Controlling Nutritional Status (CONUT) Score and Sarcopenia as Mutually Independent Prognostic Biomarkers in Advanced Urothelial Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Data Collection, and CONUT Score Calculation
2.2. Image Analysis Evaluating Sarcopenia
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
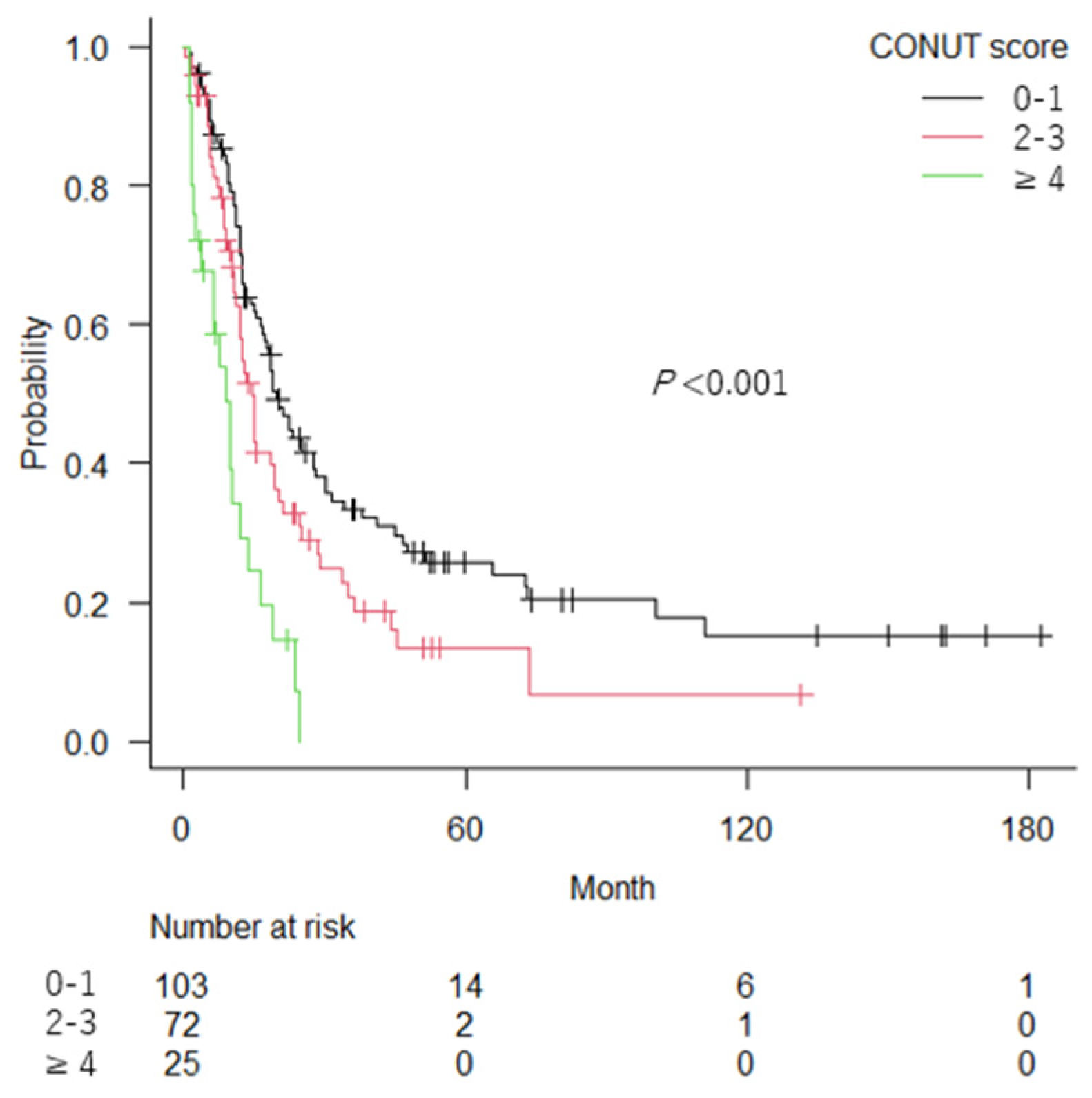
3.2. Association of the CONUT Score and Sarcopenia with OS
3.3. Role of the CONUT Score and Sarcopenia as Prognostic Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- De Santis, M.; Bellmunt, J.; Mead, G.; Kerst, J.M.; Leahy, M.; Maroto, P.; Gil, T.; Marreaud, S.; Daugaard, G.; Skoneczna, I.; et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J. Clin. Oncol. 2012, 30, 191–199. [Google Scholar] [CrossRef] [PubMed]
- von der Maase, H.; Sengelov, L.; Roberts, J.T.; Ricci, S.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Zimmermann, A.; Arning, M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J. Clin. Oncol. 2005, 23, 4602–4608. [Google Scholar] [CrossRef] [PubMed]
- Logothetis, C.J.; Dexeus, F.H.; Finn, L.; Sella, A.; Amato, R.J.; Ayala, A.G.; Kilbourn, R.G. A prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial tumors. J. Clin. Oncol. 1990, 8, 1050–1055. [Google Scholar] [CrossRef]
- Loehrer, P.J.; Einhorn, L.H.; Elson, P.J.; Crawford, E.D.; Kuebler, P.; Tannock, I.; Raghavan, D.; Stuart-Harris, R.; Sarosdy, M.F.; Lowe, B.A. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: A cooperative group study. J. Clin. Oncol. 1992, 10, 1066–1073. [Google Scholar] [CrossRef]
- Bellmunt, J.; von der Maase, H.; Mead, G.M.; Skoneczna, I.; De Santis, M.; Daugaard, G.; Boehle, A.; Chevreau, C.; Paz-Ares, L.; Laufman, L.R.; et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J. Clin. Oncol. 2012, 30, 1107–1113. [Google Scholar] [CrossRef]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef]
- Bajorin, D.F.; Dodd, P.M.; Mazumdar, M.; Fazzari, M.; McCaffrey, J.A.; Scher, H.I.; Herr, H.; Higgins, G.; Boyle, M.G. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J. Clin. Oncol. 1999, 17, 3173–3181. [Google Scholar] [CrossRef]
- Apolo, A.B.; Ostrovnaya, I.; Halabi, S.; Iasonos, A.; Philips, G.K.; Rosenberg, J.E.; Riches, J.; Small, E.J.; Milowsky, M.I.; Bajorin, D.F. Prognostic model for predicting survival of patients with metastatic urothelial cancer treated with cisplatin-based chemotherapy. J. Natl. Cancer Inst. 2013, 105, 499–503. [Google Scholar] [CrossRef]
- Takemura, K.; Yuasa, T.; Fujiwara, R.; Ito, M.; Suzuki, H.; Yonese, J.; Koga, F. Prognostic Significance of the Controlling Nutritional Status (CONUT) Score in Patients with Advanced Renal Cell Carcinoma Treated with Nivolumab after Failure of Prior Tyrosine Kinase Inhibitors. J. Urol. 2020, 204, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- de Ulíbarri, J.I.; González-Madroño, A.; de Villar, N.G.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar] [PubMed]
- González-Madroño, A.; Mancha, A.; Rodríguez, F.J.; Culebras, J.; de Ulibarri, J.I. Confirming the validity of the CONUT system for early detection and monitoring of clinical undernutrition: Comparison with two logistic regression models developed using SGA as the gold standard. Nutr. Hosp. 2012, 27, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Baysal, M.; Bas, V.; Demirci, U.; Gulsaran, S.K.; Umit, E.; Kirkizlar, H.O.; Demir, A.M. The Utility of CONUT Score in Diffuse Large B Cell Lymphoma Patients. Niger J. Clin. Pract. 2021, 24, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, D.; Sawayama, H.; Kurashige, J.; Iwatsuki, M.; Eto, T.; Tokunaga, R.; Kitano, Y.; Yamamura, K.; Ouchi, M.; Nakamura, K.; et al. Controlling Nutritional Status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2018, 21, 204–212. [Google Scholar] [CrossRef]
- Okamoto, S.; Ureshino, H.; Kidoguchi, K.; Kusaba, K.; Kizuka-Sano, H.; Sano, H.; Nishioka, A.; Yamaguchi, K.; Kamachi, K.; Itamura, H.; et al. Clinical impact of the CONUT score in patients with multiple myeloma. Ann. Hematol. 2020, 99, 113–119. [Google Scholar] [CrossRef]
- Toyokawa, T.; Kubo, N.; Tamura, T.; Sakurai, K.; Amano, R.; Tanaka, H.; Muguruma, K.; Yashiro, M.; Hirakawa, K.; Ohira, M. The pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: Results from a retrospective study. BMC Cancer 2016, 16, 722. [Google Scholar] [CrossRef]
- Suzuki, H.; Ito, M.; Takemura, K.; Nakanishi, Y.; Kataoka, M.; Sakamoto, K.; Tobisu, K.I.; Koga, F. Prognostic significance of the controlling nutritional status (CONUT) score in advanced urothelial carcinoma patients. Urol. Oncol. 2020, 38, 76.e11–76.e17. [Google Scholar] [CrossRef]
- Suzuki, H.; Ito, M.; Takemura, K.; Kobayashi, S.; Kataoka, M.; Iida, N.; Sekiya, K.; Matsumoto, T.; Koga, F. The Controlling Nutritional Status(CONUT) Score is a Prognostic Biomarkerin Advanced Urothelial Carcinoma PatientsTreated with First-Line Platinum-Based Chemotherapy. Bladder Cancer 2021, 7, 13–21. [Google Scholar] [CrossRef]
- Kim, T.N.; Choi, K.M. Sarcopenia: Definition, epidemiology, and pathophysiology. J. Bone Metab. 2013, 20, 1–10. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; van Kan, G.A.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef]
- Jang, H.Y.; Choi, G.H.; Hwang, S.H.; Jang, E.S.; Kim, J.W.; Ahn, J.M.; Choi, Y.; Cho, J.Y.; Han, H.S.; Lee, J.; et al. Sarcopenia and visceral adiposity predict poor overall survival in hepatocellular carcinoma patients after curative hepatic resection. Transl. Cancer Res. 2021, 10, 854–866. [Google Scholar] [CrossRef]
- Pekařová, A.; Pekař, M.; Daniš, D.; Nováková, Z. CT evaluated sarcopenia signals: Shorter survival for small cell lung cancer patients. Physiol. Res. 2021, 70, S381–S386. [Google Scholar] [CrossRef]
- Caan, B.J.; Cespedes Feliciano, E.M.; Prado, C.M.; Alexeeff, S.; Kroenke, C.H.; Bradshaw, P.; Quesenberry, C.P.; Weltzien, E.K.; Castillo, A.L.; Olobatuyi, T.A.; et al. Association of Muscle and Adiposity Measured by Computed Tomography With Survival in Patients With Nonmetastatic Breast Cancer. JAMA Oncol. 2018, 4, 798–804. [Google Scholar] [CrossRef]
- Fukushima, H.; Yokoyama, M.; Nakanishi, Y.; Tobisu, K.; Koga, F. Sarcopenia as a prognostic biomarker of advanced urothelial carcinoma. PLoS ONE 2015, 10, e0115895. [Google Scholar] [CrossRef]
- Mayr, R.; Fritsche, H.M.; Zeman, F.; Reiffen, M.; Siebertz, L.; Niessen, C.; Pycha, A.; van Rhijn, B.W.G.; Burger, M.; Gierth, M. Sarcopenia predicts 90-day mortality and postoperative complications after radical cystectomy for bladder cancer. World J. Urol. 2018, 36, 1201–1207. [Google Scholar] [CrossRef]
- van der Kroft, G.; Olde Damink, S.W.M.; Neumann, U.P.; Lambertz, A. Sarcopenia and Cachexia-associated Risk in Surgery. Zent. Chir. 2021, 146, 277–282. [Google Scholar] [CrossRef]
- Abe, H.; Takei, K.; Uematsu, T.; Tokura, Y.; Suzuki, I.; Sakamoto, K.; Nishihara, D.; Yamaguchi, Y.; Mizuno, T.; Nukui, A.; et al. Significance of sarcopenia as a prognostic factor for metastatic urothelial carcinoma patients treated with systemic chemotherapy. Int. J. Clin. Oncol. 2018, 23, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, R.; Honda, M.; Teraoka, S.; Yumioka, T.; Yamaguchi, N.; Kawamoto, B.; Iwamoto, H.; Morizane, S.; Hikita, K.; Takenaka, A. Sarcopenia is associated with survival in patients with urothelial carcinoma treated with systemic chemotherapy. Int. J. Clin. Oncol. 2022, 27, 175–183. [Google Scholar] [CrossRef]
- Mitsiopoulos, N.; Baumgartner, R.N.; Heymsfield, S.B.; Lyons, W.; Gallagher, D.; Ross, R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J. Appl. Physiol. 1998, 85, 115–122. [Google Scholar] [CrossRef]
- Shen, W.; Punyanitya, M.; Wang, Z.; Gallagher, D.; St-Onge, M.P.; Albu, J.; Heymsfield, S.B.; Heshka, S. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J. Appl. Physiol. 2004, 97, 2333–2338. [Google Scholar] [CrossRef]
- Buccheri, G.; Ferrigno, D.; Tamburini, M. Karnofsky and ECOG performance status scoring in lung cancer: A prospective, longitudinal study of 536 patients from a single institution. Eur. J. Cancer 1996, 32A, 1135–1141. [Google Scholar] [CrossRef]
- Chow, R.; Chiu, N.; Bruera, E.; Krishnan, M.; Chiu, L.; Lam, H.; DeAngelis, C.; Pulenzas, N.; Vuong, S.; Chow, E. Inter-rater reliability in performance status assessment among health care professionals: A systematic review. Ann. Palliat. Med. 2016, 5, 83–92. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S.P.; Santos, J.M.O.; Costa E Silva, M.P.; da Costa, R.M.G.; Medeiros, R. Cancer cachexia and its pathophysiology: Links with sarcopenia, anorexia and asthenia. J. Cachexia Sarcopenia Muscle 2020, 11, 619–635. [Google Scholar] [CrossRef] [PubMed]
- Pin, F.; Barreto, R.; Couch, M.E.; Bonetto, A.; O’Connell, T.M. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J. Cachexia Sarcopenia Muscle 2019, 10, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, J.A.; Pothuraju, R.; Jain, M.; Batra, S.K.; Nasser, M.W. Advances in cancer cachexia: Intersection between affected organs, mediators, and pharmacological interventions. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188359. [Google Scholar] [CrossRef] [PubMed]
- Vermaete, N.; Wolter, P.; Verhoef, G.; Gosselink, R. Physical activity and physical fitness in lymphoma patients before, during, and after chemotherapy: A prospective longitudinal study. Ann. Hematol. 2014, 93, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Cassie, J.; Lan-Juan, Z.; Kehao, W. Race and socioeconomic effect on sarcopenia and sarcopenic obesity in the Louisiana Osteoporosis Study (LOS). JCSM Clin. Rep. 2018, 3, 1–8. [Google Scholar] [CrossRef]
- Taguchi, S.; Nakagawa, T.; Fukuhara, H. Inconsistencies in currently used definitions of sarcopenia in oncology. Ann. Oncol. 2020, 31, 318–319. [Google Scholar] [CrossRef]
- Taguchi, S.; Nakagawa, T.; Uemura, Y.; Akamatsu, N.; Gonoi, W.; Naito, A.; Kawai, T.; Kume, H.; Fukuhara, H. Comparison of major definitions of sarcopenia based on the skeletal muscle index in patients with urothelial carcinoma. Future Oncol. 2021, 17, 197–203. [Google Scholar] [CrossRef]

| Parameter | Range of Values and Scores Per Parameter | |||
|---|---|---|---|---|
| Albumin (g/dL) | ≥3.50 | 3.00–3.49 | 2.50–2.99 | <2.50 |
| Score | 0 | 2 | 4 | 6 |
| Lymphocyte count (/μL) | ≥1600 | 1200–1599 | 800–1199 | <800 |
| Score | 0 | 1 | 2 | 3 |
| Total cholesterol (mg/dL) * | ≥180 | 140–179 | 100–139 | <100 |
| Score | 0 | 1 | 2 | 3 |
| Interpretation | ||||
| CONUT score (sum of above scores) | 0–1 | 2–4 | 5–8 | 9–12 |
| Degree of malnutrition | None | Light | Moderate | Severe |
| Variable | Total, N (%) | CONUT Score, N (%) | p | Sarcopenia, N (%) | p | ||||
|---|---|---|---|---|---|---|---|---|---|
| 0–1 | 2–3 | ≥4 | Yes | No | |||||
| Total | 200 (100) | 103 (52) | 72 (36) | 25 (12) | 168 (84) | 32 (16) | |||
| Age (years) * | 71 (38–94) | 70 (38–86) | 72 (46–90) | 72 (57–94) | 0.334 | 71 (38–91) | 69 (47–94) | 0.188 | |
| Sex | 0.426 | 0.012 | |||||||
| Male | 138 (69) | 70 (68) | 53 (74) | 15 (60) | 122 (73) | 16 (50) | |||
| Female | 62 (31) | 33 (32) | 19 (26) | 10 (40) | 46 (27) | 16 (50) | |||
| ECOG PS | <0.001 | 0.141 | |||||||
| 0 | 136 (68) | 73 (71) | 54 (75) | 9 (36) | 115 (68) | 21 (66) | |||
| 1 | 42 (21) | 24 (23) | 12 (17) | 6 (24) | 32 (19) | 10 (31) | |||
| ≥2 | 22 (11) | 6 (6) | 6 (8) | 10 (40) | 21 (13) | 1 (3) | |||
| BMI (kg/m2) * | 22 (15–36) | 23 (15–36) | 22 (15–32) | 21 (17–27) | <0.001 | 22 (15–34) | 24 (18–36) | <0.001 | |
| Primary tumor site | 0.599 | 0.019 | |||||||
| Bladder | 109 (55) | 58 (56) | 36 (50) | 15 (60) | 98 (58) | 11 (34) | |||
| UT | 91 (45) | 45 (44) | 36 (50) | 10 (40) | 70 (42) | 21 (66) | |||
| Hydronephrosis | <0.001 | 0.127 | |||||||
| No | 106 (53) | 56 (54) | 44 (61) | 6 (24) | 85 (51) | 21 (66) | |||
| Yes | 94 (47) | 47 (46) | 28 (39) | 19 (76) | 83 (49) | 11 (34) | |||
| Lymph node metastasis | 0.716 | 0.245 | |||||||
| No | 118 (59) | 62 (60) | 40 (56) | 16 (64) | 96 (57) | 22 (69) | |||
| Yes | 82 (41) | 41 (40) | 32 (44) | 9 (36) | 72 (43) | 10 (31) | |||
| Visceral metastasis | 0.072 | 0.845 | |||||||
| No | 122 (61) | 67 (65) | 45 (63) | 10 (40) | 103 (61) | 19 (59) | |||
| Yes | 78 (39) | 36 (35) | 27 (38) | 15 (60) | 65 (39) | 13 (41) | |||
| Prior curative surgery | 0.819 | 0.066 | |||||||
| No | 153 (77) | 80 (78) | 55 (76) | 18 (72) | 133 (79) | 20 (63) | |||
| Yes | 47 (23) | 23 (22) | 17 (24) | 7 (28) | 35 (21) | 12 (38) | |||
| 1st line therapy for aUC | 0.044 | 0.546 | |||||||
| Platinum-based chemotherapy | cisplatin | 117 (59) | 67 (34) | 40 (20) | 10 (5) | 0.782 | 93(47) | 24(12) | 0.009 |
| carboplatin | 31 (16) | 17 (9) | 10 (5) | 4 (2) | 30(15) | 1(1) | |||
| Chemoradiation | 15 (7.5) | 5 (33) | 8 (54) | 2 (13) | 14 (93) | 1 (7) | |||
| BSC | 37 (19) | 14 (38) | 14 (38) | 9 (24) | 30 (81) | 7 (19) | |||
| Curative surgery after diagnosis of aUC | 0.117 | 0.774 | |||||||
| No | 166 (83) | 80 (48) | 64 (39) | 22 (13) | 140 (84) | 26 (16) | |||
| Yes | 34 (17) | 23 (68) | 8 (24) | 3 (8) | 28 (82) | 6 (18) | |||
| Administration of pembrolizumab for 2nd or later line therapy | 32 (16) | 19 (59) | 12 (38) | 1 (3) | 0.206 | 31 (18) | 1 (3) | 0.030 | |
| Hemoglobin (g/dL) * | 12 (3.1–18) | 13 (7.7–18) | 11 (6.2–16) | 11 (3.1–13) | <0.001 | 12 (3.1–18) | 12 (6.2–15) | 0.109 | |
| Neutrophil (×103/μL) * | 4.9 (1.1–56) | 5.2 (1.1–31) | 4.3 (1.8–12) | 6.7 (1.7–56) | 0.011 | 6.9 (1.0–59) | 8.0 (4.2–18) | 0.060 | |
| Lymphocyte (×103/µL) * | 1.5 (0.17–4.3) | 1.8 (1.2–4.3) | 1.1 (0.64–2.7) | 1.1 (0.17–2.3) | <0.001 | 1.4 (0.17–4.0) | 1.8 (0.52–4.3) | 0.030 | |
| Creatinine (mg/dL) * | 1.0 (0.49–16) | 1.1 (0.49–4.3) | 1.0 (0.50–3.1) | 1.1 (0.50–16) | 0.298 | 1.0 (0.50–16) | 1.0 (0.50–3.1) | 0.450 | |
| Albumin (g/dL) * | 4.0 (2.7–5.0) | 4.1 (3.6–5.0) | 4.0 (3.2–4.9) | 3.2 (2.7–4.2) | <0.001 | 4 (2.7–5.0) | 4 (2.8–4.7) | 0.979 | |
| ALP (U/L) * | 261 (92–3351) | 253 (92–1539) | 258 (103–3351) | 307 (212–682) | 0.006 | 266 (92–3351) | 237 (147–586) | 0.142 | |
| LDH (U/L) * | 191 (118–2970) | 191 (119–2482) | 191 (118–2970) | 212 (129–386) | 0.382 | 192 (129–2970) | 187 (118–880) | 0.288 | |
| Corrected calcium (mg/dL) * | 8.8 (7.6–14) | 8.8 (7.6–14) | 8.7 (7.9–11) | 8.8 (7.6–11) | 0.829 | 8.8 (7.6–14) | 8.7 (7.9–9.3) | 0.168 | |
| CRP (mg/L) * | 6.0 (0.00–266) | 3.0 (0.40–115) | 5.7 (0.00–266) | 34 (0.60–139) | <0.001 | 6.4 (0.0–266) | 6.0 (0.60–65) | 0.632 | |
| Total cholesterol (mg/dL) * | 183 (98–275) | 195 (140–271) | 175 (98–275) | 167 (118–224) | <0.001 | 169 (126–240) | 187 (98–275) | 0.039 | |
| SMI (cm2/m2) * | 37 (16–64) | 38 (21–64) | 36 (16–62) | 33 (18–54) | 0.017 | 35 (16–51) | 53 (39–64) | <0.001 | |
| CONUT score * | 2 (0–8) | 1 (0–1) | 2 (2–3) | 5 (4–8) | - | 2 (0–8) | 1 (0–7) | 0.416 | |
| Sarcopenia | 0.219 | - | |||||||
| Yes | 168 (84) | 82 (80) | 64 (89) | 22 (88) | 168 (100) | 0 (0) | |||
| No | 32 (16) | 21 (20) | 8 (11) | 3 (12) | 0 (0) | 32 (100) | |||
| Definition of Sarcopenia | Sarcopenia | Median OS (Range, Months) | p | C-Index |
|---|---|---|---|---|
| Prado’s definition | Absent | 20.5 (2–183) | 0.003 | 0.541 |
| Present | 12.5 (1–162) | |||
| Martin’s definition | Absent | 18.7 (1–183) | 0.214 | 0.516 |
| Present | 12.7 (1–162) | |||
| International definition | Absent | 20.9 (2–183) | 0.020 | 0.530 |
| Present | 12.7 (1–162) |
| Variables | Univariable | Multivariable (Final Model) | ||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | p | HR | (95% CI) | p | |
| Age | 1.03 | (1.01–1.06) | 0.013 | 1.04 | (1.02–1.06) | 0.002 |
| Sex | ||||||
| Female (vs. male) | 1.38 | (0.89–2.13) | 0.134 | |||
| ECOG PS | ||||||
| 0 | ref | |||||
| 1 | 1.39 | (0.85–2.28) | 0.184 | |||
| ≥2 | 1.69 | (0.85–3.34) | 0.133 | |||
| BMI | 0.97 | (0.92–1.02) | 0.170 | |||
| Hydronephrosis | ||||||
| Yes (vs. no) | 1.50 | (1.00–2.26) | 0.049 | 1.53 | (1.08–2.17) | 0.016 |
| Primary site | ||||||
| UT (vs. bladder) | 1.90 | (1.26–2.85) | 0.034 | 1.69 | (1.18–2.41) | 0.004 |
| Lymph node metastasis | ||||||
| Yes (vs. no) | 0.94 | (0.64–1.37) | 0.758 | |||
| Visceral metastasis | ||||||
| Yes (vs. no) | 1.56 | (1.03–2.33) | 0.034 | 1.71 | (1.18–2.44) | 0.004 |
| Previous curative surgery | ||||||
| Yes (vs. no) | 1.11 | (1.26–2.85) | 0.002 | |||
| 1st line therapy for aUC | ||||||
| Chemotherapy | ref | |||||
| CRT | 1.32 | (0.61–2.67) | 0.452 | |||
| BSC | 1.05 | (0.59–1.82) | 0.862 | |||
| Curative surgery after diagnosis of aUC | ||||||
| Yes (vs. no) | 0.64 | (0.36–1.10) | 0.108 | |||
| Hemoglobin | 1.09 | (0.96–1.01) | 0.203 | |||
| Neutrophil † | 1.01 | (1.00–1.01) | 0.013 | 1.01 | (1.00–1.01) | 0.005 |
| Lymphocyte † | 1.00 | (0.96–1.03) | 0.900 | - | - | - |
| Creatinine | 1.13 | (0.97–1.29) | 0.103 | |||
| Albumin | 0.79 | (0.42–1.47) | 0.460 | - | - | - |
| ALP †† | 1.02 | (1.01–1.03) | <0.001 | 1.02 | (1.01–1.03) | <0.001 |
| LDH †† | 1.01 | (1.01–1.02) | 0.003 | 1.01 | (1.01–1.02) | 0.003 |
| Corrected calcium | 1.24 | (0.88–1.66) | 0.212 | |||
| CRP | 1.13 | (0.97–1.29) | 0.066 | 1.01 | (1.00–1.01) | 0.033 |
| Total cholesterol | 1.00 | (0.99–1.01) | 0.997 | - | - | - |
| Sarcopenia | ||||||
| Yes (vs. no) | 2.28 | (1.26–4.27) | 0.006 | 2.23 | (1.39–3.72) | <0.001 |
| CONUT score | 1.18 | (1.01–1.37) | 0.036 | 1.22 | (1.09–1.36) | <0.001 |
| Prognostic Model | C-Index | Prognostic Model | C-Index |
|---|---|---|---|
| Apolo’s model (KPS + visceral metastasis + Hb + Alb) | 0.634 | Bajorin’s model (KPS + visceral metastasis) | 0.612 |
| Apolo’s model + the CONUT score—Alb | 0.655 | Bajorin’s model + the CONUT score | 0.653 |
| Apolo’s model + sarcopenia | 0.653 | Bajorin’s model + sarcopenia | 0.631 |
| Apolo’s model + the CONUT score—Alb + sarcopenia | 0.668 | Bajorin’s model + the CONUT score + sarcopenia | 0.665 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Une, M.; Ito, M.; Suzuki, H.; Toide, M.; Kobayashi, S.; Fukushima, H.; Koga, F. Controlling Nutritional Status (CONUT) Score and Sarcopenia as Mutually Independent Prognostic Biomarkers in Advanced Urothelial Carcinoma. Cancers 2022, 14, 5075. https://doi.org/10.3390/cancers14205075
Une M, Ito M, Suzuki H, Toide M, Kobayashi S, Fukushima H, Koga F. Controlling Nutritional Status (CONUT) Score and Sarcopenia as Mutually Independent Prognostic Biomarkers in Advanced Urothelial Carcinoma. Cancers. 2022; 14(20):5075. https://doi.org/10.3390/cancers14205075
Chicago/Turabian StyleUne, Minami, Masaya Ito, Hiroaki Suzuki, Masahiro Toide, Shuichiro Kobayashi, Hiroshi Fukushima, and Fumitaka Koga. 2022. "Controlling Nutritional Status (CONUT) Score and Sarcopenia as Mutually Independent Prognostic Biomarkers in Advanced Urothelial Carcinoma" Cancers 14, no. 20: 5075. https://doi.org/10.3390/cancers14205075
APA StyleUne, M., Ito, M., Suzuki, H., Toide, M., Kobayashi, S., Fukushima, H., & Koga, F. (2022). Controlling Nutritional Status (CONUT) Score and Sarcopenia as Mutually Independent Prognostic Biomarkers in Advanced Urothelial Carcinoma. Cancers, 14(20), 5075. https://doi.org/10.3390/cancers14205075







