The Application of Magnetic Nanoparticles for Sentinel Lymph Node Detection in Clinically Node-Negative Breast Cancer Patients: A Systemic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
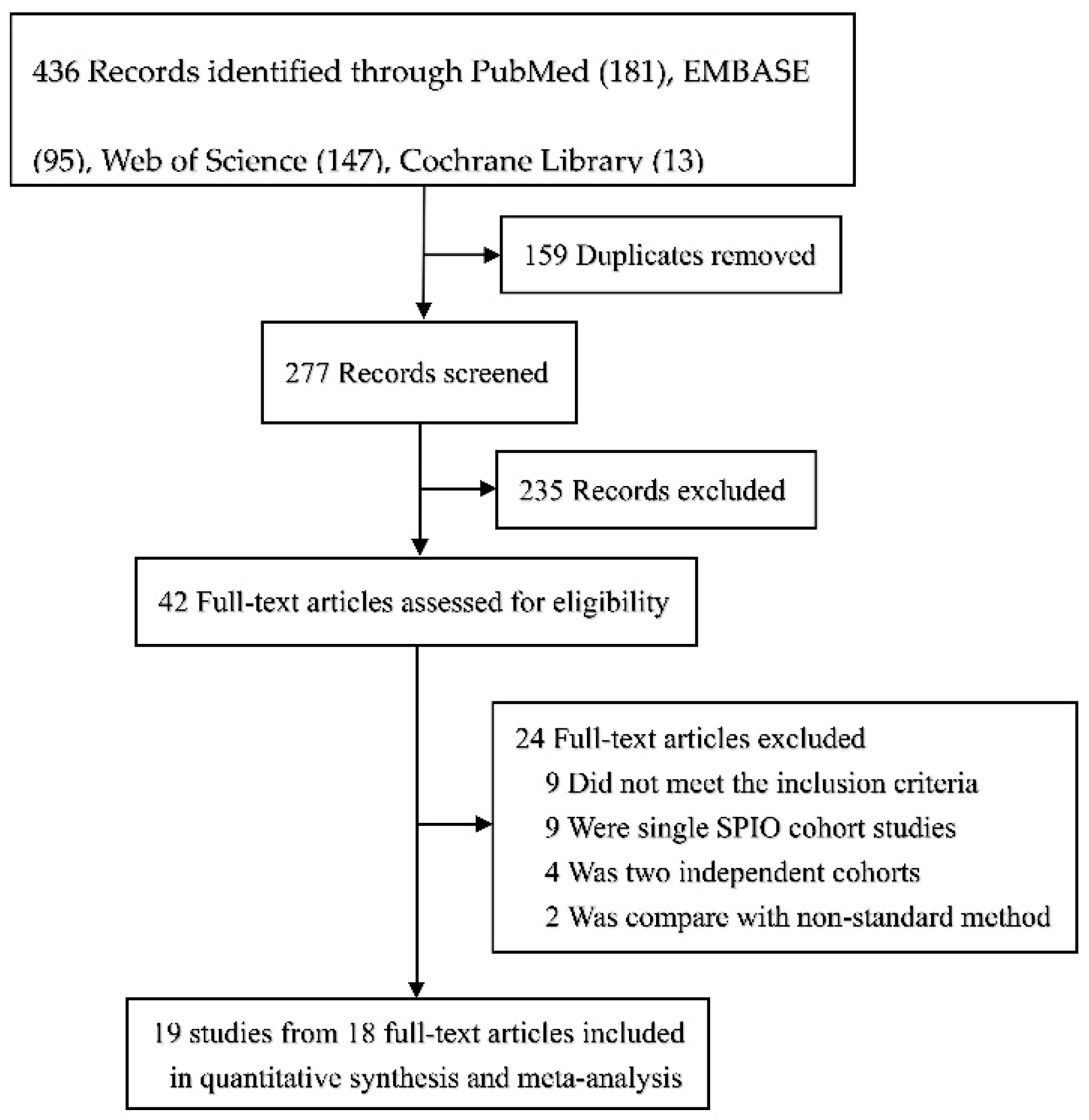
3.1. Description of Studies
3.2. Quality Assessment
Sentinel Lymph Node Biopsy Procedure
3.3. Identification and Node Retrieval
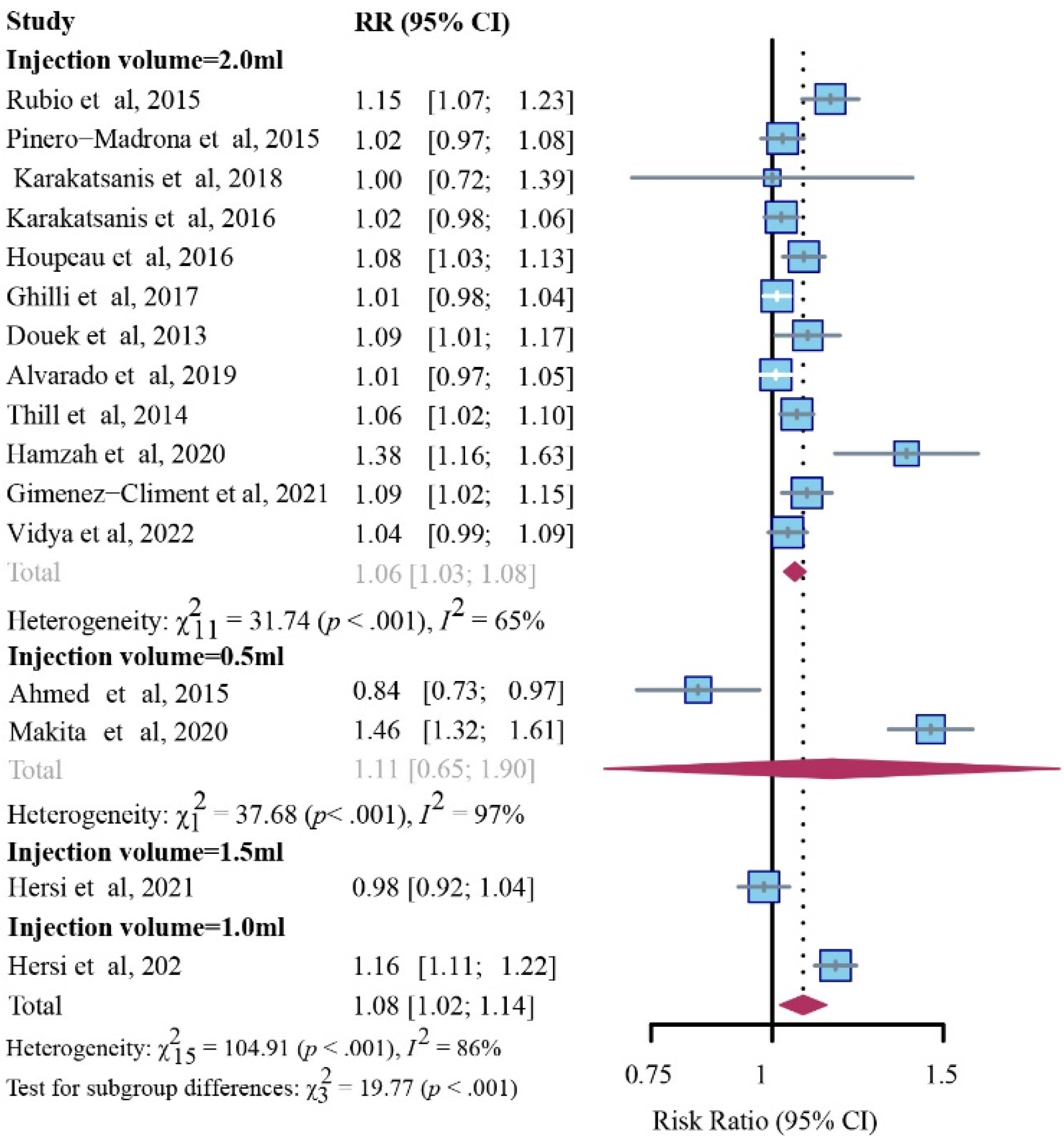
3.3.1. Detection Rate for Patient
3.3.2. Detection Rates for SLNs
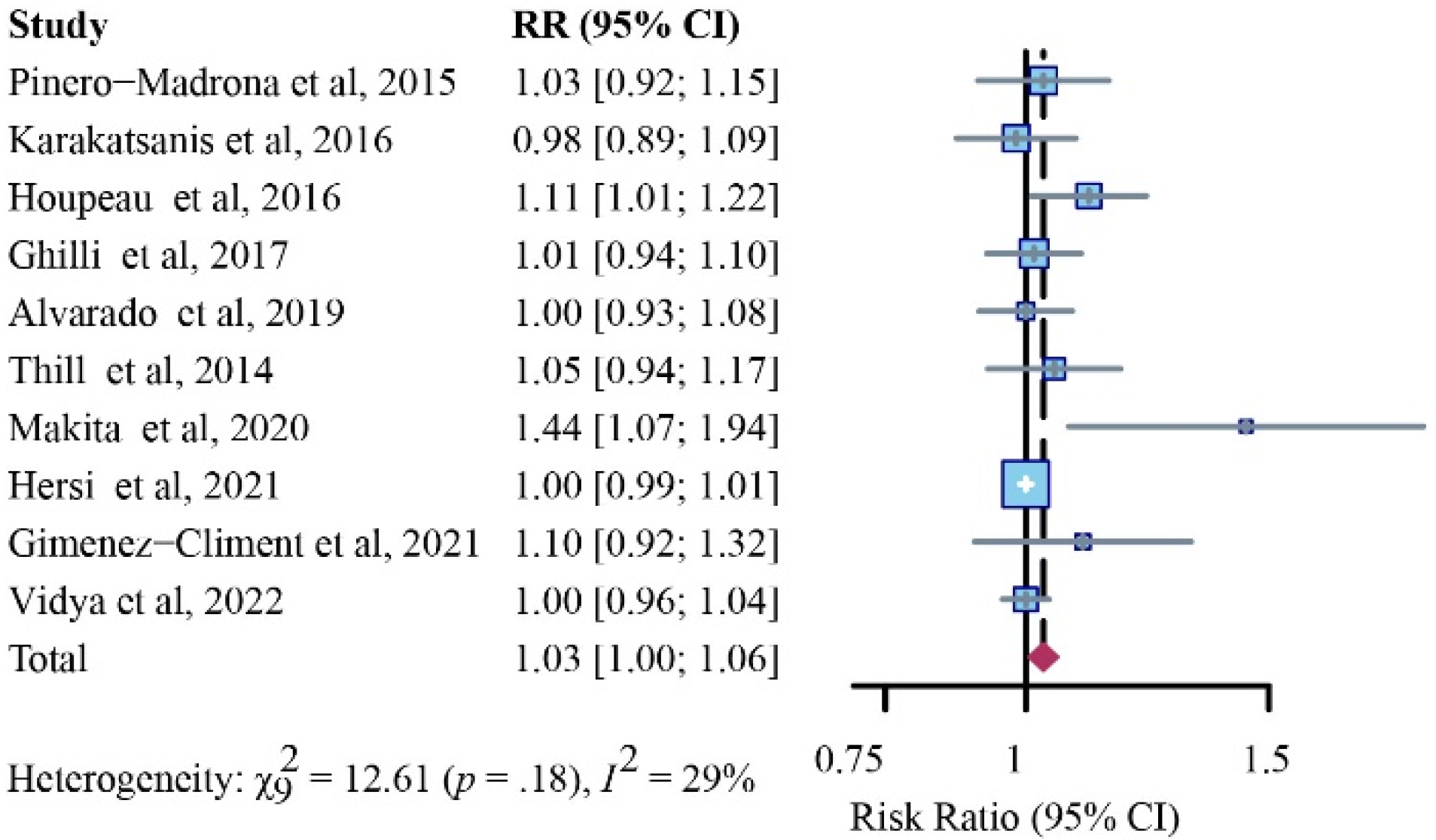
Detection Rates for Patients with Positive SLNs
Detection Rates for Positive SLNs
Complications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Krag, D.N.; Anderson, S.J.; Julian, T.B.; Brown, A.M.; Harlow, S.P.; Ashikaga, T.; Weaver, D.L.; Miller, B.J.; Jalovec, L.M.; Frazier, T.G.; et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: Results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007, 8, 881–888. [Google Scholar] [CrossRef]
- Veronesi, U.; Paganelli, G.; Viale, G.; Luini, A.; Zurrida, S.; Galimberti, V.; Intra, M.; Veronesi, P.; Robertson, C.; Maisonneuve, P.; et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N. Engl. J. Med. 2003, 349, 546–553. [Google Scholar] [CrossRef]
- Veronesi, U.; Viale, G.; Paganelli, G.; Zurrida, S.; Luini, A.; Galimberti, V.; Veronesi, P.; Intra, M.; Maisonneuve, P.; Zucca, F. Sentinel lymph node biopsy in breast cancer: Ten-year results of a randomized controlled study. Ann. Surg. 2010, 251, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Ashikaga, T.; Krag, D.N.; Land, S.R.; Julian, T.B.; Anderson, S.; Brown, A.M.; Ms, J.M.S.; Harlow, S.P.; Weaver, D.L.; Mamounas, E.P.; et al. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J. Surg. Oncol. 2010, 102, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Lucci, A.; McCall, L.M.; Beitsch, P.D.; Whitworth, P.W.; Reintgen, D.S.; Blumencranz, P.W.; Leitch, A.M.; Saha, S.; Hunt, K.K.; Giuliano, A.E. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J. Clin. Oncol. 2007, 25, 3657–3663. [Google Scholar] [CrossRef] [PubMed]
- Mansel, R.E.; Fallowfield, L.; Kissin, M.; Goyal, A.; Newcombe, R.G.; Dixon, J.M.; Yiangou, C.; Horgan, K.; Bundred, N.; Monypenny, I.; et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: The ALMANAC Trial. J. Natl. Cancer Inst. 2006, 98, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Douek, M.; Klaase, J.; Monypenny, I.; Kothari, A.; Zechmeister, K.; Brown, D.; Wyld, L.; Drew, P.; Garmo, H.; Agbaje, O.; et al. Sentinel node biopsy using a magnetic tracer versus standard technique: The SentiMAG Multicentre Trial. Ann. Surg. Oncol. 2014, 21, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Barthelmes, L.; Goyal, A.; Newcombe, R.G.; McNeill, F.; Mansel, R.E. Adverse reactions to patent blue V dye—The NEW START and ALMANAC experience. Eur. J. Surg. Oncol. 2010, 36, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Omranipour, R. Surgery for Pregnancy-Associated Breast Cancer. Adv. Exp. Med. Biol. 2020, 1252, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Purushotham, A.D.; Douek, M. Novel techniques for sentinel lymph node biopsy in breast cancer: A systematic review. Lancet Oncol. 2014, 15, e351–e362. [Google Scholar] [CrossRef]
- Ferrucci, M.; Franceschini, G.; Douek, M. New techniques for sentinel node biopsy in breast cancer. Transl. Cancer Res. 2018, 7, S405–S417. [Google Scholar] [CrossRef]
- Piñero-Madrona, A.; Torró-Richart, J.A.; de León-Carrillo, J.M.; de Castro-Parga, G.; Navarro-Cecilia, J.; Domínguez-Cunchillos, F.; Román-Santamaría, J.; Fuster-Diana, C.; Pardo-García, R. Superparamagnetic iron oxide as a tracer for sentinel node biopsy in breast cancer: A comparative non-inferiority study. Eur. J. Surg. Oncol. 2015, 41, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Pinder, S.E.; Douek, M. Deposition of superparamagnetic iron-oxide nanoparticles in axillary sentinel lymph nodes following subcutaneous injection. Histopathology 2013, 62, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Thill, M.; Kurylcio, A.; Welter, R.; van Haasteren, V.; Grosse, B.; Berclaz, G.; Polkowski, W.; Hauser, N. The Central-European SentiMag study: Sentinel lymph node biopsy with superparamagnetic iron oxide (SPIO) vs. radioisotope. Breast 2014, 23, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Teshome, M.; Wei, C.; Hunt, K.K.; Thompson, A.; Rodriguez, K.; Mittendorf, E.A. Use of a Magnetic Tracer for Sentinel Lymph Node Detection in Early-Stage Breast Cancer Patients: A Meta-analysis. Ann. Surg. Oncol. 2016, 23, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Zada, A.; Peek, M.C.L.; Ahmed, M.; Anninga, B.; Baker, R.; Kusakabe, M.; Sekino, M.; Klaase, J.M.; Haken, B.T.; Douek, M. Meta-analysis of sentinel lymph node biopsy in breast cancer using the magnetic technique. Br. J. Surg. 2016, 103, 1409–1419. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, Ed000142. [Google Scholar] [CrossRef] [PubMed]
- Hurton, S.M.S.; Hayden, J.A.; Molinari, M. Adjuvant therapy for resected pancreatic cancer. Cochrane Database Syst. Rev. 2017, 2017, CD011514. [Google Scholar] [CrossRef]
- Rücker, G.; Cates, C.J.; Schwarzer, G. Methods for including information from multi-arm trials in pairwise meta-analysis. Res. Synth. Methods 2017, 8, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Rubio, I.T.; Rodriguez-Revuelto, R.; Espinosa-Bravo, M.; Siso, C.; Rivero, J.; Esgueva, A. A randomized study comparing different doses of superparamagnetic iron oxide tracer for sentinel lymph node biopsy in breast cancer: The SUNRISE study. Eur. J. Surg. Oncol. 2020, 46, 2195–2201. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Rubio, I.T.; Diaz-Botero, S.; Esgueva, A.; Rodriguez, R.; Cortadellas, T.; Cordoba, O.; Espinosa-Bravo, M. The superparamagnetic iron oxide is equivalent to the Tc99 radiotracer method for identifying the sentinel lymph node in breast cancer. Eur. J. Surg. Oncol. 2015, 41, 46–51. [Google Scholar] [CrossRef]
- Ahmed, M.; Anninga, B.; Goyal, S.; Young, P.; Pankhurst, Q.A.; Douek, M.; Mag, S.T.G. Magnetic sentinel node and occult lesion localization in breast cancer (MagSNOLL Trial). Brit. J. Surg. 2015, 102, 646–652. [Google Scholar] [CrossRef]
- Karakatsanis, A.; Hersi, A.; Pistiolis, L.; Bagge, R.O.; Lykoudis, P.M.; Eriksson, S.; Wärnberg, F.; Nagy, G.; Mohammed, I.; Sundqvist, M.; et al. Effect of preoperative injection of superparamagnetic iron oxide particles on rates of sentinel lymph node dissection in women undergoing surgery for ductal carcinoma in situ (SentiNot study). Br. J. Surg. 2019, 106, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Karakatsanis, A.; Olofsson, H.; Stålberg, P.; Bergkvist, L.; Abdsaleh, S.; Wärnberg, F. Simplifying Logistics and Avoiding the Unnecessary in Patients with Breast Cancer Undergoing Sentinel Node Biopsy. A Prospective Feasibility Trial of the Preoperative Injection of Super Paramagnetic Iron Oxide Nanoparticles. Scand. J. Surg. 2018, 107, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Karakatsanis, A.; Christiansen, P.M.; Fischer, L.; Hedin, C.; Pistioli, L.; Sund, M.; Rasmussen, N.R.; Jørnsgård, H.; Tegnelius, D.; Eriksson, S.; et al. The Nordic SentiMag trial: A comparison of super paramagnetic iron oxide (SPIO) nanoparticles versus Tc(99) and patent blue in the detection of sentinel node (SN) in patients with breast cancer and a meta-analysis of earlier studies. Breast Cancer Res. Treat. 2016, 157, 281–294. [Google Scholar] [CrossRef]
- Houpeau, J.-L.; Chauvet, M.-P.; Guillemin, F.; Bendavid-Athias, C.; Charitansky, H.; Kramar, A.; Giard, S. Sentinel lymph node identification using superparamagnetic iron oxide particles versus radioisotope: The French Sentimag feasibility trial. J. Surg. Oncol. 2016, 113, 501–507. [Google Scholar] [CrossRef]
- Ghilli, M.; Carretta, E.; Di Filippo, F.; Battaglia, C.; Fustaino, L.; Galanou, I.; Rucci, P.; Fantini, M.; Roncella, M. The superparamagnetic iron oxide tracer: A valid alternative in sentinel node biopsy for breast cancer treatment. Eur. J. Cancer Care 2017, 26, e12385. [Google Scholar] [CrossRef]
- Alvarado, M.D.; Mittendorf, E.A.; Teshome, M.; Thompson, A.M.; Bold, R.J.; Gittleman, M.A.; Beitsch, P.D.; Blair, S.L.; Kivilaid, K.; Harmer, Q.J.; et al. SentimagIC: A Non-inferiority Trial Comparing Superparamagnetic Iron Oxide versus Technetium-99m and Blue Dye in the Detection of Axillary Sentinel Nodes in Patients with Early-Stage Breast Cancer. Ann. Surg. Oncol. 2019, 26, 3510–3516. [Google Scholar] [CrossRef]
- Taruno, K.; Kurita, T.; Kuwahata, A.; Yanagihara, K.; Enokido, K.; Katayose, Y.; Nakamura, S.; Takei, H.; Sekino, M.; Kusakabe, M. Multicenter clinical trial on sentinel lymph node biopsy using superparamagnetic iron oxide nanoparticles and a novel handheld magnetic probe. J. Surg. Oncol. 2019, 120, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Makita, M.; Manabe, E.; Kurita, T.; Takei, H.; Nakamura, S.; Kuwahata, A.; Sekino, M.; Kusakabe, M.; Ohashi, Y. Moving a neodymium magnet promotes the migration of a magnetic tracer and increases the monitoring counts on the skin surface of sentinel lymph nodes in breast cancer. BMC Med. Imaging 2020, 20, 58. [Google Scholar] [CrossRef]
- Hamzah, J.L.; Tan, B.K.T.; Tan, V.; Madhukumar, P.; Lim, S.Z.; Sim, Y.R.; Yong, W.S.; Lee, Z.J.; Ong, K.W. A pilot study comparing Sentimag/Sienna versus standard modality for sentinel lymph node identification in patients with breast cancer. Breast J. 2020, 26, 1074–1077. [Google Scholar] [CrossRef] [PubMed]
- Hersi, A.-F.; Pistiolis, L.; Luberth, C.D.; Vikhe-Patil, E.; Nilsson, F.; Mohammed, I.; Bagge, R.O.; Wärnberg, F.; Eriksson, S.; Karakatsanis, A. Optimizing Dose and Timing in Magnetic Tracer Techniques for Sentinel Lymph Node Detection in Early Breast Cancers: The Prospective Multicenter SentiDose Trial. Cancers 2021, 13, 693. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Climent, J.; Marín-Hernández, C.; Fuster-Diana, C.A.; Torró-Richart, J.A.; Navarro-Cecilia, J. Sentinel lymph node biopsy in breast cancer after neoadjuvant therapy using a magnetic tracer versus standard technique: A multicentre comparative non-inferiority study (IMAGINE-II). Int. J. Surg. Open 2021, 35, 100404. [Google Scholar] [CrossRef]
- Vidya, R.; Khosla, M.; Laws, S.; Harvey, J.; Kaushik, M.; Mullapudi, N.A.; Macmillan, D. Axillary sentinel lymph node identification using superparamagnetic iron oxide versus radioisotope in early-stage breast cancer: The UK SentiMag trial (SMART study). Surgeon 2022, in press. [CrossRef]
- Taruno, K.; Kuwahata, A.; Sekino, M.; Nakagawa, T.; Kurita, T.; Enokido, K.; Nakamura, S.; Takei, H.; Kusakabe, M. Exploratory Study of Superparamagnetic Iron Oxide Dose Optimization in Breast Cancer Sentinel Lymph Node Identification Using a Handheld Magnetic Probe and Iron Quantitation. Cancers 2022, 14, 1409. [Google Scholar] [CrossRef]
- Karakatsanis, A.; Daskalakis, K.; Stålberg, P.; Olofsson, H.; Andersson, Y.; Eriksson, S.; Bergkvist, L.; Wärnberg, F. Superparamagnetic iron oxide nanoparticles as the sole method for sentinel node biopsy detection in patients with breast cancer. Br. J. Surg. 2017, 104, 1675–1685. [Google Scholar] [CrossRef]
- Wärnberg, F.; Stigberg, E.; Obondo, C.; Olofsson, H.; Abdsaleh, S.; Wärnberg, M.; Karakatsanis, A. Long-Term Outcome after Retro-Areolar versus Peri-Tumoral Injection of Superparamagnetic Iron Oxide Nanoparticles (SPIO) for Sentinel Lymph Node Detection in Breast Cancer Surgery. Ann. Surg. Oncol. 2019, 26, 1247–1253. [Google Scholar] [CrossRef]
- Krischer, B.; Forte, S.; Niemann, T.; Kubik-Huch, R.A.; Leo, C. Feasibility of breast MRI after sentinel procedure for breast cancer with superparamagnetic tracers. Eur. J. Surg. Oncol. 2018, 44, 74–79. [Google Scholar] [CrossRef]
- Kuemmel, S.; Holtschmidt, J.; Gerber, B.; Von Der Assen, A.; Heil, J.; Thill, M.; Krug, D.; Schem, C.; Denkert, C.; Lubitz, J.; et al. Prospective, Multicenter, Randomized Phase III Trial Evaluating the Impact of Lymphoscintigraphy as Part of Sentinel Node Biopsy in Early Breast Cancer: SenSzi (GBG80) Trial. J. Clin. Oncol. 2019, 37, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Anninga, B.; White, S.H.; Moncrieff, M.; Dziewulski, P.; Geh, J.L.C.; Klaase, J.; Garmo, H.; Castro, F.; Pinder, S.; Hall-Craggs, M.A.; et al. Magnetic Technique for Sentinel Lymph Node Biopsy in Melanoma: The MELAMAG Trial. Ann. Surg. Oncol. 2016, 23, 2070–2078. [Google Scholar] [CrossRef]
- Waanders, S.; Visscher, M.; Wildeboer, R.R.; Oderkerk, T.O.B.; Krooshoop, H.J.G.; Haken, B.T. A handheld SPIO-based sentinel lymph node mapping device using differential magnetometry. Phys. Med. Biol. 2016, 61, 8120–8134. [Google Scholar] [CrossRef] [PubMed]
- Kuwahata, A.; Kaneko, M.; Chikaki, S.; Kusakabe, M.; Sekino, M. Development of device for quantifying magnetic nanoparticle tracers accumulating in sentinel lymph nodes. AIP Adv. 2018, 8, 056713. [Google Scholar] [CrossRef]
- Sekino, M.; Kuwahata, A.; Ookubo, T.; Shiozawa, M.; Ohashi, K.; Kaneko, M.; Saito, I.; Inoue, Y.; Ohsaki, H.; Takei, H.; et al. Handheld magnetic probe with permanent magnet and Hall sensor for identifying sentinel lymph nodes in breast cancer patients. Sci. Rep. 2018, 8, 1195. [Google Scholar] [CrossRef] [PubMed]
- Huizing, E.; Anninga, B.; Young, P.; Monypenny, I.; Hall-Craggs, M.; Douek, M. 4. Analysis of void artefacts in post-operative breast MRI due to residual SPIO after magnetic SLNB in SentiMAG Trial participants. Eur. J. Surg. Oncol. 2015, 41, S18. [Google Scholar] [CrossRef]
- Forte, S.; Kubik-Huch, R.; Leo, C. Improvement in breast magnetic resonance imaging after a sentinel procedure for breast cancer with superparamagnetic tracers. Eur. J. Radiol. Open 2019, 6, 215–219. [Google Scholar] [CrossRef]
- Lorek, A.; Stojčev, Z.; Zarębski, W.; Kowalczyk, M.; Szyluk, K. Analysis of Postoperative Complications after 303 Sentinel Lymph Node Identification Procedures Using the SentiMag (R) Method in Breast Cancer Patients. Med. Sci. Monit. 2019, 25, 3154–3160. [Google Scholar] [CrossRef]
- Lorek, A.; Steinhof-Radwańska, K.; Zarębski, W.; Lorek, J.; Stojčev, Z.; Zych, J.; Syrkiewicz, A.; Niemiec, P.; Szyluk, K. Comparative Analysis of Postoperative Complications of Sentinel Node Identification Using the SentiMag® Method and the Use of a Radiotracer in Patients with Breast Cancer. Curr. Oncol. 2022, 29, 2887–2894. [Google Scholar] [CrossRef]
- Bazire, L.; Alran, S.; El Bamrani, S.; Gaujal, L.; Vincent-Salomon, A.; Tardivon, A.; Kirova, Y.M. Radiation therapy after sentinel lymph node biopsy for early stage breast cancer using a magnetic tracer: Results of a single institutional prospective study of tolerance. Cancer Radiother. 2019, 23, 23–27. [Google Scholar] [CrossRef]




| Author and Year | Country | Methods Compared | Injection Site | Injection Volume | Interval Time * |
|---|---|---|---|---|---|
| Rubio et al., 2015 [23] | Spain | SPIO, RI | subareolar | 2.0 mL | 20–25 min |
| Ahmed et al., 2015 [24] | UK | SPIO, RI + BD | intratumoral | 0.5 mL | Within 24 h |
| Pinero−Madrona et al., 2015 [12] | Spain | SPIO, RI + BD | peritumoral or periareolar | 2.0 mL | 10 to 85 min |
| Karakatsanis et al., 2019 [25] | Sweden | SPIO, RI + BD | subareolar | 2.0 mL | At least 20 min |
| Karakatsanis et al., 2018 [26] | Sweden | SPIO, RI + BD | subareolar | 2.0 mL | 3–15 days (median 8 days) |
| Karakatsanis et al., 2016 [27] | Sweden | SPIO, RI + BD | subareolar | 2.0 mL | At least 20 min |
| Houpeau et al., 2016 [28] | France | SPIO, RI ± BD | periareolar | 2.0 mL | At least 20 min |
| Ghilli et al., 2017 [29] | Italy | SPIO, RI + BD | subareolar | 2.0 mL | At least 20 min |
| Douek et al., 2013 [7] | UK and Netherlands | SPIO, RI ± BD | periareolar | 2.0 mL | At least 20 min |
| Alvarado et al., 2019 [30] | America | SPIO, RI + BD | subareolar | 2.0 mL | At least 20 min |
| Thill et al., 2014 [14] | Germany | SPIO, RI | periareolar or peritumorally | 2.0 mL | At least 20 min |
| Taruno et al., 2019 [31] | Japan | SPIO, RI | subareolar | 1.0 mL | 1 day |
| Makita et al., 2020 [32] | Japan | SPIO, RI | periareolar or peritumorally | 0.5 mL | At least 20 min |
| Rubio et al., 2020 [21] | Spain | SPIO, RI | subareolar | 1.0 mL, 1.5 mL, or 2 mL | 37 min (range 35–39 min) |
| Hamzah et al., 2020 [33] | Singapore | SPIO, RI + BD | subareolar | 2.0 mL | At least 20 min |
| Hersi et al., 2021 [34] | Sweden | SPIO, RI + BD | periareolar or peritumorally | 1.5 mL | At least 20 min |
| Hersi et al., 2021 [34] | Sweden | SPIO, RI + BD | Periareolar or peritumorally | 1.0 mL | 1–7 days |
| Giménez-Climent et al., 2021 [35] | Spain | SPIO, RI | subareolar | 2 mL | At least 20 min |
| Vidya et al., 2022 [36] | UK | SPIO, RI ± BD | subareolar | 2 mL | At least 20 min |
| Author and Year | Patients | SLNs | Positive Patients | Positive SLNs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | SM | SPIO | Total | SM | SPIO | Total | SM | SPIO | Total | SM | SPIO | |
| Rubio et al., 2015 [23] | 118 | 95.7% | 98.3% | 287 | 80.1% | 92.0% | 36 | 91.7% | 94.4% | NR | NR | NR |
| Ahmed et al., 2015 [24] | 33 | 97.0% | 84.8% | 67 | 92.5% | 77.6% | 5 | 80.0% | 100.0% | NR | NR | NR |
| Pinero-Madrona et al., 2015 [12] | 181 | 97.8% | 97.2% | 321 | 89.3% | 92.5% | 60 | 91.7% | 88.3% | 76 | 88.2% | 90.8% |
| Karakatsanis et al., 2019 [25] | 40 | 65.0% | 92.5% | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Karakatsanis et al., 2018 [26] | 12 | 83.3% | 83.3% | 16 | 81.3% | 81.3% | 3 | 66.7% | 100.0% | NR | NR | NR |
| Karakatsanis et al., 2016 [27] | 206 | 97.1% | 97.6% | 402 | 91.3% | 93.3% | 54 | 99.4% | 96.3% | 68 | 92.6% | 91.2% |
| Houpeau et al., 2016 [28] | 108 | 95.4% | 97.2% | 220 | 90.2% | 97.2% | 46 | 97.8% | 95.7% | 61 | 88.5% | 98.4% |
| Ghilli et al., 2017 [29] | 193 | 99.0% | 97.9% | 308 | 94.7% | 95.8% | 57 | 96.5% | 98.3% | 77 | 93.5% | 94.8% |
| Douek et al., 2013 [7] | 160 | 95.0% | 94.4% | 404 | 73.5% | 80.0% | 35 | 97.1% | 97.1% | NR | NR | NR |
| Alvarado et al., 2019 [30] | 146 | 98.6% | 99.3% | 369 | 93.5% | 94.3% | 22 | 95.5% | 95.6% | 25 | 100.0% | 100.0% |
| Thill et al., 2014 [14] | 150 | 97.3% | 98.0% | 291 | 91.8% | 97.3% | 42 | 91.2% | 97.1% | 45 | 91.1% | 96.6% |
| Taruno et al., 2019 [31] | 210 | 98.1% | 94.8% | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Makita et al., 2020 [32] | 62 | 95.2% | 100.0% | 183 | 68.3% | 99.5% | NR | NR | NR | 19 | 68.4% | 100% |
| Rubio et al., 2020 [21] | 135 | 97.8% | 98.5% | 235 | 73.2% | 71.4% | 22 | 100.0% | 100.0% | NR | NR | NR |
| Hamzah et al., 2020 [33] | 20 | 100.0% | 95.0% | 56 | 71.4% | 98.2% | NR | NR | NR | NR | NR | NR |
| Hersi et al., 2021 [34] | 163 | 98.2% | 97.5% | 351 | 86.6% | 84.9% | 33 | 100.0% | 97.0% | NR | NR | NR |
| Hersi et al., 2021 [34] | 165 | 100.0% | 100.0% | 371 | 83.3% | 96.8% | 29 | 100.0% | 100.0% | 195 | 100.0% | 100.0% |
| Giménez-Climent et al., 2021 [35] | 89 | 96.6% | 97.8% | 129 | 90.6% | 98.4% | 21 | 85.0% | 100.0% | 23 | 86.4% | 95.4% |
| Vidya et al., 2022 [36] | 107 | 92.3% | 98.1% | 202 | 96.65% | 93.1% | 31 | 100.0% | 100.0% | 50 | 100.0% | 100.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Tan, J.; Song, Y.; Huang, K.; Zhang, Q.; Xie, H. The Application of Magnetic Nanoparticles for Sentinel Lymph Node Detection in Clinically Node-Negative Breast Cancer Patients: A Systemic Review and Meta-Analysis. Cancers 2022, 14, 5034. https://doi.org/10.3390/cancers14205034
Liu P, Tan J, Song Y, Huang K, Zhang Q, Xie H. The Application of Magnetic Nanoparticles for Sentinel Lymph Node Detection in Clinically Node-Negative Breast Cancer Patients: A Systemic Review and Meta-Analysis. Cancers. 2022; 14(20):5034. https://doi.org/10.3390/cancers14205034
Chicago/Turabian StyleLiu, Pengcheng, Jie Tan, Yuting Song, Kai Huang, Qingyi Zhang, and Huiqi Xie. 2022. "The Application of Magnetic Nanoparticles for Sentinel Lymph Node Detection in Clinically Node-Negative Breast Cancer Patients: A Systemic Review and Meta-Analysis" Cancers 14, no. 20: 5034. https://doi.org/10.3390/cancers14205034
APA StyleLiu, P., Tan, J., Song, Y., Huang, K., Zhang, Q., & Xie, H. (2022). The Application of Magnetic Nanoparticles for Sentinel Lymph Node Detection in Clinically Node-Negative Breast Cancer Patients: A Systemic Review and Meta-Analysis. Cancers, 14(20), 5034. https://doi.org/10.3390/cancers14205034






