.; Loureiro, A.I.; Fernandes-Lopes, C.; Marques, M.; Vieira-Coelho, M.A. A new role for anandamide: Defective link between the systemic and skin endocannabinoid systems in hypertrophic human wound healing. Sci. Rep. 2020, 10, 3. [Google Scholar] [CrossRef]
- Leal, E.C.; Moura, L.I.; Pirzgalska, R.M.; Marques-da-Silva, D.; Ledent, C.; Köfalvi, A.; Carvalho, E. Diabetes and Cannabinoid CB1 receptor deficiency promotes similar early onset aging-like changes in the skin. Exp. Gerontol. 2021, 15, 154. [Google Scholar]
- Robben, M.; Nasr, M.S.; Das, A.; Huber, M.; Jaworski, J.; Weidanz, J.; Luber, J.M. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2022, 28, D20–D26. [Google Scholar]
- Pati, S.; Krishna, S.; Lee, J.H.; Ross, M.K.; de La Serre, C.B.; Harn, D.A.; Wagner, J.; Filipov, N.M.; Cummings, B.S. Effects of high-fat diet and age on the blood lipidome and circulating endocannabinoids of female C57BL/6 mice. Biochim. et Biophys. Acta BBA-Mol. Cell Biol. Lipids 2017, 1863, 26–39. [Google Scholar] [CrossRef]
- Maccarrone, M.; Valverde, O.; Barbaccia, M.L.; Castañé, A.; Maldonado, R.; Ledent, C.; Parmentier, M.; Finazzi-Agrò, A. Age-related changes of anandamide metabolism in CB1cannabinoid receptor knockout mice: Correlation with behaviour. Eur. J. Neurosci. 2002, 15, 1178–1186. [Google Scholar] [CrossRef]
- Piyanova, A.; Lomazzo, E.; Bindila, L.; Lerner, R.; Albayram, O.; Ruhl, T.; Lutz, B.; Zimmer, A.; Bilkei-Gorzo, A. Age-related changes in the endocannabinoid system in the mouse hippocampus. Mech. Ageing Dev. 2015, 150, 55–64. [Google Scholar] [CrossRef]
- Romero, J.; Berrendero, F.; Garcia-Gil, L.; de la Cruz, P.; Ramos, J.; Fernandez-Ruiz, J. Loss of cannabinoid receptor binding and messenger RNA levels and cannabinoid agonist-stimulated [35s]guanylyl-5′-O-(thio)-triphosphate binding in the basal ganglia of aged rats. Neuroscience 1998, 84, 1075–1083. [Google Scholar] [CrossRef]
- Lee, T.T.-Y.; Hill, M.N.; Hillard, C.J.; Gorzalka, B.B. Temporal changes inN-acylethanolamine content and metabolism throughout the peri-adolescent period. Synapse 2012, 67, 4–10. [Google Scholar] [CrossRef]
- Sailler, S.; Schmitz, K.; Jäger, E.; Ferreiros, N.; Wicker, S.; Zschiebsch, K.; Pickert, G.; Geisslinger, G.; Walter, C.; Tegeder, I.; et al. Regulation of circulating endocannabinoids associated with cancer and metastases in mice and humans. Oncoscience 2014, 1, 272–282. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, J.; Zhao, H.; Fang, X.; Li, H. Cannabinoid receptor 2 is upregulated in melanoma. J. Cancer Res. Ther. 2012, 8, 549–554. [Google Scholar] [CrossRef]
- Baba, Y.; Funakoshi, T.; Emoto, K.; Masugi, Y.; Ekmekcioglu, S.; Amagai, M.; Mori, M.; Tanese, K. Expression of monoacylglycerol lipase as a marker of tumour invasion and progression in malignant melanoma. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 2038–2045. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhang, B.; Seviour, E.G.; Tao, K.-X.; Liu, X.-H.; Ling, Y.; Chen, J.-Y.; Wang, G.-B. Monoacylglycerol lipase (MAGL) knockdown inhibits tumor cells growth in colorectal cancer. Cancer Lett. 2011, 307, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Grill, M.; Högenauer, C.; Blesl, A.; Haybaeck, J.; Golob-Schwarzl, N.; Ferreirós, N.; Thomas, D.; Gurke, R.; Trötzmüller, M.; Köfeler, H.C.; et al. Members of the endocannabinoid system are distinctly regulated in inflammatory bowel disease and colorectal cancer. Sci. Rep. 2019, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Kargl, J.; Andersen, L.; Hasenöhrl, C.; Feuersinger, D.; Stančić, A.; Fauland, A.; Magnes, C.; El-Heliebi, A.; Lax, S.; Uranitsch, S.; et al. GPR55 promotes migration and adhesion of colon cancer cells indicating a role in metastasis. J. Cereb. Blood Flow Metab. 2015, 173, 142–154. [Google Scholar] [CrossRef]
- Hasenoehrl, C.; Feuersinger, D.; Sturm, E.M.; Bärnthaler, T.; Heitzer, E.; Graf, R.; Grill, M.; Pichler, M.; Beck, S.; Butcher, L.; et al. G protein-coupled receptor GPR55 promotes colorectal cancer and has opposing effects to cannabinoid receptor 1. Int. J. Cancer 2018, 142, 121–132. [Google Scholar] [CrossRef]
- Cianchi, F.; Papucci, L.; Schiavone, N.; Lulli, M.; Magnelli, L.; Vinci, M.C.; Messerini, L.; Manera, C.; Ronconi, E.; Romagnani, P.; et al. Cannabinoid Receptor Activation Induces Apoptosis through Tumor Necrosis Factor α–Mediated Ceramide De novo Synthesis in Colon Cancer Cells. Clin. Cancer Res. 2008, 14, 7691–7700. [Google Scholar] [CrossRef]
- Kitamura, C.; Sonoda, H.; Anzai, H.; Nagai, Y.; Abe, S.; Yokoyama, Y.; Ishii, H.; Kishikawa, J.; Emoto, S.; Murono, K.; et al. Expression of Lysophosphatidylinositol Signaling-relevant Molecules in Colorectal Cancer. Anticancer Res. 2021, 41, 2349–2355. [Google Scholar] [CrossRef]
- Milian, L.; Mata, M.; Alcacer, J.; Oliver, M.; Sancho-Tello, M.; De Llano, J.J.M.; Camps, C.; Galbis, J.; Carretero, J.; Carda, C. Cannabinoid receptor expression in non-small cell lung cancer. Effectiveness of tetrahydrocannabinol and cannabidiol inhibiting cell proliferation and epithelial-mesenchymal transition in vitro. PLoS ONE 2020, 15, e0228909. [Google Scholar] [CrossRef]
- Preet, A.; Qamri, Z.; Nasser, M.W.; Prasad, A.; Shilo, K.; Zou, X.; Groopman, J.E.; Ganju, R.K. Cannabinoid receptors, CB1 and CB2, as novel targets for inhibition of non-small cell lung cancer growth and metastasis. Cancer Prev. Res. 2011, 4, 65–75. [Google Scholar] [CrossRef]
- Winkler, K.; Ramer, R.; Dithmer, S.; Ivanov, I.; Merkord, J.; Hinz, B. Fatty acid amide hydrolase inhibitors confer anti-invasive and antimetastatic effects on lung cancer cells. Oncotarget 2016, 7, 15047–15064. [Google Scholar] [CrossRef]
- Xian, X.; Tang, L.; Wu, C.; Huang, L. MiR-23b-3p and miR-130a-5p affect cell growth, migration and invasion by targeting CB1R via the Wnt/β-catenin signaling pathway in gastric carcinoma. Onco. Targets Ther. 2018, 11, 7503–7512. [Google Scholar] [CrossRef]
- Morin-Buote, J.; Ennour-Idrissi, K.; Poirier, É.; Lemieux, J.; Furrer, D.; Burguin, A.; Durocher, F.; Diorio, C. Association of Breast Tumour Expression of Cannabinoid Receptors CBR1 and CBR2 with Prognostic Factors and Survival in Breast Cancer Patients. J. Pers. Med. 2021, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gomez, E.; Andradas, C.; Blasco-Benito, S.; Caffarel, M.M.; García-Taboada, E.; Villa-Morales, M.C.; Moreno, E.; Hamann, S.; Martin-Villar, E.; Flores, J.M.; et al. Role of Cannabinoid Receptor CB2 in HER2 Pro-oncogenic Signaling in Breast Cancer. J. Natl. Cancer Inst. 2015, 107, djv077. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, M.; Ahirwar, D.; Ravi, J.; Nasser, M.W.; Ganju, R.K. Novel role of cannabinoid receptor 2 in inhibiting EGF/EGFR and IGF-I/IGF-IR pathways in breast cancer. Oncotarget 2017, 8, 29668–29678. [Google Scholar] [CrossRef] [PubMed]
- Nasser, M.W.; Qamri, Z.; Deol, Y.S.; Smith, D.; Shilo, K.; Zou, X.; Ganju, R.K. Crosstalk between Chemokine Receptor CXCR4 and Cannabinoid Receptor CB2 in Modulating Breast Cancer Growth and Invasion. PLoS ONE 2011, 6, e23901. [Google Scholar] [CrossRef]
- Zhou, X.-L.; Guo, X.; Song, Y.-P.; Zhu, C.-Y.; Zou, W. The LPI/GPR55 axis enhances human breast cancer cell migration via HBXIP and p-MLC signaling. Acta Pharmacol. Sin. 2017, 39, 459–471. [Google Scholar] [CrossRef]
- Andradas, C.; Benito, S.B.; Castillo-Lluva, S.; Pilla, P.D.; Alarcia, R.D.; Garcia, A.J.; García-Taboada, E.; Hernando-Llorente, R.; Soriano, J.; Hamann, S.; et al. Activation of the orphan receptor GPR55 by lysophosphatidylinositol promotes metastasis in triple-negative breast cancer. Oncotarget 2016, 7, 47565–47575. [Google Scholar] [CrossRef]
- Bifulco, M.; Laezza, C.; Pisanti, S.; Gazzerro, P. Cannabinoids and cancer: Pros and cons of an antitumour strategy. J. Cereb. Blood Flow Metab. 2006, 148, 123–135. [Google Scholar] [CrossRef]
- Petersen, G.; Moesgaard, B.; Schmid, P.C.; Schmid, H.H.O.; Broholm, H.; Kosteljanetz, M.; Hansen, H.S. Endocannabinoid metabolism in human glioblastomas and meningiomas compared to human non-tumour brain tissue. J. Neurochem. 2005, 93, 299–309. [Google Scholar] [CrossRef]
- Schmid, P.C.; Wold, L.E.; Krebsbach, R.J.; Berdyshev, E.V.; Schmid, H.H.O. Anandamide and other N-acylethanolamines in human tumors. Lipids 2002, 37, 907–912. [Google Scholar] [CrossRef]
- Pagotto, U.; Marsicano, G.; Fezza, F.; Theodoropoulou, M.; Grübler, Y.; Stalla, J.; Arzberger, T.; Milone, A.; Losa, M.; Di Marzo, V.; et al. Normal Human Pituitary Gland and Pituitary Adenomas Express Cannabinoid Receptor Type 1 and Synthesize Endogenous Cannabinoids: First Evidence for a Direct Role of Cannabinoids on Hormone Modulation at the Human Pituitary Level. J. Clin. Endocrinol. Metab. 2001, 86, 2687–2696. [Google Scholar] [PubMed]
- Nithipatikom, K.; Endsley, M.P.; Isbell, M.A.; Falck, J.R.; Iwamoto, Y.; Hillard, C.J. 2-Arachidonoylglycerol: A Novel Inhibitor of Androgen-Independent Prostate Cancer Cell Invasion. Cancer Res. 2004, 64, 8826–8830. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; de Ceballos, M.L.; Gomez del Pulgar, T.; Rueda, D.; Corbacho, C.; Velasco, G.; Galve-Roperh, I.; Huffman, J.W.; Ramón y Cajal, S.; Guzmán, M. Inhibition of glioma growth in vivo by selective activation of the CB(2) cannabinoid receptor. Cancer Res. 2001, 61, 5784–5789. [Google Scholar] [PubMed]
- Ellert-Miklaszewska, A.; Grajkowska, W.; Gabrusiewicz, K.; Kaminska, B.; Konarska, L. Distinctive pattern of cannabinoid receptor type II (CB2) expression in adult and pediatric brain tumors. Brain Res. 2007, 1137, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Bashi, S.; Zali, A. The expression level of cannabinoid receptors type 1 and 2 in the different types of astrocytomas. Mol. Biol. Rep. 2020, 47, 5461–5467. [Google Scholar] [CrossRef]
- Wu, X.; Han, L.; Zhang, X.; Li, L.; Jiang, C.; Qiu, Y.; Huang, R.; Xie, B.; Lin, Z.; Ren, J.; et al. Alteration of endocannabinoid system in human gliomas. J. Neurochem. 2012, 120, 842–849. [Google Scholar] [CrossRef]
- Choucair, N.; Saker, Z.; Eddine, H.K.; Bahmad, H.F.; Fares, Y.; Zaarour, M.; Harati, H.; Nabha, S. Immunohistochemical assessment of cannabinoid type-1 receptor (CB1R) and its correlation with clinicopathological parameters in glioma. Pathologica 2022, 114, 128–137. [Google Scholar] [CrossRef]
- Piñeiro, R.; Maffucci, T.; Falasca, M. The putative cannabinoid receptor GPR55 defines a novel autocrine loop in cancer cell proliferation. Oncogene 2010, 30, 142–152. [Google Scholar] [CrossRef]
- Hu, G.; Ren, G.; Shi, Y. The putative cannabinoid receptor GPR55 promotes cancer cell proliferation. Oncogene 2010, 30, 139–141. [Google Scholar] [CrossRef]
- Benz, A.H.; Renné, C.; Maronde, E.; Koch, M.; Grabiec, U.; Kallendrusch, S.; Rengstl, B.; Newrzela, S.; Hartmann, S.; Hansmann, M.-L.; et al. Expression and Functional Relevance of Cannabinoid Receptor 1 in Hodgkin Lymphoma. PLoS ONE 2013, 8, e81675. [Google Scholar] [CrossRef]
- Islam, T.C.; Asplund, A.C.; Lindvall, J.M.; Nygren, L.; Liden, J.; Kimby, E.; Christensson, B.; Smith, C.I.; Sander, B. High level cannabinoid receptor 1, resistance of regulator G protein signaling 13 and differential expression of Cyclin D1 in mantle cell lymphoma. Leukemia 2003, 17, 1880–1890. [Google Scholar] [CrossRef] [PubMed]
- Freund, P.; Porpaczy, E.A.; Le, T.; Gruber, M.; Pausz, C.; Staber, P.; Jäger, U.; Vanura, K. Cannabinoid Receptors Are Overexpressed in CLL but of Limited Potential for Therapeutic Exploitation. PLoS ONE 2016, 11, e0156693. [Google Scholar] [CrossRef] [PubMed]
- Jordà, M.A.; Rayman, N.; Tas, M.; Verbakel, S.E.; Battista, N.; van Lom, K.; Löwenberg, B.; Maccarrone, M.; Delwel, R. The peripheral cannabinoid receptor Cb2, frequently expressed on AML blasts, either induces a neutrophilic differentiation block or confers abnormal migration properties in a ligand-dependent manner. Blood 2004, 104, 526–534. [Google Scholar] [CrossRef]
- Ronchi, A.; Grauso, F.; Zito Marino, F.; Quagliariello, V.; Maurea, N.; Facchini, G.; Montopoli, M.; Franco, R.; Berretta, M.; Messalli, E.M. Endocannabinoid system expression in ovarian epithelial tumors according to the dualistic model of ovarian carcinogenesis. Eutopean Rev. Med. Pharmacol. Sci. 2021, 25, 4678–4686. [Google Scholar]
- Messalli, E.M.; Grauso, F.; Luise, R.; Angelini, A.; Rossiello, R. Cannabinoid receptor type 1 immunoreactivity and disease severity in human epithelial ovarian tumors. Am. J. Obstet. Gynecol. 2014, 211, 234.e1–234.e6. [Google Scholar] [CrossRef]
- Carracedo, A.; Gironella, M.; Lorente, M.; Garcia, S.; Guzmán, M.; Velasco, G.; Iovanna, J.L. Cannabinoids Induce Apoptosis of Pancreatic Tumor Cells via Endoplasmic Reticulum Stress–Related Genes. Cancer Res. 2006, 66, 6748–6755. [Google Scholar] [CrossRef]
- Czifra, G.; Varga, A.; Nyeste, K.; Marincsák, R.; Tóth, B.I.; Kovács, I.; Kovács, L.; Bíró, T. Increased expressions of cannabinoid receptor-1 and transient receptor potential vanilloid-1 in human prostate carcinoma. J. Cancer Res. Clin. Oncol. 2009, 135, 507–514. [Google Scholar] [CrossRef]
- Sarfaraz, S.; Afaq, F.; Adhami, V.M.; Mukhtar, H. Cannabinoid Receptor as a Novel Target for the Treatment of Prostate Cancer. Cancer Res. 2005, 65, 1635–1641. [Google Scholar] [CrossRef]
- Chung, S.C.; Hammarsten, P.; Josefsson, A.; Stattin, P.; Granfors, T.; Egevad, L.; Mancini, G.; Lutz, B.; Bergh, A.; Fowler, C.J. A high cannabinoid CB1 receptor immunoreactivity is associated with disease severity and outcome in prostate cancer. Eur. J. Cancer 2009, 45, 174–182. [Google Scholar] [CrossRef]
- Casanova, M.L.; Blázquez, C.; Martínez-Palacio, J.; Villanueva, C.; Fernández-Aceñero, M.J.; Huffman, J.W.; Jorcano, J.L.; Guzmán, M. Inhibition of skin tumor growth and angiogenesis in vivo by activation of cannabinoid receptors. J. Clin. Investig. 2003, 111, 43–50. [Google Scholar] [CrossRef]
- Blázquez, C.; Carracedo, A.; Barrado, L.; Real, P.J.; Fernández-Luna, J.L.; Velasco, G.; Malumbres, M.; Guzmán, M. Cannabinoid receptors as novel targets for the treatment of melanoma. FASEB J. 2006, 20, 2633–2635. [Google Scholar] [CrossRef] [PubMed]
- Lakiotaki, E.; Giaginis, C.; Tolia, M.; Alexandrou, P.; Delladetsima, I.; Giannopoulou, I.; Kyrgias, G.; Patsouris, E.; Theocharis, S. Clinical Significance of Cannabinoid Receptors CB1 and CB2 Expression in Human Malignant and Benign Thyroid Lesions. BioMed Res. Int. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Guida, M.; Ligresti, A.; De Filippis, D.; D’Amico, A.; Petrosino, S.; Cipriano, M.; Bifulco, G.; Simonetti, S.; Orlando, P.; Insabato, L.; et al. The levels of the endocannabinoid receptor CB2 and its ligand 2-arachidonoylglycerol are elevated in endometrial carcinoma. Endocrinology 2010, 151, 921–928. [Google Scholar] [CrossRef]
- Hijiya, N.; Shibata, T.; Daa, T.; Hamanaka, R.; Uchida, T.; Matsuura, K.; Tsukamoto, Y.; Nakada, C.; Iha, H.; Inomata, M.; et al. Overexpression of cannabinoid receptor 1 in esophageal squamous cell carcinoma is correlated with metastasis to lymph nodes and distant organs, and poor prognosis. Pathol. Int. 2016, 67, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Nulent, T.J.; Van Diest, P.J.; Van Der Groep, P.; Leusink, F.K.; Kruitwagen, C.L.; Koole, R.; Van Cann, E.M. Cannabinoid receptor-2 immunoreactivity is associated with survival in squamous cell carcinoma of the head and neck. Br. J. Oral Maxillofac. Surg. 2013, 51, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Suk, K.-T.; Mederacke, I.; Gwak, G.-Y.; Cho, S.W.; Adeyemi, A.; Friedman, R.; Schwabe, R.F. Opposite roles of cannabinoid receptors 1 and 2 in hepatocarcinogenesis. Gut 2016, 65, 1721–1732. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Huang, S.; Liu, G.; Xie, C.; Zhou, J.; Fan, W.; Li, Q.; Wang, Q.; Zhong, D.; et al. Overexpression of cannabinoid receptors CB1 and CB2 correlates with improved prognosis of patients with hepatocellular carcinoma. Cancer Genet. Cytogenet. 2006, 171, 31–38. [Google Scholar] [CrossRef]
- Yang, J.; Tian, Y.; Zheng, R.; Li, L.; Qiu, F. Endocannabinoid system and the expression of endogenous ceramides in human hepatocellular carcinoma. Oncol. Lett. 2019, 18, 1530–1538. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Zhu, L.; Zou, Y.; Kong, W.; Dong, B.; Huang, J.; Chen, Y.; Xue, W.; Huang, Y.; et al. Cannabinoid receptor 2 as a novel target for promotion of renal cell carcinoma prognosis and progression. J. Cancer Res. Clin. Oncol. 2017, 144, 39–52. [Google Scholar] [CrossRef]
- Larrinaga, G.; Sanz, B.; Blanco, L.; Perez, I.; Candenas, M.L.; Pinto, F.M.; Irazusta, A.; Gil, J.; López, J.I. Cannabinoid CB1 receptor is expressed in chromophobe renal cell carcinoma and renal oncocytoma. Clin. Biochem. 2013, 46, 638–641. [Google Scholar] [CrossRef]
- Theocharis, S.; Giaginis, C.; Alexandrou, P.; Rodríguez, J.; Tasoulas, J.; Danas, E.; Patsouris, E.; Klijanienko, J. Evaluation of cannabinoid CB1 and CB2 receptors expression in mobile tongue squamous cell carcinoma: Associations with clinicopathological parameters and patients’ survival. Tumor Biol. 2015, 37, 3647–3656. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, S.B.; Lindgren, T.; Jonsson, M.; Jacobsson, S.O.P. Cannabinoid receptor-independent cytotoxic effects of cannabinoids in human colorectal carcinoma cells: Synergism with 5-fluorouracil. Cancer Chemother. Pharmacol. 2008, 63, 691–701. [Google Scholar] [CrossRef]
- Ramer, R.; Hinz, B. Inhibition of Cancer Cell Invasion by Cannabinoids via Increased Expression of Tissue Inhibitor of Matrix Metalloproteinases-1. JNCI J. Natl. Cancer Inst. 2008, 100, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Michalski, C.W.; Oti, F.E.; Erkan, M.; Sauliunaite, D.; Bergmann, F.; Pacher, P.; Batkai, S.; Müller, M.W.; Giese, N.A.; Friess, H.; et al. Cannabinoids in pancreatic cancer: Correlation with survival and pain. Int. J. Cancer 2007, 122, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Tutino, V.; Caruso, M.G.; De Nunzio, V.; Lorusso, D.; Veronese, N.; Gigante, I.; Notarnicola, M.; Giannelli, G. Down-Regulation of Cannabinoid Type 1 (CB1) Receptor and its Downstream Signaling Pathways in Metastatic Colorectal Cancer. Cancers 2019, 11, 708. [Google Scholar] [CrossRef]
- Wang, D.; Wang, H.; Ning, W.; Backlund, M.G.; Dey, S.K.; DuBois, R.N. Loss of Cannabinoid Receptor 1 Accelerates Intestinal Tumor Growth. Cancer Res. 2008, 68, 6468–6476. [Google Scholar] [CrossRef]
- Larrinaga, G.; Begoña, S.; Itxaro, P.; Blanco, L.; Maria, L.C.; Pinto, F.M.; Gil, J.; José, I.L. Cannabinoid CB1 receptor is downregulated in clear cell renal cell carcinoma. J. Histochem. Cytochem. 2010, 58, 1129–1134. [Google Scholar] [CrossRef]
- Li, M.; Qian, X.; Zhu, M.; Li, A.; Fang, M.; Zhu, Y.; Zhang, J. MiR-1273g-3p promotes proliferation, migration and invasion of LoVo cells via cannabinoid receptor 1 through activation of ERBB4/PIK3R3/mTOR/S6K2 signaling pathway. Mol. Med. Rep. 2018, 17, 4619–4626. [Google Scholar] [CrossRef]
- Ayakannu, T.; Taylor, A.H.; Konje, J.C. Expression of the putative cannabinoid receptor GPR55 is increased in endometrial carcinoma. Histochem. Cell Biol. 2021, 156, 449–460. [Google Scholar] [CrossRef]
- Pérez-Gómez, E.; Andradas, C.; Flores, J.M.; Quintanilla, M.; Paramio, J.M.; Guzmán, M.; Sánchez, C. The orphan receptor GPR55 drives skin carcinogenesis and is upregulated in human squamous cell carcinomas. Oncogene 2012, 32, 2534–2542. [Google Scholar] [CrossRef]
- He, D.; Wang, J.; Zhang, C.; Shan, B.; Deng, X.; Li, B.; Zhou, Y.; Chen, W.; Hong, J.; Gao, Y.; et al. Down-regulation of miR-675-5p contributes to tumor progression and development by targeting pro-tumorigenic GPR55 in non-small cell lung cancer. Mol. Cancer 2015, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Simon, V.; Cota, D. MECHANISMS IN ENDOCRINOLOGY: Endocannabinoids and metabolism: Past, present and future. Eur. J. Endocrinol. 2017, 176, R309–R324. [Google Scholar] [CrossRef] [PubMed]
- Ligresti, A.; Bisogno, T.; Matias, I.; De Petrocellis, L.; Cascio, M.G.; Cosenza, V.; D’Argenio, G.; Scaglione, G.; Bifulco, M.; Sorrentini, I.; et al. Possible endocannabinoid control of colorectal cancer growth. Gastroenterology 2003, 125, 677–687. [Google Scholar] [CrossRef]
- Chen, L.; Chen, H.; Li, Y.; Li, L.; Qiu, Y.; Ren, J. Endocannabinoid and ceramide levels are altered in patients with colorectal cancer. Oncol. Rep. 2015, 34, 447–454. [Google Scholar] [CrossRef]
- Auer, M.K.; Gebert, D.; Biedermann, S.V.; Bindila, L.; Stalla, G.; Reisch, N.; Kopczak, A.; Fuss, J. Altered endocannabinoid-dynamics in craniopharyngioma patients and their association with HPA-axis disturbances. Eur. J. Endocrinol. 2021, 1, 178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Medina-Cleghorn, D.; Bernal-Mizrachi, L.; Bracci, P.M.; Hubbard, A.; Conde, L.; Riby, J.; Nomura, D.K.; Skibola, C.F. The potential relevance of the endocannabinoid, 2-arachidonoylglycerol, in diffuse large B-cell lymphoma. Oncoscience 2016, 3, 31–41. [Google Scholar] [CrossRef][Green Version]
- Park, S.-W.; Kim, J.-E.; Oh, S.-M.; Cha, W.-J.; Hah, J.-H.; Sung, M.-W. Anticancer effects of anandamide on head and neck squamous cell carcinoma cells via the production of receptor-independent reactive oxygen species. Head Neck 2014, 37, 1187–1192. [Google Scholar] [CrossRef]
- Önay, Ö.; Köse, S.; Süslü, N.; Korkusuz, P.; Nemutlu, E.; Aydın, C.; Hosal, S. Human laryngeal squamous cell carcinoma cell line release of endogenous anandamide and 2-arachidonoylglycerol, and their antiproliferative effect via exogenous supplementation: An in vitro study. Cell Tissue Bank 2022, 23, 93–100. [Google Scholar]
- Qiu, C.; Yang, L.; Wang, B.; Cui, L.; Li, C.; Zhuo, Y.; Zhang, L.; Zhang, S.; Zhang, Q.; Wang, X. The role of 2-arachidonoylglycerol in the regulation of the tumor-immune microenvironment in murine models of pancreatic cancer. Biomed. Pharmacother. 2019, 115, 108952. [Google Scholar] [CrossRef]
- Ford, L.A.; Roelofs, A.J.; Anavi-Goffer, S.; Mowat, L.; Simpson, D.G.; Irving, A.J.; Rogers, M.J.; Rajnicek, A.M.; Ross, R.A. A role for L-α-lysophosphatidylinositol and GPR55 in the modulation of migration, orientation and polarization of human breast cancer cells. Br. J. Pharmacol. 2010, 160, 762–771. [Google Scholar] [CrossRef]
- Hofmann, N.A.; Yang, J.; Trauger, S.A.; Nakayama, H.; Huang, L.; Strunk, D.; Moses, M.A.; Klagsbrun, M.; Bischoff, J.; Graier, W.F. The GPR 55 agonist, L-α-lysophosphatidylinositol, mediates ovarian carcinoma cell-induced angiogenesis. Br. J. Pharmacol. 2015, 172, 4107–4118. [Google Scholar] [CrossRef] [PubMed]
- Akimov, M.G.; Gamisonia, A.M.; Dudina, P.V.; Gretskaya, N.M.; Gaydaryova, A.A.; Kuznetsov, A.S.; Zinchenko, G.N.; Bezuglov, V.V. GPR55 Receptor Activation by the N-acyl Dopamine Family Lipids Induces Apoptosis in Cancer Cells via the Nitric Oxide Synthase (nNOS) Over-Stimulation. Int. J. Mol. Sci. 2021, 22, 622. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Soboci, A.A.; Czarnecka, A.; Król, M.; Botta, B.; Szczylik, C. The Therapeutic Aspects of the Endocannabinoid System (ECS) for Cancer and their Development: From Nature to Laboratory. Curr. Pharm. Des. 2016, 22, 1756–1766. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, R.; Ramer, R.; Hinz, B. Targeting the endocannabinoid system as a potential anticancer approach. Drug Metab. Rev. 2018, 50, 26–53. [Google Scholar] [CrossRef] [PubMed]
- Ramer, R.; Hinz, B. Antitumorigenic targets of cannabinoids—Current status and implications. Expert Opin. Ther. Targets 2016, 20, 1219–1235. [Google Scholar] [CrossRef]
- Thors, L.; Bergh, A.; Persson, E.; Hammarsten, P.; Stattin, P.; Egevad, L.; Granfors, T.; Fowler, C.J. Fatty Acid Amide Hydrolase in Prostate Cancer: Association with Disease Severity and Outcome, CB1 Receptor Expression and Regulation by IL-4. PLoS ONE 2010, 5, e12275. [Google Scholar] [CrossRef]
- Fowler, C.J.; Josefsson, A.; Thors, L.; Chung, S.C.; Hammarsten, P.; Wikström, P.; Bergh, A. Tumour epithelial expression levels of endocannabinoid markers modulate the value of endoglin-positive vascular density as a prognostic marker in prostate cancer. Biochim. et Biophys. Acta BBA-Mol. Cell Biol. Lipids 2013, 1831, 1579–1587. [Google Scholar] [CrossRef]
- Endsley, M.P.; Thill, R.; Choudhry, I.; Williams, C.L.; Kajdacsy-Balla, A.; Campbell, W.B.; Nithipatikom, K. Expression and function of fatty acid amide hydrolase in prostate cancer. Int. J. Cancer 2008, 123, 1318–1326. [Google Scholar] [CrossRef]
- Wasilewski, A.; Krajewska, U.; Owczarek, K.; Lewandowska, U.; Fichna, J. Fatty acid amide hydrolase (FAAH) inhibitor PF-3845 reduces viability, migration and invasiveness of human colon adenocarcinoma Colo-205 cell line: An in vitro study. Acta Biochim. Pol. 2017, 64, 519–525. [Google Scholar] [CrossRef]
- Ayakannu, T.; Taylor, A.H.; Bari, M.; Mastrangelo, N.; Maccarrone, M.; Konje, J.C. Expression and Function of the Endocannabinoid Modulating Enzymes Fatty Acid Amide Hydrolase and N-Acylphosphatidylethanolamine-Specific Phospholipase D in Endometrial Carcinoma. Front. Oncol. 2019, 9, 1363. [Google Scholar] [CrossRef]
- Ayakannu, T.; Taylor, A.H.; Marczylo, T.H.; Konje, J.C. New Insights of Uterine Leiomyoma Pathogenesis: Endocannabinoid System. Med. Sci. Monit. Basic Res. 2019, 25, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, W.; Zhao, Y.; Zhou, J.; Wang, X.; Pan, Q.; Zhang, N.; Wang, L.; Wang, M.; Zhan, Z.; et al. Monoacylglycerol lipase promotes progression of hepatocellular carcinoma via NF-κB-mediated epithelial-mesenchymal transition. J. Hematol. Oncol. 2016, 25, 9. [Google Scholar]
- Liu, Y.; Yang, X.L.; Gong, J.P.; Zhang, J.Y. Monoacylglycerol lipase high expression as an independent indicator of survival for patients with hepatocellular carcinoma. Zhonghua Gan Zang Bing Za Zhi 2019, 27, 760–765. [Google Scholar] [PubMed]
- Wang, C.; Li, Z.; Zhong, L.; Chen, Y. Inhibition of monoacylglycerol lipase restrains proliferation, migration, invasion, tumor growth and induces apoptosis in cervical cancer. J. Obstet. Gynaecol. Res. 2021, 15, 110. [Google Scholar] [CrossRef]
- Li, X.; Gao, S.; Li, W.; Liu, Z.; Shi, Z.; Qiu, C.; Jiang, J. Effect of monoacylglycerol lipase on the tumor growth in endometrial cancer. J. Obstet. Gynaecol. Res. 2019, 45, 2043–2054. [Google Scholar] [CrossRef]
- Gong, X.; Zheng, X.; Huang, Y.; Song, W.; Chen, G.; Chen, T. Monoacylglycerol Lipase (MAGL) Inhibition Impedes the Osteosarcoma Progression by Regulating Epithelial Mesenchymal Transition. Tohoku J. Exp. Med. 2022, 256, 19–26. [Google Scholar] [CrossRef]
- Nomura, D.K.; Long, J.Z.; Niessen, S.; Hoover, H.S.; Ng, S.-W.; Cravatt, B.F. Monoacylglycerol Lipase Regulates a Fatty Acid Network that Promotes Cancer Pathogenesis. Cell 2010, 140, 49–61. [Google Scholar] [CrossRef]
- Carbonetti, G.; Wilpshaar, T.; Kroonen, J.; Studholme, K.; Converso, C.; D’Oelsnitz, S.; Kaczocha, M. FABP5 coordinates lipid signaling that promotes prostate cancer metastasis. Sci. Rep. 2019, 9, 18944. [Google Scholar] [CrossRef]
- Hu, W.R.; Lian, Y.F.; Peng, L.X.; Lei, J.J.; Deng, C.C.; Xu, M.; Feng, Q.S.; Chen, L.Z.; Bei, J.X.; Zeng, Y.X. Monoacylglycerol lipase promotes metastases in nasopharyngeal carcinoma. Int. J. Clin. Exp. Pathol. 2014, 7, 832. [Google Scholar]
- Sriram, K.; Insel, P.A. G protein-coupled receptors as targets for approved drugs: How many targets and how many drugs? In: Molecular Pharmacology. Mol. Pharmacol. 2018, 93, 251–258. [Google Scholar] [CrossRef]
- Insel, P.A.; Sriram, K.; Wiley, S.Z.; Wilderman, A.; Katakia, T.; McCann, T.; Yokouchi, H.; Zhang, L.; Corriden, R.; Liu, D.; et al. GPCRomics: GPCR Expression in Cancer Cells and Tumors Identifies New, Potential Biomarkers and Therapeutic Targets. Front. Pharmacol. 2018, 9, 431. [Google Scholar] [CrossRef]
- Marcu, J.P.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Horowitz, M.P.; Lee, J.; Pakdel, A.; Allison, J.; Limbad, C.; Moore, D.H.; et al. Cannabidiol Enhances the Inhibitory Effects of Δ9-Tetrahydrocannabinol on Human Glioblastoma Cell Proliferation and Survival. Mol. Cancer Ther. 2010, 9, 180–189. [Google Scholar] [CrossRef]
- Blázquez, C.; González-Feria, L.; Lvarez, L.A.´.; Haro, A.; Llanos Casanova, M.; Guzmán, M. Cannabinoids Inhibit the Vascular Endothelial Growth Factor Pathway in Gliomas. Cancer Res. 2004, 64, 5617–5623. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Hegde, V.L.; Singh, N.P.; Sisco, D.; Grant, S.; Nagarkatti, M.; Nagarkatti, P.S. Δ9-Tetrahydrocannabinol-Induced Apoptosis in Jurkat Leukemia T Cells Is Regulated by Translocation of Bad to Mitochondria. Mol. Cancer Res. 2006, 4, 549–562. [Google Scholar] [CrossRef] [PubMed]
- McKallip, R.J.; Jia, W.; Schlomer, J.; Warren, J.W.; Nagarkatti, P.S.; Nagarkatti, M. Cannabidiol-Induced Apoptosis in Human Leukemia Cells: A Novel Role of Cannabidiol in the Regulation of p22phox and Nox4 Expression. Mol. Pharmacol. 2006, 70, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Gallotta, D.; Nigro, P.; Cotugno, R.; Gazzerro, P.; Bifulco, M.; Belisario, M.A. Rimonabant-induced apoptosis in leukemia cell lines: Activation of caspase-dependent and -independent pathways. Biochem. Pharmacol. 2010, 80, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Mannino, F.; Pallio, G.; Corsaro, R.; Minutoli, L.; Altavilla, D.; Vermiglio, G.; Allegra, A.; Eid, A.H.; Bitto, A.; Squadrito, F.; et al. Beta-Caryophyllene Exhibits Anti-Proliferative Effects through Apoptosis Induction and Cell Cycle Modulation in Multiple Myeloma Cells. Cancers 2021, 13, 5741. [Google Scholar] [CrossRef]
- Gado, F.; Ferrisi, R.; Di Somma, S.; Napolitano, F.; Mohamed, K.A.; Stevenson, L.A.; Rapposelli, S.; Saccomanni, G.; Portella, G.; Pertwee, R.G.; et al. Synthesis and In Vitro Characterization of Selective Cannabinoid CB2 Receptor Agonists: Biological Evaluation against Neuroblastoma Cancer Cells. Molecules 2022, 27, 3019. [Google Scholar] [CrossRef]
- Caffarel, M.M.; Andradas, C.; Mira, E.; Pérez-Gómez, E.; Cerutti, C.; Moreno-Bueno, G.; Flores, J.M.; García-Real, I.; Palacios, J.; Mañes, S.; et al. Cannabinoids reduce ErbB2-driven breast cancer progression through Akt inhibition. Mol. Cancer 2010, 9, 196. [Google Scholar] [CrossRef]
- Melck, D.; Rueda, D.; Galve-Roperh, I.; de Petrocellis, L.; Guzmän, M.; Di Marzo, V. Involvement of the cAMP/protein kinase A pathway and of mitogen-activated protein kinase in the anti-proliferative effects of anandamide in human breast cancer cells. FEBS Lett. 1999, 463, 235–240. [Google Scholar] [CrossRef]
- Qamri, Z.; Preet, A.; Nasser, M.W.; Bass, C.E.; Leone, G.; Barsky, S.H.; Ganju, R.K. Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer. Mol. Cancer Ther. 2009, 8, 3117–3129. [Google Scholar] [CrossRef] [PubMed]
- Khunluck, T.; Lertsuwan, K.; Chutoe, C.; Sooksawanwit, S.; Inson, I.; Teerapornpuntakit, J.; Tohtong, R.; Charoenphandhu, N. Activation of cannabinoid receptors in breast cancer cells improves osteoblast viability in cancer-bone interaction model while reducing breast cancer cell survival and migration. Sci. Rep. 2022, 12, 1–15. [Google Scholar] [CrossRef]
- Greenhough, A.; Patsos, H.A.; Williams, A.C.; Paraskeva, C. The cannabinoid Δ9-tetrahydrocannabinol inhibits RAS-MAPK and PI3K-AKT survival signalling and induces BAD-mediated apoptosis in colorectal cancer cells. Int. J. Cancer 2007, 121, 2172–2180. [Google Scholar] [CrossRef] [PubMed]
- Proto, M.C.; Gazzerro, P.; Di Croce, L.; Santoro, A.; Malfitano, A.M.; Pisanti, S.; Laezza, C.; Bifulco, M. Interaction of endocannabinoid system and steroid Hormones in the control of colon cancer cell growth. J. Cell. Physiol. 2011, 227, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Refolo, M.G.; D’Alessandro, R.; Malerba, N.; Laezza, C.; Bifulco, M.; Messa, C.; Caruso, M.G.; Notarnicola, M.; Tutino, V. Anti Proliferative and Pro Apoptotic Effects of Flavonoid Quercetin Are Mediated by CB1 Receptor in Human Colon Cancer Cell Lines. J. Cell. Physiol. 2015, 230, 2973–2980. [Google Scholar] [CrossRef]
- Ding, F.; Qiu, C.; Li, W.; Liu, Z.; Kong, D.; Ma, X.; Jiang, J. CNR1 may reverse progesterone-resistance of endometrial cancer through the ERK pathway. Biochem. Biophys. Res. Commun. 2021, 548, 148–154. [Google Scholar] [CrossRef]
- Rao, M.; Chen, D.; Zhan, P.; Jiang, J. MDA19, a novel CB2 agonist, inhibits hepatocellular carcinoma partly through inactivation of AKT signaling pathway. Biol. Direct 2019, 14, 9. [Google Scholar] [CrossRef]
- Boyacıoğlu, C.; Bilgiç, E.; Varan, C.; Bilensoy, E.; Nemutlu, E.; Sevim, D.; Kocaefe, L.; Korkusuz, P. ACPA decreases non-small cell lung cancer line growth through Akt/PI3K and JNK pathways in vitro. Cell Death Dis. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Xu, S.; Ma, H.; Bo, Y.; Shao, M. The oncogenic role of CB2 in the progression of non-small-cell lung cancer. Biomed. Pharmacother. 2019, 117, 109080. [Google Scholar] [CrossRef]
- Olea-Herrero, N.; Vara, D.; Malagarie-Cazenave, S.; Diazlaviada, I. Inhibition of human tumour prostate PC-3 cell growth by cannabinoids R(+)-Methanandamide and JWH-015: Involvement of CB2. Br. J. Cancer 2009, 101, 940–950. [Google Scholar] [CrossRef]
- Sarfaraz, S.; Afaq, F.; Adhami, V.M.; Malik, A.; Mukhtar, H. Cannabinoid receptor agonist-induced apoptosis of human prostate cancer cells LNCaP proceeds through sustained activation of ERK1/2 leading to G 1 cell cycle arrest. J. Biol. Chem. 2006, 281, 39480–39491. [Google Scholar] [CrossRef]
- Pisanti, S.; Borselli, C.; Oliviero, O.; Laezza, C.; Gazzerro, P.; Bifulco, M. Antiangiogenic activity of the endocannabinoid anandamide: Correlation to its tumor-suppressor efficacy. J. Cell. Physiol. 2006, 211, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Galve-Roperh, I.; Sánchez, C.; Cortés, M.L.; Del Pulgar, T.G.; Izquierdo, M.; Guzmán, M. Anti-tumoral action of cannabinoids: Involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat. Med. 2000, 6, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, M.; Pellerito, O.; Portanova, P.; Calvaruso, G.; Santulli, A.; De Blasio, A.; Vento, R.; Tesoriere, G. Apoptosis induced in HepG2 cells by the synthetic cannabinoid WIN: Involvement of the transcription factor PPARγ. Biochimie 2009, 91, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Sarnataro, D.; Pisanti, S.; Santoro, A.; Gazzerro, P.; Malfitano, A.M.; Laezza, C.; Bifulco, M. The Cannabinoid CB1 Receptor Antagonist Rimonabant (SR141716) Inhibits Human Breast Cancer Cell Proliferation through a Lipid Raft-Mediated Mechanism. Mol. Pharmacol. 2006, 70, 1298–1306. [Google Scholar] [CrossRef]
- Li, L.-T.; Zhao, F.-F.; Jia, Z.-M.; Qi, L.-Q.; Zhang, X.-Z.; Zhang, L.; Li, Y.-Y.; Yang, J.-J.; Wang, S.-J.; Lin, H.; et al. Cannabinoid receptors promote chronic intermittent hypoxia-induced breast cancer metastasis via IGF-1R/AKT/GSK-3β. Mol. Ther.-Oncol. 2021, 23, 220–230. [Google Scholar] [CrossRef]
- Martínez-Martínez, E.; Martín-Ruiz, A.; Martín, P.; Calvo, V.; Provencio, M.; García, J.M. CB2 cannabinoid receptor activation promotes colon cancer progression via AKT/GSK3β signaling pathway. Oncotarget 2016, 7, 68781–68791. [Google Scholar] [CrossRef]
- Galve-Roperh, I.; Rueda, D.; del Pulgar, T.G.; Velasco, G.; Guzmán, M. Mechanism of Extracellular Signal-Regulated Kinase Activation by the CB1 Cannabinoid Receptor. Mol. Pharmacol. 2002, 62, 1385–1392. [Google Scholar] [CrossRef]
- Liu, C.; Sadat, S.H.; Ebisumoto, K.; Sakai, A.; Panuganti, B.A.; Ren, S.; Goto, Y.; Haft, S.; Fukusumi, T.; Ando, M.; et al. Cannabinoids Promote Progression of HPV-Positive Head and Neck Squamous Cell Carcinoma via p38 MAPK Activation. Clin. Cancer Res. 2020, 26, 2693–2703. [Google Scholar] [CrossRef]
- Andradas, C.; Caffarel, M.M.; Pérez-Gómez, E.; Salazar, M.; Lorente, M.; Velasco, G.; Guzmán, M.; Sánchez, C. The orphan G protein-coupled receptor GPR55 promotes cancer cell proliferation via ERK. Oncogene 2011, 30, 245–252. [Google Scholar] [CrossRef]
- Singh, N.S.; Bernier, M.; Wainer, I.W. Selective GPR55 antagonism reduces chemoresistance in cancer cells. Pharmacol. Res. 2016, 111, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ramirez, J.C.; A Frampton, G.; E Golden, L.; A Quinn, M.; Pae, H.Y.; Horvat, D.; Liang, L.-J.; DeMorrow, S. Anandamide exerts its antiproliferative actions on cholangiocarcinoma by activation of the GPR55 receptor. Lab. Investig. 2011, 91, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Emery, S.M.; Alotaibi, M.R.; Tao, Q.; Selley, D.E.; Lichtman, A.H.; Gewirtz, D.A. Combined Antiproliferative Effects of the Aminoalkylindole WIN55,212-2 and Radiation in Breast Cancer Cells. J. Pharmacol. Exp. Ther. 2013, 348, 293–302. [Google Scholar] [CrossRef]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol Induces Programmed Cell Death in Breast Cancer Cells by Coordinating the Cross-talk between Apoptosis and Autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Cudaback, E.; Marrs, W.; Moeller, T.; Stella, N. The Expression Level of CB1 and CB2 Receptors Determines Their Efficacy at Inducing Apoptosis in Astrocytomas. PLoS ONE 2010, 5, e8702. [Google Scholar] [CrossRef]
- Sarker, K.P.; Biswas, K.K.; Yamakuchi, M.; Lee, K.-Y.; Hahiguchi, T.; Kracht, M.; Kitajima, I.; Maruyama, I. ASK1-p38 MAPK/JNK signaling cascade mediates anandamide-induced PC12 cell death. J. Neurochem. 2003, 85, 50–61. [Google Scholar] [CrossRef]
- Soto-Mercado, V.; Mendivil-Perez, M.; Jimenez-Del-Rio, M.; E Fox, J.; Velez-Pardo, C. Cannabinoid CP55940 selectively induces apoptosis in Jurkat cells and in ex vivo T-cell acute lymphoblastic leukemia through H2O2 signaling mechanism. Leuk. Res. 2020, 95, 106389. [Google Scholar] [CrossRef]
- Elbaz, M.; Nasser, M.W.; Ravi, J.; Wani, N.A.; Ahirwar, D.K.; Zhao, H.; Oghumu, S.; Satoskar, A.R.; Shilo, K.; Carson, W.E.; et al. Modulation of the tumor microenvironment and inhibition of EGF/EGFR pathway: Novel anti-tumor mechanisms of Cannabidiol in breast cancer. Mol. Oncol. 2015, 9, 906–919. [Google Scholar] [CrossRef]
- Ivanov, V.N.; Wu, J.; Hei, T.K. Regulation of human glioblastoma cell death by combined treatment of cannabidiol, γ-radiation and small molecule inhibitors of cell signaling pathways. Oncotarget 2017, 8, 74068–74095. [Google Scholar] [CrossRef]
- Frampton, G.; Coufal, M.; Li, H.; Ramirez, J.; DeMorrow, S. Opposing actions of endocannabinoids on cholangiocarcinoma growth is via the differential activation of Notch signaling. Exp. Cell Res. 2010, 316, 1465–1478. [Google Scholar] [CrossRef]
- DeMorrow, S.; Francis, H.; Gaudio, E.; Venter, J.; Franchitto, A.; Kopriva, S.; Onori, P.; Mancinelli, R.; Frampton, G.; Coufal, M.; et al. The endocannabinoid anandamide inhibits cholangiocarcinoma growth via activation of the noncanonical Wnt signaling pathway. Am. J. Physiol. Liver Physiol. 2008, 295, G1150–G1158. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, D.; Cherkasova, V.; Gerasymchuk, M.; Narendran, A.; Kovalchuk, I.; Kovalchuk, O. Cannabinol Inhibits Cellular Proliferation, Invasion, and Angiogenesis of Neuroblastoma via Novel miR-34a/tRiMetF31/PFKFB3 Axis. Cancers 2022, 14, 1908. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, D.; Kovalchuk, I.; Apel, I.J.; Chinnaiyan, A.M.; Wóycicki, R.K.; Cantor, C.R.; Kovalchuk, O. miR-34a directly targets tRNAiMet precursors and affects cellular proliferation, cell cycle, and apoptosis. Proc. Natl. Acad. Sci. USA 2018, 115, 7392–7397. [Google Scholar] [CrossRef] [PubMed]
- Pyszniak, M.; Tabarkiewicz, J.; Łuszczki, J.J. Endocannabinoid system as a regulator of tumor cell malignancy–biological pathways and clinical significance. OncoTargets Ther. 2016, 9, 4323–4336. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, M. Cannabinoids: Potential anticancer agents. Nature Reviews Cancer. Eur. Assoc. Cardio-Thorac. Surg. 2003, 3, 745–755. [Google Scholar]
- Blázquez, C.; Casanova, M.L.; Planas, A.; del Pulgar, T.G.; Villanueva, C.; Fernández-Aceñero, M.J.; Aragones, J.; Huffman, J.W.; Jorcano, J.L.; Guzman, M. Inhibition of tumor angiogenesis by cannabinoids. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biology. 2003, 17, 529–531. [Google Scholar] [CrossRef]
- Vaccani, A.; Massi, P.; Colombo, A.; Rubino, T.; Parolaro, D. Cannabidiol inhibits human glioma cell migration through a cannabinoid receptor-independent mechanism. J. Cereb. Blood Flow Metab. 2005, 144, 1032–1036. [Google Scholar] [CrossRef]
- Ramer, R.; Bublitz, K.; Freimuth, N.; Merkord, J.; Rohde, H.; Haustein, M.; Borchert, P.; Schmuhl, E.; Linnebacher, M.; Hinz, B. Cannabidiol inhibits lung cancer cell invasion and metastasis via intercellular adhesion molecule-1. FASEB J. 2011, 26, 1535–1548. [Google Scholar] [CrossRef]
- National Toxicology Program: NTP toxicology and carcinogenesis studies of 1-trans-delta(9)-tetrahydrocannabinol (CAS No. 1972-08-3) in F344 rats and B6C3F1 mice (gavage studies). NTP Tech. Rep. 1996, 446, 1–317.
- Donadelli, M.; Dando, I.; Zaniboni, T.; Costanzo, C.; Pozza, E.D.; Scupoli, M.; Scarpa, A.; Zappavigna, S.; Marra, M.; Abbruzzese, A.; et al. Gemcitabine/cannabinoid combination triggers autophagy in pancreatic cancer cells through a ROS-mediated mechanism. Cell Death Dis. 2011, 2, e152. [Google Scholar] [CrossRef]
- Salazar, M.; Carracedo, A.; Salanueva, J.; Hernández-Tiedra, S.; Lorente, M.; Egia, A.; Vázquez, P.; Blázquez, C.; Torres, S.; García, S.; et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J. Clin. Investig. 2009, 119, 1359–1372. [Google Scholar] [CrossRef] [PubMed]
- Redlich, S.; Ribes, S.; Schütze, S.; Czesnik, D.; Nau, R. Palmitoylethanolamide stimulates phagocytosis of Escherichia coli K1 and Streptococcus pneumoniae R6 by microglial cells. J. Neuroimmunol. 2012, 244, 32–34. [Google Scholar] [CrossRef]
- Vara, D.; Salazar, M.; Olea-Herrero, N.; Guzmán, M.; Velasco, G.; Díaz-Laviada, I. Anti-tumoral action of cannabinoids on hepatocellular carcinoma: Role of AMPK-dependent activation of autophagy. Cell Death Differ. 2011, 18, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Galve-Roperh, I.; Rueda, D.; Guzmán, M. Involvement of Sphingomyelin Hydrolysis and the Mitogen-Activated Protein Kinase Cascade in the Δ9-Tetrahydrocannabinol-Induced Stimulation of Glucose Metabolism in Primary Astrocytes. Mol. Pharmacol. 1998, 54, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Ogretmen, B.; Hannun, Y.A. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat. Cancer 2004, 4, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Pellerito, O.; Notaro, A.; Sabella, S.; De Blasio, A.; Vento, R.; Calvaruso, G.; Giuliano, M. WIN induces apoptotic cell death in human colon cancer cells through a block of autophagic flux dependent on PPARγ down-regulation. Apoptosis 2014, 19, 985. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Cascio, M.G. Known Pharmacological Actions of Delta-9-Tetrahydrocannabinol and of Four Other Chemical Constituents of Cannabis that Activate Cannabinoid Receptors. In Handbook of Cannabis; Oxford University Press: Oxford, UK, 2014; Volume 115–136, p. 6. [Google Scholar] [CrossRef]
- Velasco, G.; Sánchez, C.; Guzmán, M. Towards the use of cannabinoids as antitumour agents. Nat. Rev. Cancer 2012, 12, 436–444. [Google Scholar] [CrossRef]
- Jeong, S.; Yun, H.K.; Jeong, Y.A.; Jo, M.J.; Kang, S.H.; Kim, J.L.; Kim, D.Y.; Park, S.H.; Kim, B.R.; Na, Y.J.; et al. Cannabidiol-induced apoptosis is mediated by activation of Noxa in human colorectal cancer cells. Cancer Lett. 2019, 447, 12–23. [Google Scholar] [CrossRef]
- Tubaro, A.; Giangaspero, A.; Sosa, S.; Negri, R.; Grassi, G.; Casano, S.; Della Loggia, R.; Appendino, G.B. Comparative topical anti-inflammatory activity of cannabinoids and cannabivarins. Fitoterapia 2010, 81, 816–819. [Google Scholar] [CrossRef]
- Anil, S.M.; Peeri, H.; Koltai, H. Medical Cannabis Activity Against Inflammation: Active Compounds and Modes of Action. Front. Pharmacol. 2022, 13, 908198. [Google Scholar] [CrossRef]
- Xian, X.-S.; Park, H.; Cho, Y.K.; Lee, I.S.; Kim, S.W.; Choi, M.-G.; Chung, I.-S.; Han, K.-H.; Park, J.M. Effect of a synthetic cannabinoid agonist on the proliferation and invasion of gastric cancer cells. J. Cell. Biochem. 2010, 110, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Ramer, R. Cannabinoids as anticancer drugs: Current status of preclinical Res. Br. J. Cancer 2022, 127, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Caffarel, M.M.; Sarrió, D.; Palacios, J.; Guzmán, M.; Sanchez, C. Δ9-Tetrahydrocannabinol Inhibits Cell Cycle Progression in Human Breast Cancer Cells through Cdc2 Regulation. Cancer Res. 2006, 66, 6615–6621. [Google Scholar] [CrossRef] [PubMed]
- Laezza, C.; Pisanti, S.; Crescenzi, E.; Bifulco, M. Anandamide inhibits Cdk2 and activates Chk1 leading to cell cycle arrest in human breast cancer cells. FEBS Lett. 2006, 580, 6076–6082. [Google Scholar] [CrossRef]
- Go, Y.Y.; Kim, S.R.; Kim, D.Y.; Chae, S.W.; Song, J.J. Cannabidiol enhances cytotoxicity of anti-cancer drugs in human head and neck squamous cell carcinoma. Sci. Rep. 2020, 1, 10. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, Y.; Pan, Z.; Li, M.; Liu, X.; Chen, X.; Qu, G.; Zhou, L.; Xu, M.; Zheng, Q.; et al. Cannabidiol Induces Cell Cycle Arrest and Cell Apoptosis in Human Gastric Cancer SGC-7901 Cells. Biomolecules 2019, 9, 302. [Google Scholar] [CrossRef]
- Massi, P.; Valenti, M.; Vaccani, A.; Gasperi, V.; Perletti, G.; Marras, E.; Fezza, F.; Maccarrone, M.; Parolaro, D. 5-Lipoxygenase and anandamide hydrolase (FAAH) mediate the antitumor activity of cannabidiol, a non-psychoactive cannabinoid. J. Neurochem. 2008, 104, 1091–1100. [Google Scholar] [CrossRef]
- Ramer, R.; Weinzierl, U.; Schwind, B.; Brune, K.; Hinz, B. Ceramide Is Involved in R()-Methanandamide-Induced Cyclooxygenase-2 Expression in Human Neuroglioma Cells. Mol. Pharmacol. 2003, 64, 1189–1198. [Google Scholar] [CrossRef]
- Hinz, B.; Ramer, R.; Eichele, K.; Weinzierl, U.; Brune, K. Up-Regulation of Cyclooxygenase-2 Expression Is Involved in R(+)-Methanandamide-Induced Apoptotic Death of Human Neuroglioma Cells. Mol. Pharmacol. 2004, 66, 1643–1651. [Google Scholar] [CrossRef]
- Eichele, K.; Ramer, R.; Hinz, B. R(+)-Methanandamide-Induced Apoptosis of Human Cervical Carcinoma Cells Involves A Cyclooxygenase-2-Dependent Pathway. Pharm. Res. 2008, 26, 346–355. [Google Scholar] [CrossRef]
- Eichele, K.; Ramer, R.; Hinz, B. Decisive role of cyclooxygenase-2 and lipocalin-type prostaglandin D synthase in chemotherapeutics-induced apoptosis of human cervical carcinoma cells. Oncogene 2007, 27, 3032–3044. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramer, R.; Heinemann, K.; Merkord, J.; Rohde, H.; Salamon, A.; Linnebacher, M.; Hinz, B. COX-2 and PPAR-γ Confer Cannabidiol-Induced Apoptosis of Human Lung Cancer Cells. Mol. Cancer Ther. 2013, 12, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.; Fischer, O.M.; Ullrich, A. Cannabinoids Induce Cancer Cell Proliferation via Tumor Necrosis Factor-Converting Enzyme (TACE/ADAM17)-Mediated Transactivation of the Epidermal Growth Factor Receptor. Cancer Res. 1943, 64, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Miyato, H.; Kitayama, J.; Yamashita, H.; Souma, D.; Asakage, M.; Yamada, J.; Nagawa, H. Pharmacological Synergism Between Cannabinoids and Paclitaxel in Gastric Cancer Cell Lines. J. Surg. Res. 2009, 155, 40–47. [Google Scholar] [CrossRef]
- Aviello, G.; Romano, B.; Borrelli, F.; Capasso, R.; Gallo, L.; Piscitelli, F.; Di Marzo, V.; Izzo, A.A. Chemopreventive effect of the non-psychotropic phytocannabinoid cannabidiol on experimental colon cancer. Klin. Wochenschr. 2012, 90, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Romano, B.; Borrelli, F.; Pagano, E.; Cascio, M.G.; Pertwee, R.G.; Izzo, A.A. Inhibition of colon carcinogenesis by a standardized Cannabis sativa extract with high content of cannabidiol. Phytomedicine 2013, 21, 631–639. [Google Scholar] [CrossRef]
- Ma, C.; Wu, T.-T.; Jiang, P.-C.; Li, Z.-Q.; Chen, X.-J.; Fu, K.; Wang, W.; Gong, R. Anti-carcinogenic activity of anandamide on human glioma in vitro and in vivo. Mol. Med. Rep. 2015, 13, 1558–1562. [Google Scholar] [CrossRef]
- Ramer, R.; Merkord, J.; Rohde, H.; Hinz, B. Cannabidiol inhibits cancer cell invasion via upregulation of tissue inhibitor of matrix metalloproteinases-1. Biochem. Pharmacol. 2010, 79, 955–966. [Google Scholar] [CrossRef]
- Cruz-Munoz, W.; Khokha, R. The Role of Tissue Inhibitors of Metalloproteinases in Tumorigenesis and Metastasis. Crit. Rev. Clin. Lab. Sci. 2008, 45, 291–338. [Google Scholar] [CrossRef]
- Laezza, C.; D’Alessandro, A.; Paladino, S.; Malfitano, A.M.; Proto, M.C.; Gazzerro, P.; Pisanti, S.; Santoro, A.; Ciaglia, E.; Bifulco, M. Anandamide inhibits the Wnt/β-catenin signalling pathway in human breast cancer MDA MB 231 cells. Eur. J. Cancer 2012, 48, 3112–3122. [Google Scholar] [CrossRef]
- García-Morales, L.; Castillo, A.M.; Ramírez, J.T.; Zamudio-Meza, H.; Domínguez-Robles, M.D.C.; Meza, I. CBD Reverts the Mesenchymal Invasive Phenotype of Breast Cancer Cells Induced by the Inflammatory Cytokine IL-1β. Int. J. Mol. Sci. 2020, 21, 2429. [Google Scholar] [CrossRef] [PubMed]
- Pagano, E.; Borrelli, F.; Orlando, P.; Romano, B.; Monti, M.; Morbidelli, L.; Aviello, G.; Imperatore, R.; Capasso, R.; Piscitelli, F.; et al. Pharmacological inhibition of MAGL attenuates experimental colon carcinogenesis. Pharmacol. Res. 2017, 119, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Kang, Y.; Park, P.-H.; Noh, S.K.; Lee, Y.R.; Han, S.S.; Ku, S.K.; Jung, Y.; Kim, J.-A. Anti-tumor Activity of the Novel Hexahydrocannabinol Analog LYR-8 in Human Colorectal Tumor Xenograft Is Mediated through the Inhibition of Akt and Hypoxia-Inducible Factor-1α Activation. Biol. Pharm. Bull. 2012, 35, 924–932. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Solinas, M.; Massi, P.; Cinquina, V.; Valenti, M.; Bolognini, D.; Gariboldi, M.; Monti, E.; Rubino, T.; Parolaro, D. Cannabidiol, a Non-Psychoactive Cannabinoid Compound, Inhibits Proliferation and Invasion in U87-MG and T98G Glioma Cells through a Multitarget Effect. PLoS ONE 2013, 8, e76918. [Google Scholar] [CrossRef]
- Nallathambi, R.; Mazuz, M.; Ion, A.; Selvaraj, G.; Weininger, S.; Fridlender, M.; Nasser, A.; Sagee, O.; Kumari, P.; Nemichenizer, D.; et al. Anti-Inflammatory Activity in Colon Models Is Derived from Δ9-Tetrahydrocannabinolic Acid That Interacts with Additional Compounds in Cannabis Extracts. Cannabis Cannabinoid Res. 2017, 2, 167–182. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.-J.; Huang, M.-Q.; Bao, J.-L.; Chen, X.-P.; Wang, Y.-T. Terpenoids: Natural products for cancer therapy. Exp. Opin. Investig. Drugs 2012, 21, 1801–1818. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef]
- Tomko, A.M.; Whynot, E.G.; Ellis, L.D.; Dupré, D.J. Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis. Cancers 2020, 12, 1985. [Google Scholar] [CrossRef]
- Ćulafić, D.M.; Žegura, B.; Nikolić, B.; Vuković-Gačić, B.; Knežević-Vukčević, J.; Filipič, M. Protective effect of linalool, myrcene and eucalyptol against t-butyl hydroperoxide induced genotoxicity in bacteria and cultured human cells. Food Chem. Toxicol. 2009, 47, 260–266. [Google Scholar] [CrossRef]
- Luis Da Silva, S.; Figueiredo, P.M.; Yano, T. Cytotoxic evaluation of essential oil from Zanthoxylum rhoifolium Lam. leaves. Acta Amazon. 2007, 37, 281–286. [Google Scholar] [CrossRef]
- Chung, K.-S.; Hong, J.Y.; Lee, J.-H.; Lee, H.-J.; Park, J.Y.; Choi, J.-H.; Park, H.-J.; Hong, J.; Lee, K.-T. β-Caryophyllene in the Essential Oil from Chrysanthemum Boreale Induces G1 Phase Cell Cycle Arrest in Human Lung Cancer Cells. Molecules 2019, 24, 3754. [Google Scholar] [CrossRef] [PubMed]
- Arul, S.; Rajagopalan, H.; Ravi, J.; Dayalan, H. Beta-Caryophyllene Suppresses Ovarian Cancer Proliferation by Inducing Cell Cycle Arrest and Apoptosis. Anticancer Agents Med. Chem. 2020, 20, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, V.; Kotakonda, M.; Periyannan, V. JAK1/STAT3 regulatory effect of β-caryophyllene on MG-63 osteosarcoma cells via ROS-induced apoptotic mitochondrial pathway by DNA fragmentation. J. Biochem. Mol. Toxicol. 2020, 1, 34. [Google Scholar] [CrossRef]
- di Giacomo, S.; di Sotto, A.; Mazzanti, G.; Wink, M. Chemosensitizing properties of β-caryophyllene and β-caryophyllene oxide in combination with doxorubicin in human cancer cells. Anticancer Res. 2017, 37, 1191–1196. [Google Scholar] [PubMed]
- di Sotto, A.; Irannejad, H.; Eufemi, M.; Mancinelli, R.; Abete, L.; Mammola, C.L.; Altieri, F.; Mazzanti, G.; Di Giacomo, S. Potentiation of low-dose doxorubicin cytotoxicity by affecting p-glycoprotein through caryophyllane sesquiterpenes in hepg2 cells: An in vitro and in silico study. Int. J. Mol. Sci. 2020, 21, 633. [Google Scholar] [CrossRef]
- Ambrož, M.; Matoušková, P.; Skarka, A.; Zajdlová, M.; Žáková, K.; Skálová, L. The effects of selected sesquiterpenes from myrica rubra essential oil on the efficacy of doxorubicin in sensitive and resistant cancer cell lines. Molecules 2017, 22, 1021. [Google Scholar] [CrossRef] [PubMed]
- Hanušová, V.; Caltová, K.; Svobodová, H.; Ambrož, M.; Skarka, A.; Murínová, N.; Králová, V.; Tomšík, P.; Skálová, L. The effects of β-caryophyllene oxide and trans-nerolidol on the efficacy of doxorubicin in breast cancer cells and breast tumor-bearing mice. Biomed. Pharmacother. 2017, 95, 828–836. [Google Scholar] [CrossRef]
- Meeran, M.N.; Al Taee, H.; Azimullah, S.; Tariq, S.; Adeghate, E.; Ojha, S. β-Caryophyllene, a natural bicyclic sesquiterpene attenuates doxorubicin-induced chronic cardiotoxicity via activation of myocardial cannabinoid type-2 (CB2) receptors in rats. Chem. Interactions 2019, 304, 158–167. [Google Scholar] [CrossRef]
- Ambrož, M.; Šmatová, M.; Šadibolová, M.; Pospíšilová, E.; Hadravská, P.; Kašparová, M.; Hanusova Skarkova, V.; Kralova, V.; Skalova, L. Sesquiterpenes α-humulene and β-caryophyllene oxide enhance the efficacy of 5-fluorouracil and oxaliplatin in colon cancer cells. Acta Pharm. 2019, 69, 121–128. [Google Scholar] [CrossRef]
- di Giacomo, S.; Briz, O.; Monte, M.J.; Sanchez-Vicente, L.; Abete, L.; Lozano, E.; Mazzanti, G.; Di Sotto, A.; Marin, J.J.G. Chemosensitization of hepatocellular carcinoma cells to sorafenib by β-caryophyllene oxide-induced inhibition of ABC export pumps. Arch Toxicol. 2019, 93, 623–634. [Google Scholar] [CrossRef]
- Legault, J.; Dahl, W.; Debiton, E.; Pichette, A.; Madelmont, J.-C. Antitumor Activity of Balsam Fir Oil: Production of Reactive Oxygen Species Induced by α-Humulene as Possible Mechanism of Action. Planta Med. 2003, 69, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F.; Dodaro, D.; Passalacqua, N.G.; Statti, G.; Menichini, F. In vitro cytotoxic effects of Senecio stabianus Lacaita (Asteraceae) on human cancer cell lines. Nat. Prod. Res. 2009, 23, 1707–1718. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yuan, J.; Hao, J.; Wen, Y.; Lv, Y.; Chen, L.; Yang, X. α-Humulene inhibits hepatocellular carcinoma cell proliferation and induces apoptosis through the inhibition of Akt signaling. Food Chem. Toxicol. 2019, 134, 110830. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Liang, Z.; Mi, Q.; Guo, Y. Limonene terpenoid obstructs human bladder cancer cell (T24 cell line) growth by inducing cellular apoptosis, caspase activation, G2/M phase cell cycle arrest and stops cancer metastasis. J. Buon Off. J. Balk. Union Oncol. 2020, 25, 280–285. [Google Scholar]
- Jia, S.-S.; Xi, G.-P.; Zhang, M.; Chen, Y.-B.; Lei, B.; Dong, X.-S.; Yang, Y.-M. Induction of apoptosis by D-limonene is mediated by inactivation of Akt in LS174T human colon cancer cells. Oncol. Rep. 2012, 29, 349–354. [Google Scholar] [CrossRef]
- Berliocchi, L.; Chiappini, C.; Adornetto, A.; Gentile, D.; Cerri, S.; Russo, R.; Bagetta, G.; Corasaniti, M.T. Early LC3 lipidation induced by d -limonene does not rely on mTOR inhibition, ERK activation and ROS production and it is associated with reduced clonogenic capacity of SH-SY5Y neuroblastoma cells. Phytomedicine 2018, 40, 98–105. [Google Scholar] [CrossRef]
- Russo, R.; Cassiano, M.G.V.; Ciociaro, A.; Adornetto, A.; Varano, G.P.; Chiappini, C.; Berliocchi, L.; Tassorelli, C.; Bagetta, G.; Corasaniti, M.T. Role of D-Limonene in Autophagy Induced by Bergamot Essential Oil in SH-SY5Y Neuroblastoma Cells. PLoS ONE 2014, 9, e113682. [Google Scholar] [CrossRef]
- Yu, X.; Lin, H.; Wang, Y.; Lv, W.; Zhang, S.; Qian, Y.; Deng, X.; Feng, N.; Yu, H.; Qian, B. D-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. OncoTargets Ther. 2018, 11, 1833–1847. [Google Scholar] [CrossRef]
- Haag, J.D.; Lindstrom, M.J.; Gould, M.N. Limonene-induced regression of mammary carcinomas. Cancer Res. 1992, 52, 4021–4027. [Google Scholar]
- Gould, M.N.; Moore, C.J.; Zhang, R.; Wang, B.; Kennan, W.S.; Haag, J.D. Limonene chemoprevention of mammary carcinoma induction following direct in situ transfer of v-Ha-ras. Cancer Res. 1994, 54, 3540–3543. [Google Scholar]
- Elegbede, J.A.; Elson, C.E.; Qureshi, A.; Tanner, M.A.; Gould, M.N. Regression of Rat Primary Mammary Tumors Following Dietary d-Limonene2. JNCI: J. Natl. Cancer Inst. 1986, 76, 323. [Google Scholar] [CrossRef]
- Nakaizumi, A.; Baba, M.; Uehara, H.; Iishi, H.; Tatsuta, M. d-Limonene inhibits N-nitrosobis(2-oxopropyl)amine induced hamster pancreatic carcinogenesis. Cancer Lett. 1997, 117, 99–103. [Google Scholar] [CrossRef]
- Manuele, M.G.; Arcos Barreiro, M.L.; Davicino, R.; Ferraro, G.; Cremaschi, G.; Anesini, C. Limonene exerts antiproliferative effects and increases nitric oxide levels on a lymphoma cell line by dual mechanism of the ERK pathway: Relationship with oxidative stress. Cancer Investig. 2010, 28, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.G.; Zhan, L.B.; Feng, B.A.; Qu, M.Y.; Yu, L.H.; Xie, J.H. Inhibition of growth and metastasis of human gastric cancer implanted in nude mice by d-limonene. World J. Gastroenterol. 2004, 10, 2140–2144. Available online: https://pubmed.ncbi.nlm.nih.gov/15237454 (accessed on 1 October 2022). [CrossRef] [PubMed]
- Uedo, N.; Tatsuta, M.; Iishi, H.; Baba, M.; Sakai, N.; Yano, H.; Otani, T. Inhibition by d-limonene of gastric carcinogenesis induced by N-methyl-N H-nitro-N-nitrosoguanidine in Wistar rats. Cancer Lett. 1999, 137, 131–136. [Google Scholar] [CrossRef]
- Kumar Giri, R.; Paru, T.; Das, B.R. d-limonene chemoprevention of hepatocarcinogenesis in AKR mice: Inhibition of c-jun and c-myc. Oncol. Rep. 1999, 6, 1123–1127. [Google Scholar]
- Miller, J.A.; Pappan, K.; Thompson, P.A.; Want, E.J.; Siskos, A.P.; Keun, H.C.; Wulff, J.; Hu, C.; Lang, J.E.; Chow, H.-H.S. Plasma Metabolomic Profiles of Breast Cancer Patients after Short-term Limonene Intervention. Cancer Prev. Res. 2015, 8, 86–93. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, Y.; Zhu, Y.; Zhou, B.; Ren, C.; Liang, S.; Guo, Y. α-Pinene Induces Apoptotic Cell Death via Caspase Activation in Human Ovarian Cancer Cells. Med. Sci. Monit. 2019, 25, 6631–6638. [Google Scholar] [CrossRef]
- Xu, Q.; Li, M.; Yang, M.; Yang, J.; Xie, J.; Lu, X.; Wang, F.; Chen, W. α-pinene regulates miR-221 and induces G2/M phase cell cycle arrest in human hepatocellular carcinoma cells. Biosci. Rep. 2018, 11, 38. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, R.; Wang, Y.; Yang, Y. α-pinene inhibits human prostate cancer growth in a mouse xenograft model. Chemotherapy 2018, 63, 1–7. [Google Scholar] [CrossRef]
- Li, Y.-L.; Yeung, C.-M.; Chiu, L.C.M.; Cen, Y.-Z.; Ooi, V.E.C. Chemical composition and antiproliferative activity of essential oil from the leaves of a medicinal herb, Schefflera heptaphylla. Phytotherapy Res. 2008, 23, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, S.; Liu, X.; Gao, X. Synergistic Antitumor Effect of α-pinene and β-pinene with Paclitaxel against Non-small-cell Lung Carcinoma (NSCLC). Drug Res. 2014, 65, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-Q.; Xu, B.; Mao, J.-W.; Wei, F.-X.; Li, M.; Liu, T.; Jin, X.-B.; Zhang, L.-R. Inhibitory Effects of α-Pinene on Hepatoma Carcinoma Cell Proliferation. Asian Pac. J. Cancer Prev. 2014, 15, 3293–3297. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Li, M.; Mao, J.; Zhang, L.; Huang, R.; Jin, X.; Ye, L. Anti-tumor effect of α-pinene on human hepatoma cell lines through inducing G2/M cell cycle arrest. J. Pharm. Sci. 2015, 127, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, A.L.; Figueiredo, C.R.; Arruda, D.C.; Pereira, F.V.; Scutti, J.A.B.; Massaoka, M.H.; Travassos, L.R.; Sartorelli, P.; Lago, J.H.G. α-Pinene isolated from Schinus terebinthifolius Raddi (Anacardiaceae) induces apoptosis and confers antimetastatic protection in a melanoma model. Biochem. Biophys. Res. Commun. 2011, 411, 449–454. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef]
- Mangal, N.; Erridge, S.; Habib, N.; Sadanandam, A.; Reebye, V.; Sodergren, M.H. Cannabinoids in the landscape of cancer. J. Cancer Res. Clin. Oncol. 2021, 147, 2507–2534. [Google Scholar] [CrossRef]
- McKallip, R.J.; Lombard, C.; Fisher, M.; Martin, B.R.; Ryu, S.; Grant, S.; Nagarkatti, P.S.; Nagarkatti, M. Targeting CB2 cannabinoid receptors as a novel therapy to treat malignant lymphoblastic disease. Blood 2002, 100, 627–634. [Google Scholar] [CrossRef]
- Preet, A.; Ganju, R.K.; Groopman, J.E. Δ9-Tetrahydrocannabinol inhibits epithelial growth factor-induced lung cancer cell migration in vitro as well as its growth and metastasis in vivo. Oncogene 2008, 27, 339–346. [Google Scholar] [CrossRef]
- McAllister, S.D.; Murase, R.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Allison, J.; Almanza, C.; Pakdel, A.; Lee, J.; Limbad, C.; et al. Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Cancer Res. Treat. 2011, 129, 37–47. [Google Scholar] [CrossRef]
- Zhu, L.X.; Sharma, S.; Stolina, M.; Gardner, B.; Roth, M.D.; Tashkin, D.P.; Dubinett, S.M. Δ-9-Tetrahydrocannabinol Inhibits Antitumor Immunity by a CB2 Receptor-Mediated, Cytokine-Dependent Pathway. J. Immunol. 2000, 165, 373–380. [Google Scholar] [CrossRef] [PubMed]
- McKallip, R.J.; Nagarkatti, M.; Nagarkatti, P.S. Δ-9-Tetrahydrocannabinol Enhances Breast Cancer Growth and Metastasis by Suppression of the Antitumor Immune Response. J. Immunol. 2005, 174, 3281–3289. [Google Scholar] [CrossRef] [PubMed]
- Darmani, N. ?9-Tetrahydrocannabinol and Synthetic Cannabinoids Prevent Emesis Produced by the Cannabinoid CB1 Receptor Antagonist/Inverse Agonist SR 141716A. Neuropsychopharmacology 2001, 24, 198–203. [Google Scholar] [CrossRef]
- A Darmani, N. Delta-9-tetrahydrocannabinol differentially suppresses cisplatin-induced emesis and indices of motor function via cannabinoid CB1 receptors in the least shrew. Pharmacol. Biochem. Behav. 2001, 69, 239–249. [Google Scholar] [CrossRef]
- Parker, L.A.; Kwiatkowska, M.; Burton, P.; Mechoulam, R. Effect of cannabinoids on lithium-induced vomiting in the Suncus murinus (house musk shrew). Psychopharmacology 2004, 171, 156–161. [Google Scholar] [CrossRef]
- Fride, E.; Bregman, T.; Kirkham, T.C. Endocannabinoids and Food Intake: Newborn Suckling and Appetite Regulation in Adulthood. Exp. Biol. Med. 2005, 230, 225–234. [Google Scholar] [CrossRef]
- Mechoulam, R.; Berry, E.M.; Avraham, Y.; di Marzo, V.; Fride, E. Endocannabinoids, feeding and suckling—From our perspective. Int. J. Obes. 2006, 30, S24–S28. [Google Scholar] [CrossRef]
- Walker, J.; Hohmann, A.G.; Martin, W.J.; Strangman, N.M.; Huang, S.M.; Tsou, K. The neurobiology of cannabinoid analgesia. Life Sci. 1999, 65, 665–673. [Google Scholar] [CrossRef]
- Meng Ian, D.; Manning Barton, H.; Martin William, J.; Fields Howard, L. An analgesia circuit activated by cannabinoids. Nature 1998, 395, 381–383. [Google Scholar] [CrossRef]
- Khasabova, I.A.; Gielissen, J.; Chandiramani, A.; Harding-Rose, C.; Abu Odeh, D.; Simone, D.A.; Seybold, V.S. CB1 and CB2 receptor agonists promote analgesia through synergy in a murine model of tumor pain. Behav. Pharmacol. 2011, 22, 607–616. [Google Scholar] [CrossRef]
- Ward, S.J.; McAllister, S.D.; Kawamura, R.; Murase, R.; Neelakantan, H.; A Walker, E. Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5- HT 1A receptors without diminishing nervous system function or chemotherapy efficacy. J. Cereb. Blood Flow Metab. 2014, 171, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Rahn, E.J.; Makriyannis, A.; Hohmann, A.G. Activation of cannabinoid CB1 and CB2 receptors suppresses neuropathic nociception evoked by the chemotherapeutic agent vincristine in rats. J. Cereb. Blood Flow Metab. 2007, 152, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Guimarães, F.S. Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology 2008, 199, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Díaz, M.; Caynas-Rojas, S.; Santacruz, V.A.; Ruiz-Contreras, A.E.; Aguilar-Roblero, R.; Prospéro-García, O. Entopeduncular nucleus endocannabinoid system modulates sleep–waking cycle and mood in rats. Pharmacol. Biochem. Behav. 2013, 107, 29–35. [Google Scholar] [CrossRef]
- Guzmán, M.; Duarte, M.J.; Blázquez, C.; Ravina, J.; Rosa, M.C.; Galve-Roperh, I.; Sanchez, C.; Velasco, G.; González-Feria, L. A pilot clinical study of Δ9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br. J. Cancer 2006, 95, 197–203. [Google Scholar] [CrossRef]
- Twelves, C.; Sabel, M.; Checketts, D.; Miller, S.; Tayo, B.; Jove, M.; Brazil, L.; Short, S.C. A phase 1b randomised, placebo-controlled trial of nabiximols cannabinoid oromucosal spray with temozolomide in patients with recurrent glioblastoma. Br. J. Cancer 2021, 124, 1379–1387. [Google Scholar] [CrossRef]
- Yeshurun, M.; Shpilberg, O.; Herscovici, C.; Shargian, L.; Dreyer, J.; Peck, A.; Israeli, M.; Levy-Assaraf, M.; Gruenewald, T.; Mechoulam, R.; et al. Cannabidiol for the Prevention of Graft-versus-Host-Disease after Allogeneic Hematopoietic Cell Transplantation: Results of a Phase II Study. Biol. Blood Marrow Transplant. 2015, 21, 1770–1775. [Google Scholar] [CrossRef]
- Bar-Lev Schleider, L.; Mechoulam, R.; Lederman, V.; Hilou, M.; Lencovsky, O.; Betzalel, O.; Shbiro, L.; Novack, V. Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. Eur. J. Intern. Med. 2018, 49, 37–43. [Google Scholar] [CrossRef]
- Weiss, M.C.; Hibbs, J.E.; Buckley, M.E.; Danese, S.R.; Leitenberger, A.; Bollmann-Jenkins, M.; Meske, S.W.; Aliano-Ruiz, K.E.; McHugh, T.W.; Larson, S.L.; et al. A Coala-T-Cannabis Survey Study of breast cancer patients’ use of cannabis before, during, and after treatment. Cancer 2022, 128, 160–168. [Google Scholar] [CrossRef]
- Ahmedzai, S.; Carlyle, D.L.; Calder, I.T.; Moran, F. Anti-emetic efficacy and toxicity of nabilone, a synthetic cannabinoid, in lung cancer chemotherapy. Br. J. Cancer 1983, 48, 657–663. [Google Scholar] [CrossRef]
- Chan, H.S.; Correia, J.A.; MacLeod, S.M. Nabilone versus prochlorperazine for control of cancer chemotherapy-induced emesis in children: A double-blind, crossover trial. Pediatrics 1987, 79, 946–952. [Google Scholar] [PubMed]
- Johansson, R.; Kilkku, P.; Groenroost, M. A double-bllnd, controlled trial of nabHone vs. procldorperazine for refractory emesis induced by cancer chemotherapy. Cancer Treat. Rev. 1982, 9, 25–33. [Google Scholar] [CrossRef]
- Aila, N.; Karin, M. A cross-over comparison of nabilone and prochlorperazine for emesis induced by cancer chemotherapy. Ann. J. Clin. Oncol. 1985, 8, 336–340. [Google Scholar]
- Brisbois, T.D.; de Kock, I.H.; Watanabe, S.M.; Mirhosseini, M.; Lamoureux, D.C.; Chasen, M.; MacDonald, N.; Baracos, V.E.; Wismer, W.V. Delta-9-tetrahydrocannabinol may palliate altered chemosensory perception in cancer patients: Results of a randomized, double-blind, placebo-controlled pilot trial. Ann. Oncol. 2011, 22, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Turcott, J.G.; del Rocío Guillen Núñez, M.; Flores-Estrada, D.; Oñate-Ocaña, L.F.; Zatarain-Barrón, Z.L.; Barrón, F.; Arrieta, O. The effect of nabilone on appetite, nutritional status, and quality of life in lung cancer patients: A randomized, double-blind clinical trial. Supportive Care Cancer 2018, 26, 3029–3038. [Google Scholar] [CrossRef]
- Noyes, R.; Brunk, S.F.; Baram, D.A.; Canter, A. Analgesic Effect of Delta-9-Tetrahydrocannabinol. J. Clin. Pharmacol. 1975, 15, 139–143. [Google Scholar] [CrossRef]
- Noyes, R.; Brunk, S.F.; Avery, D.H.; Canter, A. The analgesic properties of delta-9-tetrahydrocannabinol and codeine. Clin. Pharmacol. Ther. 1975, 18, 84–89. [Google Scholar] [CrossRef]
- Johnson, J.R.; Burnell-Nugent, M.; Lossignol, D.; Ganae-Motan, E.D.; Potts, R.; Fallon, M.T. Multicenter, Double-Blind, Randomized, Placebo-Controlled, Parallel-Group Study of the Efficacy, Safety, and Tolerability of THC:CBD Extract and THC Extract in Patients with Intractable Cancer-Related Pain. J. Pain Symptom Manag. 2010, 39, 167–179. [Google Scholar] [CrossRef]
- Maida, V.; Ennis, M.; Irani, S.; Corbo, M.; Dolzhykov, M. Adjunctive nabilone in cancer pain and symptom management: A prospective observational study using propensity scoring. J. Support Oncol. 2008, 6, 119–124. [Google Scholar]
- Waissengrin, B.; Mirelman, D.; Pelles, S.; Bukstein, F.; Blumenthal, D.T.; Wolf, I.; Geva, R. Effect of cannabis on oxaliplatin-induced peripheral neuropathy among oncology patients: A retrospective analysis. Ther. Adv. Med. Oncol. 2021, 13, 203. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, M.; Archibald, S.D.; Jackson, B.S.; Gupta, M.K. Association of Marijuana Use with Psychosocial and Quality of Life Outcomes Among Patients With Head and Neck Cancer. JAMA Otolaryngol. Neck Surg. 2018, 144, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, R.C.; Allebeck, P.; Akre, O.; McGlynn, K.A.; Sidorchuk, A. Cannabis Use and Incidence of Testicular Cancer: A 42-Year Follow-up of Swedish Men between 1970 and 2011. Cancer Epidemiol. Biomarkers Prev. 2017, 26, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Wallner, L.; Quinn, V.; Slezak, J.; Chien, G.; Jacobsen, S. Association between cannabis use and the risk of bladder cancer: Results from the California Men’s Health Study. J. Urol. 2013, 189, 527. [Google Scholar] [CrossRef]
- Hashibe, M.; Morgenstern, H.; Cui, Y.; Tashkin, D.P.; Zhang, Z.-F.; Cozen, W.; Mack, T.M.; Greenland, S. Marijuana Use and the Risk of Lung and Upper Aerodigestive Tract Cancers: Results of a Population-Based Case-Control Study. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1829–1834. [Google Scholar] [CrossRef]
- Marks, M.A.; Chaturvedi, A.K.; Kelsey, K.; Straif, K.; Berthiller, J.; Schwartz, S.M.; Smith, E.; Wyss, A.; Brennan, P.; Olshan, A.F.; et al. Association of Marijuana Smoking with Oropharyngeal and Oral Tongue Cancers: Pooled Analysis from the INHANCE Consortium. Cancer Epidemiol. Biomark. Prev. 2014, 23, 160–171. [Google Scholar] [CrossRef]
- Mehra, R.; Moore, B.A.; Crothers, K.; Tetrault, J.; Fiellin, D.A. The Association Between Marijuana Smoking and Lung Cancer a Systematic Review. Arch. Intern. Med. 2006, 166, 1359–1367. [Google Scholar] [CrossRef]
- Taha, T.; Meiri, D.; Talhamy, S.; Wollner, M.; Peer, A.; Bar-Sela, G. Cannabis Impacts Tumor Response Rate to Nivolumab in Patients with Advanced Malignancies. Oncologist 2019, 24, 549–554. [Google Scholar] [CrossRef]
- Bar-Sela, G.; Cohen, I.; Campisi-Pinto, S.; Lewitus, G.M.; Oz-Ari, L.; Jehassi, A.; Peer, A.; Turgeman, I.; Vernicova, O.; Berman, P.; et al. Cannabis consumption used by cancer patients during immunotherapy correlates with poor clinical outcome. Cancers 2020, 12, 2447. [Google Scholar] [CrossRef]
- Foroughi, M.; Hendson, G.; Sargent, M.A.; Steinbok, P. Spontaneous regression of septum pellucidum/forniceal pilocytic astrocytomas—Possible role of Cannabis inhalation. Child’s Nerv. Syst. 2011, 27, 671–679. [Google Scholar] [CrossRef]
- Torres, S.; Lorente, M.; Rodríguez-Fornés, F.; Hernández-Tiedra, S.; Salazar, M.; García-Taboada, E.; Barcia, J.; Guzmán, M.; Velasco, G. A Combined Preclinical Therapy of Cannabinoids and Temozolomide against Glioma. Mol. Cancer Ther. 2011, 10, 90–103. [Google Scholar] [CrossRef]
- Scott, K.A.; Dalgleish, A.G.; Liu, W.M. The Combination of Cannabidiol and Δ9-Tetrahydrocannabinol Enhances the Anticancer Effects of Radiation in an Orthotopic Murine Glioma Model. Mol. Cancer Ther. 2014, 13, 2955–2967. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, B.G.; Kim, D.Y.; Kim, B.R.; Kim, J.L.; Park, S.H.; Na, Y.J.; Jo, M.J.; Yun, H.K.; Jeong, Y.A.; et al. Cannabidiol Overcomes Oxaliplatin Resistance by Enhancing NOS3- and SOD2-Induced Autophagy in Human Colorectal Cancer Cells. Cancers 2019, 11, 781. [Google Scholar] [CrossRef]
- Nabissi, M.; Morelli, M.B.; Santoni, M.; Santoni, G. Triggering of the TRPV2 channel by cannabidiol sensitizes glioblastoma cells to cytotoxic chemotherapeutic agents. Carcinogenesis 2012, 34, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Yamaori, S.; Okamoto, Y.; Yamamoto, I.; Watanabe, K. Cannabidiol, a Major Phytocannabinoid, As a Potent Atypical Inhibitor for CYP2D6. Drug Metab. Dispos. 2011, 39, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Yamaori, S.; Okamoto, Y.; Yamamoto, I.; Watanabe, K. Cannabidiol Is a Potent Inhibitor of the Catalytic Activity of Cytochrome P450 2C19. Drug Metab. Pharmacokinet. 2013, 28, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Engels, F.K.; de Jong, F.A.; Sparreboom, A.; Mathot, R.A.A.; Loos, W.J.; Kitzen, J.J.E.M.; de Bruijn, P.; Verweij, J.; Mathijssen, R.H.J. Medicinal Cannabis Does Not Influence the Clinical Pharmacokinetics of Irinotecan and Docetaxel. Oncologist 2007, 12, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Sexton, M.; Garcia, J.M.; Jatoi, A.; Clark, C.S.; Wallace, M.S. The Management of Cancer Symptoms and Treatment-Induced Side Effects with Cannabis or Cannabinoids. J. Natl. Cancer Inst. Monogr. 2021, 2021, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Pergam, S.A.; Bs, M.C.W.; Lee, C.M.; Cheng, G.-S.; Ms, K.K.B.; Marquis, S.R.; Fann, J.R. Cannabis use among patients at a comprehensive cancer center in a state with legalized medicinal and recreational use. Cancer 2017, 123, 4488–4497. [Google Scholar] [CrossRef]
- Page, R.; Blanchard, E. Opioids and Cancer Pain: Patients’ Needs and Access Challenges. J. Oncol. Pr. 2019, 15, 229–231. [Google Scholar] [CrossRef]
- Cichewicz, D.L. Synergistic interactions between cannabinoid and opioid analgesics. Life Sci. 2003, 74, 1317–1324. [Google Scholar] [CrossRef]
- Hohmann, A.G.; Briley, E.M.; Herkenham, M. Pre- and postsynaptic distribution of cannabinoid and mu opioid receptors in rat spinal cord. Brain Res. 1999, 822, 17–25. [Google Scholar] [CrossRef]
- Mackie, K.; Hillet, B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells (aminoalkylindole/cyclic AMP/guanine nucleotide-binding protein/w-conotoxln/pertussis toxin). Neurobiology 1992, 89, 3825–3829. [Google Scholar]
- Deadwyler, S.A.; Hampson, R.E.; Bennett, B.A.; Edwards, T.A.; Mu, J.; Pacheco, M.A.; Ward, S.J.; Childers, S.R. Cannabinoids modulate potassium current in cultured hippocampal neurons. Recept. Channels 1993, 1, 121–134. [Google Scholar] [PubMed]
- Ibrahim, M.M.; Rude, M.L.; Stagg, N.J.; Mata, H.P.; Lai, J.; Vanderah, T.W.; Porreca, F.; Buckley, N.E.; Makriyannis, A.; Malan, T.F., Jr.; et al. CB2 cannabinoid receptor mediation of antinociception. Pain 2006, 122, 36–42. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.H.; di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for medical use: A systematic review and meta-analysis. JAMA-Journal of the American Medical Association. Am. Med. Assoc. 2015, 313, 2456–2473. [Google Scholar] [CrossRef]
- Lynch, M.E.; Campbell, F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials Keywords cannabinoids, chronic non-cancer pain, neuropathic pain, systematic review. Br. J. Clin. Pharmacol. 2011, 72, 735–744. [Google Scholar] [CrossRef]
- Ellis, R.J.; Toperoff, W.; Vaida, F.; Brande, G.V.D.; Gonzales, J.; Gouaux, B.; Bentley, H.; Atkinson, J.H. Smoked Medicinal Cannabis for Neuropathic Pain in HIV: A Randomized, Crossover Clinical Trial. Neuropsychopharmacology 2008, 34, 672–680. [Google Scholar] [CrossRef]
- Wilsey, B.; Marcotte, T.; Deutsch, R.; Gouaux, B.; Sakai, S.; Donaghe, H. Low-Dose Vaporized Cannabis Significantly Improves Neuropathic Pain. J. Pain 2012, 14, 136–148. [Google Scholar] [CrossRef]
- Wilsey, B.; Marcotte, T.D.; Deutsch, R.; Zhao, H.; Prasad, H.; Phan, A. An Exploratory Human Laboratory Experiment Evaluating Vaporized Cannabis in the Treatment of Neuropathic Pain from Spinal Cord Injury and Disease. J. Pain 2016, 17, 982–1000. [Google Scholar] [CrossRef]
- Wallace, M.S.; Marcotte, T.D.; Umlauf, A.; Gouaux, B.; Atkinson, J.H. Efficacy of Inhaled Cannabis on Painful Diabetic Neuropathy. J. Pain 2015, 16, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Sabioni, P.; Trigo, J.M.; A Ware, M.; Betz-Stablein, B.; Murnion, B.; Lintzeris, N.; Khor, K.E.; Farrell, M.; Smith, A.; et al. Opioid-Sparing Effect of Cannabinoids: A Systematic Review and Meta-Analysis. Neuropsychopharmacology 2017, 42, 1752–1765. [Google Scholar] [CrossRef] [PubMed]
- Narang, S.; Gibson, D.; Wasan, A.D.; Ross, E.L.; Michna, E.; Nedeljkovic, S.S.; Jamison, R.N. Efficacy of Dronabinol as an Adjuvant Treatment for Chronic Pain Patients on Opioid Therapy. J. Pain 2008, 9, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Portenoy, R.K.; Ganae-Motan, E.D.; Allende, S.; Yanagihara, R.; Shaiova, L.; Weinstein, S.; McQuade, R.; Wright, S.; Fallon, M.T. Nabiximols for Opioid-Treated Cancer Patients with Poorly-Controlled Chronic Pain: A Randomized, Placebo-Controlled, Graded-Dose Trial. J. Pain 2012, 13, 438–449. [Google Scholar] [CrossRef]
- Callaghan, B.C.; Price, R.S.; Feldman, E.L. Distal symmetric polyneuropathy a review. JAMA-J. Am. Med. Assoc. 2015, 314, 2172–2181. [Google Scholar] [CrossRef]
- Martín-Sánchez, E.; Furukawa, T.; Taylor, J.; Martin, J.L.R. Systematic Review and Meta-analysis of Cannabis Treatment for Chronic Pain. Pain Med. 2009, 10, 1353–1368. [Google Scholar] [CrossRef]
- Khasabova, I.A.; Khasabov, S.; Paz, J.; Harding-Rose, C.; Simone, N.A.; Seybold, V.S. Cannabinoid Type-1 Receptor Reduces Pain and Neurotoxicity Produced by Chemotherapy. J. Neurosci. 2012, 32, 7091–7101. [Google Scholar] [CrossRef]
- Lynch, M.E.; Cesar-Rittenberg, P.; Hohmann, A.G. A Double-Blind, Placebo-Controlled, Crossover Pilot Trial with Extension Using an Oral Mucosal Cannabinoid Extract for Treatment of Chemotherapy-Induced Neuropathic Pain. J. Pain Symptom Manag. 2014, 47, 166–173. [Google Scholar] [CrossRef]
- Bloechl-Daum, B.; Deuson, R.R.; Mavros, P.; Hansen, M.; Herrstedt, J. Delayed Nausea and Vomiting Continue to Reduce Patients’ Quality of Life After Highly and Moderately Emetogenic Chemotherapy Despite Antiemetic Treatment. J. Clin. Oncol. 2006, 24, 4472–4478. [Google Scholar] [CrossRef]
- Sanger, G.J. Endocannabinoids and the gastrointestinal tract: What are the key questions? Br. J. Pharmacol. 2007, 152, 663–670. [Google Scholar] [CrossRef]
- Pacher, P.; Bátkai, S.; Kunos, G. The Endocannabinoid System as an Emerging Target of Pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef] [PubMed]
- Roswell, F.; Todaro, B.; York, N. Cannabinoids in the Treatment of Chemotherapy-Induced Nausea and Vomiting. J. Natl. Compr. Cancer Netw. 2012, 10, 487–492. [Google Scholar]
- Tramèr, M.R.; Carroll, D.; A Campbell, F.; Reynolds, D.J.M.; Moore, R.A.; McQuay, H.J. Cannabinoids for control of chemotherapy induced nausea and vomiting: Quantitative systematic. BMJ 2001, 323, 16. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.E.; Shiling, D.J.; Stillman, R.C.; Goldberg, N.H.; Seipp, C.A.; Barofsky, I.; Simon, R.M.; Rosenberg, S.A. Delta-9-Tetrahydrocannabinol as an Antiemetic in Cancer Patients Receiving High-Dose Methotrexate a Prospective, Randomized Evaluation. Ann. Intern. Med. 1979, 91, 819–824. Available online: http://annals.org/pdfaccess.ashx?url=/data/journals/aim/19553/ (accessed on 1 October 2022). [CrossRef]
- Chang, A.E.; Shiling, D.J.; Stillman, R.C.; Goldberg, N.H.; Seipp, C.A.; Barofsky, I.; Simon, R.M.; Rosenberg, S.A. A prospective evaluation of delta-9-tetrahydrocannabinol as an antiemetic in patients receiving adriamycin and cytoxan chemotherapy. Cancer 1981, 47, 1746–1751. [Google Scholar]
- ben Amar, M. Cannabinoids in medicine: A review of their therapeutic potential. J. Ethnopharmacol. 2006, 105, 1–25. [Google Scholar] [CrossRef]
- Strasser, F.; Luftner, D.; Possinger, K.; Ernst, G.; Ruhstaller, T.; Meissner, W.; Ko, Y.D.; Schnelle, M.; Reif, M.; Cerny, T. Comparison of orally administered cannabis extract and delta-9- tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: A multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J. Clin. Oncol. 2006, 24, 3394–3400. [Google Scholar]
- Nelson, K.; Walsh, D.; Deeter, P.; Sheehan, F. A phase II study of delta-9-tetrahydrocannabinol for appetite stimulation in cancer-associated anorexia. J. Palliat. Care 1994, 10, 14–18. [Google Scholar]
- Jatoi, A.; Windschitl, H.E.; Loprinzi, C.L.; Sloan, J.A.; Dakhil, S.R.; Mailliard, J.A.; Pundaleeka, S.; Kardinal, C.G.; Fitch, T.R.; Krook, J.E.; et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: A North Central Cancer Treatment Group study. J. Clin. Oncol. 2002, 20, 567–573. [Google Scholar] [CrossRef]
- Massa, F.; Marsicano, G.; Hermann, H.; Cannich, A.; Monory, K.; Cravatt, B.F.; Ferri, G.-L.; Sibaev, A.; Storr, M.; Lutz, B. The endogenous cannabinoid system protects against colonic inflammation. J. Clin. Investig. 2004, 113, 1202–1209. [Google Scholar] [CrossRef]
- Patsos, H.; Hicks, D.; Greenhough, A.; Williams, A.; Paraskeva, C. Cannabinoids and cancer: Potential for colorectal cancer therapy. Biochem. Soc. Trans. 2005, 33, 712–714. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.W.; Dalgleish, A.G. Cannabis-Derived Substances in Cancer Therapy—An Emerging Anti-Inflammatory Role for the Cannabinoids. Curr. Clin. Pharmacol. 2010, 5, 281–287. [Google Scholar] [CrossRef]
- Malfitano, A.M.; Ciaglia, E.; Gangemi, G.; Gazzerro, P.; Laezza, C.; Bifulco, M. Update on the endocannabinoid system as an anticancer target. Expert Opin. Ther. Targets 2011, 15, 297–308. [Google Scholar] [CrossRef]
- Sarfaraz, S.; Adhami, V.M.; Syed, D.N.; Afaq, F.; Mukhtar, H. Cannabinoids for Cancer Treatment: Progress and Promise. Cancer Res. 2008, 68, 339–342. [Google Scholar] [CrossRef]
- Cherkasova, V.; Kovalchuk, O.; Kovalchuk, I. Cannabinoids and Endocannabinoid System Changes in Intestinal Inflammation and Colorectal Cancer. Cancers 2021, 13, 4353. [Google Scholar] [CrossRef]
- Kim, H.I.; Lim, H.; Moon, A. Sex differences in cancer: Epidemiology, genetics and therapy. Biomol. Therapeutics. Korean Soc. Appl. Pharmacol. 2018, 26, 335–342. [Google Scholar] [CrossRef]
- Tevfik Dorak, M.; Karpuzoglu, E. Gender differences in cancer susceptibility: An inadequately addressed issue. Front. Genet. 2012, 3, 1–11. [Google Scholar]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef]
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L.; et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study Global Burden of Disease Cancer Collaboration. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [PubMed]
- Blanton, H.L.; Barnes, R.C.; McHann, M.C.; Bilbrey, J.A.; Wilkerson, J.L.; Guindon, J. Sex differences and the endocannabinoid system in pain. Pharmacol. Biochem. Behav. 2021, 202, 173107. [Google Scholar] [CrossRef]
- Levine, A.; Liktor-Busa, E.; Lipinski, A.A.; Couture, S.; Balasubramanian, S.; Aicher, S.A.; Langlais, P.R.; Vanderah, T.W.; Largent-Milnes, T.M. Sex differences in the expression of the endocannabinoid system within V1M cortex and PAG of Sprague Dawley rats. Biol. Sex Differ. 2021, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Henderson-Redmond, A.N.; Crawford, L.C.; Sepulveda, D.E.; Hale, D.E.; Lesperance, J.J.; Morgan, D.J. Sex Differences in Tolerance to Delta-9-Tetrahydrocannabinol in Mice with Cisplatin-Evoked Chronic Neuropathic Pain. Front. Mol. Biosci. 2021, 8, 4115. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Iemolo, A.; Montilla-Perez, P.; Li, H.-R.; Yang, X.; Telese, F. Chronic adolescent exposure to cannabis in mice leads to sex-biased changes in gene expression networks across brain regions. Neuropsychopharmacology 2022, 2, 1–10. [Google Scholar] [CrossRef]
- Rock, E.M.; Limebeer, C.L.; Smoum, R.; Mechoulam, R.; Parker, L.A. Evaluation of Sex Differences in the Potential of Δ 9-Tetrahydrocannabinol, Cannabidiol, Cannabidiolic Acid, and Oleoyl Alanine to Reduce Nausea-Induced Conditioned Gaping Reactions in Sprague-Dawley Rats. Cannabis Cannabinoid Res. 2022, 2022, 158. [Google Scholar] [CrossRef]
- Wyrofsky, R.R.; Reyes, B.A.S.; Yu, D.; Kirby, L.G.; Van Bockstaele, E.J. Sex differences in the effect of cannabinoid type 1 receptor deletion on locus coeruleus-norepinephrine neurons and corticotropin releasing factor-mediated responses. Eur. J. Neurosci. 2018, 48, 2118–2138. [Google Scholar] [CrossRef]
- Ney, L.J.; Matthews, A.; Bruno, R.; Felmingham, K.L. Modulation of the endocannabinoid system by sex hormones: Implications for posttraumatic stress disorder. Neurosci. Biobehav. Rev. 2018, 94, 302–320. [Google Scholar] [CrossRef]
- Morena, M.; Patel, S.; Bains, J.; Hill, M.N. Neurobiological Interactions Between Stress and the Endocannabinoid System. Neuropsychopharmacology 2015, 41, 80–102. [Google Scholar] [CrossRef]
- Koenen, K.C.; Ratanatharathorn, A.; Ng, L.; McLaughlin, K.A.; Bromet, E.J.; Stein, D.J.; Karam, E.G.; Meron Ruscio, A.; Benjet, C.; Scott, K.; et al. Posttraumatic stress disorder in the World Mental Health Surveys. Psychol. Med. 2017, 47, 2260–2274. [Google Scholar] [CrossRef]
- Hill, M.N.; Campolongo, P.; Yehuda, R.; Patel, S. Integrating Endocannabinoid Signaling and Cannabinoids into the Biology and Treatment of Posttraumatic Stress Disorder. Neuropsychopharmacology 2017, 43, 80–102. [Google Scholar] [CrossRef] [PubMed]
- Wilker, S.; Pfeiffer, A.; Elbert, T.; Ovuga, E.; Karabatsiakis, A.; Krumbholz, A.; Thieme, D.; Schelling, G.; Kolassa, I.-T. Endocannabinoid concentrations in hair are associated with PTSD symptom severity. Psychoneuroendocrinology 2016, 67, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Ney, L.J.; Matthews, A.; Bruno, R.; Felmingham, K.L. Commentary on “Sex differences in the effect of cannabinoid type 1 receptor deletion on locus coeruleus-norepinephrine neurons and corticotropin releasing factor-mediated responses”. Eur. J. Neurosci. 2019, 49, 1210–1211. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Lossignol, D.; Burnell-Nugent, M.; Fallon, M.T. An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. J. Pain Symptom Manag. 2013, 46, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Fallon, M.T.; Lux, E.A.; McQuade, R.; Rossetti, S.; Sanchez, R.; Sun, W.; Wright, S.; Lichtman, A.H.; Kornyeyeva, E. Sativex oromucosal spray as adjunctive therapy in advanced cancer patients with chronic pain unalleviated by optimized opioid therapy: Two double-blind, randomized, placebo-controlled phase 3 studies. Br. J. Pain 2017, 11, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, A.H.; Lux, E.A.; McQuade, R.; Rossetti, S.; Sanchez, R.; Sun, W.; Wright, S.; Kornyeyeva, E.; Fallon, M.T. Results of a Double-Blind, Randomized, Placebo-Controlled Study of Nabiximols Oromucosal Spray as an Adjunctive Therapy in Advanced Cancer Patients with Chronic Uncontrolled Pain. J. Pain Symptom Manag. 2018, 55, 179–188.e1. [Google Scholar] [CrossRef]
- Arkell, T.R.; Kevin, R.C.; Vinckenbosch, F.; Lintzeris, N.; Theunissen, E.; Ramaekers, J.G.; McGregor, I.S. Sex differences in acute cannabis effects revisited: Results from two randomized, controlled trials. Addict. Biol. 2021, 27, e13125. [Google Scholar] [CrossRef]
- Aviram, J.; Lewitus, G.M.; Vysotski, Y.; Berman, P.; Shapira, A.; Procaccia, S.; Meiri, D. Sex differences in medical cannabis-related adverse effects. Pain 2021, 163, 975–983. [Google Scholar] [CrossRef]
- Kasvis, P.; Canac-Marquis, M.; Aprikian, S.; Vigano, M.; Vigano, A. Sex differences exist in the perceived relief of cancer symptoms with medical cannabis: Results from the Quebec Cannabis Registry. Support. Care Cancer 2022, 1, 1–9. [Google Scholar] [CrossRef]
- Doherty, G.J.; de Paula, B.H.R. Cannabinoids in glioblastoma multiforme—Hype or hope? Br. J. Cancer 2021, 124, 1341–1343. [Google Scholar] [CrossRef]
- Chapman, S.; Protudjer, J.; Bourne, C.; Kelly, L.E.; Oberoi, S.; Vanan, M.I. Medical cannabis in pediatric oncology: A survey of patients and caregivers. Support. Care Cancer 2021, 29, 6589–6594. [Google Scholar] [CrossRef] [PubMed]
- Malach, M.; Kovalchuk, I.; Kovalchuk, O. Medical Cannabis in Pediatric Oncology: Friend or Foe? Pharmaceuticals 2022, 15, 359. [Google Scholar] [CrossRef] [PubMed]
- Solomon, H.V.; Greenstein, A.P.; DeLisi, L.E. Cannabis Use in Older Adults: A Perspective. Harv. Rev. Psychiatry 2021, 29, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Manning, L.; Bouchard, L. Medical Cannabis Use: Exploring the Perceptions and Experiences of Older Adults with Chronic Conditions. Clin. Gerontol. 2020, 44, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Skliamis, K.; Benschop, A.; Korf, D.J. Cannabis users and stigma: A comparison of users from European countries with different cannabis policies. Eur. J. Criminol. 2020, 23, 3560. [Google Scholar] [CrossRef]
- Bottorff, J.L.; Bissell, L.J.; Balneaves, L.G.; Oliffe, J.L.; Capler, N.R.; Buxton, J. Perceptions of cannabis as a stigmatized medicine: A qualitative descriptive study. Harm Reduct. J. 2013, 10, 2. [Google Scholar] [CrossRef]
- Reid, M. A qualitative review of cannabis stigmas at the twilight of prohibition. J. Cannabis Res. 2020, 2, 46. [Google Scholar] [CrossRef]
- Leos-Toro, C.; Shiplo, S.; Hammond, D. Perceived support for medical cannabis use among approved medical cannabis users in Canada. Drug Alcohol Rev. 2018, 37, 627–636. [Google Scholar] [CrossRef]
- Davis, B.A. Discrimination: A Social Determinant of Health Inequities. Health Aff. 2020. [Google Scholar] [CrossRef]
- Barry, C.L.; McGinty, E.E.; Pescosolido, B.A.; Goldman, H.H. Stigma, Discrimination, Treatment Effectiveness, and Policy: Public Views About Drug Addiction and Mental Illness. Psychiatr. Serv. 2014, 65, 1269–1272. [Google Scholar] [CrossRef]
- Atmanli, A.; İleri, E.; Yüksel, B. Experimental investigation of engine performance and exhaust emissions of a diesel engine fueled with diesel–n-butanol–vegetable oil blends. Energy Convers Manag. 2014, 81, 312–321. [Google Scholar] [CrossRef]
- Victorson, D.; McMahon, M.; Horowitz, B.; Glickson, S.; Parker, B.; Mendoza-Temple, L. Exploring cancer survivors’ attitudes, perceptions, and concerns about using medical cannabis for symptom and side effect management: A qualitative focus group study. Complement. Ther. Med. 2019, 47, 102204. [Google Scholar] [CrossRef] [PubMed]
- Rudski, J.M. Treatment Acceptability, Stigma, and Legal Concerns of Medical Marijuana Are Affected by Method of Administration. J. Drug Issues 2013, 44, 308–320. [Google Scholar] [CrossRef]
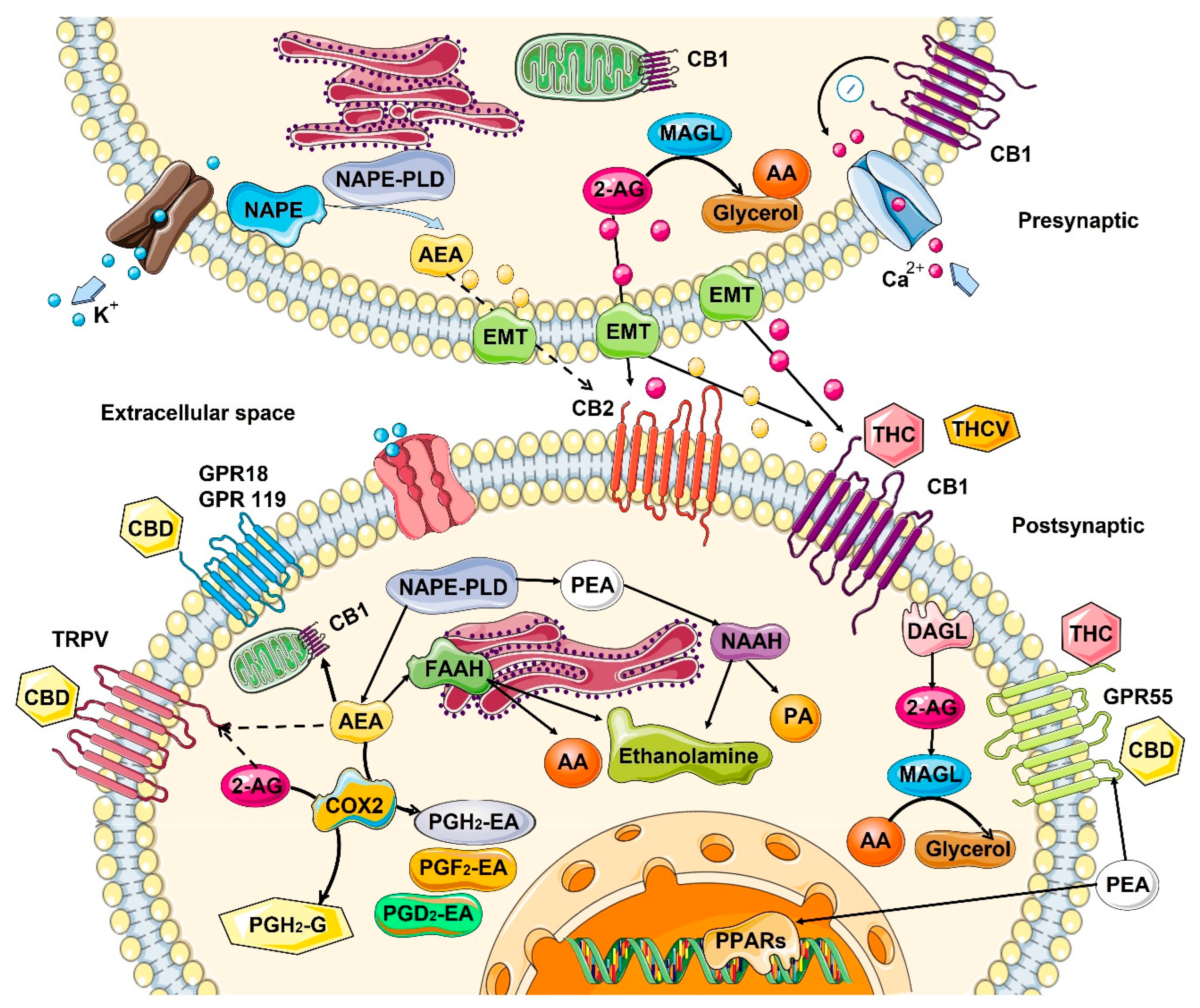
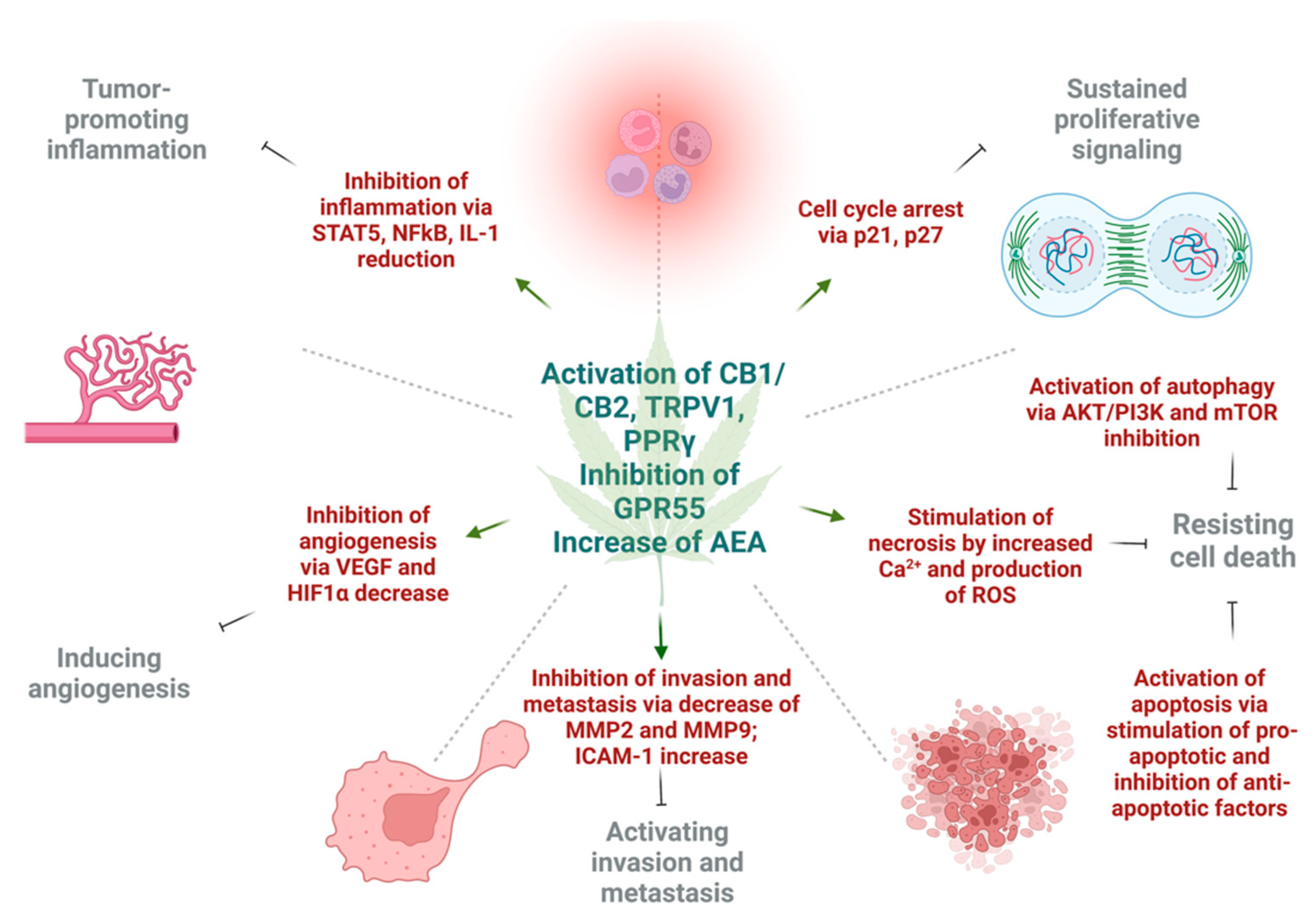
| Tissues/Organs | Endocannabinoids | Receptors | Metabolizing Enzymes |
|---|---|---|---|
| Skin | No reliable data | ↓ in CB1 expression [13] | FAAH tends to ↓ with age [45] |
| Lung | 2-AG ↓ and AEA ↑ in mice [46] | No reliable data | No reliable data |
| Brain | From no change [40] to a ↓ in AEA [47] ↓ in 2-AG levels in mice [48] | From ↑ in humans [41] to no change [39] to a ↓ [38,49] in mice/rats in CB1 expression, brain area-specific | ↓ FAAH activity in rats [50] ↑ in MAGL levels in mice [48] |
| Blood | Small ↑ in 2-AG and AEA in mice [46] | No reliable data | No reliable data |
| Tissues/Organs | Endocannabinoids | Receptors | Metabolizing Enzymes |
|---|---|---|---|
| Skin | Decreased AEA and increased 2-AG in melanoma [51] | ↑ CB2 in melanoma [52] | ↑ MAGL [53], and ↑ FAAH in melanoma [45] |
| Intestine (colorectal) | ↑ AEA and 2-AG [54,55] ↑ LPI [55,56] | ↓ CB1 [57,58], ↑ GPR55 [57,59], ↑ CB2 and ↓ CB1 [58] | ↑ MAGL [54], ↑ FAAH [45] |
| Lung | ↑ CB1 and CB2 [60,61] | ↑ FAAH [62], ↑ FAAH [45] | |
| Breast | ↑ LPI [63] | ↑ CB1 and CB2 [64,65,66,67], ↑ GPR55 [68,69] | ↑ FAAH [45] |
| Brain | ↑ AEA and 2-AG in many cancers [70,71,72,73,74] | ↑ CNR1, ↑ CNR2, ↑ CB1 and CB2 in glioma [75,76,77,78], ↓ CB1 in glioma [79] | ↓ FAAH [80] in glioma, ↑ FAAH in glioma [45] |
| Cannabis Drugs Used | Cancer Types/Preclinical Models of Diseases | Experimental Models | Effects in Cancer | Citation |
|---|---|---|---|---|
| Delta-9-THC | Hepatic adenomas, hepatocellular carcinoma, decreased incidence in adenomas and papillomas in mammary glands, uterus, pituitary gland, testicles, pancreas | In vivo, in vitro | Cancer prevention | [191] |
| Delta-9-THC, delta-8-THC, Selective CB2 agonist JWH-133, Co-administration of CBD and THC | Lewis lung adenocarcinoma, glioblastoma multiforme | In vivo, in vitro | Reduced tumor growth | [70,75,144,145] |
| Delta-9-THC, HU-210, anandamide, CB2 agonist JWH-015 | Malignant lymphoblastic diseases | In vivo, in vitro | Reduced tumor growth | [271] |
| CB1/CB2 agonist WIN-55,212-2, CB2 agonist JWH-133 | Non-melanoma skin tumors | In vivo, in vitro | Reduced tumor growth | [92] |
| Delta-9-THC, CB2 agonist JWH-015 | Hepatocellular carcinoma | In vitro, in vivo | Reduced tumor growth | [195] |
| Delta-9-THC, WIN55,212-2, JWH-015 | Non-small cell lung carcinoma | In vitro, in vivo (immunodeficient mice) | Reduced tumor growth | [61,272] |
| CBD, THC, JWH-015 | Breast cancer | In vitro, in vivo | Reduced tumor growth | [67,151,273] |
| CBD | Colorectal cancer | In vitro, in vivo | Reduced tumor growth | [218,219] |
| Delta-9-THC | Non-small cell lung carcinoma, breast cancer | In vitro, in vivo (immunocompetent mice) | Increased tumor growth | [274,275] |
| Delta-9-THC, CP-55,940, WIN55,212-2, CBD | Animal model of emesis | In vivo | Antiemetic effect, inhibition of chemotherapy-induced nausea and vomiting | [276,277,278] |
| Anandamide, Delta-9-THC | Changes in appetite in animals | In vivo | Increased food intake | [279,280] |
| WIN55,212-2, arachidonylcyclopropylamide, AM1241 | Animal models of pain induction | In vivo | Analgetic effect | [281,282,283] |
| CBD, WIN55,212-2 | Animal models of chemotherapy-induced neuropathic pain | In vivo | Analgetic effect | [284,285] |
| CBD | Animal models of stress, recording sleep-walking cycles in rats | In vivo | Reduction of anxiety and improvement of sleep | [286,287] |
| Drugs Used | Cancer Types/Participant Groups | Primary/Anticancer Effects | Palliative Care | Adverse Effects | Citation/Clinical Trial # |
|---|---|---|---|---|---|
| Delta-9-THC | Intra-tumoral injection in patients with recurrent glioblastoma multiforme | No significant clinical benefit | - | - | [200,288] |
| Sativex and Temozolomide | Glioblastoma multiforme | Increased 1-year survival rate in 39% | - | - | NCT01812603; NCT01812616 [289] |
| Dexanabinol | Solid tumors | Progression-free survival increased | - | - | NCT01489826 |
| CBD | Acute leukemia and myelodysplastic syndrome | Lower incidence rate of acute graft-versus-host disease after allogenic hematopoietic stem cell transplantation | - | - | NCT01385124 NCT01596075 [290] |
| Government-issued Cannabis | Cancer patients | - | Improvement of symptoms related to nausea and vomiting, sleep disorders, restlessness, anxiety and depression, pruritus, headaches | - | [291] |
| Cannabis use | Breast cancer patients | - | Relief symptoms: of pain, insomnia, anxiety, stress, nausea, and vomiting | - | [292] |
| Dronabinol, Nabilone * | Patients with different cancers | - | Treatment of chemotherapy-induced nausea and vomiting | - | [293,294,295,296] |
| Delta-9-THC | Patients with advanced cancers | - | Appetite stimulation | - | [296,297,298] |
| Delta-9-THC, THC:CBD extracts, Nabilone | Patients with different cancers | - | Analgetic effect | - | [299,300,301,302] |
| Cannabis | Patients with chemotherapy-induced peripheral neuropathy | - | Analgetic effect | - | [303] |
| Delta-9-THC, Dronabinol * | Patients with different cancers | - | Anxiolytic effect, increased quality of sleep | - | [296,302] |
| Cannabis use | Patients with head and neck cancers | - | Decreased anxiety and depression scores | - | [304] |
| Marijuana | Chronic marijuana smokers | - | - | Increased risk for testicular germ cell tumors in “Heavy” Cannabis users | [305] |
| Smoking Cannabis | Healthy subjects and Cannabis users | - | - | Cannabis use was associated with 45% reduction in bladder cancer incidence | [306] |
| Smoking Cannabis | Healthy individuals, lung cancer patients | - | - | Smoking cannabis is not associated with lung cancer or head and neck cancers | [307,308,309] |
| Cannabis use during nivolumab immunotherapy | Patients with advanced melanoma, non-small cell lung carcinoma, renal cell carcinoma | - | - | Cannabis use reduced the response rate to immunotherapy by 21.25%. Cannabis use was correlated with poor clinical outcome | [310,311] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherkasova, V.; Wang, B.; Gerasymchuk, M.; Fiselier, A.; Kovalchuk, O.; Kovalchuk, I. Use of Cannabis and Cannabinoids for Treatment of Cancer. Cancers 2022, 14, 5142. https://doi.org/10.3390/cancers14205142
Cherkasova V, Wang B, Gerasymchuk M, Fiselier A, Kovalchuk O, Kovalchuk I. Use of Cannabis and Cannabinoids for Treatment of Cancer. Cancers. 2022; 14(20):5142. https://doi.org/10.3390/cancers14205142
Chicago/Turabian StyleCherkasova, Viktoriia, Bo Wang, Marta Gerasymchuk, Anna Fiselier, Olga Kovalchuk, and Igor Kovalchuk. 2022. "Use of Cannabis and Cannabinoids for Treatment of Cancer" Cancers 14, no. 20: 5142. https://doi.org/10.3390/cancers14205142
APA StyleCherkasova, V., Wang, B., Gerasymchuk, M., Fiselier, A., Kovalchuk, O., & Kovalchuk, I. (2022). Use of Cannabis and Cannabinoids for Treatment of Cancer. Cancers, 14(20), 5142. https://doi.org/10.3390/cancers14205142








