1. Introduction
The reprogramming of cellular metabolism is a hallmark of cancer allowing tumor cells to survive and proliferate. The Warburg effect is an established signature of cancer metabolism and refers to the observation that tumor cells preferentially use glucose via glycolysis pathway rather than oxidative phosphorylation (OXPHOS) in mitochondria for energy production [
1]. The reliance on glycolysis in cancer cells has been previously attributed to dysfunctional mitochondria [
2,
3]. However, recent data revealed that many tumor types have functional mitochondria and are not solely dependent on glycolysis for their energy requirements but also derive energy from OXPHOS [
4,
5,
6]. For instance, subsets of ovarian cancers with metastatic phenotype or cancer stem cell-like features have been shown to be highly metabolically active with increased mitochondrial respiration [
7,
8,
9]. A better understanding of tumor bioenergetics can reveal metabolic dependencies of cancer cells and facilitate development of new therapies.
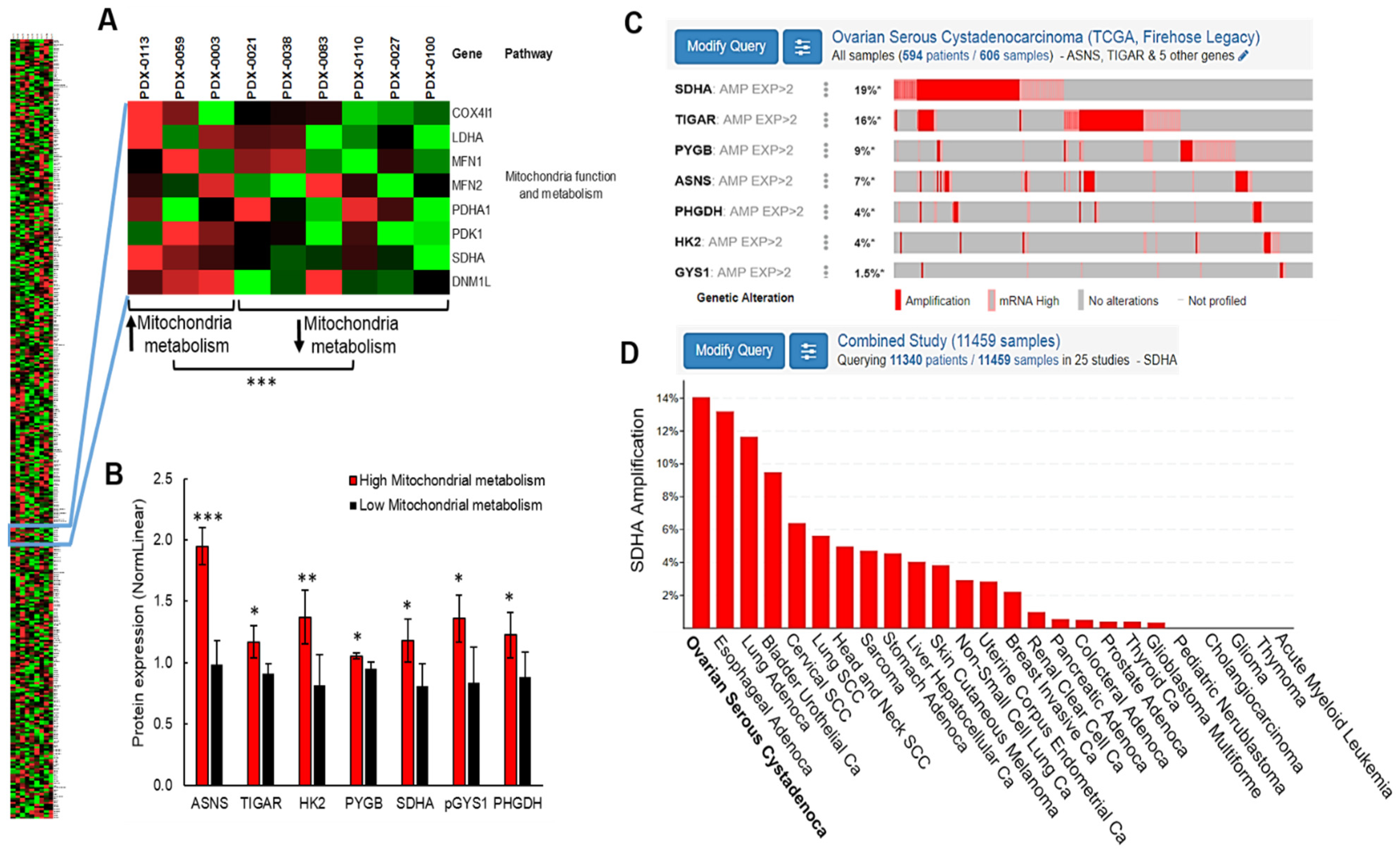
To gain a greater insight into the ovarian cancer metabolism, we interrogated metabolic phenotypes of patient-derived xenografts (PDXs) developed from patients’ ovarian tumors [
10]. We used proteomic approaches and discovered that the overexpressed subunit A of succinate dehydrogenase enzyme (SDHA) is associated with elevated mitochondrial metabolism in ovarian PDX models. The genomic data demonstrated that the
SDHA gene amplification or overexpression is highly prevalent in ovarian carcinoma patients (19% of all cases) compared to other tumor types, which indicates its potential importance in reprogramming of ovarian cancer metabolism.
Succinate dehydrogenase (SDH) also known as mitochondrial complex II, couples oxidation of succinate to fumarate with reduction of ubiquinone to ubiquinol, thus directly connecting the mitochondrial electron transport chain (ETC) with the tricarboxylic acid (TCA) cycle [
11]. SDH consists of four subunits, SDHA flavoprotein and SDHB iron-sulfur protein that function as the catalytic core, which is bound to SDHC and SDHD proteins that anchor the whole protein complex to the inner mitochondrial membrane [
12]. It has been shown that a correct assembly of succinate dehydrogenase complex is critical for its function [
13]. For instance, genetic mutation of SDH subunits results in severe assembly defects leading to a number of clinical pathologies including neurodevelopmental disorders or some rare malignancies [
11,
14,
15,
16,
17]. Previous studies demonstrated that the mechanism of tumor development in patients with succinate dehydrogenase deficiency is associated with succinate accumulation. An excess amount of succinate inhibits HIF1α prolyl hydroxylase activity leading to HIF-1α stabilization and increased transcription of HIF1α regulated genes promoting tumorigenesis [
11]. In addition, defective complex II assembly often results in accumulation of a free SDHA flavoprotein in the mitochondrial matrix [
18]. While mutations of SDH subunits are very rare in ovarian cancer, the amplification or overexpression of a SDHA subunit is highly prevalent in this tumor type (~20% tumors). However, the role of SDHA upregulation and its impact on ovarian cancer metabolism has never been investigated, emphasizing the need for further research.
In this work, using various in vitro and in vivo ovarian cancer models, we investigated the biological consequences of SDHA overexpression and its impact on tumor phenotype. Our findings revealed that SDHA overexpression is associated with the improved ability of cells to survive and generate colonies in anchorage-independent conditions. This is an important feature of metastatic ovarian tumor cells that are able to survive and spread in peritoneal fluid (ascites) within the abdominal cavity. Further, we showed that the overexpression of SDHA enhanced ovarian cancer metabolism reflected as increased mitochondrial respiration and ATP production rate. Lastly, we performed a drug screening and identified an anti-metabolic compound shikonin known to disrupt glycolysis and amino acid metabolism [
19,
20]. We showed that shikonin exhibited a profound anti-tumor efficacy and selectivity towards SDHA overexpressing tumor cells in vitro superior to that observed with traditional chemotherapy.
Altogether, our study demonstrated that SDHA upregulation frequently occurs in ovarian cancer and contributes to reprogramming of energy metabolism towards highly metabolically active phenotype. Importantly, we showed here that SDHA overexpressing tumor cells can be effectively targetable by anti-metabolic compounds such as shikonin, which represents a new opportunity for therapeutic intervention in ovarian cancer.
2. Materials and Methods
2.1. Source of Ovarian Cancer Cells and PDX Tumor Models
The OVCAR3 cell line was purchased from ATCC (Manassas, VA, USA). The OVCAR4 cell line was a gift from Dr. Jones [
21]. Caov3, TYKnu, and OVSAHO cell lines were purchased from JCRB Cell Bank (Osaka, Japan). OVCAR3 and OVCAR4 cell lines were authenticated by short tandem repeat (STR) profiling by ATCC. To ensure the identity and validity of our cell lines and to prevent potential problems associated with cell culture, such as cell line misidentification, contamination and genetic drift, we purchase cell lines form validated, reliable source and cryopreserve 20 vials of each cell line at low passage (passage 1–3). The cell line vials are kept protected in a lab cell line bank and are distributed to lab members according to the experimental needs. All cell lines were maintained in RPMI 1640 Medium containing L-glutamine 300 mg/L and D-glucose 2 g/L (#11875, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS) (#F0926, Sigma-Aldrich, St. Louis, MO, USA), in a standard humidified incubator at 37 °C in 5% CO
2 and 95% O
2 atmosphere. Cell lines were tested for Mycoplasma by Idexx BioAnalytics and were found negative for any contamination. Human fallopian tube tissues and established PDX tumor models were obtained from the PDX-PCT core facility at OMRF [
10].
2.2. Animal Experiments
All animal procedures were approved by the OMRF’s Institutional Animal Care and Use Committee. For in vivo experiments, 6 week-old female NOD/scid mice (#1303, Jackson Laboratory, Bar Harbor, ME, USA) were implanted subcutaneously into the flank with tumor cells (5 × 106 OVCAR4-SDHAi cells or 5 × 106 OVCAR3-SDHAi cells). Mice with established subcutaneous tumors of ~100 mm3 volume were randomized and fed with doxycycline-supplemented food (pellets containing 200 mg/kg doxycycline, #S3888, Bio-Serv, Flemington, NJ, USA) or respective control diet (#S3888, Bio-Serv, Flemington, NJ, USA). Subcutaneous tumor volumes were calculated using the formula ½ (Length × Width2). Mice were monitored weekly for body weight, development and progression of ovarian tumors, and any symptoms of physical distress or illness. At indicated time points tumors were harvested from mice and assessed for SDHA expression levels. At the endpoint, animals were humanely euthanized by CO2 inhalation as described in the IACUC-approved animal use protocol (#22-01).
2.3. Reverse Phase Protein Array
RPPA experiments were performed in accordance with instructions from the RPPA Core facility at the MD Anderson Cancer Center, University of Texas. We prepared cell pellets containing 2 × 106 of cells derived from 9 HGSOC PDXs that were sent to the RPPA core facility. Our PDX samples were analyzed for 367 validated targets. Linear values of normalized RPPA data were used to determine protein expression.
2.4. In Silico Analysis of TCGA Datasets
The current study used publicly available genomic datasets generated by the TCGA and processed these data in accordance with the TCGA publication guidelines [
22]. The cBioPortal online analytical tool (
http://www.cbioportal.org/public-portal/, accessed on 15 January 2020) was used to analyze TCGA data matrix [
23]. To assess genomic alterations of metabolic genes, we used an ovarian cancer dataset that encompassed 617 ovarian serous cystadenocarcinoma specimens in the “Firehose Legacy” category [
24]. We queried tumors with copy-number variance (CNV) and mRNA expression data presented by z-scores detected with U133 microarray only, which included 606 samples. To determine the occurrence of SDHA amplification in ovarian cancer vs. other tumors, we compared the SDHA amplification frequency in 25 studies representing diverse malignancies, which included 11,459 samples. The results were visualized in OncoPrint.
2.5. Quantitative Real-Time PCR
Total RNA was extracted with the Qiagen RNeasy Mini kit (Qiagen, Valencia, CA, USA), according to the Manufacturer’s protocol. cDNA was synthesized using iScript cDNA synthesis kit (#1708840, Bio-Rad, Hercules, CA, USA) following the manufacturer’s instructions. A 20 μL reaction volume was prepared with 1 μg purified RNA. cDNA was diluted 1:20 in nuclease-free water and 1μL was analyzed in triplicates by real-time PCR using the LightCycler 96 System (Roche) and PowerUp™ SYBR® Green Master Mix (#A25742, Thermo Fisher Scientific, Waltham, MA, USA), with 0.2 μM of each primer in a total volume of 25 μL reaction mixture. Primers were ordered from Integrated DNA Technologies, Inc. (IDT) (Coralville, IA, USA). Primers were based on published sequences or designed using Prime 3 software (Prime 3), which are listed as the following sequences: forward primer and reverse primer. Human SDHA: 5′ AGG CTT GCG AGC TGC ATT TG 3′, and 5′ AGC CCT TCA CGG TGT CGT AG 3′; human β-actin (ACTB): 5′ TGA CCC AGA TCA TGT TTG AGA CC 3′, and 5′ GTC CAG ACG CAG GAT GGC ATT 3′.
2.6. WES (ProteinSimple)
Cells or tumors were homogenized and lysed in Buffer B (25 mM Tris-HCl, pH 7.5, 0.42 M NaCl, 1.5 mM MgCl2, 0.5 mM EDTA, 1 mM DTT, 25% sucrose, 1 mM Na3VO4, and 1× protease inhibitor cocktail) on ice for 15 min, followed by centrifugal clearing at 4 °C for 10 min at 10,000 rpm to recover whole cell lysates. For analysis of proteins using a capillary electrophoresis-based protein analysis system (WES; ProteinSimple, San Jose, CA, USA), cellular proteins (0.5 mg/mL) were separated and visualized using the standard instrument protocol. Primary antibodies used were: GAPDH (1:300, #sc-25778) from Santa Cruz Biotechnology (Santa Cruz, CA, USA); α-Tubulin (1:25, #2144S); SDHA (1:25, #11998S) from Cell Signaling Technology (Danvers, MA, USA). Anti-rabbit secondary antibodies were included in the Wes-Rabbit (12-230 kDa) Master Kit (#PS-MK14, ProteinSimple, San Jose, CA, USA).
2.7. Succinate Dehydrogenase Activity
The activity of succinate dehydrogenase was assessed by the Succinate Dehydrogenase Activity Colorimetric Assay (#K660-100, BioVision, Waltham, MA, USA). Ovarian cancer cell lines were stimulated with 100 ng/mL dox for 24 h to induce SDHA overexpression and their corresponding control cells were harvested and assayed immediately for SDH activity following the manufacturer’s instructions. The reaction was initiated by adding a blue colored artificial probe to accept electrons from the succinate to fumarate oxidation. Absorbance at 600 nm was recorded in kinetic mode at RT. The decrease in absorbance per unit time (slope) was compared to a standard curve obtained with known protein concentrations and reported as a relative fold change of SDH activity between examined cell lines [
25].
2.8. Fumarate Levels
Fumarate levels were assessed by the Fumarate Assay Kit (cat. no. MAK060, Sigma-Aldrich, St. Louis, MO, USA). Ovarian cancer cell lines were stimulated with 100 ng/mL dox for 24 h to induce SDHA overexpression and their corresponding control cells were harvested and assayed immediately for fumarate levels following the manufacturer’s instructions. Briefly, 1 mM fumarate standard solution was serially diluted and plated into a 96-well plate, generating fumarate standard curve. Fumarate assay buffer was then added to each well to bring the total volume up to 50 μL. Ovarian cancer cells were harvested (106 cells per well in triplicates), washed with cold PBS, then resuspended and homogenized with 100 μL of ice cold Fumarate Assay Buffer, and centrifuged 10,000× g for 5 min to remove precipitate. Next, 50 μL of sample and 100 μL of an appropriate master reaction mix was added to each well and incubated for 30 min at 37 °C protected from light. Absorbance was then measured at 450 nm using a microplate reader. To measure fumarate levels, a standard curve was prepared in a 96-well plate using fumarate standard solution. Concentration (mM) of fumarate in ovarian cancer samples was calculated as follows: Fumarate = (Sa/Sv) × D; where Sa is an amount of fumarate from standard curve (mM), Sv is a sample volume added into each well (μL), and D represents sample dilution factor.
2.9. Generation of Lentiviruses and Cell Transduction
To generate ovarian cancer cell lines conditionally overexpressing SDHA, we used cell lines with low SDHA expression and conditionally overexpress the SDHA coding sequence by transducing the cells with pLentiTRE/rtTA-SDHA lentivirus. To construct the pLentiTRE/rtTA-SDHA lentivirus, the coding sequence of SDHA gene was amplified by PCR from human ovarian cancer cells and flanked by restriction sites for the HpaI and PacI enzymes. Next, the sequence was subcloned into HpaI and PacI restriction sites of the tetracycline inducible (Tet-On) lentiviral expression vector pLentiTRE/rtTA carrying both TRE and rtTA cassettes. The SDHA presence was verified by sequencing. Recombinant lentiviruses were produced in HEK293T cells according to our standard protocols [
26]. Ovarian cancer cell lines were then infected with lentivirus containing the tetracycline-inducible SDHA, followed by selection with blasticidin. The SDHA expression was induced by the addition of 100 ng/mL of doxycycline (dox) for 24 h. To knockdown SDHA gene, we used validated shRNA clone TRCN0000028085 from Sigma-Aldrich. The shRNA was cloned into pLKO.1-puro lentiviral vector (Sigma-Aldrich, St. Louis, MO, USA). Recombinant lentiviruses were produced in HEK293T cells according to standard protocols [
26]. Ovarian cancer cell lines endogenously overexpressing SDHA were then infected with lentiviruses containing the shRNA against SDHA, or control shRNA with a scrambled sequence, followed by selection with puromycin, as described [
26].
2.10. 3T5 Cell Proliferation Assay
The 3T5 cell proliferation assay was performed by plating 5 × 105 cells per 10 cm tissue culture plate (each cell line was set up in triplicate), followed by counting and re-plating at the same density every 3 days for 13 days. Population doubling time was calculated using the formula ln(post-3-day cell count/5 × 105)/ln(2). The given population doubling time was added to the cumulative doubling time of the previous count.
2.11. Soft Agar Colony Formation Assay
Soft agar colony formation assay was performed as previously published [
27]. Briefly, cells were cultured in 6-well plates (4 × 10
4 cells per well) in a mixture of 0.6% DifcoTM Noble Agar (#214220, BD Biosciences, Franklin Lakes, NJ, USA) and their respective media, which was added on top of a layer of 1% noble agar in culture medium. To induce SDHA overexpression, fresh doxycycline solution in culture medium (100 ng/mL final concentration) was added every two days to respective wells. Control cells received the same volume of culture medium. Cells were cultured for 10 days and then stained overnight with 200 μL of nitroblue tetrazolium chloride (#VWR0329, VWR Chemicals, Radnor, PA, USA). The visible colonies were photographed using a Leica 205 FCA microscope. Colony number and was estimated using Image J, version 1.52a.
2.12. Metabolic Flux Analysis
The Seahorse XFe24 Extracellular Flux Analyzer (Agilent Technologies, Santa Clara, CA, USA) was used to assess bioenergetic profiles of ovarian cancer cell lines [
28]. To run seahorse assay, cells were evenly seeded (60,000 cells/well; following optimization of cell seeding number) into the XF24 cell culture plate (#102340-100, Seahorse XFe24 FluxPaks, Agilent Technologies, Santa Clara, CA, USA) and incubated for 24 h at 37 °C and 5% CO
2. After 24 h of incubation, cell culture medium was replaced with Seahorse XF Base Medium (#103335-100, Agilent Technologies, Santa Clara, CA, USA) supplemented with 2 mM L-Glutamine (#G7513-100ML, Sigma-Aldrich, St. Louis, MO, USA), 1 mM Sodium Pyruvate (#13-115E, Lonza Bioscience, Basel, Switzerland), 10 mM Glucose (#G8270-100G, Sigma-Aldrich, St. Louis, MO, USA) with pH adjusted to 7.4. Then, the cells were placed in a non-CO
2 incubator for 1 h at 37 °C required for cells to reach an optimal pH and temperature conditions prior to the start of the experiment. Following 1 h incubation, the XF24 cell culture plate was loaded into the Seahorse instrument, which measured OCR/ECAR at intervals of approximately 5–8 min. Depending on the seahorse assay, various pharmacological compounds interrupting mitochondrial respiration were injected via ports to determine their effects on mitochondria function. The plate included also control blank wells containing only media to which various reagents were added similar to experimental wells. The blanks were automatically subtracted from experimental wells by the instrument software. Three measurements of OCR/ECAR were obtained following injection of each compound modulating cellular respiration. Compounds used in Seahorse assays included 1 uM Oligomycin A (ATP synthase inhibitor, #495455-10MG, MilliporeSigma, Burlington, MA, USA), 1 uM of FCCP (protonophoric uncoupler, #C2920-10MG, MilliporeSigma, Burlington, MA, USA) and 1 uM of Antimycin A (complex III inhibitor, #A8674-25MG, MilliporeSigma, Burlington, MA, USA), 10 mM glucose (#G8270-100G, Sigma-Aldrich, St. Louis, MO, USA), 50 mM 2-deoxy-D-Glucose (#14325, Cayman Chemical, Ann Arbor, MI, USA). Compound concentrations were optimized prior to experiments. The measurements were normalized with cell number and total protein levels (Bradford protein assay).
2.13. Seahorse XF Real-Time ATP Rate Assay Calculations
We performed calculations (validated by Agilent [
29]) based on known reaction stoichiometry, where OCR, ECAR, and PER data were converted to mitochondrial or glycolytic ATP production rates. The calculated parameters included: mitochondrial OCR (pmol O
2/min) = basal OCR—non-mitochondrial OCR after antimycin A (AA) injection; mitochondrial PER (mitoPER, pmol H
+/min) = CO
2 conversion factor × mitochondrial OCR, in which the CO
2 conversion factor for XFe24 well plates is defined as 0.60 [
30]; basal glycolysis (glycoPER, pmol H
+/min) = total PER − mitochondrial PER; glycoATP Production Rate (pmol ATP/min) = glycoPER; OCR
ATP (pmol O
2/min) = basal OCR basal—OCR after oligomycin injection; mito ATP Production Rate (pmol ATP/min) = OCRATP × 2 (pmol O/pmol O
2) × P/O (pmol ATP/pmol O), where P/O value of 2.75 derives from the stoichiometry of reaction representing the number of molecules of ATP synthesized per atom of O reduced by an electron pair; and total ATP Production Rate (pmol ATP/min) = glycol ATP Production Rate + mito ATP Production Rate [
31].
2.14. Glycolytic Rate Assay Calculations
We performed the Glycolytic Rate Assay, which measures a Proton Efflux Rate (PER). The obtained PER values allowed to calculate determinants of glycolysis: mitochondrial PER (mitoPER) = CO
2 conversion factor × (OCR − non-mitochondrial OCR after antimycin A injection), in which the CO
2 conversion factor for XFe24 well plates is defined as 0.60 [
30]; basal glycolysis (glycoPER) = (total PER -2-DG PER, which includes other sources of extracellular acidification that are not attributed to glycolysis or mitochondrial TCA activity)− mitochondrial PER; maximal glycolysis = PER after Antimycin A injection—2-DG PER (post-2-DG acidification); and glycolytic reserve = Maximal glycolysis—glycoPER.
2.15. Nutrient Deprivation Assay
For glucose deprivation conditions, respective cells were cultured in glucose free RPMI 1640 medium (Gibco #11879020), supplemented with 1 mM sodium pyruvate and low concentration of D-glucose (200 mg/L). Glutamine deficient medium was prepared using glutamine free RPMI 1640 medium (Gibco #21870076) supplemented with 1 mM sodium pyruvate and low concentration of glutamine (30 mg/L). To inhibit glycolysis, galactose medium was generated using glucose free RPMI 1640 medium (Gibco #11879020), supplemented with 1 mM sodium pyruvate and optimal concentration of galactose (2 g/L). The cells were cultured in complete medium or nutrient deprived medium for 48 h prior metabolic flux analysis (as previously described [
32]).
2.16. Anti-Metabolic Compound Library and Drug Screening Assay
A customized anti-metabolic compound library was generated from Selleck Chemicals inventory (#L3000, #L3700, #L5700, and #L6900), and consisted of 64 selective agents targeting various aspects of cellular metabolism (
Supplementary Table S2). The in vitro drug screening assay was carried out by treating exponentially growing ovarian cancer cells with 64 individual anti-metabolic agents followed by an MTT assay to measure cell viability. The cell viability was assessed by the Quick Cell Proliferation Assay kit II (BioVision, Milpitas, CA, USA). Briefly, cells were seeded in a 96-well plate in 100 μL of their respective media at a density of 2000–5000 cells per well. After cells were cultured for 24 h, the medium was aspirated and cells were exposed to desired drug concentration(s) for 4 days. In addition, a vehicle control corresponding to the highest DMSO concentration, which did not exceed 0.2%, was also included. Next, cells were incubated with the WST reagent for 2 h and absorbance was determined at 450 nm. Absorbance measurements were normalized to the DMSO control wells. Normalized values were plotted as an average ± SD of three wells per condition and these data were analyzed to determine the changes in cell viability.
2.17. Statistical Analysis
All in vitro experiments were performed three times and in triplicate when applicable. Values are presented as mean ± SD, or as mean ± SEM. Statistical analysis of in vitro assays or in vivo data was done using unpaired t-test, multiple t-test, or analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test whenever applicable. p < 0.05 was considered significant. Statistical analysis was performed using GraphPad Prism 6.0 Software (San Diego, CA, USA).
4. Discussion
The reprograming of cellular metabolism is an essential mechanism of tumorigenesis. The metabolic plasticity allows cancer cells to adapt to various unfavorable microenvironmental conditions and sustain uncontrolled cell proliferation, which promotes tumor progression and metastasis [
1,
41]. In this study, we identified a mitochondrial enzyme succinate dehydrogenase SDHA as a key protein associated with an elevated mitochondrial metabolism in ovarian tumors. The SDHA gene amplification is highly prevalent in ovarian cancer, and has been reported in a considerably higher frequency in ovarian tumors than in many other malignancies (TCGA data) indicating its potential role in reprogramming of ovarian cancer metabolism.
Succinate dehydrogenase has been previously studied in the context of its deficiency in some rare disorders and malignancies [
11,
14,
15,
16,
17]. However, the SDHA gain-of-function studies are sparse. Increased SDHA function has been associated with elevated mitochondrial respiration and fumarate accumulation driving pathological metabolism in certain diseases [
42,
43,
44]. For instance, in patients with primary antibody deficiency syndrome, the SDHA gain-of-function phenotype leads to an inflammatory reprogramming of lymphocytes B promoting systemic inflammation and worsening the severity of the disease [
42]. In metastatic uveal melanoma, elevated SDHA contributes to a metabolic dysregulation with increased mitochondrial respiration, which leads to resistance to therapy, and a significantly shorter time to metastasis and death of patients [
44].
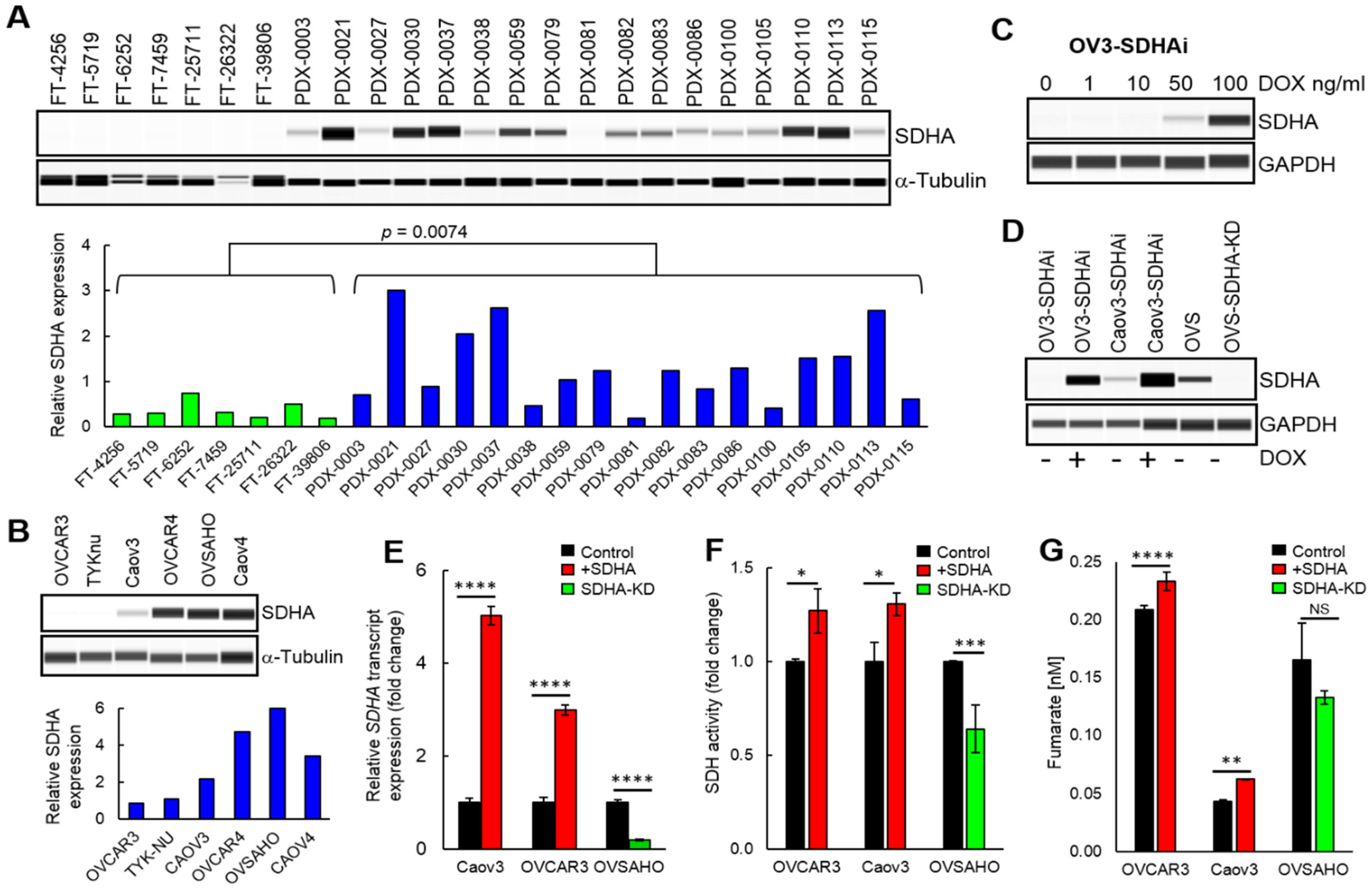
In the present study, our initial findings highlighted a potential importance of SDHA upregulation in ovarian cancer and set the stage for further more rigorous research. First, we performed an analysis of SDHA expression in a collection of healthy fallopian tubes and HGSOC PDX models, which showed that SDHA protein levels are significantly lower in fallopian tubes than in PDX tumors. Further, we observed that ovarian PDXs and established ovarian cancer cell lines exhibit a range of SDHA expressions. Importantly, the percentage (23.5%) of SDHA-high PDX tumors in our data set is consistent with the percentage (19%) of ovarian tumors harboring amplification and/or overexpression of SDHA in patients population (TGCA data set) [
24]. Our findings are also in agreement with the study by Guo et al., who reported that SDHA is commonly upregulated in ovarian cancer cell lines [
43]. As a next step, we functionally validated our ovarian cancer models and showed that the conditional overexpression of SDHA in ovarian cancer cell lines significantly increased succinate dehydrogenase enzyme activity, while SDHA knockdown suppressed its activity. In addition, the increased succinate dehydrogenase activity in those cells was associated with fumarate accumulation. Fumarate accumulation in cells with upregulated SDHA has been also reported in other diseases. For instance, Burgener et al., showed an increased fumarate levels in lymphocytes B with SDHA gain-of-function phenotype in patients with primary antibody deficiency syndrome [
42].
To evaluate the SDHA biological functions in ovarian cancer, we assessed the effect of SDHA overexpression on cell proliferation and in vivo tumor growth. The in vitro experiments revealed that SDHA upregulation is associated with a reduced cell proliferation in some cell lines, while in other cell lines the proliferation of cells is only marginally affected. In vivo data showed a tendency towards reduced tumor growth rate in mice bearing OVCAR3 tumor model. Our findings are in agreement with published studies. For instance, Xu et al., reported that the overexpression of SDHA in renal carcinoma cells inhibited cell proliferation in vitro and suppressed tumor growth in a nude mouse model in vivo [
45]. Different study showed that a high expression of SDHA inhibited cell proliferation and invasion of multiple myeloma cell lines in vitro [
46].
Further, our work demonstrated that SDHA overexpression is associated with a generation of significantly more and larger cancer cell colonies in anchorage-independent conditions. It has been shown that aggressive and metastatic tumor cells are able to survive and rapidly propagate in the absence of anchorage to the extracellular matrix, while less tumorigenic or normal cells undergo growth inhibition and/or apoptosis (a process known as anoikis) [
47]. These data indicate that while SDHA overexpressing cells proliferate slower, these cells have improved ability to survive and generate colonies in suspension within a semi-solid matrix. This is highly relevant to ovarian cancer biology, since this tumor type develops large amounts of ascitic fluid in the peritoneal cavity containing floating cancer cells that have to acquire the ability to survive, propagate and metastasize in order to promote disease progression. Other studies reported that the upregulation of SDHA inhibited migration and invasion of renal carcinoma cells [
45]. Similarly, Sun et al., reported that SDHA overexpressing multiple myeloma cells are less invasive than control cells [
46]. Based on this collective knowledge, we propose that SDHA overexpression could promote cell survival and ability to proliferate in anchorage-independent conditions, which is in contrast to SDHA-low tumor cells that more efficiently proliferate, migrate and invade in adherent cell cultures.
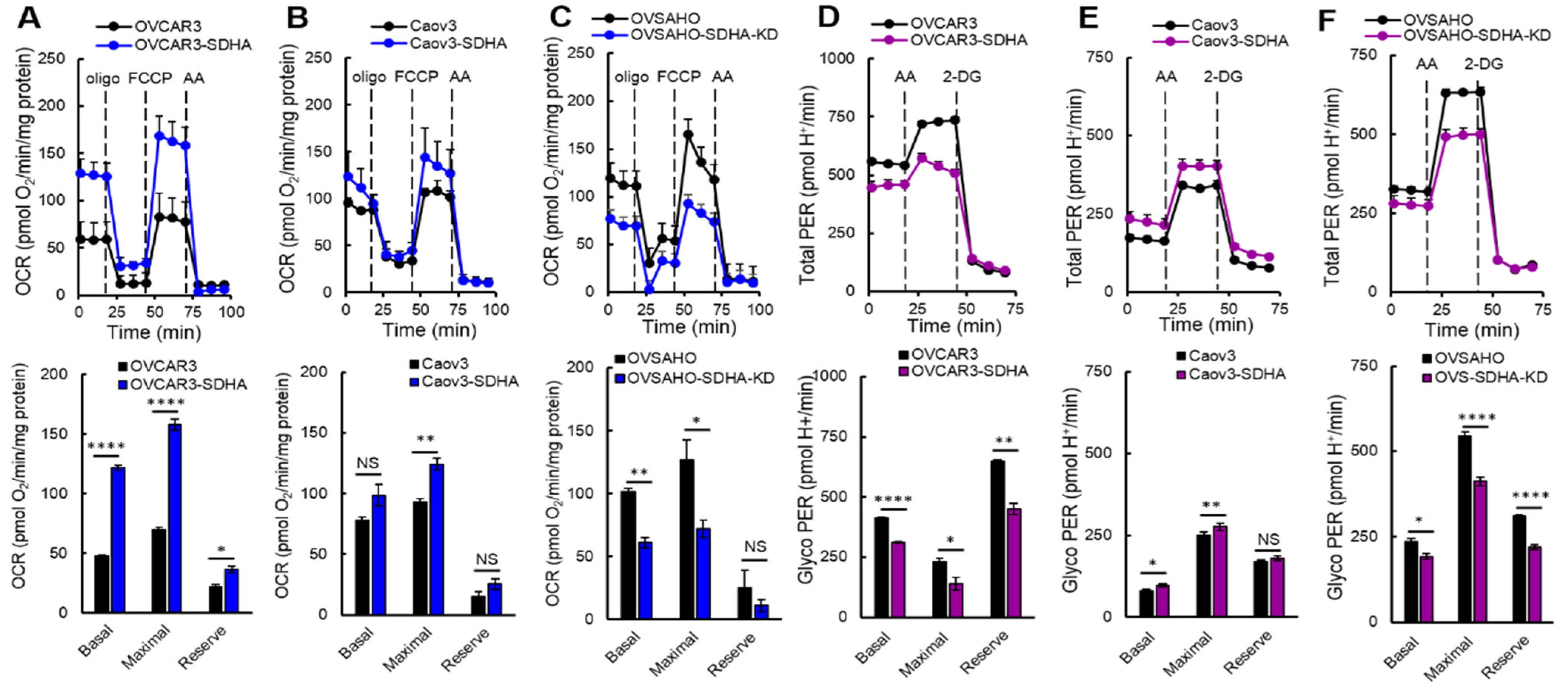
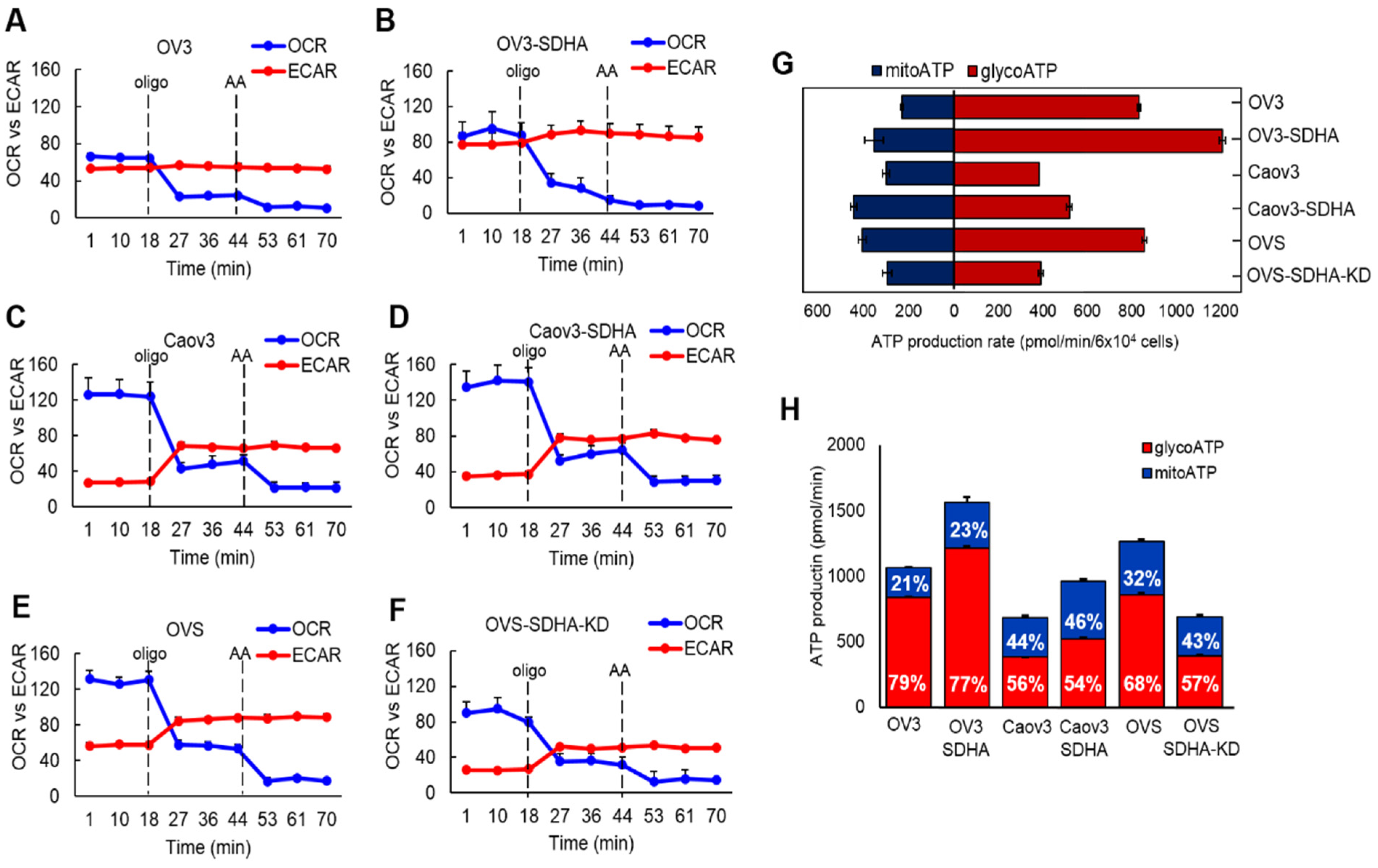
In the present study, we set out to investigate the differences in bioenergetic profiles of ovarian cancer cell lines overexpressing SDHA vs. their respective SDHA-low counterparts. The cell lines showed a substantial diversity in their bioenergetic requirements, which was associated with the SDHA overexpression status. Our data consistently demonstrated that the cell lines with SDHA overexpression showed a significantly higher mitochondrial respiration, and tended to have higher maximal and reserve glycolytic capacity. This highly metabolically active phenotype of SDHA overexpressing cells was reflected by significantly increased total ATP yield. Variability in bioenergetic profiles and metabolic plasticity have been previously described in ovarian cancer and other tumor types by others [
8,
21,
32,
48,
49]. For instance, Dar et al., reported that metabolic changes in ovarian cancer occur as a result of chemotherapy, where the cells acquire metabolic flexibility to survive a chemotherapy insult [
32]. Other studies identified a subgroup of highly metabolically active ovarian cancer cells characterized by enhanced OXPHOS, high glucose uptake, and high glycolysis. The high bioenergetic signature of these cells improved cell ability to form spheroids and survive in anchorage-independent conditions [
8,
21], similarly to our current findings. In addition, high energy phenotype seems to be a feature of ovarian tumor cells, since normal ovarian epithelial cells exhibit low mitochondrial respiration and glycolytic rates when compared with several distinct ovarian cancer cell lines [
21].
In our study, we also noted that all cell lines regardless of SDHA status preferentially used glycolysis pathway to fulfill their energy requirements as assessed by higher rates of ATP production from glycolysis than from OXPHOS, which is also in agreement with published data [
50,
51,
52]. Sun et al., reported that the growth of ovarian cancer cells relies primarily on glucose and glycolysis, which is the most important pathway for energy generation and cell survival [
52]. In a different study, Creekmore et al., observed that the metabolic phenotype of ovarian cancer cells changed to a more glycolytic as the cell phenotype progressed from a benign to a highly aggressive [
50]. Similarly, in a murine ovarian cancer model, the late stage tumor cells showed an increased utilization of glycolysis pathway [
51]. In agreement with the above, our findings support the concept that ovarian cancer cells with high glycolysis are also able to efficiently utilize OXPHOS following SDHA overexpression, which is manifested by enhanced energy metabolism.
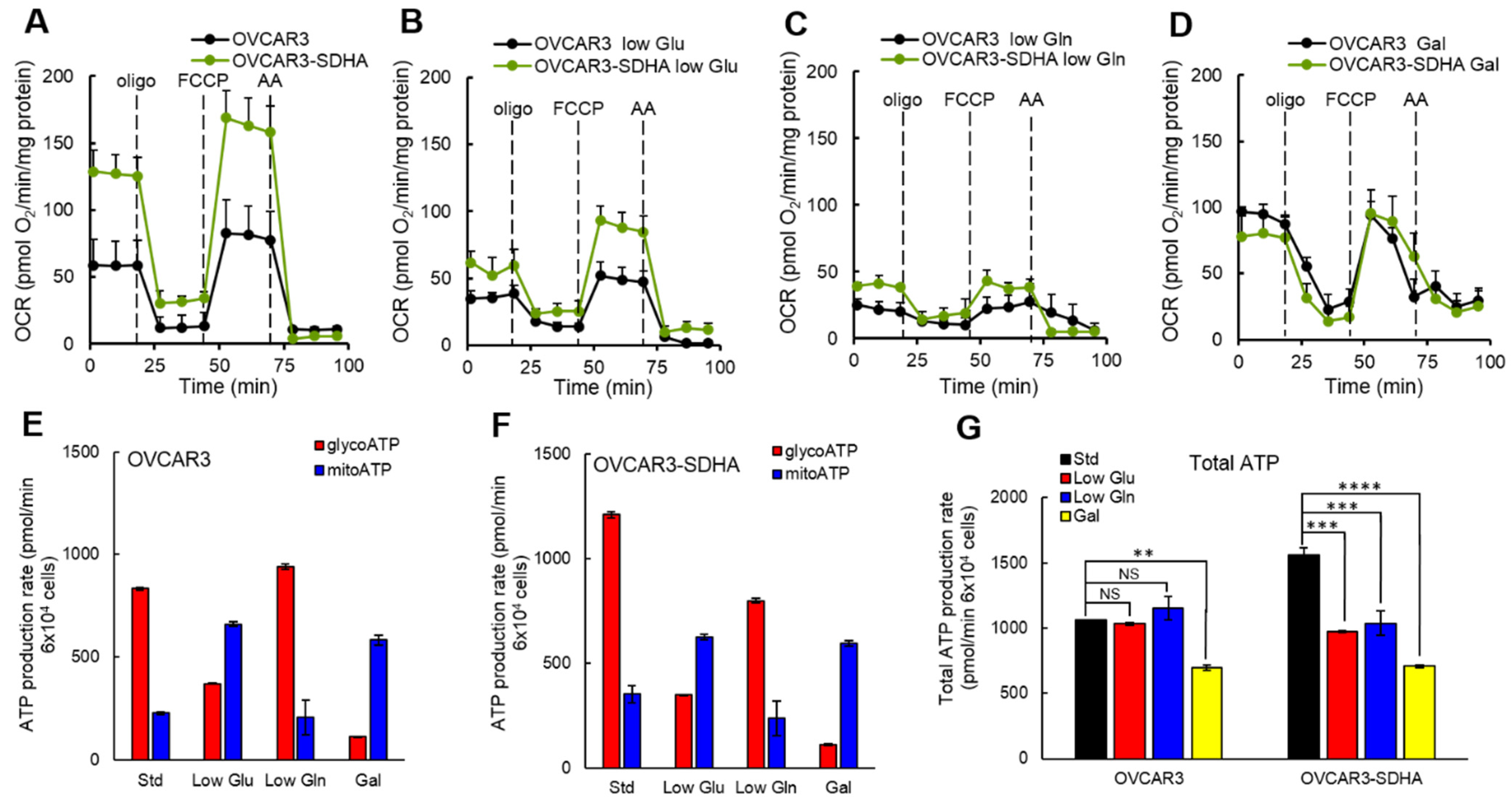
In different sets of experiments, we deprived ovarian cancer cells of glucose or glutamine to determine if SDHA overexpression redirects cellular metabolism in response to nutrient deficiency. The cells showed substantial metabolic plasticity switching between glycolysis and oxidative phosphorylation to adapt to changing nutrient conditions. We observed that glucose limitation significantly reduced ATP production from glycolysis that has been compensated with a higher ATP yield from OXPHOS, while glutamine deprivation increased glycolytic ATP yield suppressing ATP production via mitochondrial respiration. Such metabolic flexibility allows cells to become more resilient in variable conditions of tumor microenvironment [
8,
9,
32,
53]. In a study by Anderson et al., the authors reported a highly adaptable metabolic phenotype of mouse ovarian tumor cells that were enriched in the stem cell population. The cells were able to increase OXPHOS and glycolysis under appropriate stress, whereas parental tumor cells lacking stem cell features exhibited the glycolytic Warburg effect and were not able to efficiently modulate cellular metabolism under stress [
53]. In our study, however, all ovarian cancer cell lines showed metabolic plasticity to some degree when exposed to metabolic stress (limitation of glucose or glutamine). We propose that well-established human ovarian cancer cell lines have already acquired sufficient metabolic plasticity to adapt to limited nutrients availability, similarly as mouse ovarian tumor cells with stem cell features. However, our data showed that the ability to compensate for the loss of ATP production following deprivation of respective nutrients is different between cell lines with and without SDHA overexpression. Our findings indicate that SDHA overexpressing cell lines are not able to fully compensate for the loss of ATP production following glucose or glutamine deprivation, which results in a significant drop in a total ATP yield. In contrast, the corresponding control cells demonstrated an improved metabolic capacity allowing them to better maintain a constant ATP production rate in nutrient deprived conditions. These data suggest that SDHA overexpressing cells characterized by elevated energy metabolism can be more sensitive to a deficiency in essential nutrients or cellular stress. Indeed, it has been shown that ovarian cancer cells with high glycolytic and mitochondrial activity displayed strong sensitivity to inhibition of either glycolysis or OXPHOS that led to a bioenergetic dysfunction and cell death [
21]. Different study revealed that high-invasive ovarian cancer cell lines with enhanced metabolism were dependent on glutamine, which deprivation was more detrimental to those cells than to the low-invasive counterparts [
7]. However, others reported that a highly adaptable metabolic phenotype of cancer cells allow them to survive cellular stress or substrate deficiency [
8,
32]. Nevertheless, our data and previous studies indicate that certain cancer cells with highly metabolic phenotype and increased energy demands may have diminished ability to cope with nutrients deficiency. Thus, we reasoned that SDHA overexpressing cells could be particularly vulnerable to drugs disrupting glucose and/or glutamine metabolism. We carried out a screen of anti-metabolic agents, and identified a potent drug shikonin that more effectively kills SDHA overexpressing ovarian cancer cells than SDHA-low counterparts. Moreover, the SDHA overexpression strongly sensitized cancer cells to shikonin, which exhibited a profound anti-tumor efficacy superior to that seen with traditional chemotherapy. Shikonin is a traditional Chinese medicine with various biological activities, among which inhibition of glycolysis and glutamine metabolism was reported [
19,
20]. We propose that SDHA overexpressing cells carry out high energy metabolism and depend on both glucose to perform glycolysis and glutamine to support TCA cycle flux and OXPHOS in mitochondria, thus disruption of both glycolysis and glutamine metabolism could be detrimental for survival of those cells. In fact, it has been previously observed that the combination of glycolysis and glutaminolysis supports rapid proliferation of cancer cells through production of ATP and other biosynthetic precursors, and a simultaneous inhibition of both pathways could be a promising therapeutic strategy [
52,
54]. For instance, Sun et al., demonstrated that the inhibition of both glutaminolysis and glycolysis (by aminooxyacetate and by 2-DG, respectively) led to a strong synergistic cytotoxic effect on ovarian cancer cell viability [
52]. A similar therapeutic approach has been also explored by others, where a combination of metformin (OXPHOS inhibitor) and 2-DG inhibited mitochondrial respiration and glycolysis in prostate [
55] and ovarian [
56] cancer cells leading to a severe depletion of ATP and cell death.