Event-Free Survival in Patients with Early HER2-Positive Breast Cancer with a Pathological Complete Response after HER2-Targeted Therapy: A Pooled Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Populations
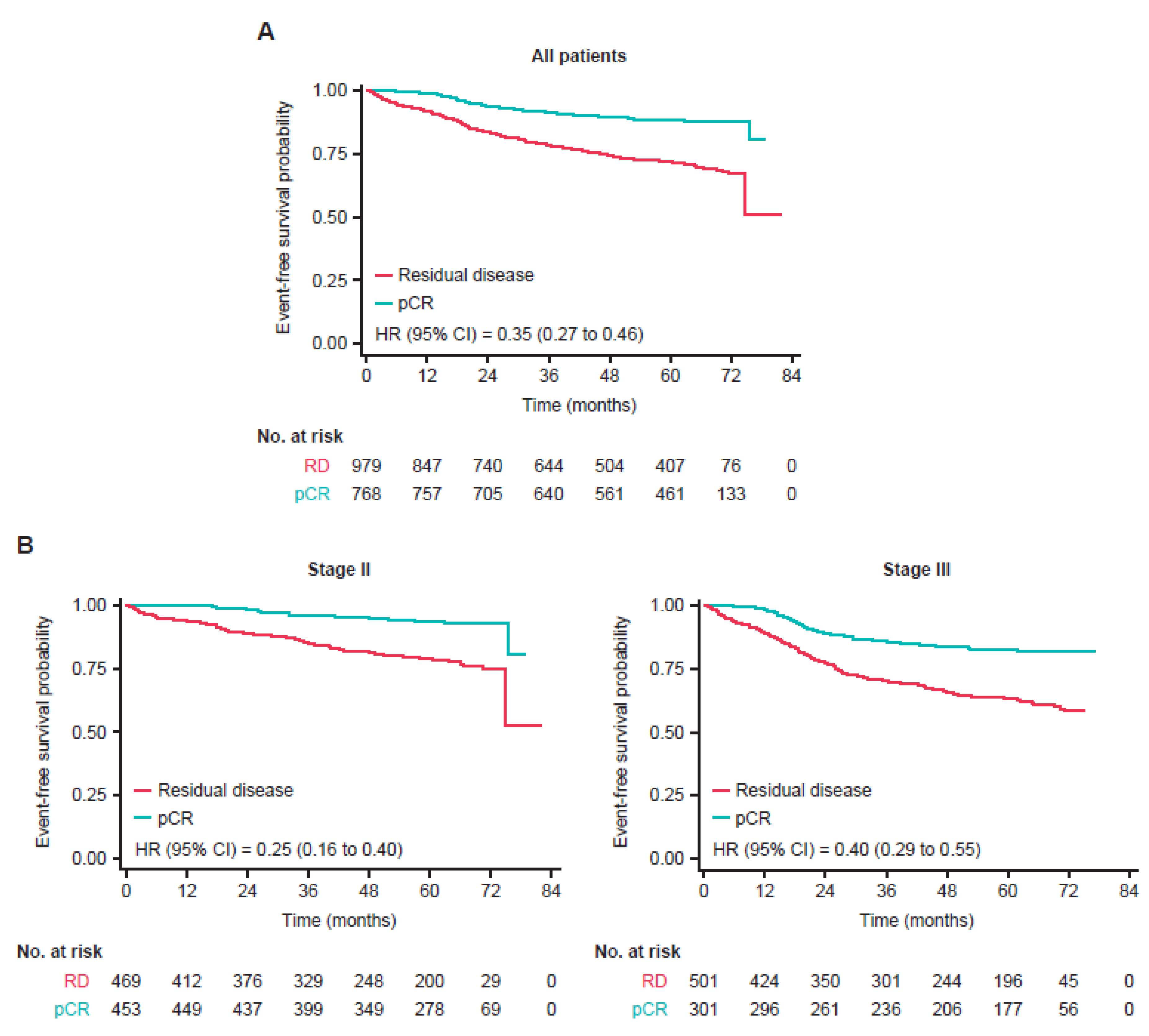
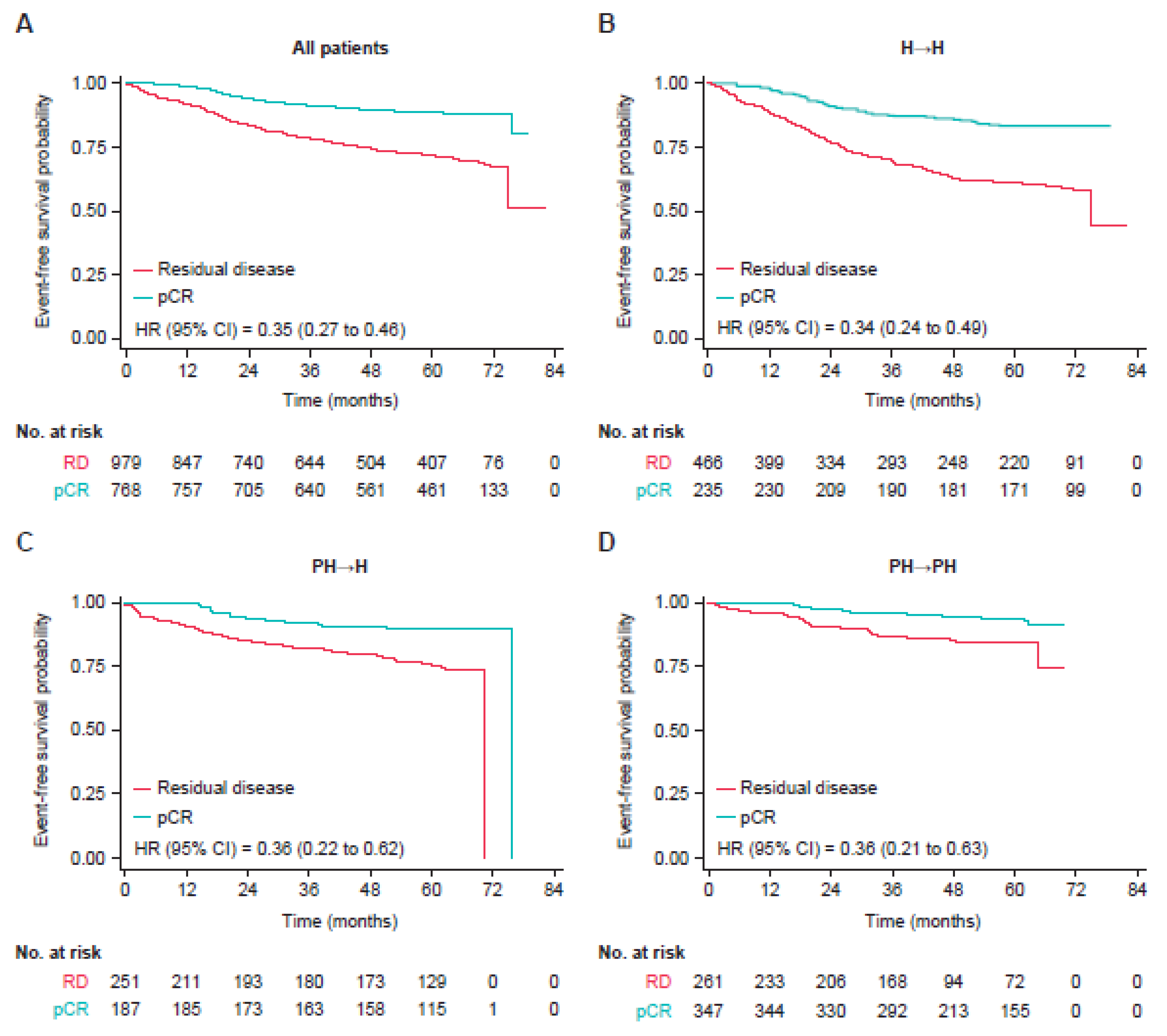
3.2. Event-Free Survival
3.3. Type of Recurrence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Untch, M.; Fasching, P.A.; Konecny, G.E.; Hasmüller, S.; Lebeau, A.; Kreienberg, R.; Camara, O.; Müller, V.; du Bois, A.; Kühn, T.; et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: Results from the TECHNO trial of the AGO and GBG study groups. J. Clin. Oncol. 2011, 29, 3351–3357. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- de Azambuja, E.; Holmes, A.P.; Piccart-Gebhart, M.; Holmes, E.; Di Cosimo, S.; Swaby, R.F.; Untch, M.; Jackisch, C.; Lang, I.; Smith, I.; et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): Survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014, 15, 1137–1146. [Google Scholar] [CrossRef]
- Gianni, L.; Eiermann, W.; Semiglazov, V.; Lluch, A.; Tjulandin, S.; Zambetti, M.; Moliterni, A.; Vazquez, F.; Byakhov, M.; Lichinitser, M.; et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): Follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014, 15, 640–647. [Google Scholar] [CrossRef]
- Schneeweiss, A.; Chia, S.; Hickish, T.; Harvey, V.; Eniu, A.; Waldron-Lynch, M.; Eng-Wong, J.; Kirk, S.; Cortés, J. Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: Evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. Eur. J. Cancer 2018, 89, 27–35. [Google Scholar] [PubMed]
- Loibl, S.; Untch, M.; Buyse, M.; Robidoux, A.; Gianni, L.; Schneeweiss, A.; Conte, P.; Piccart, M.; Bonnefoi, H.; Jackisch, C.; et al. Pathologic complete response (pCR) and prognosis following neoadjuvant chemotherapy plus anti-HER2 therapy of HER2-positive early breast cancer (EBC). Cancer Res. 2020, 80, P5–06. [Google Scholar] [CrossRef]
- Spring, L.M.; Fell, G.; Are, A.; Sharma, C.; Greenup, R.; Reynolds, K.L.; Smith, B.L.; Alexander, B.; Moy, B.; Isakoff, S.J.; et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: A comprehensive meta-analysis. Clin. Cancer Res. 2020, 26, 2838–2848. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1674. [Google Scholar] [CrossRef]
- Ditsch, N.; Untch, M.; Thill, M.; Müller, V.; Janni, W.; Albert, U.-S.; Bauerfeind, I.; Blohmer, J.; Budach, W.; Dall, P.; et al. AGO recommendations for he diagnosis and treatment of patients with early breast cancer: Update 2019. Breast Care 2019, 14, 224–245. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Breast Cancer.Version 7.2021. 23 August 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 25 July 2022).
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Thomssen, C.; Balic, M.; Harbeck, N.; Gnant, M.S. Gallen/Vienna 2021: A brief summary of the consensus discussion on customizing therapies for women with early breast cancer. Breast Care 2021, 16, 135–143. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Pienkowski, T.; Im, Y.-H.; Roman, L.; Tseng, L.-M.; Liu, M.-C.; Lluch, A.; Staroslawska, E.; de la Haba-Rodriguez, J.; Im, S.-A.; et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012, 13, 25–32. [Google Scholar] [CrossRef]
- Ismael, G.; Hegg, R.; Muehlbauer, S.; Heinzmann, D.; Lum, B.; Kim, S.-B.; Pienkowski, T.; Lichinitser, M.; Semiglazov, V.; Melichar, B.; et al. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): A phase 3, open-label, multicentre, randomised trial. Lancet Oncol. 2012, 13, 869–878. [Google Scholar] [CrossRef]
- Schneeweiss, A.; Chia, S.; Hickish, T.; Harvey, V.; Eniu, A.; Hegg, R.; Tausch, J.; Seo, J.H.; Tsai, Y.-F.; Ratnayake, J.; et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann. Oncol. 2013, 24, 2278–2284. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Pienkowski, T.; Im, Y.-H.; Tseng, L.-M.; Liu, M.-C.; Lluch, A.; Staroslawska, E.; de la Haba-Rodriguez, J.; Im, S.A.; Pedrini, J.L.; et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): A multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016, 17, 791–800. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Martin, M.; Symmans, W.F.; Jung, K.H.; Huang, C.-S.; Thompson, A.M.; Harbeck, N.; Valero, V.; Stroyakovskiy, D.; Wildiers, H.; et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018, 19, 115–126. [Google Scholar] [CrossRef]
- Swain, S.M.; Ewer, M.S.; Viale, G.; Delaloge, S.; Ferrero, J.-M.; Verrill, M.; Colomer, R.; Vieira, C.; Werner, T.L.; Douthwaite, H.; et al. Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): A phase II, open-label, multicenter, multinational cardiac safety study. Ann. Oncol. 2018, 29, 646–653. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Martin, M.; Jung, K.H.; Huang, C.-S.; Harbeck, N.; Valero, V.; Stoyakovskiy, D.; Wildiers, H.; Campone, M.; Boileau, J.-F.; et al. Neoadjuvant trastuzumab emtansine and pertuzumab in human epidermal growth factor receptor 2-positive breast cancer: Three-year outcomes from the phase III KRISTINE study. J. Clin. Oncol. 2019, 37, 2206–2216. [Google Scholar] [CrossRef]
- Jackisch, C.; Stroyakovskiy, D.; Pivot, X.; Ahn, J.S.; Melichar, B.; Chen, S.-C.; Meyenberg, C.; Al-Sakaff, N.; Heinzmann, D.; Hegg, R. Subcutaneous vs intravenous trastuzumab for patients with ERBB2-positive early breast cancer: Final analysis of the HannaH phase 3 randomized clinical trial. JAMA. Oncol. 2019, 5, e190339. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Procter, M.; de Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N. Engl. J. Med. 2017, 377, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Piccart, M.; Procter, M.; Fumagalli, D.; de Azambuja, E.; Clark, E.; Ewer, M.S.; Restuccia, E.; Jerusalem, G.; Dent, S.; Reaby, L.; et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer in the APHINITY trial: 6 years’ follow-up. J. Clin. Oncol. 2021, 39, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.; Ewer, M.S.; Delaloge, S.; Ferrero, J.-M.; Colomer, R.; de la Cruz-Merino, L.; Werner, T.L.; Dadswell, K.; Verrill, M.; Eiger, D.; et al. BERENICE final analysis: Cardiac safety study of neoadjuvant pertuzumab, trastuzumab, and chemotherapy followed by adjuvant pertuzumab and trastuzumab in HER2-positive early breast cancer. Cancers 2022, 14, 2596. [Google Scholar] [CrossRef] [PubMed]
- Hajage, D.; Chauvet, G.; Belin, L.; Lafourcade, A.; Tubach, F.; De Rycke, Y. Closed-form variance estimator for weighted propensity score estimators with survival outcome. Biom. J. 2018, 60, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J.; Robert, N.; Annavarapu, S.; Zhou, J.; Sussell, J.; Cheng, A.; Fung, A. Recurrence rates in patients with HER2+ breast cancer who achieved a pathological complete response after neoadjuvant pertuzumab plus trastuzumab followed by adjuvant trastuzumab: A real-world evidence study. Breast Cancer Res. Treat. 2021, 187, 903–913. [Google Scholar] [CrossRef]
- Bines, J.; Clark, E.; Barton, C.; Restuccia, E.; Procter, M.; Sonnenblick, A.; Fumagalli, D.; Parlier, D.; Arahmani, A.; Baselga, J.; et al. Patient-reported function, health-related quality of life, and symptoms in APHINITY: Pertuzumab plus trastuzumab and chemotherapy in HER2-positive early breast cancer. Br. J. Cancer 2021, 125, 38–47. [Google Scholar] [CrossRef]
- Weiss, A.; Campbell, J.; Ballman, K.V.; Sikov, W.M.; Carey, L.A.; Hwang, E.S.; Poppe, M.M.; Partridge, A.H.; Ollila, D.W.; Golshan, M. Factors associated with nodal pathologic complete response among breast cancer patients treated with neoadjuvant chemotherapy: Results of CALGB 40601 (HER2+) and 40603 (triple-negative) (Alliance). Ann. Surg. Oncol. 2021, 28, 5960–5971. [Google Scholar] [CrossRef]

| Characteristic, n (%) | Overall (n = 1763) | H→H (n = 703) | PH→H (n = 439) | PH→PH (n = 621) |
|---|---|---|---|---|
| Age (years) | ||||
| <40 | 314 (17.8) | 115 (16.4) | 73 (16.6) | 126 (20.3) |
| 40−60 | 1302 (73.9) | 531 (75.5) | 336 (76.5) | 435 (70.0) |
| >60 | 147 (8.3) | 57 (8.1) | 30 (6.8) | 60 (9.7) |
| Clinical stage | ||||
| I | 22 (1.2) | 21 (3.0) | 0 | 1 (0.2) |
| II | 933 (52.9) | 285 (40.5) | 204 (46.5) | 444 (71.5) |
| III | 807 (45.8) | 397 (56.5) | 235 (53.5) | 175 (28.2) |
| Unknown | 1 (0.1) | 0 | 0 | 1 (0.2) |
| Hormone receptor status | ||||
| Negative | 783 (44.4) | 328 (46.7) | 223 (50.8) | 232 (37.4) |
| Positive | 963 (54.6) | 372 (52.9) | 215 (49.0) | 376 (60.5) |
| Unknown | 17 (1.0) | 3 (0.4) | 1 (0.2) | 13 (2.1) |
| Tumor stage at study entry | ||||
| T1 | 72 (4.1) | 43 (6.1) | 0 | 29 (4.7) |
| T2 | 926 (52.5) | 304 (43.2) | 201 (45.8) | 421 (67.8) |
| T3 | 406 (23.0) | 138 (19.6) | 144 (32.8) | 124 (20.0) |
| T4 | 354 (20.1) | 217 (30.9) | 91 (20.7) | 46 (7.4) |
| Unknown | 5 (0.3) | 1 (0.1) | 3 (0.7) | 1 (0.2) |
| Clinical nodal stage at study entry | ||||
| Node-positive | 536 (30.4) | 164 (23.3) | 128 (29.2) | 244 (39.3) |
| Node-negative | 1206 (68.4) | 538 (76.5) | 308 (70.2) | 360 (58.0) |
| Unknown | 21 (1.2) | 1 (0.1) | 3 (0.7) | 17 (2.7) |
| n (%) | pCR | Residual Disease | Total |
|---|---|---|---|
| Tumor stage | |||
| T1 | 36 (50.0) | 36 (50.0) | 72 |
| T2 | 447 (48.3) | 479 (51.7) | 926 |
| T3 | 177 (43.6) | 229 (56.4) | 406 |
| T4 | 111 (31.4) | 243 (68.6) | 354 |
| Unknown | 2 (40.0) | 3 (60.0) | 5 |
| Clinical stage | |||
| 0 | 0 (0) | 1 (100.0) | 1 |
| I | 11 (50.0) | 11 (50.0) | 22 |
| II | 456 (48.9) | 477 (51.1) | 933 |
| III | 306 (37.9) | 501 (62.1) | 807 |
| Hormone receptor status | |||
| Negative | 434 (55.4) | 349 (44.6) | 783 |
| Positive | 331 (34.4) | 632 (65.6) | 963 |
| Unknown | 8 (47.1) | 9 (52.9) | 17 |
| Treatment modality | |||
| H→H | 236 (33.6) | 467 (66.4) | 703 |
| PH→H | 185 (42.1) | 254 (57.9) | 439 |
| PH→PH | 352 (56.7) | 269 (43.3) | 621 |
| All Patients, Regardless of Pathological Complete Response Status | ||||||
| H→H (n = 703) | PH→H (n = 439) | PH→PH (n = 621) | ||||
| Patients with EFS event, % | 217 (30.9) | 79 (18.0) | 53 (8.5) | |||
| Patients without EFS event, % | 486 (69.1) | 360 (82.0) | 568 (91.5) | |||
| Hazard ratio versus H→H (95% CI) | 0.56 (0.43–0.73) | 0.36 (0.26–0.49) | ||||
| Hazard ratio versus PH→H (95% CI) | 0.67 (0.47–0.96) | |||||
| pCR | Residual Disease | |||||
| H→H (n = 236) | PH→H (n = 185) | PH→PH (n = 352) | H→H (n = 467) | PH→H (n = 254) | PH→PH (n = 269) | |
| Patients with EFS event, % | 41 (17.4) | 19 (10.3) | 20 (5.7) | 176 (37.7) | 60 (23.6) | 33 (12.3) |
| Patients without EFS event, % | 195 (82.6) | 166 (89.7) | 332 (94.3) | 291 (62.3) | 194 (76.4) | 236 (87.7) |
| Time to event (months) | ||||||
| Hazard ratio versus H→H (95% CI) | 0.59 (0.34–1.01) | 0.46 (0.26–0.82) | 0.60 (0.45–0.81) | 0.43 (0.30–0.63) | ||
| Hazard ratio versus PH→H (95% CI) | 0.85 (0.44–1.65) | 0.75 (0.49–1.16) | ||||
| 4-Year Event-Free Survival Rate in Patients with pCR | |||
| H→H (n = 236) | PH→H (n = 185) | PH→PH (n = 352) | |
| Patients remaining at risk, n | 179 | 155 | 219 |
| 4-year event-free survival rate, % (95% CI) | 86 (81–89) | 90 (85–94) | 95 (92–97) |
| 4-Year Event-Free Survival Rate in Patients with Residual Disease | |||
| H→H (n = 467) | PH→H (n = 254) | PH→PH (n = 269) | |
| Patients remaining at risk, n | 251 | 176 | 107 |
| 4-year event-free survival rate, % (95% CI) | 64 (59–68) | 80 (75–85) | 87 (82–91) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swain, S.M.; Macharia, H.; Cortes, J.; Dang, C.; Gianni, L.; Hurvitz, S.A.; Jackisch, C.; Schneeweiss, A.; Slamon, D.; Valagussa, P.; et al. Event-Free Survival in Patients with Early HER2-Positive Breast Cancer with a Pathological Complete Response after HER2-Targeted Therapy: A Pooled Analysis. Cancers 2022, 14, 5051. https://doi.org/10.3390/cancers14205051
Swain SM, Macharia H, Cortes J, Dang C, Gianni L, Hurvitz SA, Jackisch C, Schneeweiss A, Slamon D, Valagussa P, et al. Event-Free Survival in Patients with Early HER2-Positive Breast Cancer with a Pathological Complete Response after HER2-Targeted Therapy: A Pooled Analysis. Cancers. 2022; 14(20):5051. https://doi.org/10.3390/cancers14205051
Chicago/Turabian StyleSwain, Sandra M., Harrison Macharia, Javier Cortes, Chau Dang, Luca Gianni, Sara A. Hurvitz, Christian Jackisch, Andreas Schneeweiss, Dennis Slamon, Pinuccia Valagussa, and et al. 2022. "Event-Free Survival in Patients with Early HER2-Positive Breast Cancer with a Pathological Complete Response after HER2-Targeted Therapy: A Pooled Analysis" Cancers 14, no. 20: 5051. https://doi.org/10.3390/cancers14205051
APA StyleSwain, S. M., Macharia, H., Cortes, J., Dang, C., Gianni, L., Hurvitz, S. A., Jackisch, C., Schneeweiss, A., Slamon, D., Valagussa, P., du Toit, Y., Heinzmann, D., Knott, A., Song, C., & Cortazar, P. (2022). Event-Free Survival in Patients with Early HER2-Positive Breast Cancer with a Pathological Complete Response after HER2-Targeted Therapy: A Pooled Analysis. Cancers, 14(20), 5051. https://doi.org/10.3390/cancers14205051









