Does Aggressive Surgery Mean Worse Quality of Life and Functional Capacity in Retroperitoneal Sarcoma Patients?—A Retrospective Study of 161 Patients from China
Abstract
Simple Summary
Abstract
1. Background
2. Methods
2.1. Subject
2.2. Statistical Methods
3. Results
3.1. Baseline Characteristics
3.2. Summary of GH Scores
3.3. Comparison of EORTC Scores between MVR and Non-MVR Groups
3.3.1. Across Functional Domains and Symptom Scales
3.3.2. Across Time
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mastrangelo, G.; Coindre, J.M.; Ducimetière, F.; Dei, T.A.; Fadda, E.; Blay, J.Y.; Buja, A.; Fedeli, U.; Cegolon, L.; Frasson, A.; et al. Incidence of soft tissue sarcoma and beyond: A population-based prospective study in 3 European regions. Cancer-Am. Cancer Soc. 2012, 118, 5339–5348. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Lo, V.S.; Fiore, M.; Mussi, C.; Stacchiotti, S.; Collini, P.; Lozza, L.; Pennacchioli, E.; Mariani, L.; Casali, P.G. Aggressive surgical policies in a retrospectively reviewed single-institution case series of retroperitoneal soft tissue sarcoma patients. J. Clin. Oncol. 2009, 27, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Bonvalot, S.; Rivoire, M.; Castaing, M.; Stoeckle, E.; Le Cesne, A.; Blay, J.Y.; Laplanche, A. Primary retroperitoneal sarcomas: A multivariate analysis of surgical factors associated with local control. J. Clin. Oncol. 2009, 27, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Macneill, A.J.; Gronchi, A.; Miceli, R.; Bonvalot, S.; Swallow, C.J.; Hohenberger, P.; Van Coevorden, F.; Rutkowski, P.; Callegaro, D.; Hayes, A.; et al. Postoperative morbidity after radical resection of primary retroperitoneal sarcoma: A report from the transatlantic RPS working group. Ann. Surg. 2018, 267, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Strauss, D.C.; Miceli, R.; Bonvalot, S.; Swallow, C.J.; Hohenberger, P.; Van Coevorden, F.; Rutkowski, P.; Callegaro, D.; Hayes, A.; et al. Variability in patterns of recurrence after resection of primary retroperitoneal sarcoma (RPS): A report on 1007 patients from the multi-institutional collaborative RPS working group. Ann. Surg. 2016, 263, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services FDA Center for Drug Evaluation and Research Laurie. Guidance for industry: Patient-reported outcome measures: Use in medical product development to support labeling claims: Draft guidance. Health Qual. Life Outcomes 2006, 4, 79. [Google Scholar] [CrossRef] [PubMed]
- Kluetz, P.G.; Slagle, A.; Papadopoulos, E.J.; Johnson, L.L.; Donoghue, M.; Kwitkowski, V.E.; Chen, W.-H.; Sridhara, R.; Farrell, A.T.; Keegan, P.; et al. Focusing on core Patient-Reported outcomes in cancer clinical trials: Symptomatic adverse events, physical function, and Disease-Related symptoms. Clin. Cancer Res. 2016, 22, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.; Kassam, Z.; Springer, A.N.; Gladdy, R.; Chung, P.; Ringash, J.; Catton, C. Long-Term quality of life of retroperitoneal sarcoma patients treated with Pre-Operative radiotherapy and surgery. Cureus 2017, 9, e1764. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Ong, C.J.; Skanthakumar, T.; Mak, L.; Wasudevan, S.D.; Tan, J.W.-S.; Chia, C.S.; Tan, G.H.C.; Teo, M.C.C. Retrospective quality of life study in patients with retroperitoneal sarcoma in an Asian population. Health Qual. Life Outcomes 2020, 18, 270. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Brunelli, C.; Miceli, R.; Manara, M.; Lenna, S.; Rampello, N.N.; Callegaro, D.; Colombo, C.; Radaelli, S.; Pasquali, S.; et al. A prospective observational study of multivisceral resection for retroperitoneal sarcoma: Clinical and Patient-Reported outcomes 1 year after surgery. Ann. Surg. Oncol. 2021, 28, 3904–3916. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; De Haes, J.C.J.M.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- King, M.T. The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Qual. Life Res. 1996, 5, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Swallow, C.J.; Strauss, D.C.; Bonvalot, S.; Rutkowski, P.; Desai, A.; Gladdy, R.A.; Gonzalez, R.; Gyorki, D.E.; Fairweather, M.; van Houdt, W.J.; et al. Management of primary retroperitoneal sarcoma (RPS) in the adult: An updated consensus approach from the transatlantic australasian RPS working group. Ann. Surg. Oncol. 2021, 28, 7873–7888. [Google Scholar] [CrossRef] [PubMed]
- Chia, C.S.; Tan, W.J.; Wong, J.F.; Tan, G.H.; Lim, C.; Wang, W.; Sin, E.-L.; Tham, C.; Soo, K.; Teo, M. Quality of life in patients with peritoneal surface malignancies after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur. J. Surg. Oncol. 2014, 40, 909–916. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | MVR (n = 77) | Non-MVR (n = 84) | p |
|---|---|---|---|
| Gender | 0.141 | ||
| Male | 41 (53.2) | 35 (41.7) | |
| Female | 36 (46.8) | 49 (58.3) | |
| Age, years mean (SD) | 55.9 (±12.3) | 54.5 (±14.1) | 0.494 |
| ASA score | 0.210 | ||
| 1 | 54 (70.1) | 51 (60.7) | |
| >1 | 23 (29.9) | 33 (39.3) | |
| Symptoms | 0.637 | ||
| Yes | 23 (29.9) | 28 (33.3) | |
| No | 54 (70.1) | 56 (66.7) | |
| Tumor burden, cm mean (SD) | 21.0 (±8.9) | 11.9 (±7.1) | <0.001 |
| Histologic subtypes | <0.001 | ||
| WDLPS | 43 (55.8) | 29 (34.5) | |
| DDLPS | 26 (33.8) | 6 (7.1) | |
| LMS | 4 (5.2) | 19 (22.6) | |
| SFT | 1 (5.6) | 17 (20.2) | |
| Others | 3 (3.9) | 13 (15.5) | |
| FNCLCC | 0.216 | ||
| Grade 1 | 29 (37.7) | 40 (47.6) | |
| Grade 2 | 29 (37.7) | 22 (26.2) | |
| Grade 3 | 12 (15.6) | 18 (21.4) | |
| Unknow | 7 (9.1) | 4 (4.8) | |
| Location | 0.681 | ||
| Left | 41 (53.2) | 42 (50.0) | |
| Right | 36 (46.8) | 42 (50.0) | |
| Multifocality | 0.644 | ||
| Yes | 6 (7.8) | 5 (6.0) | |
| No | 71 (92.2) | 79 (94.0) | |
| Radiation | 0.162 | ||
| Yes | 5 (6.5) | 11 (13.1) | |
| No | 72 (93.5) | 73 (86.9) | |
| Chemotherapy | 0.655 | ||
| Yes | 9 (11.7) | 8 (9.5) | |
| No | 68 (88.3) | 76 (90.5) | |
| Operation | 0.370 | ||
| Laparoscopic surgery | 1 (1.3) | 4 (4.8) | |
| Open surgery | 76 (98.7) | 80 (95.2) | |
| Complete resection | 1.000 | ||
| Yes | 83 (98.7) | 83 (98.8) | |
| No | 1 (1.3) | 1 (1.2) | |
| Major vascular surgery | 0.077 | ||
| Yes | 11 (14.3) | 5 (6.0) | |
| No | 66 (85.7) | 79 (94.0) | |
| Pancreaticoduodenectomy | 0.068 | ||
| Yes | 3 (3.9) | 0 (0.0) | |
| No | 74 (96.1) | 84 (100.0) | |
| Number of combined resections median, (IQR) | 3 (2–4) | 0 (0–1) | <0.001 |
| Resected organs | |||
| Colon | 58 (75.3) | 15 (17.9) | <0.001 |
| Kidney | 60 (77.9) | 9 (10.7) | <0.001 |
| Adrenal gland | 36 (46.8) | 1 (1.2) | <0.001 |
| Spleen | 16 (20.8) | 0 (0) | <0.001 |
| Pancreas | 16 (20.8) | 0 (0) | <0.001 |
| Small intestine | 13 (16.9) | 2 (2.4) | 0.002 |
| Diaphragm | 6 (7.8) | 2 (2.4) | 0.154 |
| Abdominal Wall | 3 (3.9) | 1 (1.2) | 0.350 |
| Operative time, hours mean (SD) | 4.4(±1.7) | 2.7 (±1.1) | <0.001 |
| Estimated blood loss, ml median, (IQR) | 780.6 (±713.3) | 424.6 (±780.5) | 0.004 |
| Packed RBC transfusion | 0.011 | ||
| Yes | 25 (32.5) | 13 (15.5) | |
| No | 52 (67.5) | 71 (84.5) | |
| ICU Stay | <0.001 | ||
| Yes | 57 (74.0) | 28 (33.3) | |
| No | 20 (26.0) | 56 (66.7) | |
| Severe postoperative adverse events | 0.088 | ||
| Yes | 7 (9.1) | 2 (2.4) | |
| No | 70 (90.9) | 82 (97.6) | |
| Postoperative Hospital Stay, days mean (SD) | 19.1 (±11.2) | 13.5 (±9.1) | 0.002 |
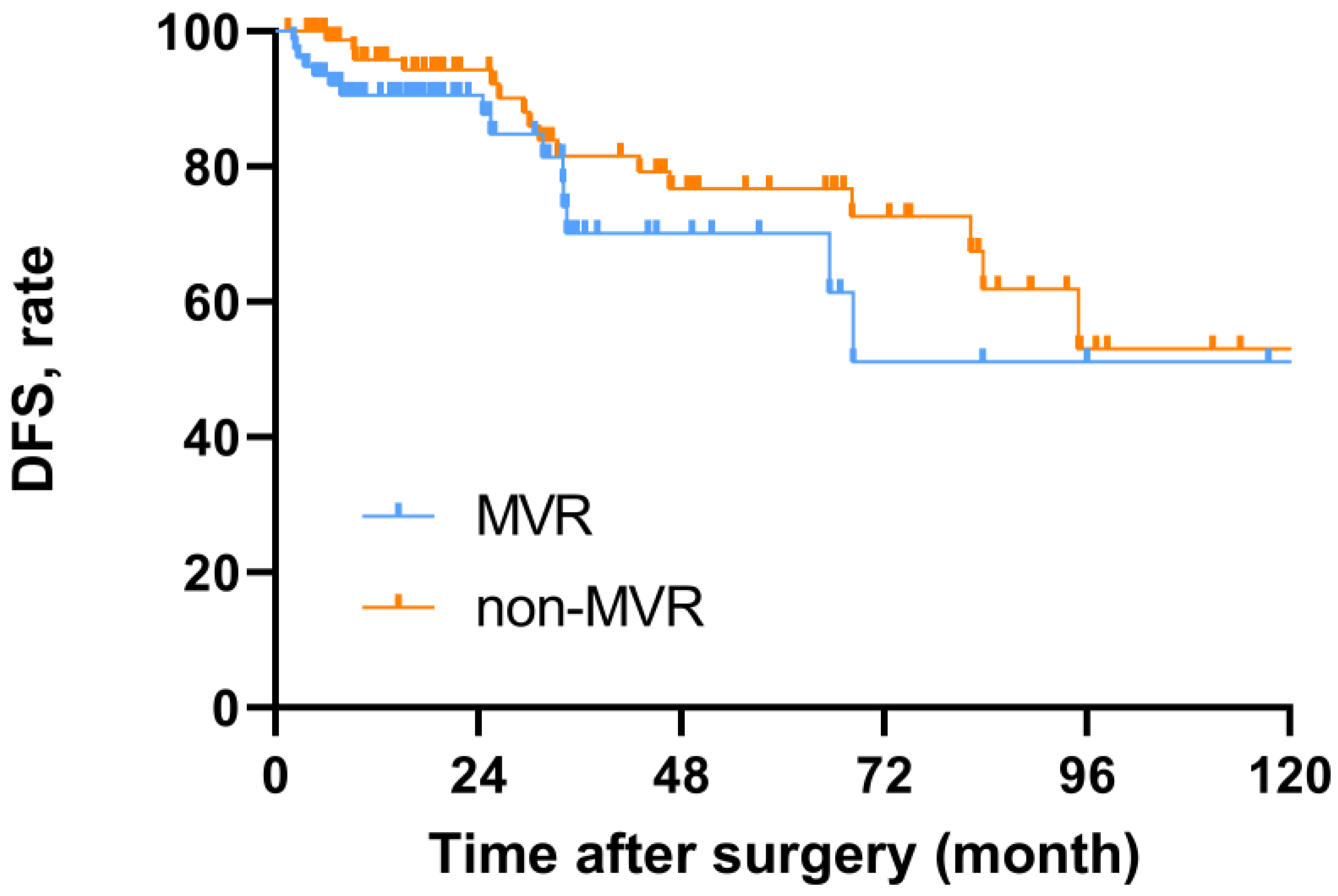
| Disease recurrence | 0.792 | ||
| Yes | 15 (19.5) | 16 (19.0) | |
| No | 62 (80.5) | 68 (81.0) |
| Characteristics | Mean | Standard Deviation | Median | IQR | p |
|---|---|---|---|---|---|
| Gender | 0.672 | ||||
| Male | 70.7 | 17.2 | 70.8 | 58.3–83.3 | |
| Female | 71.2 | 20.6 | 75.0 | 58.3–83.3 | |
| Age, years median | 0.101 | ||||
| ≤60 | 72.8 | 19.4 | 75.0 | 58.3–83.3 | |
| >60 | 67.9 | 18.0 | 66.7 | 58.3–83.3 | |
| ASA score | 0.088 | ||||
| 1 | 73.2 | 18.1 | 75.0 | 58.3–83.3 | |
| >1 | 66.7 | 20.2 | 66.7 | 50.0–83.3 | |
| Symptoms | 0.119 | ||||
| Yes | 67.3 | 20.9 | 66.7 | 50.0–83.3 | |
| No | 72.7 | 17.9 | 75.0 | 58.3–83.3 | |
| Tumor burden | 0.243 | ||||
| 0–10 | 73.1 | 19.9 | 75.0 | 58.3–83.3 | |
| >10 | 69.8 | 18.4 | 66.7 | 58.3–83.3 | |
| Histologic subtypes | 0.963 | ||||
| WDLPS | 71.6 | 17.8 | 75.0 | 58.3–83.3 | |
| DDLPS | 70.1 | 18.7 | 75.0 | 58.3–83.3 | |
| LMS | 68.8 | 24.0 | 66.7 | 50.0–83.3 | |
| SFT | 69.4 | 18.3 | 66.7 | 50.0–83.3 | |
| Others | 74.5 | 19.6 | 70.8 | 58.3–100.0 | |
| FNCLCC | 0.550 | ||||
| Grade 1 | 71.5 | 19.5 | 75.0 | 58.3–83.3 | |
| Grade 2 | 71.6 | 20.4 | 75.0 | 50.0–83.3 | |
| Grade 3 | 71.1 | 18.3 | 75.0 | 56.3–83.3 | |
| Unknow | 65.2 | 10.4 | 66.7 | 58.3–66.7 | |
| Location | 0.510 | ||||
| Left | 72.5 | 17.4 | 75.0 | 58.3–83.3 | |
| Right | 69.4 | 20.5 | 66.7 | 58.3–83.3 | |
| Multifocality | 0.001 | ||||
| Yes | 51.5 | 20.7 | 50.0 | 50.0–66.7 | |
| No | 72.4 | 18.1 | 75.0 | 58.3–83.3 | |
| Radiation | 0.165 | ||||
| Yes | 76.6 | 17.0 | 83.3 | 66.7–89.6 | |
| No | 70.4 | 19.2 | 66.7 | 58.3–83.3 | |
| Chemotherapy | 0.841 | ||||
| Yes | 72.5 | 13.4 | 66.7 | 66.7–83.3 | |
| No | 70.8 | 19.6 | 75.0 | 58.3–83.3 | |
| Severe postoperative adverse events | |||||
| Yes | 54.6 | 16.2 | 58.3 | 37.5–70.8 | 0.008 |
| No | 72.0 | 18.8 | 75.0 | 58.3–83.3 | |
| Recurrence | |||||
| Yes | 61.4 | 20.4 | 66.7 | 50.0–75.0 | 0.005 |
| No | 73.2 | 18.1 | 75.0 | 58.3–83.3 |
| Characteristics | MVR (n = 62) | non-MVR (n = 69) | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | IQR | Mean | SD | Median | IQR | ||
| Global health | 75.9 | 16.0 | 75.0 | 64.6–83.3 | 70.8 | 19.5 | 75.0 | 52.1–83.3 | 0.213 |
| Functional Scales | |||||||||
| Physical functioning | 87.0 | 14.4 | 90.0 | 80.0–100.0 | 89.4 | 12.9 | 93.3 | 80.0–100.0 | 0.282 |
| Role functioning | 82.8 | 18.8 | 83.3 | 66.7–100.0 | 83.3 | 22.7 | 100.0 | 66.7–100.0 | 0.424 |
| Emotional functioning | 82.8 | 16.3 | 87.5 | 72.95–93.8 | 79.2 | 20.0 | 83.3 | 66.7–91.7 | 0.360 |
| Cognitive functioning | 87.9 | 14.5 | 100.0 | 83.3–100.0 | 84.3 | 15.6 | 83.3 | 66.7–100.0 | 0.179 |
| Social functioning | 83.3 | 20.9 | 83.3 | 66.7–100.0 | 84.5 | 20.7 | 91.7 | 83.3–100.0 | 0.549 |
| Symptom Scales | |||||||||
| Fatigue | 24.4 | 20.9 | 22.2 | 11.1–33.3 | 27.1 | 22.6 | 33.3 | 11.1–33.3 | 0.495 |
| Nausea and vomiting | 6.2 | 16.6 | 0 | 0–0 | 3.6 | 8.5 | 0.0 | 0.0–0.0 | 0.831 |
| Pain | 11.8 | 20.1 | 0 | 0–16.7 | 15.2 | 18.9 | 16.7 | 0.0–16.7 | 0.149 |
| Dyspnoea | 11.3 | 19.0 | 0 | 0–33.3 | 16.4 | 21.1 | 0.0 | 0–33.3 | 0.150 |
| Insomnia | 28.0 | 29.7 | 33.3 | 0–33.3 | 27.1 | 32.5 | 33.3 | 0–33.3 | 0.597 |
| Appetite loss | 22.0 | 24.1 | 33.3 | 0–33.3 | 23.7 | 24.3 | 33.3 | 0–33.3 | 0.721 |
| Constipation | 14.0 | 22.2 | 0 | 0–33.3 | 29.5 | 34.1 | 33.3 | 0–58.3 | 0.011 |
| Diarrhoea | 20.4 | 26.6 | 0 | 0–33.3 | 15.9 | 26.0 | 0.0 | 0–33.3 | 0.214 |
| Financial difficulties | 26.9 | 33.5 | 0 | 0–33.3 | 28.0 | 33.6 | 0.0 | 0–58.3 | 0.955 |
| Number | Global QOL | Physical Functioning | Role Functioning | Emotional Functioning | Cognitive Functioning | Social Functioning | Fatigue | Nausea and Vomiting | Pain | Dyspnoea | Insomnia | Appetite Loss | Constipation | Diarrhoea | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | MVR | Non MVR | MVR | Non MVR | p * | MVR | Non MVR | p * | MVR | Non MVR | p * | MVR | Non MVR | p * | MVR | Non MVR | p * | MVR | Non MVR | p * | MVR | Non MVR | p * | MVR | Non MVR | p * | MVR | Non MVR | p * | MVR | Non MVR | p * | MVR | Non MVR | p * | MVR | Non MVR | p * | MVR | Non MVR | p * | MVR | Non MVR | p * |
| 1 | 5 | 6 | 58.3 | 76.4 | 0.082 | 69.3 | 84.4 | 0.177 | 63.3 | 72.2 | 0.662 | 75.0 | 77.8 | 1.000 | 83.3 | 91.7 | 0.792 | 70.0 | 80.6 | 1.000 | 42.2 | 31.5 | 0.329 | 16.7 | 2.8 | 0.429 | 36.7 | 8.3 | 0.329 | 13.3 | 38.9 | 0.126 | 46.7 | 22.2 | 0.126 | 33.3 | 27.8 | 0.792 | 13.3 | 22.2 | 0.792 | 33.3 | 11.1 | 0.662 |
| 2 | 13 | 10 | 64.7 | 59.2 | 0.522 | 86.2 | 80.7 | 0.522 | 76.9 | 76.7 | 1.000 | 83.3 | 77.5 | 0.257 | 93.6 | 81.7 | 0.131 | 75.6 | 81.7 | 0.738 | 30.8 | 28.9 | 0.693 | 5.1 | 5.0 | 0.879 | 19.2 | 16.7 | 0.738 | 25.6 | 13.3 | 0.284 | 43.6 | 26.7 | 0.284 | 28.2 | 26.7 | 0.927 | 12.8 | 36.7 | 0.166 | 28.2 | 13.3 | 0.232 |
| 3 | 12 | 4 | 75.7 | 64.6 | 0.170 | 90.6 | 91.7 | 1.000 | 93.1 | 83.3 | 0.212 | 85.4 | 68.8 | 0.078 | 84.7 | 70.8 | 0.212 | 84.7 | 75.0 | 0.684 | 22.2 | 44.4 | 0.170 | 12.5 | 4.2 | 1.000 | 8.3 | 20.8 | 0.078 | 2.8 | 8.3 | 0.684 | 25.0 | 50.0 | 0.684 | 25.0 | 41.7 | 0.262 | 8.3 | 25.0 | 0.379 | 33.3 | 50.0 | 0.521 |
| 4 | 8 | 11 | 82.3 | 66.7 | 0.129 | 91.7 | 85.5 | 0.600 | 89.6 | 74.2 | 0.717 | 77.1 | 65.2 | 0.492 | 89.6 | 78.8 | 0.238 | 93.8 | 72.7 | 0.075 | 20.8 | 35.4 | 0.657 | 4.2 | 9.1 | 0.442 | 6.3 | 18.2 | 0.238 | 8.3 | 18.2 | 0.310 | 20.8 | 33.3 | 0.778 | 12.5 | 30.3 | 0.545 | 16.7 | 36.4 | 0.272 | 20.8 | 21.2 | 0.840 |
| 5 | 18 | 18 | 81.0 | 79.6 | 0.938 | 85.9 | 95.6 | 0.134 | 80.6 | 93.5 | 0.047 | 84.3 | 88.9 | 0.239 | 87.0 | 90.7 | 0.606 | 86.1 | 94.4 | 0.074 | 21.6 | 14.8 | 0.239 | 2.8 | 0.0 | 0.584 | 7.4 | 5.6 | 0.938 | 9.3 | 9.3 | 0.839 | 18.5 | 22.2 | 0.719 | 20.4 | 11.1 | 0.406 | 16.7 | 24.1 | 0.424 | 9.3 | 7.4 | 0.791 |
| 6 | 6 | 19 | 91.7 | 71.5 | 0.036 | 93.3 | 90.9 | 0.780 | 88.9 | 86.8 | 0.877 | 86.1 | 81.6 | 0.555 | 86.1 | 83.3 | 0.780 | 86.1 | 87.7 | 0.555 | 13.0 | 26.9 | 0.106 | 0.0 | 3.5 | 0.598 | 2.8 | 22.8 | 0.176 | 5.6 | 17.5 | 0.437 | 22.2 | 24.6 | 0.926 | 11.1 | 24.6 | 0.246 | 16.7 | 28.1 | 0.733 | 0.0 | 17.5 | 0.274 |
| p # | - | - | <0.001 | 0.278 | - | 0.070 | 0.048 | - | 0.101 | 0.071 | - | 0.340 | 0.070 | - | 0.861 | 0.674 | - | 0.222 | 0.083 | - | 0.015 | 0.338 | - | 0.207 | 0.226 | - | 0.018 | 0.672 | - | 0.100 | 0.405 | - | 0.107 | 0.476 | - | 0.039 | 0.404 | - | 0.441 | 0.691 | - | 0.004 | 0.488 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, A.; Fang, Y.; Ma, L.; Yang, H.; Lu, W.; Zhou, Y.; Zhang, Y.; Tong, H. Does Aggressive Surgery Mean Worse Quality of Life and Functional Capacity in Retroperitoneal Sarcoma Patients?—A Retrospective Study of 161 Patients from China. Cancers 2022, 14, 5126. https://doi.org/10.3390/cancers14205126
Zhuang A, Fang Y, Ma L, Yang H, Lu W, Zhou Y, Zhang Y, Tong H. Does Aggressive Surgery Mean Worse Quality of Life and Functional Capacity in Retroperitoneal Sarcoma Patients?—A Retrospective Study of 161 Patients from China. Cancers. 2022; 14(20):5126. https://doi.org/10.3390/cancers14205126
Chicago/Turabian StyleZhuang, Aobo, Yuan Fang, Lijie Ma, Hua Yang, Weiqi Lu, Yuhong Zhou, Yong Zhang, and Hanxing Tong. 2022. "Does Aggressive Surgery Mean Worse Quality of Life and Functional Capacity in Retroperitoneal Sarcoma Patients?—A Retrospective Study of 161 Patients from China" Cancers 14, no. 20: 5126. https://doi.org/10.3390/cancers14205126
APA StyleZhuang, A., Fang, Y., Ma, L., Yang, H., Lu, W., Zhou, Y., Zhang, Y., & Tong, H. (2022). Does Aggressive Surgery Mean Worse Quality of Life and Functional Capacity in Retroperitoneal Sarcoma Patients?—A Retrospective Study of 161 Patients from China. Cancers, 14(20), 5126. https://doi.org/10.3390/cancers14205126




