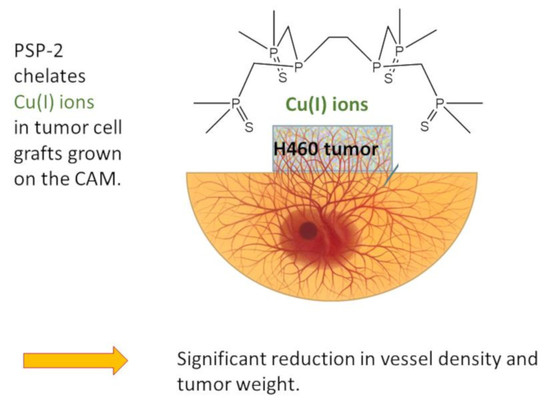
High-Affinity Cu(I)-Chelator with Potential Anti-Tumorigenic Action—A Proof-of-Principle Experimental Study of Human H460 Tumors in the CAM Assay
Abstract
Simple Summary
Abstract
1. Introduction
- Topical PSP-2 application reduces H460 tumor weight.
- PSP-2 treatment reduces vessel density in H460 tumors.
- Application of PSP-2 reduces the proliferation of H460 tumor cells and enhances their HIF-1α+ fraction.
- Copper amount per weight of tumor decreases in tumors treated with PSP-2.
2. Materials and Methods
2.1. Chemicals
2.2. Cells, Cell Culture, and Viability
2.3. Histology
2.4. MRI of the Living Chicken Embryo and CAM
2.5. Copper Content in Whole Tumors
2.6. Statistics
3. Results
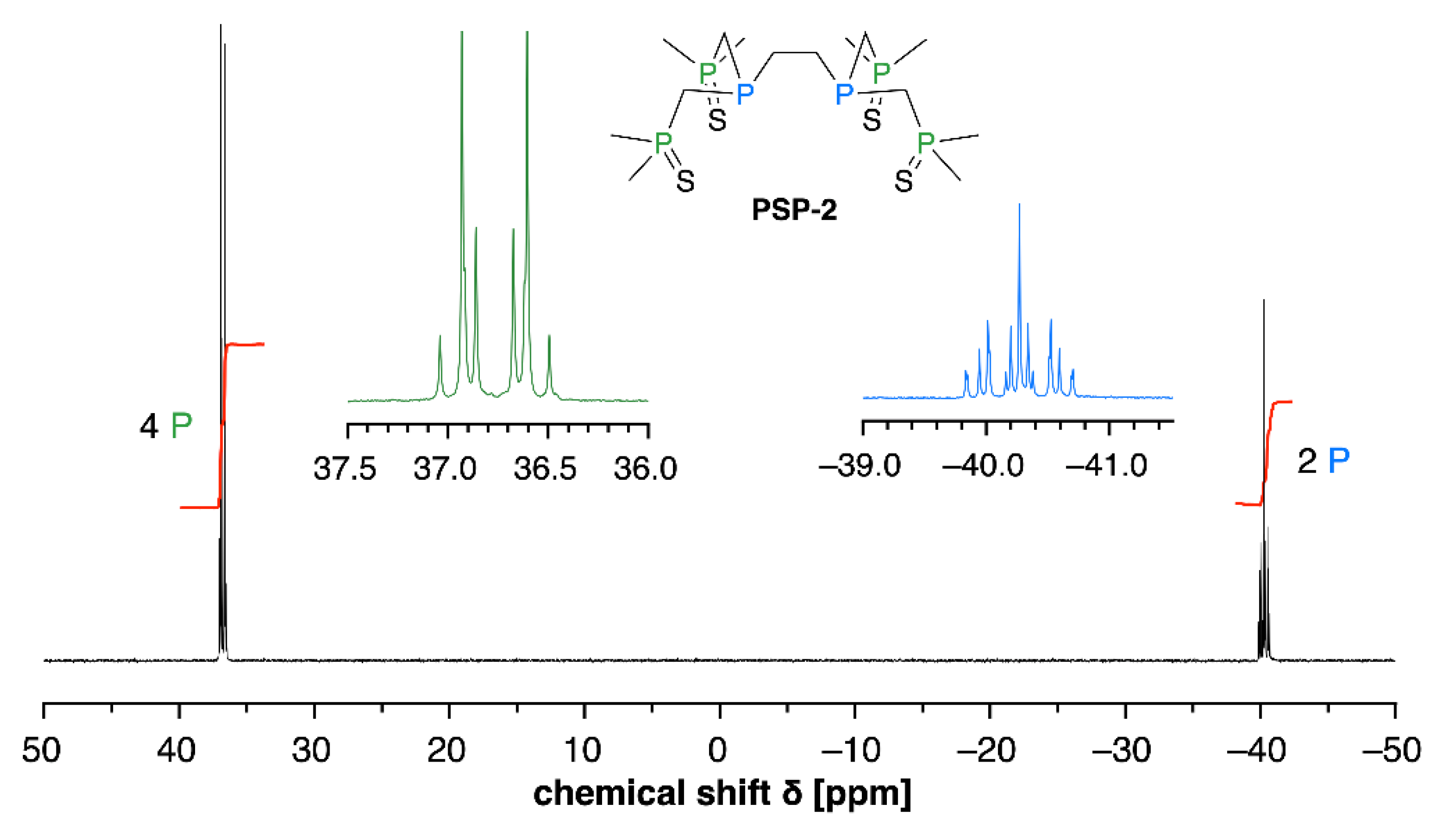
3.1. Oxidative Stability of PSP-2
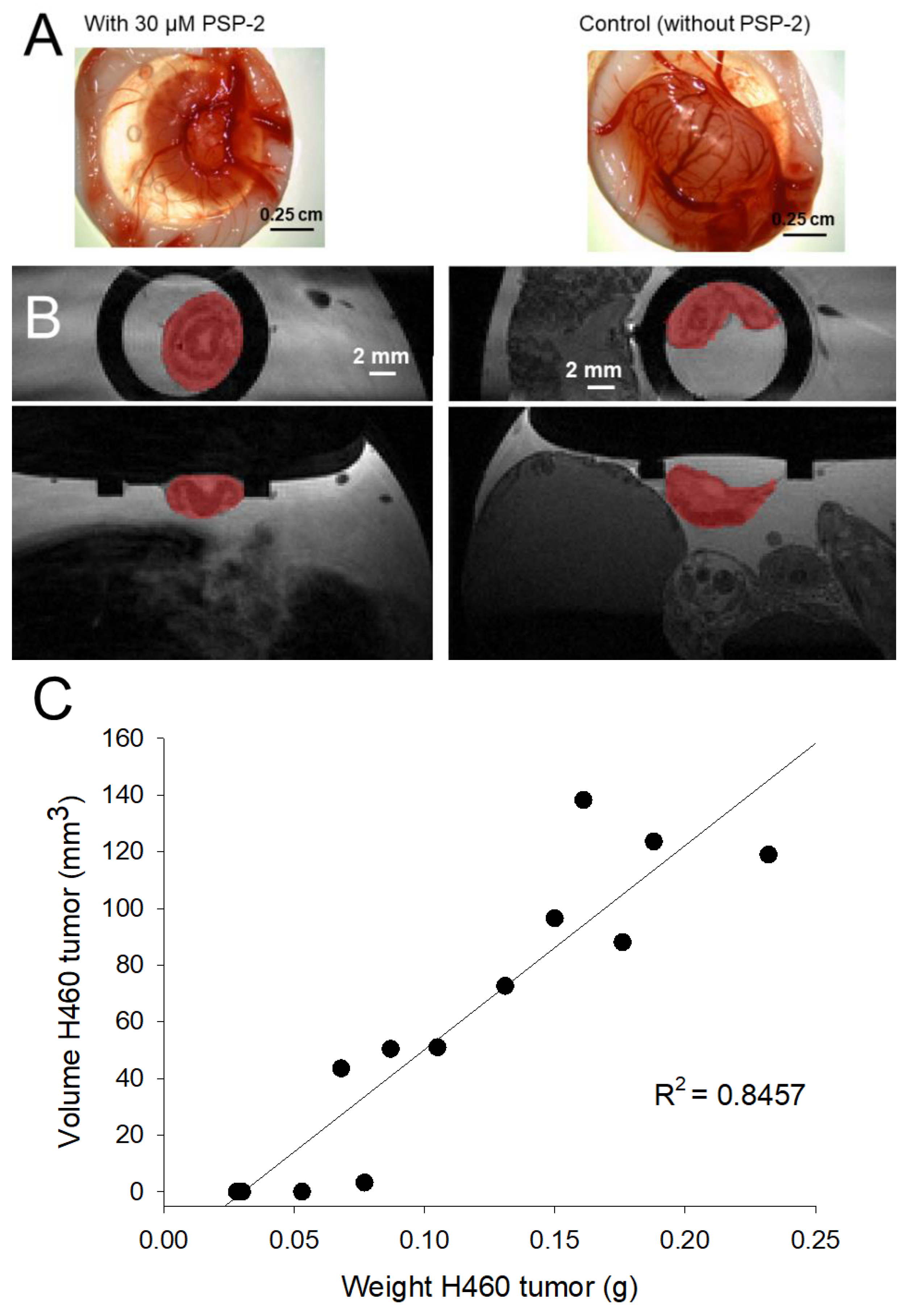
3.2. Weight and Volume of H460 Tumors Grown on the CAM
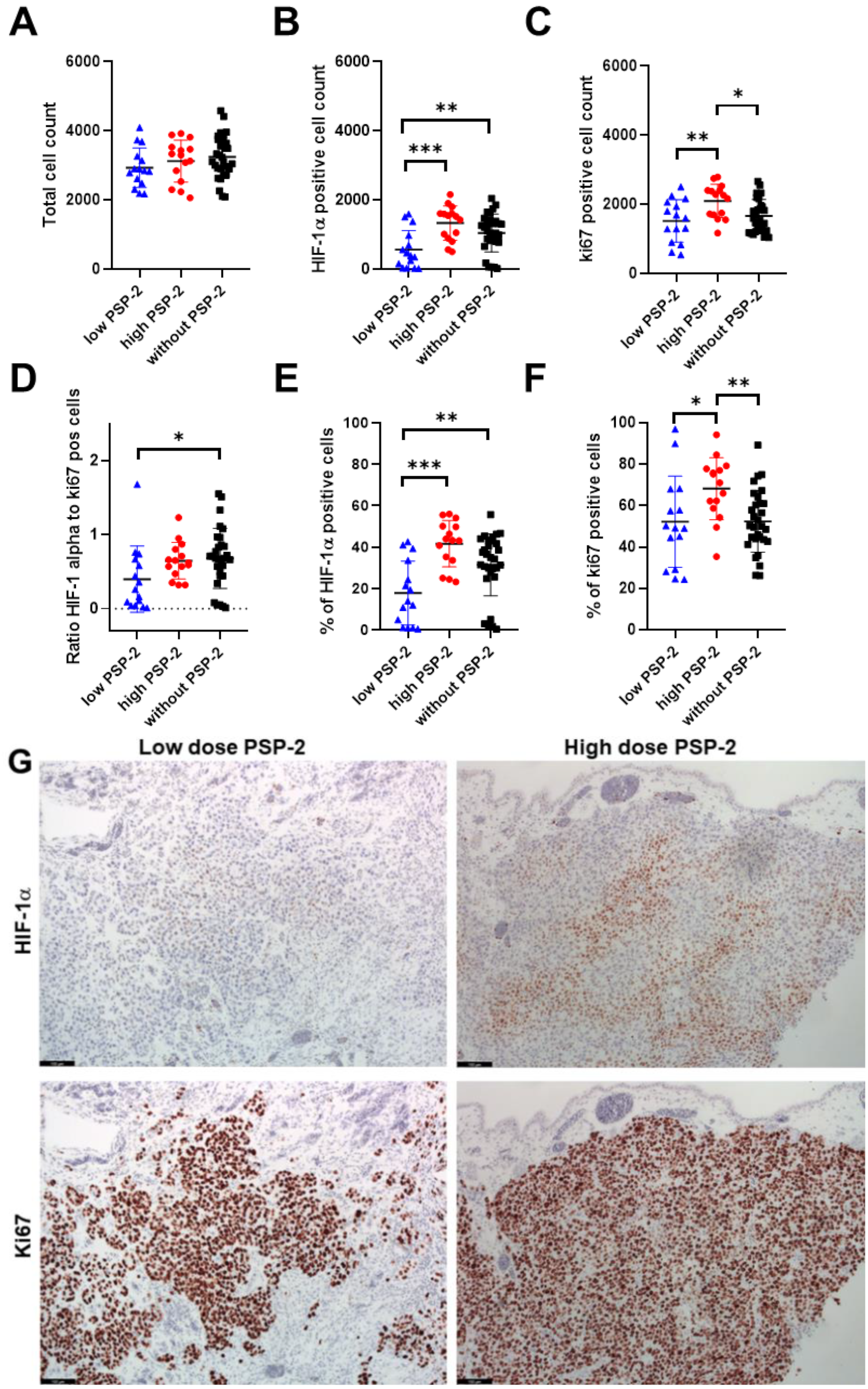
3.3. Vessel Density and Immunohistochemistry of H460 Tumors with and without PSP-2
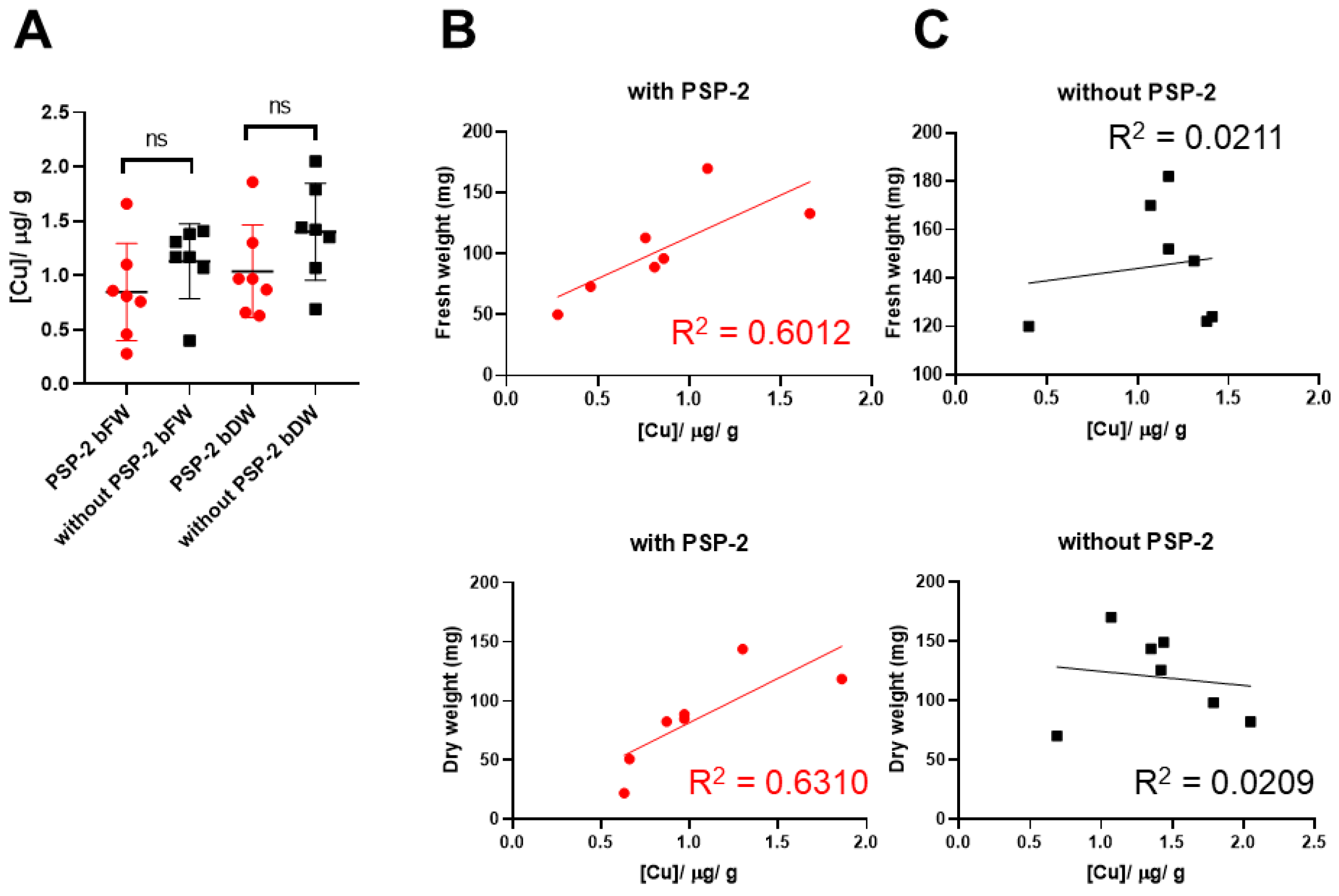
3.4. Copper Content of H460 Tumors with and without PSP-2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, J.; Cheng, J.; Zheng, N.; Zhang, X.; Dai, X.; Zhang, L.; Hu, C.; Wu, X.; Jiang, Q.; Wu, D.; et al. Copper Promotes Tumorigenesis by Activating the PDK1-AKT Oncogenic Pathway in a Copper Transporter 1 Dependent Manner. Adv. Sci. 2021, 8, e2004303. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Andreux, P.; Poitry-Yamate, C.; Auwerx, J.; Hanahan, D. Bioavailable copper modulates oxidative phosphorylation and growth of tumors. Proc. Natl. Acad. Sci. USA 2013, 110, 19507–19512. [Google Scholar] [CrossRef] [PubMed]
- Urso, E.; Maffia, M. Behind the Link between Copper and Angiogenesis: Established Mechanisms and an Overview on the Role of Vascular Copper Transport Systems. J. Vasc. Res. 2015, 52, 172–196. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhou, Y.C.; Zhou, B.; Huang, Y.C.; Wang, G.Z.; Zhou, G.B. Systematic analysis of concentrations of 52 elements in tumor and counterpart normal tissues of patients with non-small cell lung cancer. Cancer Med. 2019, 8, 7720–7727. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zeng, J.W.; Ma, Q.; Zhang, S.; Tang, J.; Feng, J.F. Serum copper and zinc levels in breast cancer: A meta-analysis. J. Trace Elem. Med. Biol. 2020, 62, 126629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, Q. Association between serum copper levels and lung cancer risk: A meta-analysis. J. Int. Med. Res. 2018, 46, 4863–4873. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Luo, J.; Chen, X.; Ma, K.; He, H.; Li, W.; Cui, J. Serum Copper Level and the Copper-to-Zinc Ratio Could Be Useful in the Prediction of Lung Cancer and Its Prognosis: A Case-Control Study in Northeast China. Nutr. Cancer 2021, 73, 1908–1915. [Google Scholar] [CrossRef]
- Crowe, A.; Jackaman, C.; Beddoes, K.M.; Ricciardo, B.; Nelson, D.J. Rapid copper acquisition by developing murine mesothelioma: Decreasing bioavailable copper slows tumor growth, normalizes vessels and promotes T cell infiltration. PLoS ONE 2013, 8, e73684. [Google Scholar] [CrossRef]
- Rieber, M. Cancer Pro-oxidant Therapy Through Copper Redox Cycling: Repurposing Disulfiram and Tetrathiomolybdate. Curr. Pharm. Des. 2020, 26, 4461–4466. [Google Scholar] [CrossRef]
- Arredondo, M.; Núñez, M.T. Iron and copper metabolism. Mol. Asp. Med. 2005, 26, 313–327. [Google Scholar] [CrossRef]
- Lowndes, S.A.; Sheldon, H.V.; Cai, S.J.; Taylor, J.M.; Harris, A.L. Copper chelator ATN-224 inhibits endothelial function by multiple mechanisms. Microvasc. Res. 2009, 77, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Herchenhan, A.; Uhlenbrock, F.; Eliasson, P.; Weis, M.; Eyre, D.; Kadler, K.E.; Magnusson, S.P.; Kjaer, M. Lysyl Oxidase Activity Is Required for Ordered Collagen Fibrillogenesis by Tendon Cells. J. Biol. Chem. 2015, 290, 16440–16450. [Google Scholar] [CrossRef] [PubMed]
- Helsel, M.E.; Franz, K.J. Pharmacological activity of metal binding agents that alter copper bioavailability. Dalton Trans. 2015, 44, 8760–8770. [Google Scholar] [CrossRef] [PubMed]
- Poier, N.; Hochstöger, J.; Hackenberg, S.; Scherzad, A.; Bregenzer, M.; Schopper, D.; Kleinsasser, N. Effects of Zinc Oxide Nanoparticles in HUVEC: Cyto- and Genotoxicity and Functional Impairment After Long-Term and Repetitive Exposure in vitro. Int. J. Nanomed. 2020, 15, 4441–4452. [Google Scholar] [CrossRef]
- Kim, Y.W.; Byzova, T.V. Oxidative stress in angiogenesis and vascular disease. Blood 2014, 123, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Bharathi Devi, S.R.; Dhivya, M.A.; Sulochana, K.N. Copper transporters and chaperones: Their function on angiogenesis and cellular signalling. J. Biosci. 2016, 41, 487–496. [Google Scholar] [CrossRef]
- Paterson, T.E.; Bari, A.; Bullock, A.J.; Turner, R.; Montalbano, G.; Fiorilli, S.; Vitale-Brovarone, C.; MacNeil, S.; Shepherd, J. Multifunctional Copper-Containing Mesoporous Glass Nanoparticles as Antibacterial and Proangiogenic Agents for Chronic Wounds. Front Bioeng. Biotechnol. 2020, 8, 246. [Google Scholar] [CrossRef]
- Zhang, P.L.; Hou, X.X.; Liu, M.R.; Huang, F.P.; Qin, X.Y. Two novel chiral tetranucleate copper-based complexes: Crystal structures, nanoparticles, and inhibiting angiogenesis and the growth of human breast cancer by regulating the VEGF/VEGFR2 signal pathway in vitro. Dalton Trans. 2020, 49, 6043–6055. [Google Scholar] [CrossRef]
- Zhang, W.J.; Chang, Q.; Xu, L.; Li, G.L.; Yang, G.Z.; Ding, X.; Wang, X.S.; Cui, D.X.; Jiang, X.Q. Graphene Oxide-Copper Nanocomposite-Coated Porous CaP Scaffold for Vascularized Bone Regeneration via Activation of Hif-1. Adv. Healthc. Mat. 2016, 5, 1299–1309. [Google Scholar] [CrossRef]
- Peña, Q.; Sciortino, G.; Maréchal, J.D.; Bertaina, S.; Simaan, A.J.; Lorenzo, J.; Capdevila, M.; Bayón, P.; Iranzo, O.; Palacios, Ò. Copper(II) N,N,O-Chelating Complexes as Potential Anticancer Agents. Inorg. Chem. 2021, 60, 2939–2952. [Google Scholar] [CrossRef]
- Park, K.C.; Fouani, L.; Jansson, P.J.; Wooi, D.; Sahni, S.; Lane, D.J.; Palanimuthu, D.; Lok, H.C.; Kovačević, Z.; Huang, M.L.; et al. Copper and conquer: Copper complexes of di-2-pyridylketone thiosemicarbazones as novel anti-cancer therapeutics. Metallomics 2016, 8, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Gandin, V.; Trenti, A.; Porchia, M.; Tisato, F.; Giorgetti, M.; Zanusso, I.; Trevisi, L.; Marzano, C. Homoleptic phosphino copper(I) complexes with in vitro and in vivo dual cytotoxic and anti-angiogenic activity. Metallomics 2015, 7, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Brady, D.C.; Crowe, M.S.; Turski, M.L.; Hobbs, G.A.; Yao, X.; Chaikuad, A.; Knapp, S.; Xiao, K.; Campbell, S.L.; Thiele, D.J.; et al. Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature 2014, 509, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Bao, L.W.; Kleer, C.G.; Brewer, G.J.; Merajver, S.D. Antiangiogenic tetrathiomolybdate enhances the efficacy of doxorubicin against breast carcinoma. Mol. Cancer Ther. 2003, 2, 617–622. [Google Scholar]
- Pan, Q.T.; Bao, L.W.; Merajver, S.D. Tetrathiomolybdate inhibits angiogenesis and metastasis through suppression of the NF kappa B signaling cascade. Mol. Cancer Res. 2003, 1, 701–706. [Google Scholar]
- Khan, R.A.; Usman, M.; Dhivya, R.; Balaji, P.; Alsalme, A.; AlLohedan, H.; Arjmand, F.; AlFarhan, K.; Akbarsha, M.A.; Marchetti, F.; et al. Heteroleptic Copper(I) Complexes of “Scorpionate” Bis-pyrazolyl Carboxylate Ligand with Auxiliary Phosphine as Potential Anticancer Agents: An Insight into Cytotoxic Mode. Sci. Rep. 2017, 7, 45229. [Google Scholar] [CrossRef]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting copper in cancer therapy: ‘Copper That Cancer’. Metallomics 2015, 7, 1459–1476. [Google Scholar] [CrossRef]
- Gaur, K.; Vázquez-Salgado, A.; Duran-Camacho, G.; Dominguez-Martinez, I.; Benjamín-Rivera, J.; Fernández-Vega, L.; Carmona Sarabia, L.; Cruz García, A.; Pérez-Deliz, F.; Méndez Román, J.; et al. Iron and Copper Intracellular Chelation as an Anticancer Drug Strategy. Inorganics 2018, 6, 126. [Google Scholar] [CrossRef]
- Denoyer, D.; Clatworthy, S.A.S.; Cater, M.A. Copper Complexes in Cancer Therapy. Met. Ions Life Sci. 2018, 18, 469–506. [Google Scholar] [CrossRef]
- Brewer, G.J. Copper lowering therapy with tetrathiomolybdate as an Antiangiogenic strategy in cancer. Curr. Cancer Drug Targ. 2005, 5, 195–202. [Google Scholar] [CrossRef]
- Khan, M.K.; Miller, M.W.; Taylor, J.; Gill, N.K.; Dick, R.D.; Van Golen, K.; Brewer, G.J.; Merajver, S.D. Radiotherapy and antiangiogenic TM in lung cancer. Neoplasia 2002, 4, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.T.; Yang, B.; Harankhedkar, S.; Nabatilan, A.; Bourassa, D.; McCallum, A.M.; Sun, F.X.; Wu, R.H.; Forest, C.R.; Fahrni, C.J. Stabilization of Aliphatic Phosphines by Auxiliary Phosphine Sulfides Offers Zeptomolar Affinity and Unprecedented Selectivity for Probing Biological Cu-I. Angew. Chem. Int. Ed. Eng. 2018, 57, 9711–9715. [Google Scholar] [CrossRef] [PubMed]
- Heuberger, D.M.; Harankhedkar, S.; Morgan, T.; Wolint, P.; Calcagni, M.; Lai, B.; Fahrni, C.J.; Buschmann, J. High-affinity Cu(I) chelator PSP-2 as potential anti-angiogenic agent. Sci. Rep. 2019, 9, 14055. [Google Scholar] [CrossRef]
- Aubert, L.; Nandagopal, N.; Steinhart, Z.; Lavoie, G.; Nourreddine, S.; Berman, J.; Saba-El-Leil, M.K.; Papadopoli, D.; Lin, S.; Hart, T.; et al. Copper bioavailability is a KRAS-specific vulnerability in colorectal cancer. Nat. Commun. 2020, 11, 3701. [Google Scholar] [CrossRef]
- Ge, E.J.; Bush, A.I.; Casini, A.; Cobine, P.A.; Cross, J.R.; DeNicola, G.M.; Dou, Q.P.; Franz, K.J.; Gohil, V.M.; Gupta, S.; et al. Connecting copper and cancer: From transition metal signalling to metalloplasia. Nat. Rev. Cancer 2022, 22, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef]
- Jang, J.H.; Baerts, L.; Waumans, Y.; De Meester, I.; Yamada, Y.; Limani, P.; Gil-Bazo, I.; Weder, W.; Jungraithmayr, W. Suppression of lung metastases by the CD26/DPP4 inhibitor Vildagliptin in mice. Clin. Exp. Metastasis 2015, 32, 677–687. [Google Scholar] [CrossRef]
- Wolint, P.; Bopp, A.; Woloszyk, A.; Tian, Y.; Evrova, O.; Hilbe, M.; Giovanoli, P.; Calcagni, M.; Hoerstrup, S.P.; Buschmann, J.; et al. Cellular self-assembly into 3D microtissues enhances the angiogenic activity and functional neovascularization capacity of human cardiopoietic stem cells. Angiogenesis 2018, 22, 37–52. [Google Scholar] [CrossRef]
- Finney, L.; Mandava, S.; Ursos, L.; Zhang, W.; Rodi, D.; Vogt, S.; Legnini, D.; Maser, J.; Ikpatt, F.; Olopade, O.I.; et al. X-ray fluorescence microscopy reveals large-scale relocalization and extracellular translocation of cellular copper during angiogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 2247–2252. [Google Scholar] [CrossRef]
- Lopez, J.; Ramchandani, D.; Vahdat, L. Copper Depletion as a Therapeutic Strategy in Cancer. Met. Ions Life Sci. 2019, 19, 303–330. [Google Scholar] [CrossRef]
- Nasulewicz, A.; Mazur, A.; Opolski, A. Role of copper in tumour angiogenesis—Clinical implications. J. Trace Elem. Med. Biol. 2004, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cukierman, D.S.; Lázaro, D.F.; Sacco, P.; Ferreira, P.R.; Diniz, R.; Fernández, C.O.; Outeiro, T.F.; Rey, N.A. X1INH, an improved next-generation affinity-optimized hydrazonic ligand, attenuates abnormal copper(I)/copper(II)-α-Syn interactions and affects protein aggregation in a cellular model of synucleinopathy. Dalton Trans. 2020, 49, 16252–16267. [Google Scholar] [CrossRef] [PubMed]
- Tapia, L.; González-Agüero, M.; Cisternas, M.F.; Suazo, M.; Cambiazo, V.; Uauy, R.; González, M. Metallothionein is crucial for safe intracellular copper storage and cell survival at normal and supra-physiological exposure levels. Biochem. J. 2004, 378, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.T.; Bourassa, D.; Harankhedkar, S.; McCallum, A.M.; Zlatic, S.A.; Calvo, J.S.; Meloni, G.; Faundez, V.; Fahrni, C.J. Ratiometric two-photon microscopy reveals attomolar copper buffering in normal and Menkes mutant cells. Proc. Natl. Acad. Sci. USA 2019, 116, 12167–12172. [Google Scholar] [CrossRef] [PubMed]
- Kivrak Pfiffner, F.; Waschkies, C.; Tian, Y.; Woloszyk, A.; Calcagni, M.; Giovanoli, P.; Rudin, M.; Buschmann, J. A new in vivo MRI method to non-invasively monitor and quantify the perfusion capacity of 3D-biomaterials grown on the chorioallantoic membrane of chick embryos. Tissue Eng. C 2014, 21, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, D.; Ikeda, Y.; Nakazawa, S. Copper chelation inhibits tumor angiogenesis in the experimental 9L gliosarcoma model. Neurosurgery 1995, 37, 287–292, discussion 292–283. [Google Scholar] [CrossRef]
- Liang, S.; Gao, Y.; Liu, Y.; Qiu, C.; Chen, Y.; Zhu, S. Contrast-enhanced Ultrasound in evaluating of angiogenesis and tumor staging of nasopharyngeal carcinoma in nude mice. PLoS ONE 2019, 14, e0221638. [Google Scholar] [CrossRef]
- Rzepakowska, A.; Żurek, M.; Grzybowski, J.; Pihowicz, P.; Górnicka, B.; Niemczyk, K.; Osuch-Wójcikiewicz, E. Microvascular density and hypoxia-inducible factor in intraepithelial vocal fold lesions. Eur. Arch. Otorhinolaryngol. 2019, 276, 1117–1125. [Google Scholar] [CrossRef]
- Boyd, N.H.; Tran, A.N.; Bernstock, J.D.; Etminan, T.; Jones, A.B.; Gillespie, G.Y.; Friedman, G.K.; Hjelmeland, A.B. Glioma stem cells and their roles within the hypoxic tumor microenvironment. Theranostics 2021, 11, 665–683. [Google Scholar] [CrossRef]
- Triner, D.; Shah, Y.M. Hypoxia-inducible factors: A central link between inflammation and cancer. J. Clin. Investig. 2016, 126, 3689–3698. [Google Scholar] [CrossRef] [PubMed]
- Shahrzad, S.; Shirasawa, S.; Sasazuki, T.; Rak, J.W.; Coomber, B.L. Low-dose metronomic cyclophosphamide treatment mediates ischemia-dependent K-ras mutation in colorectal carcinoma xenografts. Oncogene 2008, 27, 3729–3738. [Google Scholar] [CrossRef] [PubMed]
- Mudassar, F.; Shen, H.; O’Neill, G.; Hau, E. Targeting tumor hypoxia and mitochondrial metabolism with anti-parasitic drugs to improve radiation response in high-grade gliomas. J. Exp. Clin. Cancer Res. 2020, 39, 208. [Google Scholar] [CrossRef]
- Macklin, P.S.; McAuliffe, J.; Pugh, C.W.; Yamamoto, A. Hypoxia and HIF pathway in cancer and the placenta. Placenta 2017, 56, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Brekken, R.A.; Hyder, S.M. Vascular endothelial growth factor induces proliferation of breast cancer cells and inhibits the anti-proliferative activity of anti-hormones. Endocr. Relat. Cancer 2006, 13, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Devery, A.M.; Wadekar, R.; Bokobza, S.M.; Weber, A.M.; Jiang, Y.; Ryan, A.J. Vascular endothelial growth factor directly stimulates tumour cell proliferation in non-small cell lung cancer. Int. J. Oncol. 2015, 47, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Shukla, V.K.; Vaidya, M.P.; Roy, S.K.; Gupta, S. Serum trace elements and Cu/Zn ratio in breast cancer patients. J. Surg. Oncol. 1991, 46, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Apelgot, S.; Coppey, J.; Fromentin, A.; Guille, E.; Poupon, M.F.; Roussel, A. Altered distribution of copper (64Cu) in tumor-bearing mice and rats. Anticancer Res. 1986, 6, 159–164. [Google Scholar]
- Samoszuk, M.; Nguyen, V. In vitro and in vivo interactions of D-penicillamine with tumors. Anticancer Res. 1996, 16, 1219–1223. [Google Scholar] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heuberger, D.M.; Wolint, P.; Jang, J.-H.; Itani, S.; Jungraithmayr, W.; Waschkies, C.F.; Meier-Bürgisser, G.; Andreoli, S.; Spanaus, K.; Schuepbach, R.A.; et al. High-Affinity Cu(I)-Chelator with Potential Anti-Tumorigenic Action—A Proof-of-Principle Experimental Study of Human H460 Tumors in the CAM Assay. Cancers 2022, 14, 5122. https://doi.org/10.3390/cancers14205122
Heuberger DM, Wolint P, Jang J-H, Itani S, Jungraithmayr W, Waschkies CF, Meier-Bürgisser G, Andreoli S, Spanaus K, Schuepbach RA, et al. High-Affinity Cu(I)-Chelator with Potential Anti-Tumorigenic Action—A Proof-of-Principle Experimental Study of Human H460 Tumors in the CAM Assay. Cancers. 2022; 14(20):5122. https://doi.org/10.3390/cancers14205122
Chicago/Turabian StyleHeuberger, Dorothea M., Petra Wolint, Jae-Hwi Jang, Saria Itani, Wolfgang Jungraithmayr, Conny F. Waschkies, Gabriella Meier-Bürgisser, Stefano Andreoli, Katharina Spanaus, Reto A. Schuepbach, and et al. 2022. "High-Affinity Cu(I)-Chelator with Potential Anti-Tumorigenic Action—A Proof-of-Principle Experimental Study of Human H460 Tumors in the CAM Assay" Cancers 14, no. 20: 5122. https://doi.org/10.3390/cancers14205122
APA StyleHeuberger, D. M., Wolint, P., Jang, J.-H., Itani, S., Jungraithmayr, W., Waschkies, C. F., Meier-Bürgisser, G., Andreoli, S., Spanaus, K., Schuepbach, R. A., Calcagni, M., Fahrni, C. J., & Buschmann, J. (2022). High-Affinity Cu(I)-Chelator with Potential Anti-Tumorigenic Action—A Proof-of-Principle Experimental Study of Human H460 Tumors in the CAM Assay. Cancers, 14(20), 5122. https://doi.org/10.3390/cancers14205122