Alcohol as a Non-UV Social-Environmental Risk Factor for Melanoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Alcohol and Melanoma
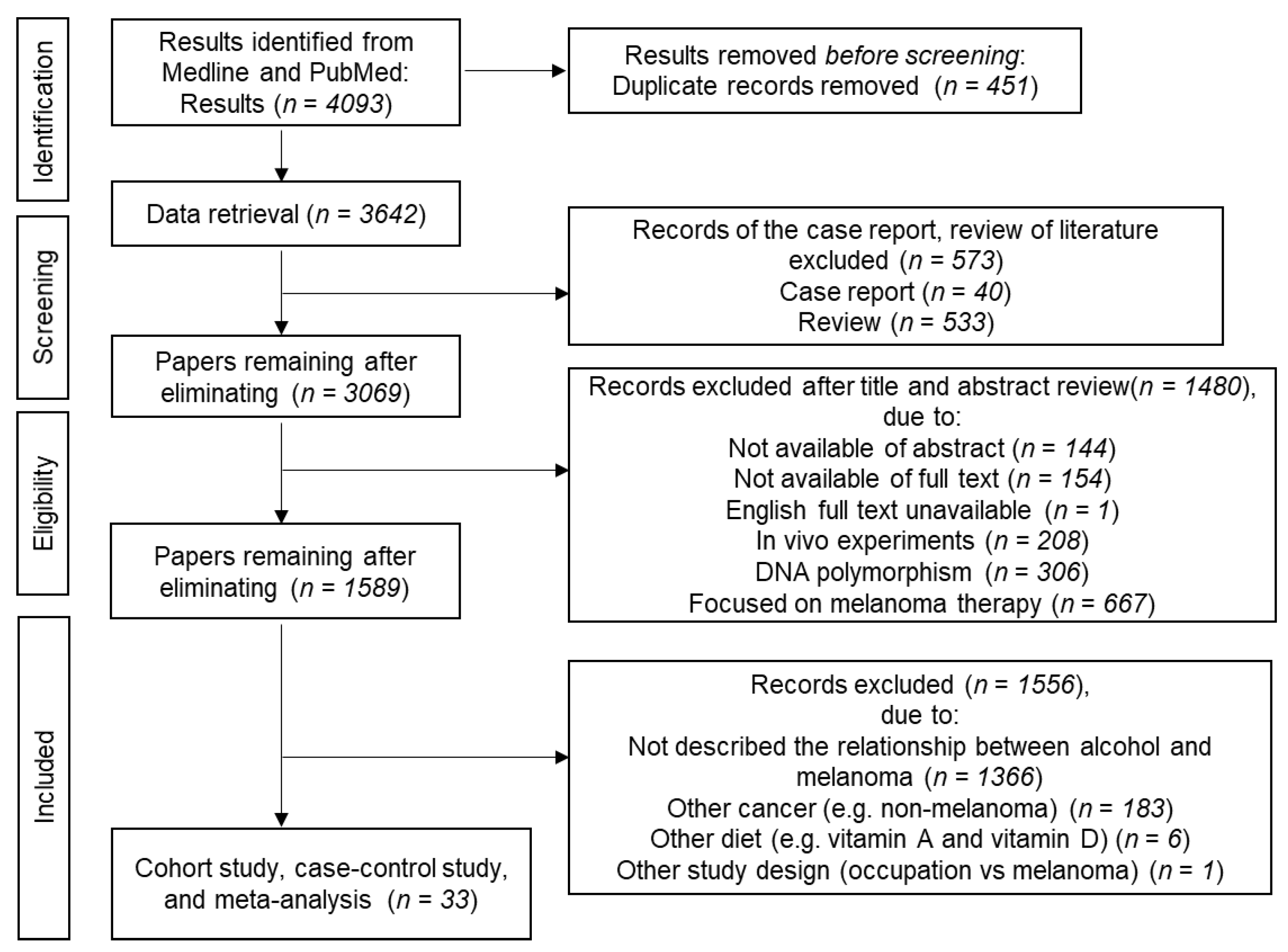
2.1. Methods
2.1.1. Literature Search
2.1.2. Study Selection and Data Extraction
2.2. Cohort and Case-Control Studies
2.3. Meta-Analyses
2.4. Dose-Dependent Effects of Alcohol on Melanoma
2.5. Effects of UV Exposure on the Association between Alcohol and Melanoma
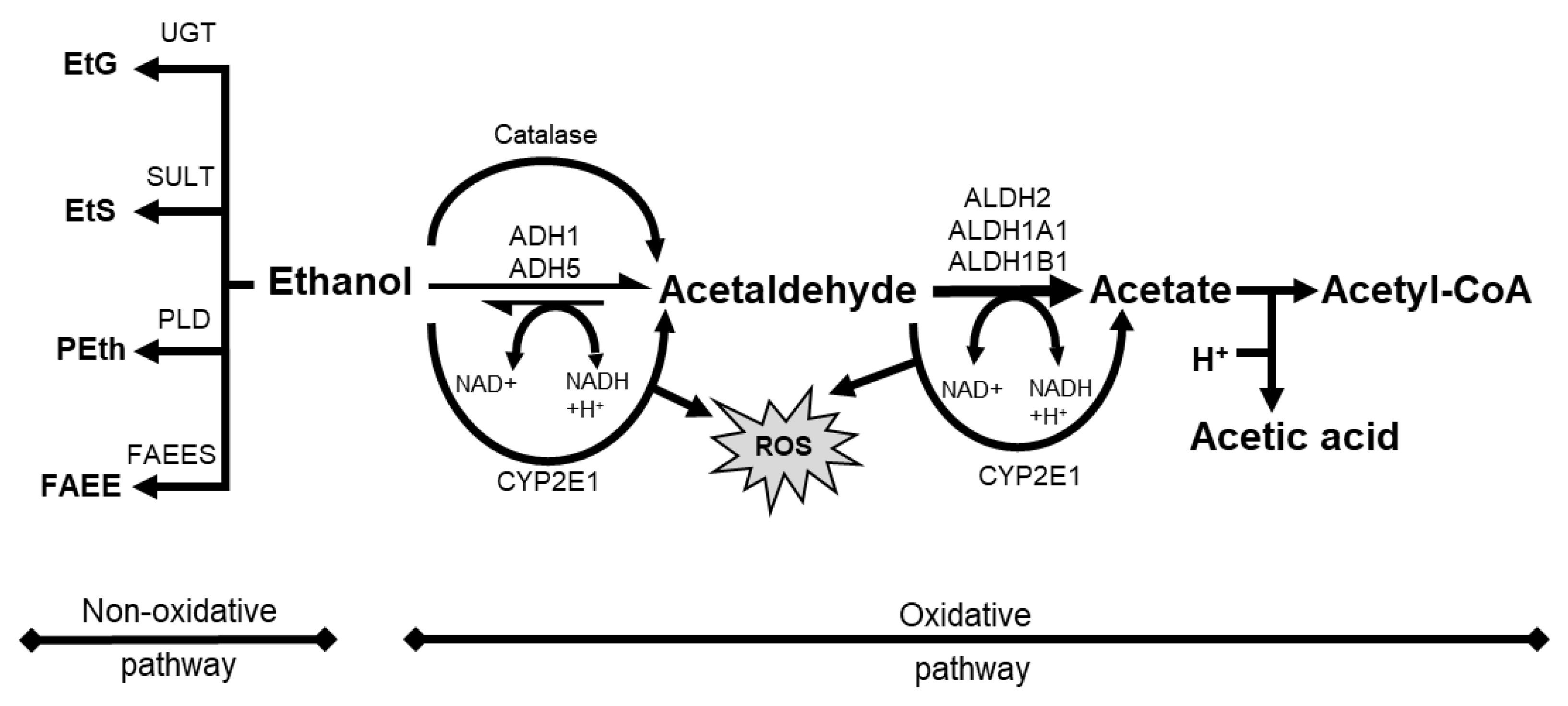
3. Potential Roles of Ethanol on Melanoma Initiation and Progression
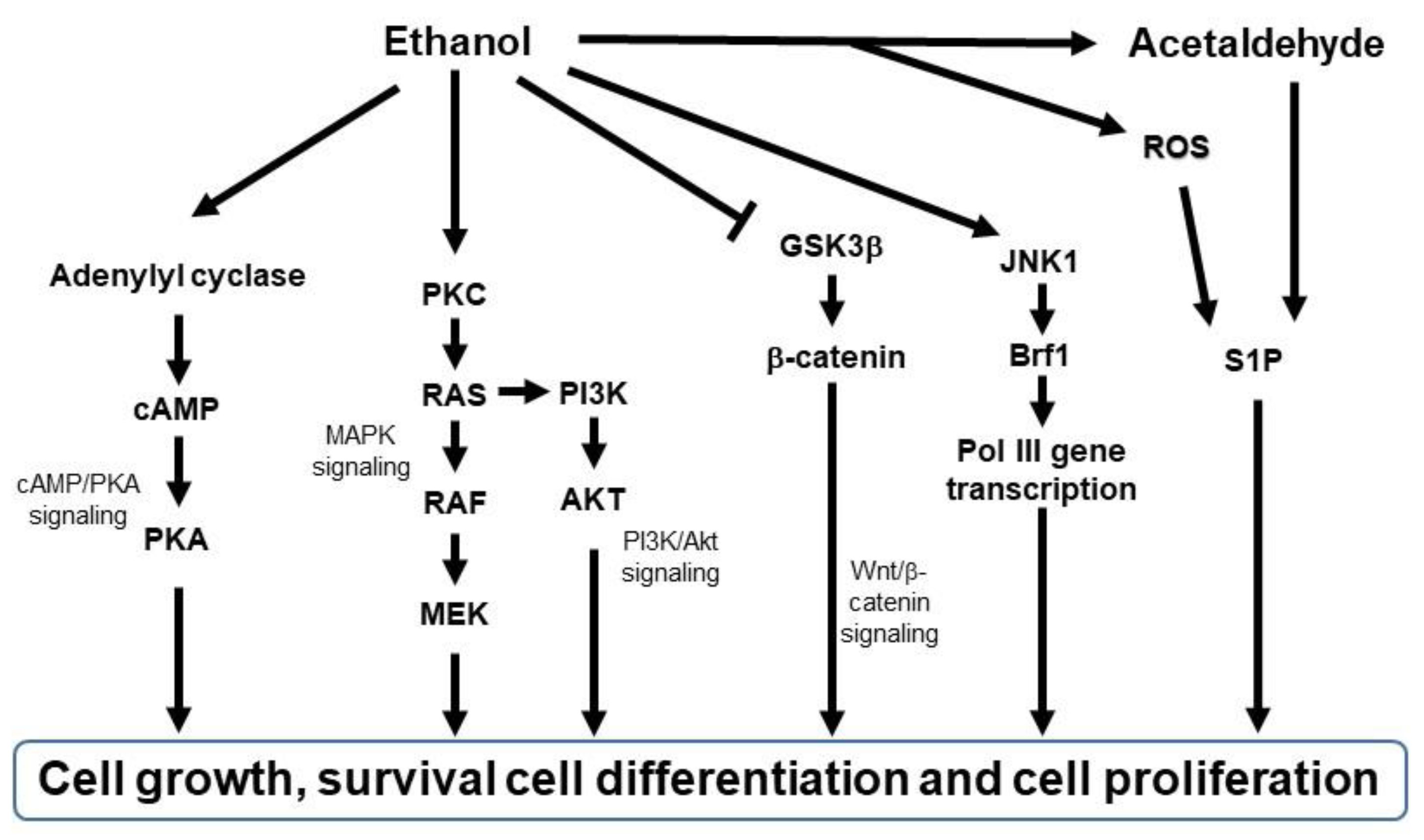
3.1. Roles of Ethanol or AcAH in Cellular Biology
3.2. Roles of Ethanol or AcAH in Tumor Biology
3.3. Roles of Ethanol or AcAH in Skin Biology
3.4. Does Ethanol or AcAH Affect Melanoma Initiation?
3.5. Does Ethanol or AcAH Affect Melanoma Progression?
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bell, K.J.L.; Cust, A.E. Beyond country-specific incidence and mortality: The global burden of melanoma. Br. J. Dermatol. 2018, 178, 315–316. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.; Smith, C.; Wernberg, J. Epidemiology and risk factors of melanoma. Surg. Clin. N. Am. 2020, 100, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Roush, G.C.; McKay, L.; Holford, T.R. A reversal in the long-term increase in deaths attributable to malignant melanoma. Cancer 1992, 69, 1714–1720. [Google Scholar] [CrossRef]
- Linos, E.; Swetter, S.M.; Cockburn, M.G.; Colditz, G.A.; Clarke, C.A. Increasing burden of melanoma in the United States. J. Investig. Dermatol. 2009, 129, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Welch, H.G.; Mazer, B.L.; Adamson, A.S. The rapid rise in cutaneous melanoma diagnoses. N. Engl. J. Med. 2021, 384, 72–79. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Jemal, A.; Thomas, A.; Murray, T.; Thun, M. Cancer statistics, 2002. CA Cancer J. Clin. 2002, 52, 23–47. [Google Scholar] [CrossRef]
- Guy, G.P., Jr.; Machlin, S.R.; Ekwueme, D.U.; Yabroff, K.R. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011. Am. J. Prev. Med. 2015, 48, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Herbert, A.; Koo, M.M.; Barclay, M.E.; Greenberg, D.C.; Abel, G.A.; Levell, N.J.; Lyratzopoulos, G. Stage-specific incidence trends of melanoma in an English region, 1996–2015: Longitudinal analyses of population-based data. Melanoma Res. 2020, 30, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Hubner, J.; Waldmann, A.; Eisemann, N.; Noftz, M.; Geller, A.C.; Weinstock, M.A.; Volkmer, B.; Greinert, R.; Breitbart, E.W.; Katalinic, A. Association between risk factors and detection of cutaneous melanoma in the setting of a population-based skin cancer screening. Eur. J. Cancer Prev. 2018, 27, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Bolognia, J.L.; Jorizzo, J.L.; Schaffer, J.V. Dermatology E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Read, J.; Wadt, K.A.; Hayward, N.K. Melanoma genetics. J. Med. Genet. 2016, 53, 1–14. [Google Scholar] [CrossRef]
- Hawkes, J.E.; Truong, A.; Meyer, L.J. Genetic predisposition to melanoma. Semin. Oncol. 2016, 43, 591–597. [Google Scholar] [CrossRef]
- Tagliabue, E.; Gandini, S.; Bellocco, R.; Maisonneuve, P.; Newton-Bishop, J.; Polsky, D.; Lazovich, D.; Kanetsky, P.A.; Ghiorzo, P.; Gruis, N.A.; et al. MC1R variants as melanoma risk factors independent of at-risk phenotypic characteristics: A pooled analysis from the M-SKIP project. Cancer Manag. Res. 2018, 10, 1143–1154. [Google Scholar] [CrossRef]
- Wei, E.X.; Li, X.; Nan, H. Having a first-degree relative with melanoma increases lifetime risk of melanoma, squamous cell carcinoma, and basal cell carcinoma. J. Am. Acad. Dermatol. 2019, 81, 489–499. [Google Scholar] [CrossRef]
- Liang, X.S.; Pfeiffer, R.M.; Wheeler, W.; Maeder, D.; Burdette, L.; Yeager, M.; Chanock, S.; Tucker, M.A.; Goldstein, A.M.; Yang, X.R. Genetic variants in DNA repair genes and the risk of cutaneous malignant melanoma in melanoma-prone families with/without CDKN2A mutations. Int. J. Cancer 2012, 130, 2062–2066. [Google Scholar] [CrossRef]
- Bataille, V. It’s Not All Sunshine: Non-sun-related Melanoma risk-factors. Acta Derm. Venereol. 2020, 100, adv00137. [Google Scholar] [CrossRef]
- Rigel, D.S.; Carucci, J.A. Malignant melanoma: Prevention, early detection, and treatment in the 21st century. CA Cancer J. Clin. 2000, 50, 215–236; quiz 237–240. [Google Scholar] [CrossRef]
- Raimondi, S.; Sera, F.; Gandini, S.; Iodice, S.; Caini, S.; Maisonneuve, P.; Fargnoli, M.C. MC1R variants, melanoma and red hair color phenotype: A meta-analysis. Int. J. Cancer 2008, 122, 2753–2760. [Google Scholar] [CrossRef] [PubMed]
- Belbasis, L.; Stefanaki, I.; Stratigos, A.J.; Evangelou, E. Non-genetic risk factors for cutaneous melanoma and keratinocyte skin cancers: An umbrella review of meta-analyses. J. Dermatol. Sci. 2016, 84, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Pion, I.A.; Rigel, D.S.; Garfinkel, L.; Silverman, M.K.; Kopf, A.W. Occupation and the risk of malignant melanoma. Cancer 1995, 75, 637–644. [Google Scholar] [CrossRef]
- Ting, W.; Schultz, K.; Cac, N.N.; Peterson, M.; Walling, H.W. Tanning bed exposure increases the risk of malignant melanoma. Int. J. Dermatol. 2007, 46, 1253–1257. [Google Scholar] [CrossRef]
- Rockley, P.F.; Trieff, N.; Wagner, R.F., Jr.; Tyring, S.K. Nonsunlight risk factors for malignant melanoma. Part I: Chemical agents, physical conditions, and occupation. Int. J. Dermatol. 1994, 33, 398–406. [Google Scholar] [CrossRef]
- Hodis, E.; Watson, I.R.; Kryukov, G.V.; Arold, S.T.; Imielinski, M.; Theurillat, J.P.; Nickerson, E.; Auclair, D.; Li, L.; Place, C.; et al. A landscape of driver mutations in melanoma. Cell 2012, 150, 251–263. [Google Scholar] [CrossRef]
- Sanchez, M.I.; Grichnik, J.M. Melanoma’s high C>T mutation rate: Is deamination playing a role? Exp. Dermatol. 2014, 23, 551–552. [Google Scholar] [CrossRef]
- Okura, R.; Yoshioka, H.; Yoshioka, M.; Hiromasa, K.; Nishio, D.; Nakamura, M. Expression of AID in malignant melanoma with BRAF(V600E) mutation. Exp. Dermatol. 2014, 23, 347–348. [Google Scholar] [CrossRef]
- Larese Filon, F.; Buric, M.; Fluehler, C. UV exposure, preventive habits, risk perception, and occupation in NMSC patients: A case-control study in Trieste (NE Italy). Photodermatol. Photoimmunol. Photomed. 2019, 35, 24–30. [Google Scholar] [CrossRef]
- D’Orazio, J.A.; Marsch, A.; Lagrew, J.; Veith, W.B. Skin pigmentation and melanoma risk. In Advances in Malignant Melanoma—Clinical and Research Perspectives; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Lombardo, N.; Della Corte, M.; Pelaia, C.; Piazzetta, G.; Lobello, N.; Del Duca, E.; Bennardo, L.; Nistico, S.P. Primary mucosal melanoma presenting with a unilateral nasal obstruction of the left inferior turbinate. Medicina 2021, 57, 359. [Google Scholar] [CrossRef]
- Jhaveri, M.B.; Driscoll, M.S.; Grant-Kels, J.M. Melanoma in pregnancy. Clin. Obstet. Gynecol. 2011, 54, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, M.S.; Martires, K.; Bieber, A.K.; Pomeranz, M.K.; Grant-Kels, J.M.; Stein, J.A. Pregnancy and melanoma. J. Am. Acad. Dermatol. 2016, 75, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Roberts, C.L.; Dobbins, T.; Stavrou, E.; Black, K.; Morris, J.; Young, J. Incidence and outcomes of pregnancy-associated cancer in Australia, 1994–2008: A population-based linkage study. BJOG 2012, 119, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Bannister-Tyrrell, M.; Roberts, C.L.; Hasovits, C.; Nippita, T.; Ford, J.B. Incidence and outcomes of pregnancy-associated melanoma in New South Wales 1994–2008. Aust. N. Zeal. J. Obstet. Gynaecol. 2015, 55, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Lens, M.; Bataille, V. Melanoma in relation to reproductive and hormonal factors in women: Current review on controversial issues. Cancer Causes Control 2008, 19, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Donahue, T.; Lee, C.Y.; Sanghvi, A.; Obregon, R.; Sidiropoulos, M.; Cooper, C.; Merkel, E.A.; Yelamos, O.; Ferris, L.; Gerami, P. Immunosuppression is an independent prognostic factor associated with aggressive tumor behavior in cutaneous melanoma. J. Am. Acad. Dermatol. 2015, 73, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Wang, Y.; Dang, Y.; Gagel, A.; Ross, M.I.; Gershenwald, J.E.; Cormier, J.N.; Wargo, J.; Haydu, L.E.; Davies, M.A.; et al. Association between body mass index, C-reactive protein levels, and Melanoma patient outcomes. J. Investig. Dermatol. 2017, 137, 1792–1795. [Google Scholar] [CrossRef]
- McQuade, J.L.; Daniel, C.R.; Hess, K.R.; Mak, C.; Wang, D.Y.; Rai, R.R.; Park, J.J.; Haydu, L.E.; Spencer, C.; Wongchenko, M.; et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: A retrospective, multicohort analysis. Lancet Oncol. 2018, 19, 310–322. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K.; International Agency for Research on Cancer Handbook Working Group. Body fatness and cancer--Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Secretan, B.; Straif, K.; Baan, R.; Grosse, Y.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part E: Tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009, 10, 1033–1034. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Williams, R.R.; Horm, J.W. Association of cancer sites with tobacco and alcohol consumption and socioeconomic status of patients: Interview study from the Third National Cancer Survey. J. Natl. Cancer. Inst. 1977, 58, 525–547. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; Bain, C.; McLennan, R.; Siskind, V. Risk factors for cutaneous melanoma in Queensland. Recent Results Cancer Res. 1986, 102, 76–97. [Google Scholar] [CrossRef] [PubMed]
- Holman, C.D.; Armstrong, B.K.; Heenan, P.J.; Blackwell, J.B.; Cumming, F.J.; English, D.R.; Holland, S.; Kelsall, G.R.; Matz, L.R.; Rouse, I.L.; et al. The causes of malignant melanoma: Results from the West Australian Lions Melanoma Research Project. Recent Results Cancer Res. 1986, 102, 18–37. [Google Scholar] [CrossRef]
- Osterlind, A.; Tucker, M.A.; Stone, B.J.; Jensen, O.M. The Danish case-control study of cutaneous malignant melanoma. IV. No association with nutritional factors, alcohol, smoking or hair dyes. Int. J. Cancer 1988, 42, 825–828. [Google Scholar] [CrossRef] [PubMed]
- Stryker, W.S.; Stampfer, M.J.; Stein, E.A.; Kaplan, L.; Louis, T.A.; Sober, A.; Willett, W.C. Diet, plasma levels of beta-carotene and alpha-tocopherol, and risk of malignant melanoma. Am. J. Epidemiol. 1990, 131, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Adami, H.O.; McLaughlin, J.K.; Hsing, A.W.; Wolk, A.; Ekbom, A.; Holmberg, L.; Persson, I. Alcoholism and cancer risk: A population-based cohort study. Cancer Causes Control. 1992, 3, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.; Green, A.; Siskind, V.; Alexander, J.; Harvey, P. Diet and melanoma. An exploratory case-control study. Ann. Epidemiol. 1993, 3, 235–238. [Google Scholar] [CrossRef]
- Kirkpatrick, C.S.; White, E.; Lee, J.A. Case-control study of malignant melanoma in Washington State. II. Diet, alcohol, and obesity. Am. J. Epidemiol. 1994, 139, 869–880. [Google Scholar] [CrossRef]
- Bataille, V.; Bishop, J.A.; Sasieni, P.; Swerdlow, A.J.; Pinney, E.; Griffiths, K.; Cuzick, J. Risk of cutaneous melanoma in relation to the numbers, types and sites of naevi: A case-control study. Br. J. Cancer 1996, 73, 1605–1611. [Google Scholar] [CrossRef]
- Sigvardsson, S.; Hardell, L.; Przybeck, T.R.; Cloninger, R. Increased cancer risk among Swedish female alcoholics. Epidemiology 1996, 7, 140–143. [Google Scholar] [CrossRef]
- Westerdahl, J.; Olsson, H.; Masback, A.; Ingvar, C.; Jonsson, N. Risk of malignant melanoma in relation to drug intake, alcohol, smoking and hormonal factors. Br. J. Cancer 1996, 73, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Rolon, P.A.; Kramarova, E.; Rolon, H.I.; Khlat, M.; Parkin, D.M. Plantar melanoma: A case-control study in Paraguay. Cancer Causes Control 1997, 8, 850–856. [Google Scholar] [CrossRef]
- Veierod, M.B.; Thelle, D.S.; Laake, P. Diet and risk of cutaneous malignant melanoma: A prospective study of 50,757 Norwegian men and women. Int. J. Cancer 1997, 71, 600–604. [Google Scholar] [CrossRef]
- Freedman, D.M.; Sigurdson, A.; Doody, M.M.; Rao, R.S.; Linet, M.S. Risk of melanoma in relation to smoking, alcohol intake, and other factors in a large occupational cohort. Cancer Causes Control 2003, 14, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Stang, A.; Ahrens, W.; Anastassiou, G.; Jockel, K.H. Phenotypical characteristics, lifestyle, social class and uveal melanoma. Ophthalmic. Epidemiol. 2003, 10, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Millen, A.E.; Tucker, M.A.; Hartge, P.; Halpern, A.; Elder, D.E.; Guerry, D.t.; Holly, E.A.; Sagebiel, R.W.; Potischman, N. Diet and melanoma in a case-control study. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1042–1051. [Google Scholar] [CrossRef]
- Naldi, L.; Gallus, S.; Tavani, A.; Imberti, G.L.; La Vecchia, C.; on behalf of the Oncology Study Group of the Italian Group for Epidemiologic Research in Dermatology (GISED). Risk of melanoma and vitamin A, coffee and alcohol: A case-control study from Italy. Eur. J. Cancer Prev. 2004, 13, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Pellacani, G.; Malagoli, C.; Bassissi, S.; Sieri, S.; Bonvicini, F.; Krogh, V.; Seidenari, S. A population-based case-control study of diet and melanoma risk in northern Italy. Public Health Nutr. 2005, 8, 1307–1314. [Google Scholar] [CrossRef]
- Le Marchand, L.; Saltzman, B.S.; Hankin, J.H.; Wilkens, L.R.; Franke, A.A.; Morris, S.J.; Kolonel, L.N. Sun exposure, diet, and melanoma in Hawaii Caucasians. Am. J. Epidemiol. 2006, 164, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Fortes, C.; Mastroeni, S.; Melchi, F.; Pilla, M.A.; Antonelli, G.; Camaioni, D.; Alotto, M.; Pasquini, P. A protective effect of the Mediterranean diet for cutaneous melanoma. Int. J. Epidemiol. 2008, 37, 1018–1029. [Google Scholar] [CrossRef]
- Gogas, H.; Trakatelli, M.; Dessypris, N.; Terzidis, A.; Katsambas, A.; Chrousos, G.P.; Petridou, E.T. Melanoma risk in association with serum leptin levels and lifestyle parameters: A case-control study. Ann. Oncol. 2008, 19, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.E.; Beral, V.; Casabonne, D.; Kan, S.W.; Reeves, G.K.; Brown, A.; Green, J.; Million Women Study, C. Moderate alcohol intake and cancer incidence in women. J. Natl. Cancer Inst. 2009, 101, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, A.; Parent, M.E.; Siemiatycki, J. Lifetime consumption of alcoholic beverages and risk of 13 types of cancer in men: Results from a case-control study in Montreal. Cancer Detect. Prev. 2009, 32, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Asgari, M.M.; Brasky, T.M.; White, E. Association of vitamin A and carotenoid intake with melanoma risk in a large prospective cohort. J. Investig. Dermatol. 2012, 132, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- de Vries, E.; Trakatelli, M.; Kalabalikis, D.; Ferrandiz, L.; Ruiz-de-Casas, A.; Moreno-Ramirez, D.; Sotiriadis, D.; Ioannides, D.; Aquilina, S.; Apap, C.; et al. Known and potential new risk factors for skin cancer in European populations: A multicentre case-control study. Br. J. Dermatol. 2012, 167 (Suppl. S2), 1–13. [Google Scholar] [CrossRef]
- Kubo, J.T.; Henderson, M.T.; Desai, M.; Wactawski-Wende, J.; Stefanick, M.L.; Tang, J.Y. Alcohol consumption and risk of melanoma and non-melanoma skin cancer in the Women’s Health Initiative. Cancer Causes Control 2014, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Klatsky, A.L.; Li, Y.; Nicole Tran, H.; Baer, D.; Udaltsova, N.; Armstrong, M.A.; Friedman, G.D. Alcohol intake, beverage choice, and cancer: A cohort study in a large kaiser permanente population. Perm. J. 2015, 19, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Mahamat-Saleh, Y.; Cervenka, I.; Al Rahmoun, M.; Savoye, I.; Mancini, F.R.; Trichopoulou, A.; Boutron-Ruault, M.C.; Kvaskoff, M. Mediterranean dietary pattern and skin cancer risk: A prospective cohort study in French women. Am. J. Clin. Nutr. 2019, 110, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Malagoli, C.; Malavolti, M.; Farnetani, F.; Longo, C.; Filippini, T.; Pellacani, G.; Vinceti, M. Food and beverage consumption and Melanoma risk: A population-based case-control study in Northern Italy. Nutrients 2019, 11, 2206. [Google Scholar] [CrossRef]
- Sanford, N.N.; Sher, D.J.; Xu, X.; Ahn, C.; D’Amico, A.V.; Aizer, A.A.; Mahal, B.A. Alcohol use among patients with cancer and survivors in the United States, 2000–2017. J. Natl. Compr. Cancer Netw. 2020, 18, 69–79. [Google Scholar] [CrossRef]
- Rota, M.; Pasquali, E.; Bellocco, R.; Bagnardi, V.; Scotti, L.; Islami, F.; Negri, E.; Boffetta, P.; Pelucchi, C.; Corrao, G.; et al. Alcohol drinking and cutaneous melanoma risk: A systematic review and dose-risk meta-analysis. Br. J. Dermatol. 2014, 170, 1021–1028. [Google Scholar] [CrossRef]
- Miura, K.; Zens, M.S.; Peart, T.; Holly, E.A.; Berwick, M.; Gallagher, R.P.; Mack, T.M.; Elwood, J.M.; Karagas, M.R.; Green, A.C. Alcohol consumption and risk of melanoma among women: Pooled analysis of eight case-control studies. Arch. Dermatol. Res. 2015, 307, 819–828. [Google Scholar] [CrossRef]
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacere, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E.; et al. Alcohol consumption and site-specific cancer risk: A comprehensive dose-response meta-analysis. Br. J. Cancer 2015, 112, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.; Nan, H.; Li, T.; Qureshi, A.; Cho, E. Alcohol Intake and Risk of Incident Melanoma: A Pooled Analysis of Three Prospective Studies in the United States. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1550–1558. [Google Scholar] [CrossRef]
- Mehta, A.; Nayak, R.; Hasija, Y. Melanoma risk prediction with respect to modifiable lifestyle factors by meta-analysis aided machine learning technique. In Proceedings of the 2020 IEEE International Conference on Computing, Power and Communication Technologies (GUCON), Greater Noida, India, 2–4 October 2020; pp. 223–226. [Google Scholar]
- Gandini, S.; Masala, G.; Palli, D.; Cavicchi, B.; Saieva, C.; Ermini, I.; Baldini, F.; Gnagnarella, P.; Caini, S. Alcohol, alcoholic beverages, and melanoma risk: A systematic literature review and dose-response meta-analysis. Eur. J. Nutr. 2018, 57, 2323–2332. [Google Scholar] [CrossRef] [PubMed]
- Le Dare, B.; Lagente, V.; Gicquel, T. Ethanol and its metabolites: Update on toxicity, benefits, and focus on immunomodulatory effects. Drug Metab. Rev. 2019, 51, 545–561. [Google Scholar] [CrossRef]
- Batta, N.; Shangraw, S.; Nicklawsky, A.; Yamauchi, T.; Zhai, Z.; Ravindran Menon, D.; Gao, D.; Dellavalle, R.P.; Fujita, M. Global Melanoma correlations with obesity, smoking, and alcohol consumption. JMIR Dermatol. 2021, 4, e31275. [Google Scholar] [CrossRef]
- Warthan, M.M.; Sewell, D.S.; Marlow, R.A.; Warthan, M.L.; Wagner, R.F., Jr. The economic impact of acute sunburn. Arch. Dermatol. 2003, 139, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Mukamal, K.J. Alcohol consumption and self-reported sunburn: A cross-sectional, population-based survey. J. Am. Acad. Dermatol. 2006, 55, 584–589. [Google Scholar] [CrossRef]
- Cederbaum, A.I. Alcohol metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Xu, F.; Meng, L.; Wei, S.; Wang, J.; Hao, P.; Bian, Y.; Zhang, Y.; Chen, Y. Acetylation-dependent regulation of mitochondrial ALDH2 activation by SIRT3 mediates acute ethanol-induced eNOS activation. FEBS Lett. 2012, 586, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.D.; Hanna-Khalil, B.; Carson, R. A Review of the potential health benefits of low alcohol and alcohol-free beer: Effects of ingredients and craft brewing processes on potentially bioactive metabolites. Beverages 2020, 6, 25. [Google Scholar] [CrossRef]
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martinez, P.; Medina-Remon, A.; Lamuela-Raventos, R.M.; Estruch, R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients 2012, 4, 759–781. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Ande, A.; Kumar, A.; Kumar, S. Regulation of cytochrome P450 2e1 expression by ethanol: Role of oxidative stress-mediated pkc/jnk/sp1 pathway. Cell Death Dis. 2013, 4, e554. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Alcohol consumption and ethyl carbamate. IARC Monogr. Eval. Carcinog. Risks Hum. 2010, 96, 3–1383. [Google Scholar]
- Cogliano, V.J.; Baan, R.; Straif, K.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; et al. Preventable exposures associated with human cancers. J. Natl. Cancer Inst. 2011, 103, 1827–1839. [Google Scholar] [CrossRef]
- Koivisto, T.; Salaspuro, M. Acetaldehyde alters proliferation, differentiation and adhesion properties of human colon adenocarcinoma cell line Caco-2. Carcinogenesis 1998, 19, 2031–2036. [Google Scholar] [CrossRef][Green Version]
- Homann, N.; Karkkainen, P.; Koivisto, T.; Nosova, T.; Jokelainen, K.; Salaspuro, M. Effects of acetaldehyde on cell regeneration and differentiation of the upper gastrointestinal tract mucosa. J. Natl. Cancer Inst. 1997, 89, 1692–1697. [Google Scholar] [CrossRef]
- Li, K.; Guo, W.; Li, Z.; Wang, Y.; Sun, B.; Xu, D.; Ling, J.; Song, H.; Liao, Y.; Wang, T.; et al. ALDH2 repression promotes lung tumor progression via accumulated acetaldehyde and DNA damage. Neoplasia 2019, 21, 602–614. [Google Scholar] [CrossRef]
- Na, H.K.; Lee, J.Y. Molecular basis of alcohol-related gastric and colon cancer. Int. J. Mol. Sci. 2017, 18, 1116. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, A.; Ohashi, S.; Hirohashi, K.; Amanuma, Y.; Matsuda, T.; Muto, M. Molecular mechanisms of acetaldehyde-mediated carcinogenesis in squamous epithelium. Int. J. Mol. Sci. 2017, 18, 1943. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Millonig, G.; Nair, J.; Patsenker, E.; Stickel, F.; Mueller, S.; Bartsch, H.; Seitz, H.K. Ethanol-induced cytochrome P4502E1 causes carcinogenic etheno-DNA lesions in alcoholic liver disease. Hepatology 2009, 50, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Brooks, P.J.; Theruvathu, J.A. DNA adducts from acetaldehyde: Implications for alcohol-related carcinogenesis. Alcohol 2005, 35, 187–193. [Google Scholar] [CrossRef]
- Linhart, K.; Bartsch, H.; Seitz, H.K. The role of reactive oxygen species (ROS) and cytochrome P-450 2E1 in the generation of carcinogenic etheno-DNA adducts. Redox. Biol. 2014, 3, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, H.; Sonohara, Y.; Tohashi, K.; Aoki Shioi, N.; Iwai, S.; Kuraoka, I. Effects of acetaldehyde-induced DNA lesions on DNA metabolism. Genes Environ. 2020, 42, 2. [Google Scholar] [CrossRef]
- Setshedi, M.; Wands, J.R.; Monte, S.M. Acetaldehyde adducts in alcoholic liver disease. Oxid. Med. Cell Longev. 2010, 3, 178–185. [Google Scholar] [CrossRef]
- Abbas, O.; Mahalingam, M. Epidermal stem cells: Practical perspectives and potential uses. Br. J. Dermatol. 2009, 161, 228–236. [Google Scholar] [CrossRef]
- Dohrman, D.P.; Diamond, I.; Gordon, A.S. Ethanol causes translocation of cAMP-dependent protein kinase catalytic subunit to the nucleus. Proc. Natl. Acad. Sci. USA 1996, 93, 10217–10221. [Google Scholar] [CrossRef]
- Carlson, S.L.; Kumar, S.; Werner, D.F.; Comerford, C.E.; Morrow, A.L. Ethanol activation of protein kinase A regulates GABAA alpha1 receptor function and trafficking in cultured cerebral cortical neurons. J. Pharmacol. Exp. Ther. 2013, 345, 317–325. [Google Scholar] [CrossRef]
- Kumar, S.; Ren, Q.; Beckley, J.H.; O’Buckley, T.K.; Gigante, E.D.; Santerre, J.L.; Werner, D.F.; Morrow, A.L. Ethanol activation of protein kinase A regulates GABA(A) receptor subunit expression in the cerebral cortex and contributes to ethanol-induced hypnosis. Front. Neurosci. 2012, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Aroor, A.R.; Shukla, S.D. MAP kinase signaling in diverse effects of ethanol. Life Sci. 2004, 74, 2339–2364. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Zhang, C.L.; Song, F.Y.; Zhao, X.L.; Yu, L.H.; Zhu, Z.P.; Xie, K.Q. PI3K/Akt pathway activation was involved in acute ethanol-induced fatty liver in mice. Toxicology 2012, 296, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Neasta, J.; Ben Hamida, S.; Yowell, Q.V.; Carnicella, S.; Ron, D. AKT signaling pathway in the nucleus accumbens mediates excessive alcohol drinking behaviors. Biol. Psychiatry 2011, 70, 575–582. [Google Scholar] [CrossRef]
- Mercer, K.E.; Hennings, L.; Ronis, M.J. Alcohol consumption, Wnt/beta-catenin signaling, and hepatocarcinogenesis. Adv. Exp. Med. Biol. 2015, 815, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kong, Q.; Wang, J.; Jiang, Y.; Hua, H. Complex roles of cAMP-PKA-CREB signaling in cancer. Exp. Hematol. Oncol. 2020, 9, 32. [Google Scholar] [CrossRef]
- Wand, G.; Levine, M.; Zweifel, L.; Schwindinger, W.; Abel, T. The cAMP-protein kinase A signal transduction pathway modulates ethanol consumption and sedative effects of ethanol. J. Neurosci. 2001, 21, 5297–5303. [Google Scholar] [CrossRef]
- Mantovani, G.; Bondioni, S.; Lania, A.G.; Rodolfo, M.; Peverelli, E.; Polentarutti, N.; Veliz Rodriguez, T.; Ferrero, S.; Bosari, S.; Beck-Peccoz, P.; et al. High expression of PKA regulatory subunit 1A protein is related to proliferation of human melanoma cells. Oncogene 2008, 27, 1834–1843. [Google Scholar] [CrossRef]
- Finger, E.C.; Castellini, L.; Rankin, E.B.; Vilalta, M.; Krieg, A.J.; Jiang, D.; Banh, A.; Zundel, W.; Powell, M.B.; Giaccia, A.J. Hypoxic induction of AKAP12 variant 2 shifts PKA-mediated protein phosphorylation to enhance migration and metastasis of melanoma cells. Proc. Natl. Acad. Sci. USA 2015, 112, 4441–4446. [Google Scholar] [CrossRef]
- Messing, R.O.; Petersen, P.J.; Henrich, C.J. Chronic ethanol exposure increases levels of protein kinase C delta and epsilon and protein kinase C-mediated phosphorylation in cultured neural cells. J. Biol. Chem. 1991, 266, 23428–23432. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.H.; Yap, T.A.; Yan, L.; Cunningham, D. Targeting the PI3K-AKT-mTOR signaling network in cancer. Chin. J. Cancer 2013, 32, 253–265. [Google Scholar] [CrossRef]
- Xu, M.; Wang, S.; Qi, Y.; Chen, L.; Frank, J.A.; Yang, X.H.; Zhang, Z.; Shi, X.; Luo, J. Role of MCP-1 in alcohol-induced aggressiveness of colorectal cancer cells. Mol. Carcinog. 2016, 55, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Fang, Z.; Lei, J.; Shi, G.; Zhang, Y.; He, Z.; Li, B.W.; Zhong, S. The significance of Runx2 mediating alcohol-induced Brf1 expression and RNA Pol III gene transcription. Chem. Biol. Interact. 2020, 323, 109057. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Huang, C.; Zhang, Y.; Tian, S.; Lei, J.; Chen, S.; Shi, G.; Wu, Z.; Xia, N.; Zhong, S. Exploring a common mechanism of alcohol-induced deregulation of RNA Pol III genes in liver and breast cells. Gene 2017, 626, 309–318. [Google Scholar] [CrossRef]
- Barron, K.A.; Jeffries, K.A.; Krupenko, N.I. Sphingolipids and the link between alcohol and cancer. Chem. Biol. Interact. 2020, 322, 109058. [Google Scholar] [CrossRef] [PubMed]
- Serio, R.N.; Gudas, L.J. Modification of stem cell states by alcohol and acetaldehyde. Chem. Biol. Interact. 2020, 316, 108919. [Google Scholar] [CrossRef]
- Di Rocco, G.; Baldari, S.; Pani, G.; Toietta, G. Stem cells under the influence of alcohol: Effects of ethanol consumption on stem/progenitor cells. Cell Mol. Life Sci. 2019, 76, 231–244. [Google Scholar] [CrossRef]
- Subramaniyan, V.; Chakravarthi, S.; Jegasothy, R.; Seng, W.Y.; Fuloria, N.K.; Fuloria, S.; Hazarika, I.; Das, A. Alcohol-associated liver disease: A review on its pathophysiology, diagnosis and drug therapy. Toxicol. Rep. 2021, 8, 376–385. [Google Scholar] [CrossRef]
- Bode, C.; Bode, J.C. Alcohol’s role in gastrointestinal tract disorders. Alcohol Health Res. World 1997, 21, 76–83. [Google Scholar]
- Brooks, P.J.; Enoch, M.A.; Goldman, D.; Li, T.K.; Yokoyama, A. The alcohol flushing response: An unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med. 2009, 6, e50. [Google Scholar] [CrossRef] [PubMed]
- Slutske, W.S.; Heath, A.C.; Madden, P.A.; Bucholz, K.K.; Dinwiddie, S.H.; Dunne, M.P.; Statham, D.S.; Whitfield, J.B.; Martin, N.G. Is alcohol-related flushing a protective factor for alcoholism in Caucasians? Alcohol Clin. Exp. Res. 1995, 19, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Gill, K.; Eagle Elk, M.; Liu, Y.; Deitrich, R.A. An examination of ALDH2 genotypes, alcohol metabolism and the flushing response in Native Americans. J. Stud. Alcohol 1999, 60, 149–158. [Google Scholar] [CrossRef]
- Higgins, E.; du Vivier, A. Alcohol intake and other skin disorders. Clin. Dermatol. 1999, 17, 437–441. [Google Scholar] [CrossRef]
- Liu, S.W.; Lien, M.H.; Fenske, N.A. The effects of alcohol and drug abuse on the skin. Clin. Dermatol. 2010, 28, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Nyman, E.; Palmlöv, A. The elimination of ethyl alcohol in sweat 1. Skand. Arch. Für Physiol. 1936, 74, 155–159. [Google Scholar] [CrossRef]
- Pawan, G.L.; Grice, K. Distribution of alcohol in urine and sweat after drinking. Lancet 1968, 2, 1016. [Google Scholar] [CrossRef]
- Baker, L.B.; Wolfe, A.S. Physiological mechanisms determining eccrine sweat composition. Eur. J. Appl. Physiol. 2020, 120, 719–752. [Google Scholar] [CrossRef]
- Kwak, S.; Brief, E.; Langlais, D.; Kitson, N.; Lafleur, M.; Thewalt, J. Ethanol perturbs lipid organization in models of stratum corneum membranes: An investigation combining differential scanning calorimetry, infrared and (2)H NMR spectroscopy. Biochim. Biophys. Acta 2012, 1818, 1410–1419. [Google Scholar] [CrossRef]
- Hook-Nikanne, J.; Kariniemi, A.L.; Renkonen, O.V.; Mustakallio, K.; Salaspuro, M. Could bacterial acetaldehyde production explain the deleterious effect of alcohol on skin diseases? Acta Derm. Venereol. 1995, 75, 330. [Google Scholar] [CrossRef]
- Destek, S.; Gul, V.O.; Ahioglu, S.; Erbil, Y. A rare disease of the digestive tract: Esophageal Melanosis. Gastroenterol. Res. 2016, 9, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, A.; Chaudhary, F.S.; Naqvi, H.A.; Saleh, N.; Farooqi, R.; Yousaf, M.N. Black esophagus: A syndrome of acute esophageal necrosis associated with active alcohol drinking. BMJ Open Gastroenterol. 2020, 7, e000466. [Google Scholar] [CrossRef] [PubMed]
- Nagra, N.; Tolentino, L.; Singhvi, G. Esophageal Melanosis: A rare condition of undetermined significance. Clin. Gastroenterol. Hepatol. 2020, 18, e59. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Chen, J.; Chen, L.; Histen, G.; Lin, Z.; Gross, S.; Hixon, J.; Chen, Y.; Kung, C.; Chen, Y.; et al. ALDH2(E487K) mutation increases protein turnover and promotes murine hepatocarcinogenesis. Proc. Natl. Acad. Sci. USA 2015, 112, 9088–9093. [Google Scholar] [CrossRef]
- Matsumoto, A.; Ito, S.; Wakamatsu, K.; Ichiba, M.; Vasiliou, V.; Akao, C.; Song, B.J.; Fujita, M. Ethanol induces skin hyperpigmentation in mice with aldehyde dehydrogenase 2 deficiency. Chem. Biol. Interact. 2019, 302, 61–66. [Google Scholar] [CrossRef]
- Matsumura, Y.; Li, N.; Alwaseem, H.; Pagovich, O.E.; Crystal, R.G.; Greenblatt, M.B.; Stiles, K.M. Systemic adeno-associated virus-mediated gene therapy prevents the multiorgan disorders associated with Aldehyde Dehydrogenase 2 deficiency and chronic ethanol ingestion. Hum. Gene Ther. 2020, 31, 163–182. [Google Scholar] [CrossRef]
- Heier, C.; Xie, H.; Zimmermann, R. Nonoxidative ethanol metabolism in humans-from biomarkers to bioactive lipids. IUBMB Life 2016, 68, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Appenzeller, B.M.; Agirman, R.; Neuberg, P.; Yegles, M.; Wennig, R. Segmental determination of ethyl glucuronide in hair: A pilot study. Forensic Sci. Int. 2007, 173, 87–92. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Fu, X.; Reisz, J.A.; Stone, M.; Kleinman, S.; Zimring, J.C.; Busch, M.; for the Recipient Epidemiology and Donor Evaluation Study-III (REDS III). Ethyl glucuronide, a marker of alcohol consumption, correlates with metabolic markers of oxidant stress but not with hemolysis in stored red blood cells from healthy blood donors. Transfusion 2020, 60, 1183–1196. [Google Scholar] [CrossRef]
- Lewis, S.S.; Hutchinson, M.R.; Zhang, Y.; Hund, D.K.; Maier, S.F.; Rice, K.C.; Watkins, L.R. Glucuronic acid and the ethanol metabolite ethyl-glucuronide cause toll-like receptor 4 activation and enhanced pain. Brain Behav. Immun. 2013, 30, 24–32. [Google Scholar] [CrossRef]
- Mandrekar, P.; Catalano, D.; Dolganiuc, A.; Kodys, K.; Szabo, G. Inhibition of myeloid dendritic cell accessory cell function and induction of T cell anergy by alcohol correlates with decreased IL-12 production. J. Immunol. 2004, 173, 3398–3407. [Google Scholar] [CrossRef] [PubMed]
- Ness, K.J.; Fan, J.; Wilke, W.W.; Coleman, R.A.; Cook, R.T.; Schlueter, A.J. Chronic ethanol consumption decreases murine Langerhans cell numbers and delays migration of Langerhans cells as well as dermal dendritic cells. Alcohol Clin. Exp. Res. 2008, 32, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Parlet, C.P.; Schlueter, A.J. Mechanisms by which chronic ethanol feeding impairs the migratory capacity of cutaneous dendritic cells. Alcohol Clin. Exp. Res. 2013, 37, 2098–2107. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, E.; Grolier, P.; Herbeth, B.; Pirollet, P.; Musse, N.; Paille, F.; Braesco, V.; Siest, G.; Artur, Y. The relation of alcohol consumption to serum carotenoid and retinol levels. Effects of withdrawal. Int. J. Vitam. Nutr. Res. 1994, 64, 170–175. [Google Scholar]
- Stahl, W.; Sies, H. Carotenoids and protection against solar UV radiation. Skin Pharmacol. Appl. Skin Physiol. 2002, 15, 291–296. [Google Scholar] [CrossRef]
- Saladi, R.N.; Nektalova, T.; Fox, J.L. Induction of skin carcinogenicity by alcohol and ultraviolet light. Clin. Exp. Dermatol. 2010, 35, 7–11. [Google Scholar] [CrossRef]
- Darvin, M.E.; Sterry, W.; Lademann, J.; Patzelt, A. Alcohol consumption decreases the protection efficiency of the antioxidant network and increases the risk of sunburn in human skin. Skin Pharmacol. Physiol. 2013, 26, 45–51. [Google Scholar] [CrossRef]
- Darvin, M.E.; Patzelt, A.; Knorr, F.; Blume-Peytavi, U.; Sterry, W.; Lademann, J. One-year study on the variation of carotenoid antioxidant substances in living human skin: Influence of dietary supplementation and stress factors. J. Biomed. Opt. 2008, 13, 044028. [Google Scholar] [CrossRef] [PubMed]
- Leo, M.A.; Lieber, C.S. Alcohol, vitamin A, and beta-carotene: Adverse interactions, including hepatotoxicity and carcinogenicity. Am. J. Clin. Nutr. 1999, 69, 1071–1085. [Google Scholar] [CrossRef]
- Brand, R.M.; Stottlemyer, J.M.; Paglia, M.C.; Carey, C.D.; Falo, L.D., Jr. Ethanol consumption synergistically increases ultraviolet radiation induced skin damage and immune dysfunction. J. Dermatol. Sci. 2021, 101, 40–48. [Google Scholar] [CrossRef]
- Manzo-Avalos, S.; Saavedra-Molina, A. Cellular and mitochondrial effects of alcohol consumption. Int. J. Environ. Res. Public Health 2010, 7, 4281–4304. [Google Scholar] [CrossRef] [PubMed]
- Ji, C. Mechanisms of alcohol-induced endoplasmic reticulum stress and organ injuries. Biochem. Res. Int. 2012, 2012, 216450. [Google Scholar] [CrossRef] [PubMed]
- Paluncic, J.; Kovacevic, Z.; Jansson, P.J.; Kalinowski, D.; Merlot, A.M.; Huang, M.L.; Lok, H.C.; Sahni, S.; Lane, D.J.; Richardson, D.R. Roads to melanoma: Key pathways and emerging players in melanoma progression and oncogenic signaling. Biochim. Biophys. Acta 2016, 1863, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Strickland, F.M.; Muller, H.K.; Stephens, L.C.; Bucana, C.D.; Donawho, C.K.; Sun, Y.; Pelley, R.P. Induction of primary cutaneous melanomas in C3H mice by combined treatment with ultraviolet radiation, ethanol and aloe emodin. Photochem. Photobiol. 2000, 72, 407–414. [Google Scholar] [CrossRef]
- Strickland, F.M.; Pathak, S.; Multani, A.S.; Pelley, R.P.; Donawho, C.K. Molecular characterization of new melanoma cell lines from C3H mice induced by ethanol plus ultraviolet radiation. Cancer Res. 2003, 63, 3503–3510. [Google Scholar] [PubMed]
- Wikonkal, N.M.; Brash, D.E. Ultraviolet radiation induced signature mutations in photocarcinogenesis. J. Investig. Dermatol. Symp. Proc. 1999, 4, 6–10. [Google Scholar] [CrossRef]
- Davis, E.J.; Johnson, D.B.; Sosman, J.A.; Chandra, S. Melanoma: What do all the mutations mean? Cancer 2018, 124, 3490–3499. [Google Scholar] [CrossRef]
- Vu, H.L.; Aplin, A.E. Targeting mutant NRAS signaling pathways in melanoma. Pharmacol. Res. 2016, 107, 111–116. [Google Scholar] [CrossRef]
- Fargnoli, M.C.; Chimenti, S.; Keller, G.; Soyer, H.P.; Dal Pozzo, V.; Hofler, H.; Peris, K. CDKN2a/p16INK4a mutations and lack of p19ARF involvement in familial melanoma kindreds. J. Investig. Dermatol. 1998, 111, 1202–1206. [Google Scholar] [CrossRef]
- Meadows, G.G.; Zhang, H. Effects of alcohol on tumor growth, metastasis, immune response, and host survival. Alcohol Res. 2015, 37, 311–322. [Google Scholar]
- Hartman, M.L.; Czyz, M. MITF in melanoma: Mechanisms behind its expression and activity. Cell Mol. Life Sci. 2015, 72, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Rambow, F.; Marine, J.C.; Goding, C.R. Melanoma plasticity and phenotypic diversity: Therapeutic barriers and opportunities. Genes Dev. 2019, 33, 1295–1318. [Google Scholar] [CrossRef] [PubMed]
- Garraway, L.A.; Widlund, H.R.; Rubin, M.A.; Getz, G.; Berger, A.J.; Ramaswamy, S.; Beroukhim, R.; Milner, D.A.; Granter, S.R.; Du, J.; et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature 2005, 436, 117–122. [Google Scholar] [CrossRef]
- Bertolotto, C.; Lesueur, F.; Giuliano, S.; Strub, T.; de Lichy, M.; Bille, K.; Dessen, P.; d’Hayer, B.; Mohamdi, H.; Remenieras, A.; et al. A SUMOylation-defective MITF germline mutation predisposes to melanoma and renal carcinoma. Nature 2011, 480, 94–98. [Google Scholar] [CrossRef]
- Hemesath, T.J.; Price, E.R.; Takemoto, C.; Badalian, T.; Fisher, D.E. MAP kinase links the transcription factor Microphthalmia to c-Kit signalling in melanocytes. Nature 1998, 391, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Hauge, C.; Frodin, M. RSK and MSK in MAP kinase signalling. J. Cell Sci. 2006, 119, 3021–3023. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, G.S.; Madhunapantula, S.V.; Robertson, G.P. Targeting the MAPK pathway in melanoma: Why some approaches succeed and other fail. Biochem. Pharmacol. 2010, 80, 624–637. [Google Scholar] [CrossRef]
- Kashyap, T.; Pramanik, K.K.; Nath, N.; Mishra, P.; Singh, A.K.; Nagini, S.; Rana, A.; Mishra, R. Crosstalk between Raf-MEK-ERK and PI3K-Akt-GSK3beta signaling networks promotes chemoresistance, invasion/migration and stemness via expression of CD44 variants (v4 and v6) in oral cancer. Oral Oncol. 2018, 86, 234–243. [Google Scholar] [CrossRef]
- Kim, J.H.; Hong, A.R.; Kim, Y.H.; Yoo, H.; Kang, S.W.; Chang, S.E.; Song, Y. JNK suppresses melanogenesis by interfering with CREB-regulated transcription coactivator 3-dependent MITF expression. Theranostics 2020, 10, 4017–4029. [Google Scholar] [CrossRef]
- Tan, W.; Bailey, A.P.; Shparago, M.; Busby, B.; Covington, J.; Johnson, J.W.; Young, E.; Gu, J.W. Chronic alcohol consumption stimulates VEGF expression, tumor angiogenesis and progression of melanoma in mice. Cancer Biol. Ther. 2007, 6, 1211–1217. [Google Scholar] [CrossRef]
- Blank, S.E.; Meadows, G.G. Ethanol modulates metastatic potential of B16BL6 melanoma and host responses. Alcohol Clin. Exp. Res. 1996, 20, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Kushiro, K.; Nunez, N.P. Ethanol inhibits B16-BL6 melanoma metastasis and cell phenotypes associated with metastasis. Vivo 2012, 26, 47–58. [Google Scholar]
- Zhang, H.; Zhu, Z.; McKinley, J.M.; Meadows, G.G. IFN-gamma is essential for the inhibition of B16BL6 melanoma lung metastasis in chronic alcohol drinking mice. Clin. Exp. Metastasis 2011, 28, 301–307. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meadows, G.G.; Elstad, C.A.; Blank, S.E.; Gallucci, R.M.; Pfister, L.J. Alcohol consumption suppresses metastasis of B16-BL6 melanoma in mice. Clin. Exp. Metastasis 1993, 11, 191–199. [Google Scholar] [CrossRef]
- Abdallah, M.A.; Singal, A.K. Mitochondrial dysfunction and alcohol-associated liver disease: A novel pathway and therapeutic target. Signal Transduct. Target Ther. 2020, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Bonet-Ponce, L.; Saez-Atienzar, S.; da Casa, C.; Flores-Bellver, M.; Barcia, J.M.; Sancho-Pelluz, J.; Romero, F.J.; Jordan, J.; Galindo, M.F. On the mechanism underlying ethanol-induced mitochondrial dynamic disruption and autophagy response. Biochim. Biophys. Acta 2015, 1852, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Dal Yontem, F.; Kim, S.H.; Ding, Z.; Grimm, E.; Ekmekcioglu, S.; Akcakaya, H. Mitochondrial dynamic alterations regulate melanoma cell progression. J. Cell Biochem. 2018, 120, 2098–2108. [Google Scholar] [CrossRef] [PubMed]
- From the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO); Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef] [PubMed]
- Sadikot, R.T.; Bedi, B.; Li, J.; Yeligar, S.M. Alcohol-induced mitochondrial DNA damage promotes injurious crosstalk between alveolar epithelial cells and alveolar macrophages. Alcohol 2019, 80, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Liu, W.; Luo, Y.; Tanaka, A.; Cai, X.; Norris, D.A.; Dinarello, C.A.; Fujita, M. Constitutively active inflammasome in human melanoma cells mediating autoinflammation via caspase-1 processing and secretion of interleukin-1beta. J. Biol. Chem. 2010, 285, 6477–6488. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Liu, W.; Kaur, M.; Luo, Y.; Domenico, J.; Samson, J.M.; Shellman, Y.G.; Norris, D.A.; Dinarello, C.A.; Spritz, R.A.; et al. NLRP1 promotes tumor growth by enhancing inflammasome activation and suppressing apoptosis in metastatic melanoma. Oncogene 2017, 36, 3820–3830. [Google Scholar] [CrossRef]
- Tengesdal, I.W.; Menon, D.R.; Osborne, D.G.; Neff, C.P.; Powers, N.E.; Gamboni, F.; Mauro, A.G.; D’Alessandro, A.; Stefanoni, D.; Henen, M.A.; et al. Targeting tumor-derived NLRP3 reduces melanoma progression by limiting MDSCs expansion. Proc. Natl. Acad. Sci. USA 2021, 118, e2000915118. [Google Scholar] [CrossRef] [PubMed]
- Molina, P.E.; Happel, K.I.; Zhang, P.; Kolls, J.K.; Nelson, S. Focus on: Alcohol and the immune system. Alcohol Res. Health 2010, 33, 97–108. [Google Scholar] [PubMed]
- Zhang, H.; Zhu, Z.; Meadows, G.G. Chronic alcohol consumption impairs distribution and compromises circulation of B cells in B16BL6 melanoma-bearing mice. J. Immunol. 2012, 189, 1340–1348. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, Z.; Meadows, G.G. Chronic alcohol consumption decreases the percentage and number of NK cells in the peripheral lymph nodes and exacerbates B16BL6 melanoma metastasis into the draining lymph nodes. Cell Immunol. 2011, 266, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Meadows, G.G. Chronic alcohol consumption enhances myeloid-derived suppressor cells in B16BL6 melanoma-bearing mice. Cancer Immunol. Immunother. 2010, 59, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Rappa, G.; Anzanello, F.; Lorico, A. Ethanol induces upregulation of the nerve growth factor receptor CD271 in human melanoma cells via nuclear factor-kappaB activation. Oncol. Lett. 2015, 10, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Civenni, G.; Walter, A.; Kobert, N.; Mihic-Probst, D.; Zipser, M.; Belloni, B.; Seifert, B.; Moch, H.; Dummer, R.; van den Broek, M.; et al. Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res. 2011, 71, 3098–3109. [Google Scholar] [CrossRef]
- Restivo, G.; Diener, J.; Cheng, P.F.; Kiowski, G.; Bonalli, M.; Biedermann, T.; Reichmann, E.; Levesque, M.P.; Dummer, R.; Sommer, L. Publisher Correction: The low affinity neurotrophin receptor CD271 regulates phenotype switching in melanoma. Nat. Commun. 2018, 9, 314. [Google Scholar] [CrossRef]
- Ravindran Menon, D.; Das, S.; Krepler, C.; Vultur, A.; Rinner, B.; Schauer, S.; Kashofer, K.; Wagner, K.; Zhang, G.; Bonyadi Rad, E.; et al. A stress-induced early innate response causes multidrug tolerance in melanoma. Oncogene 2015, 34, 4448–4459. [Google Scholar] [CrossRef]
- Lehraiki, A.; Cerezo, M.; Rouaud, F.; Abbe, P.; Allegra, M.; Kluza, J.; Marchetti, P.; Imbert, V.; Cheli, Y.; Bertolotto, C.; et al. Increased CD271 expression by the NF-kB pathway promotes melanoma cell survival and drives acquired resistance to BRAF inhibitor vemurafenib. Cell Discov. 2015, 1, 15030. [Google Scholar] [CrossRef] [PubMed]
- Rambow, F.; Rogiers, A.; Marin-Bejar, O.; Aibar, S.; Femel, J.; Dewaele, M.; Karras, P.; Brown, D.; Chang, Y.H.; Debiec-Rychter, M.; et al. Toward minimal residual disease-directed therapy in Melanoma. Cell 2018, 174, 843–855.e19. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, S.M.; Dunagin, M.C.; Torborg, S.R.; Torre, E.A.; Emert, B.; Krepler, C.; Beqiri, M.; Sproesser, K.; Brafford, P.A.; Xiao, M.; et al. Rare cell variability and drug-induced reprogramming as a mode of cancer drug resistance. Nature 2017, 546, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, J.; Liu, B. Targeting VEGF/VEGFR to modulate antitumor immunity. Front. Immunol. 2018, 9, 978. [Google Scholar] [CrossRef] [PubMed]
- Boshuizen, J.; Vredevoogd, D.W.; Krijgsman, O.; Ligtenberg, M.A.; Blankenstein, S.; de Bruijn, B.; Frederick, D.T.; Kenski, J.C.N.; Parren, M.; Bruggemann, M.; et al. Reversal of pre-existing NGFR-driven tumor and immune therapy resistance. Nat. Commun. 2020, 11, 3946. [Google Scholar] [CrossRef]
- Furuta, J.; Inozume, T.; Harada, K.; Shimada, S. CD271 on melanoma cell is an IFN-gamma-inducible immunosuppressive factor that mediates downregulation of melanoma antigens. J. Investig. Dermatol. 2014, 134, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Kim, Y.J.; Robert, L.; Tsoi, J.; Comin-Anduix, B.; Berent-Maoz, B.; Cochran, A.J.; Economou, J.S.; Tumeh, P.C.; Puig-Saus, C.; et al. Immunotherapy resistance by inflammation-induced dedifferentiation. Cancer Discov. 2018, 8, 935–943. [Google Scholar] [CrossRef]
| Study # | Authors (Year) | Study Design | No. (MM) | No. (Control) | Alcohol Consumption | Risk Ratio | 95% CI | p (Single or Trend *) | Correlation to Alcohol | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| C1 | Green et al. (1986) | case-control | 91 | 89 | Any | RR = 0.74 | 0.39–1.40 | null | [45] | |
| ≤1 drink/day | RR = 0.70 | 0.24–2.04 | ||||||||
| >1 drink/day | RR = 0.76 | 0.34–1.70 | ||||||||
| C2 | Holman et al. (1986) | case-control | 511 | 511 | Any | RR = 1.37 | 1.09–1.71 | positive | [46] | |
| ≤1 drink/day | RR = 1.16 | 0.79–1.71 | ||||||||
| >1 drink/day | RR = 1.48 | 1.13–1.95 | ||||||||
| C3 | Osterlind et al. (1988) | case-control | 474 | 924 | Any | RR = 0.75 | 0.57–0.99 | null | [47] | |
| ≤1 drink/day | RR = 0.80 | 0.59–1.08 | ||||||||
| >1 drink/day | RR = 0.69 | 0.50–0.96 | ||||||||
| C4 | Stryker et al. (1990) | case-control | 204 | 248 | 10 g/day | OR = 1.8 | 1.0–3.3 | 0.03 | positive | [48] |
| C5 | Adami et al. (1992) | cohort | 10,350 (individuals) | Any | SIR = 0.9 | 0.3–1.9 | N/A | null | [49] | |
| C6 | Bain et al. (1993) | case-control | 41 (women) | 297 (women) | 0.1–9.9 g/day | OR = 0.78 | 0.32–1.94 | 0.12 (trend) | positive | [50] |
| 10.0–19.9 g/day | OR = 1.40 | 0.46–4.3 | ||||||||
| ≥20 g/day | OR = 2.5 | 0.87–7.4 | ||||||||
| C7 | Kirkpatrick et al. (1994) | case-control | 234 | 248 | Any | RR = 1.23 | 0.72–2.12 | null | [51] | |
| C8 | Bataille et al. (1996) | case-control | 255 | 253 | Any | pOR = 2.5 | 1.7–3.7 | N/A | positive | [52] |
| C9 | Sigvardsson et al. (1996) | cohort | 15,508 (women) | Registered alcoholics | RR = 0.5 | 0.3–1.0 | N/A | negative | [53] | |
| C10 | Wesrerdahl et al. (1996) | case-control | 400 | 640 | 1–9 g/day | OR = 0.8 | 0.6–1.1 | >0.05 (trend) | null | [54] |
| 10–19 g/day | OR = 0.9 | 0.5–1.5 | ||||||||
| ≥20 g/day | OR = 0.9 | 0.5–1.8 | ||||||||
| C11 | Rolon et al. (1997) | case-control (male) | 41 (plantar MM) | 168 (hospital control) | Current or ex-drinkers | OR = 2.5 | 1.3–5.1 | N/A | positive | [55] |
| C12 | Veierod et al. (1997) | cohort | 25,708 (men) 25,049 (women) | Beer | IRR = 0.7 | 0.3–1.4 | N/A | null | [56] | |
| Wine/liquor | IRR = 0.6 | 0.3–1.2 | ||||||||
| Beer | IRR = 1.4 | 0.6–3.4 | ||||||||
| Wine/liquor | IRR = 1.7 | 0.9–3.2 | ||||||||
| C13 | Freedman et al. (2003) | cohort | 68,588 (white) | <1–6 drinks/week | RR = 1.2 | 0.8–1.8 | 0.08 (trend) | positive | [57] | |
| 7–14 drinks/week | RR = 1.4 | 0.8–2.5 | ||||||||
| >14 drinks/week | RR = 2.1 | 0.9–4.8 | ||||||||
| C14 | Stang et al. (2003) | case-control | 118 | 475 | 1–15 g/day | OR = 1.0 | 0.5–1.8 | null | [58] | |
| 16–27 g/day | OR = 0.7 | 0.3–1.4 | ||||||||
| >28 g/day | OR = 1.0 | 0.5–2.1 | ||||||||
| C15 | Millen et al. (2004) | case-control | 502 | 565 | 0.2–1 % kcal | OR = 0.97 | 0.62–1.50 | 0.003 (trend) | positive | [59] |
| 1–4 % kcal | OR = 1.16 | 0.76–1.77 | ||||||||
| 4–10 % kcal | OR = 1.86 § | 1.24–2.78 | ||||||||
| ≥10 % kcal | OR = 1.65 § | 1.09–2.49 | ||||||||
| 0.7 drinks/week | OR = 1.04 | 0.69–1.57 | 0.04 (trend) | positive | ||||||
| 1.4–7.0 drinks/week | OR = 1.55 § | 1.09–2.20 | ||||||||
| 7.7–59 drinks/week | OR = 1.53 § | 1.03–2.29 | ||||||||
| C16 | Naldi et al. (2004) | case-control | 542 | 538 | <1 drinks/week | OR = 0.81 | 0.53–1.22 | N/A | null | [60] |
| 1–13 drinks/week | OR = 0.91 | 0.62–1.33 | ||||||||
| 14–27 drinks/week | OR = 1.26 | 0.83–1.91 | ||||||||
| ≥28 drinks/week | OR = 0.83 | 0.49–1.40 | ||||||||
| C17 | Vinceti et al. (2004) | case-control | 59 | 59 | Energy-adjusted tertiles | RR = 1.86 | 0.64–5.42 | 0.978 | null | [61] |
| C18 | Le Marchand et al. (2006) | case-control | 177 (males) | 177 (males) | 45,421–265,001 g/lifetime ≥265,002 g/lifetime | OR = 1.2 | 0.6–2.2 | 0.01 (trend) | positive (male) null (female) | [62] |
| OR = 2.3 | 1.2–4.4 | |||||||||
| 111 (females) | 111 (females) | 45,421–265,001 g/lifetime | OR = 1.1 | 0.5–2.4 | 0.19 (trend) | |||||
| ≥265,002 g/lifetime | OR = 1.7 | 0.7–3.8 | ||||||||
| C19 | Fortes et al. (2008) | case-control | 304 | 305 | Wine (weekly) | OR = 1.28 | 0.80–2.04 | 0.73 (trend) | null | [63] |
| (daily and more) | OR = 0.83 | 0.49–1.42 | ||||||||
| Exclusive wine (weekly) | OR = 0.79 | 0.34–1.84 | 0.36 (trend) | null | ||||||
| (daily and more) | OR = 0.64 | 0.22–1.88 | ||||||||
| Beer (less than weekly) | OR = 1.05 | 0.67–1.64 | 0.99 (trend) | null | ||||||
| (more than weekly) | OR = 0.98 | 0.53–1.79 | ||||||||
| Spirits | OR = 1.15 | 0.72–1.83 | null | |||||||
| C20 | Gogas et al. (2008) | case-control | 55 | 165 | >1 drink/day | OR = 2.45 | 1.00–6.13 | 0.05 | positive | [64] |
| C21 | Allen et al. (2009) | cohort | 1,280,296 (middle-aged women) | <2 drinks/week | RR = 1.0 | 0.93–1.07 | 0.3 (trend) | null | [65] | |
| 3–6 drinks/week | RR = 1.0 | 0.92–1.08 | ||||||||
| 7–14 drinks/week | RR = 0.96 | 0.88–1.05 | ||||||||
| >15 drinks/week | RR = 1.17 | 1.00–1.37 | ||||||||
| C22 | Benedetti et al. (2009) | case-control | 107 | 507 | 1–6/week | OR = 0.93 | 0.50–1.72 | N/A | null | [66] |
| 7+/week | OR = 1.21 | 0.68–2.18 | ||||||||
| 7+/wk (0–71 drinks/yr) | OR = 1.32 | 0.69–2.52 | 0.586 (trend) | null | ||||||
| 7+/wk (72–179 drinks/yr) | OR = 0.71 | 0.31–1.63 | ||||||||
| 7+/wk (180+ drinks/yr) | OR = 1.65 | 0.71–3.83 | ||||||||
| C23 | Asgari et al. (2012) | cohort | 69,635 (individuals) | <1 drink/day | HR = 1.19 | 0.96–1.48 | 0.05 (trend) | positive | [67] | |
| 1–1.9 drinks/day | HR = 1.33 | 1.01–1.76 | ||||||||
| ≥2 drinks/day | HR = 1.28 | 0.97–1.70 | ||||||||
| C24 | de Vries et al. (2012) | case-control | 360 | 1550 | Regular alcohol consumption | OR = 1.32 | 1.01–1.74 | 0.04 | positive | [68] |
| C25 | Kubo et al. (2014) | cohort | 59,575 (white women) | Past drinker | HR = 0.99 | 0.64–1.52 | 0.0013 (trend) | positive | [69] | |
| <1 drink/month | HR = 0.88 | 0.55–1.40 | ||||||||
| <1 drink/week | HR = 1.10 | 0.74–1.66 | ||||||||
| 1–7 drinks/week | HR = 1.40 | 0.95–2.06 | ||||||||
| ≥7 drinks/ week | HR = 1.64 | 1.09–2.49 | ||||||||
| >0–5 drink-yr/lifetime | HR = 1.35 | 0.99–1.83 | 0.0046 (trend) | positive | ||||||
| >5–10 drink-yr/lifetime | HR = 1.66 | 1.19–2.33 | ||||||||
| >10–20 drink-yr/lifetime | HR = 1.55 | 1.09–2.21 | ||||||||
| >20–50 drink-yr/lifetime | HR = 1.79 | 1.29–2.50 | ||||||||
| >50–200 drink-yr/lifetime | HR = 1.98 | 1.32–2.95 | ||||||||
| C26 | Klatsky et al. (2015) | cohort | 124,193 (individuals) | Ex-drinker | HR = 1.4 | 0.9–2.2 | [70] | |||
| <1 drink/day | HR = 1.6 § | 1.2–2.1 | < 0.01 | positive | ||||||
| 1–2 drinks/day | HR = 1.9 § | 1.4–2.6 | < 0.001 | positive | ||||||
| ≥3 drinks/day | HR = 2.2 § | 1.6–3.1 | < 0.001 | positive | ||||||
| C27 | Mahamat-Saleh et al. (2019) | cohort | 404 | 67,332 | Any (median 7.9 g/day, mostly wine)) | HR = 0.89 | 0.73–1.09 | negative | [71] | |
| (Women) | ||||||||||
| C28 | Malagoli et al. (2019) | case-control | 380 | 719 | Red wine (2nd tertile) | OR = 0.94 † | 0.64–1.36 | null | [72] | |
| Red wine (3rd tertile) | OR = 0.83 † | 0.58–1.19 | null | |||||||
| White wine (2nd tertile) | OR = 1.44 † | 1.01–2.06 | positive | |||||||
| White wine (3rd tertile) | OR = 1.03 † | 0.73–1.45 | null | |||||||
| Aperitif wines and beer (2nd tertile) | OR = 0.94 † | 0.66–1.36 | null | |||||||
| Aperitif wines and beer (3rd tertile) | OR = 0.83 † | 0.58–1.19 | null | |||||||
| Spirits and liqueurs (2nd tertile) | OR = 0.93 † | 0.64–1.36 | null | |||||||
| Spirits and liqueurs (3rd tertile) | OR = 0.92 † | 0.63–1.35 | null | |||||||
| C29 | Sanford et al. (2020) | cohort | 3100 (MM patients) | Current drinking | OR = 1.59 | 1.45–1.75 | positive | [73] | ||
| Exceeding moderate drinking | OR = 0.95 | 0.84–1.08 | ||||||||
| Binge drinking | OR = 1.20 | 1.05–1.38 | positive | |||||||
| Cohort Study (n = 10) | Case-Control Study (n = 19) | |
|---|---|---|
| Melanoma correlation | 10/10 papers | 19/19 papers |
| Positive | 4 (40%) | 10 (52.6%) |
| Negative | 2 (20%) | 0 (0%) |
| Null | 3 (30%) | 9 (47.4%) |
| Dose-dependent effects | 6/10 papers | 14/19 papers |
| Positive | 5 (83.3%) | 8 (57.1%) |
| Negative | 0 (0%) | 0 (0%) |
| Null | 1 (12.7%) | 6 (42.9%) |
| Link to UV/Sun exposure | 4/10 papers | 8/19 papers |
| Positive | 3 (75.0%) | 5 (62.5%) |
| Negative | 0 (0%) | 0 (0%) |
| Null | 1 (25.0%) | 3 (37.5%) |
| Study # | Authors (Year) | Study Cases and Types | No. (MM) | No. (Control) | Alcohol Consumption | Risk Ratio | 95% CI | p (Single or Trend *) | Ref |
|---|---|---|---|---|---|---|---|---|---|
| M1 | Rota et al. (2014) | 16 studies (2 cohort and 14 case-control studies) | 6251 | N/A | Any (case-control, 14) | pRR = 1.2 | 1.01–1.44 | 0.003 | [74] |
| Any (cohort, 2) | pRR = 1.26 | 1.19–1.35 | 0.657 | ||||||
| Any (overall, 16) | pRR = 1.2 | 1.06–1.37 | 0.006 | ||||||
| ≤1 drink (≤12.5 g) /day | pRR = 1.10 | 0.96–1.26 | 0.045 | ||||||
| >1 drink (≥12.5 g) /day | pRR = 1.18 | 1.01–1.40 | 0.021 | ||||||
| M2 | Miura et al. (2015) | 8 case-control studies | 1886 | 2113 | Any | pOR = 1.30 | 1.1–1.5 | <0.05 | [75] |
| 0.5–<3.5 g/day | pOR = 1.30 | 1.0–1.7 | |||||||
| 3.5–<6.8 g/day | pOR = 1.30 | 1.0–1.7 | |||||||
| 6.8–<14.4 g/day | pOR = 1.30 | 0.9–1.7 | |||||||
| 14.4–127.3 g/day | pOR = 1.0 | 0.7–1.3 | |||||||
| M3 | Bagnardi et al. (2015) | 14 studies (2 cohort and 12 case-control studies) | 4631 | 1465 | ≤12.5 g/day | pRR = 1.11 | 0.97–1.27 | 0.156 | [76] |
| >12.5–≤50 g/day | pRR = 1.20 | 1.03–1.41 | |||||||
| >50 g/day | n.e. | ||||||||
| M4 | Rivera et al. (2016) | 3 prospective cohort studies | 1374 Invasive MM | N/A | 0.1–4.9 g/day | mHR a = 1.13 | 0.91–1.41 | 0.04 (trend) | [77] |
| 5–9.9 g/day | mHR a = 1.02 | 0.81–1.28 | |||||||
| 10–19.9 g/day | mHR a = 1.21 | 0.97–1.49 | |||||||
| 20+ g/day | mHR a = 1.23 | 0.96–1.59 | |||||||
| Per drink (12.8 g)/day | mHR a = 1.14 | 1.00–1.29 | |||||||
| 835 MIS | 0.1–4.9 g/day | mRR a = 1.13 | 1.19–2.00 | <0.0001 (trend) | |||||
| 5–9.9 g/day | mRR a = 1.54 | 0.81–1.28 | |||||||
| 10–19.9 g/day | mRR a = 1.75 | 1.14–2.70 | |||||||
| 20+ g/day | mRR a = 1.57 | 1.12–2.22 | |||||||
| Per drink (12.8 g)/day | mRR a = 1.46 | 1.24–1.72 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamauchi, T.; Shangraw, S.; Zhai, Z.; Ravindran Menon, D.; Batta, N.; Dellavalle, R.P.; Fujita, M. Alcohol as a Non-UV Social-Environmental Risk Factor for Melanoma. Cancers 2022, 14, 5010. https://doi.org/10.3390/cancers14205010
Yamauchi T, Shangraw S, Zhai Z, Ravindran Menon D, Batta N, Dellavalle RP, Fujita M. Alcohol as a Non-UV Social-Environmental Risk Factor for Melanoma. Cancers. 2022; 14(20):5010. https://doi.org/10.3390/cancers14205010
Chicago/Turabian StyleYamauchi, Takeshi, Sarah Shangraw, Zili Zhai, Dinoop Ravindran Menon, Nisha Batta, Robert P. Dellavalle, and Mayumi Fujita. 2022. "Alcohol as a Non-UV Social-Environmental Risk Factor for Melanoma" Cancers 14, no. 20: 5010. https://doi.org/10.3390/cancers14205010
APA StyleYamauchi, T., Shangraw, S., Zhai, Z., Ravindran Menon, D., Batta, N., Dellavalle, R. P., & Fujita, M. (2022). Alcohol as a Non-UV Social-Environmental Risk Factor for Melanoma. Cancers, 14(20), 5010. https://doi.org/10.3390/cancers14205010






