Longitudinal CT Imaging to Explore the Predictive Power of 3D Radiomic Tumour Heterogeneity in Precise Imaging of Mantle Cell Lymphoma (MCL)
Abstract
Simple Summary
Abstract
1. Introduction
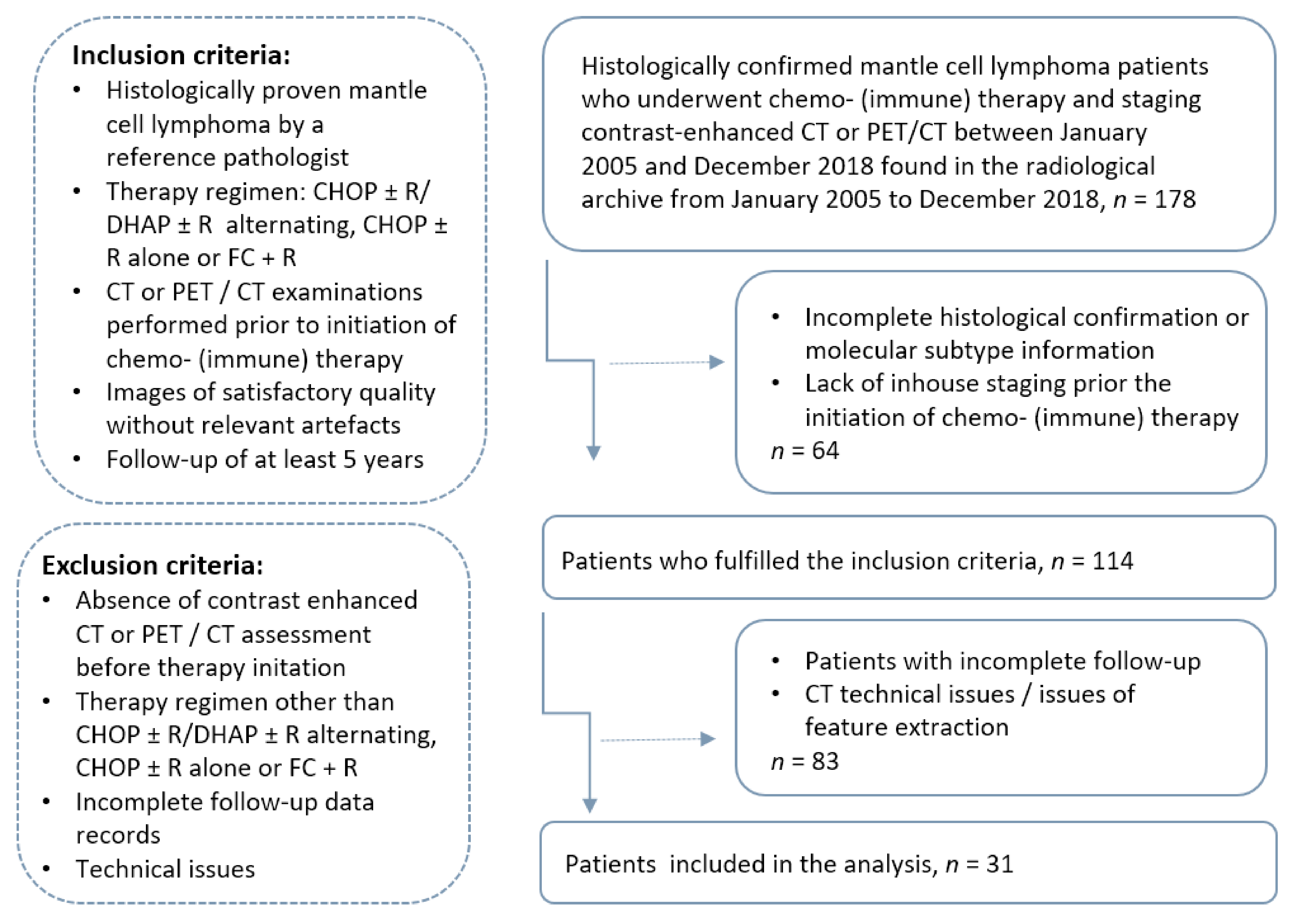
2. Materials and Methods
2.1. Patients and Imaging Protocol
2.2. Image Processing and Feature Extraction
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
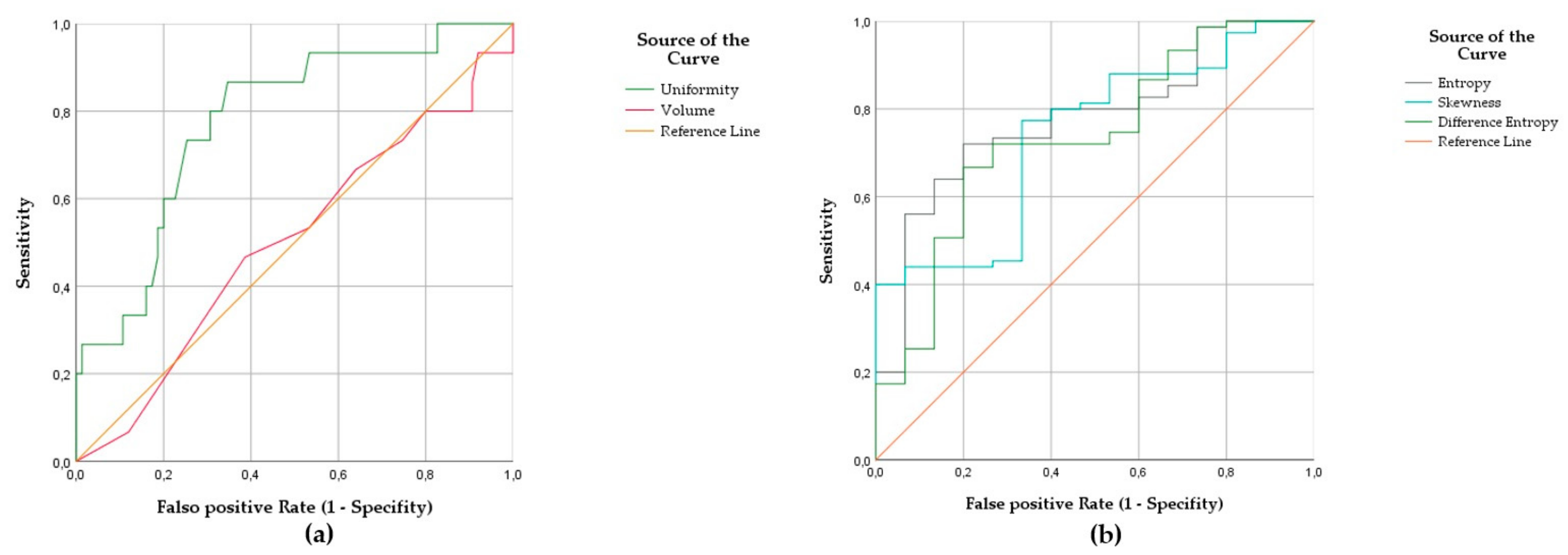
3.2. Radiomic Fingerprint of MCL at High Risk of Relapse
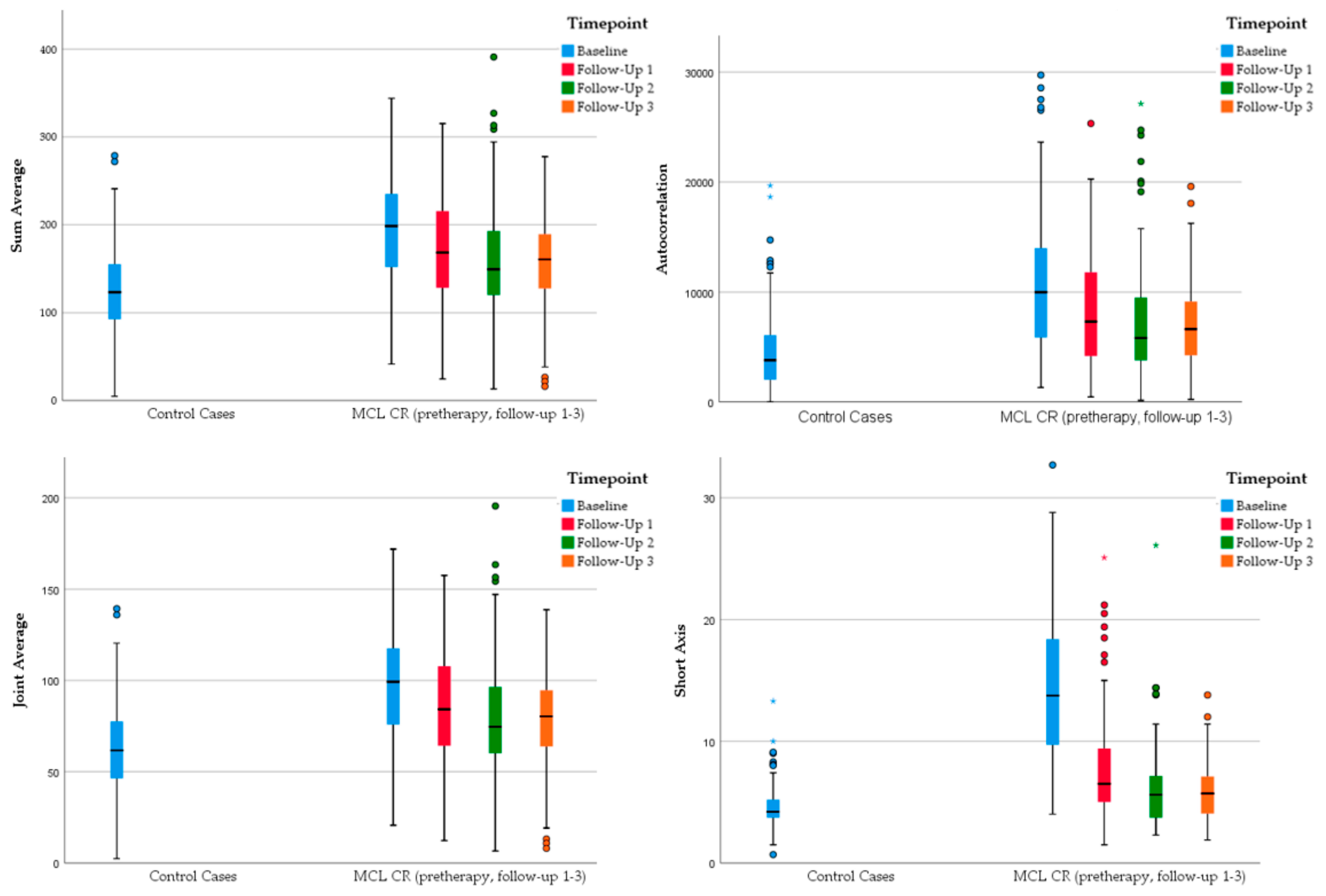
3.3. Radiomic Changes of MCL Lymph Nodes Characteristic for Sustained Remission
- Sum average
- Autocorrelation
- Joint average
- Short axis
- Volume
- P90th
- Skewness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Setting | Determination |
|---|---|
| Bin Method | FBS |
| Bin Amount | 1 |
| LoG Filter | 0 |
| LoG Sigma | 1 |
| Matrix Aggregation Method | 3D Average Directions |
| Resample Filter | 0 |
| Resample Spacing X | 1 |
| Resample Spacing Y | 1 |
| Resample Spacing Z | 0 |
| Second-Order Distance | 1 |
| Threshold Filter | 0 |
| Threshold Filter Min | −1000 |
| Threshold Filter Max | 3000 |
| Radiomic Features of First-Order: Histogram | Radiomic Features of Second-Order: Gray Level Co-Occurrence Matrix (GLCM) |
|---|---|
| Coefficient Variation Value | Angular Second Moment |
| Entropy | Autocorrelation |
| Kurtosis | Contrast |
| Maximum Histogram Gradient | Difference Entropy |
| Minimum Histogram Gradient | Information Correlation Difference |
| P 10th (10th percentile) | Inverse Difference Normalised |
| P 90th (90th percentile) | Joint Average |
| Quartile Coefficient Dispersion | Joint Entropy |
| Skewness | Sum Average |
References
- Hoster, E.; Unterhalt, M.; Woörmann, B.; Duührsen, U.; Metzner, B.; Eimermacher, H.; Neubauer, A.; Wandt, H.; Steinhauer, H.; Martin, S.; et al. The Addition of Rituximab to First-Line Chemotherapy (R-CHOP) Results in Superior Response Rates, Time to Treatment Failure and Response Duration in Patients with Advanced Stage Mantle Cell Lymphoma: Long Term Results of a Randomized GLSG Trial. Blood 2008, 112, 3049. [Google Scholar] [CrossRef]
- Ghielmini, M.; Zucca, E. How I treat mantle cell lymphoma. Blood J. Am. Soc. Hematol. 2009, 114, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Dreyling, M.; Lenz, G.; Hoster, E.; Van Hoof, A.; Gisselbrecht, C.; Schmits, R.; Metzner, B.; Truemper, L.; Reiser, M.; Steinhauer, H. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: Results of a prospective randomized trial of the European MCL Network. Blood 2005, 105, 2677–2684. [Google Scholar] [PubMed]
- Hoster, E.; Rosenwald, A.; Berger, F.; Bernd, H.-W.; Hartmann, S.; Loddenkemper, C.; Barth, T.F.; Brousse, N.; Pileri, S.; Rymkiewicz, G.; et al. Prognostic Value of Ki-67 Index, Cytology, and Growth Pattern in Mantle-Cell Lymphoma: Results from Randomized Trials of the European Mantle Cell Lymphoma Network. J. Clin. Oncol. 2016, 34, 1386–1394. [Google Scholar] [CrossRef]
- Nadeu, F.; Martin-Garcia, D.; Clot, G.; Díaz-Navarro, A.; Duran-Ferrer, M.; Navarro, A.; Vilarrasa-Blasi, R.; Kulis, M.; Royo, R.; Gutiérrez-Abril, J.; et al. Genomic and epigenomic insights into the origin, pathogenesis, and clinical behavior of mantle cell lymphoma subtypes. Blood 2020, 136, 1419–1432. [Google Scholar] [CrossRef] [PubMed]
- Hill, H.A.; Qi, X.; Jain, P.; Nomie, K.; Wang, Y.; Zhou, S.; Wang, M.L. Genetic mutations and features of mantle cell lymphoma: A systematic review and meta-analysis. Blood Adv. 2020, 4, 2927–2938. [Google Scholar] [CrossRef]
- Titov, A.; Zmievskaya, E.; Ganeeva, I.; Valiullina, A.; Petukhov, A.; Rakhmatullina, A.; Miftakhova, R.; Fainshtein, M.; Rizvanov, A.; Bulatov, E. Adoptive Immunotherapy beyond CAR T-Cells. Cancers 2021, 13, 743. [Google Scholar] [CrossRef] [PubMed]
- Kienle, D.; Katzenberger, T.; Ott, G.; Saupe, D.; Benner, A.; Kohlhammer, H.; Barth, T.F.; Höller, S.; Kalla, J.; Rosenwald, A.; et al. Quantitative Gene Expression Deregulation in Mantle-Cell Lymphoma: Correlation with Clinical and Biologic Factors. J. Clin. Oncol. 2007, 25, 2770–2777. [Google Scholar] [CrossRef] [PubMed]
- Rosenwald, A.; Wright, G.; Wiestner, A.; Chan, W.C.; Connors, J.M.; Campo, E.; Gascoyne, R.D.; Grogan, T.M.; Muller-Hermelink, H.; Smeland, E.B.; et al. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer Cell 2003, 3, 185–197. [Google Scholar] [CrossRef]
- Salaverria, I.; Zettl, A.; Beà, S.; Moreno, V.; Valls, J.; Hartmann, E.; Ott, G.; Wright, G.; Lopez-Guillermo, A.; Chan, W.C.; et al. Specific Secondary Genetic Alterations in Mantle Cell Lymphoma Provide Prognostic Information Independent of the Gene Expression–Based Proliferation Signature. J. Clin. Oncol. 2007, 25, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, M.; Schrader, C.; Klapper, W.; Dreyling, M.H.; Campo, E.; Norton, A.; Berger, F.; Kluin, P.; Ott, G.; Pileri, S.; et al. Histopathology, cell proliferation indices and clinical outcome in 304 patients with mantle cell lymphoma (MCL): A clinicopathological study from the European MCL Network. Br. J. Haematol. 2005, 131, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Vardiman, J.W. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; WHO Classification of Tumours; WHO: Geneva, Switzerland, 2017; Volume 2. [Google Scholar]
- Hoster, E.; Dreyling, M.; Klapper, W.; Gisselbrecht, C.; Van Hoof, A.; Kluin-Nelemans, J.C.; Pfreundschuh, M.; Reiser, M.; Metzner, B.; Einsele, H.; et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood 2008, 111, 558–565. [Google Scholar] [CrossRef]
- Roschewski, M.; Rossi, D.; Kurtz, D.M.; Alizadeh, A.A.; Wilson, W.H. Circulating Tumor DNA in Lymphoma: Principles and Future Directions. Blood Cancer Discov. 2021. [Google Scholar] [CrossRef]
- Lakhotia, R.; Melani, C.; Pittaluga, S.; Dunleavy, K.; Saba, N.S.; Lucas, R.A.N.; Jacob, M.A.; Yusko, E.; Steinberg, S.M.; Jaffe, E.S.; et al. Circulating Tumor DNA Dynamics during Therapy Predict Outcomes in Mantle Cell Lymphoma. Blood 2018, 132, 147. [Google Scholar] [CrossRef]
- Dreyling, M.; Thieblemont, C.; Gallamini, A.; Arcaini, L.; Campo, E.; Hermine, O.; Kluin-Nelemans, J.C.; Ladetto, M.; Le Gouill, S.; Iannitto, E.; et al. ESMO Consensus conferences: Guidelines on malignant lymphoma. Part 2: Marginal zone lymphoma, mantle cell lymphoma, peripheral T-cell lymphoma. Ann. Oncol. 2013, 24, 857–877. [Google Scholar] [CrossRef] [PubMed]
- Dreyling, M.; Campo, E.; Hermine, O.; Jerkeman, M.; le Gouill, S.; Rule, S.; Shpilberg, O.; Walewski, J.; Ladetto, M. Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv62–iv71. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.P.M.; Granton, P.; Zegers, C.M.L.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef]
- Patyk, M.; Silicki, J.; Mazur, R.; Kręcichwost, R.; Sokołowska-Dąbek, D.; Zaleska-Dorobisz, U. Radiomics–the value of the numbers in present and future radiology. Pol. J. Radiol. 2018, 83, e171. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
- Davnall, F.; Yip, C.S.P.; Ljungqvist, G.; Selmi, M.; Ng, F.; Sanghera, B.; Ganeshan, B.; Miles, K.A.; Cook, G.; Goh, V. Assessment of tumor heterogeneity: An emerging imaging tool for clinical practice? Insights Imaging 2012, 3, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Miles, K.A. How to use CT texture analysis for prognostication of non-small cell lung cancer. Cancer Imaging 2016, 16, 1–6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ganeshan, B.; Miles, K.A. Quantifying tumour heterogeneity with CT. Cancer Imaging 2013, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.A.; Tan, T.-T.; Rabson, A.B.; Anderson, D.; Degenhardt, K.; White, E. Hypoxia and defective apoptosis drive genomic instability and tumorigenesis. Genes Dev. 2004, 18, 2095–2107. [Google Scholar] [CrossRef] [PubMed]
- Stanta, G.; Bonin, S. Overview on Clinical Relevance of Intra-Tumor Heterogeneity. Front. Med. 2018, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Schürch, C.M.; Federmann, B.; Quintanilla-Martinez, L.; Fend, F. Tumor Heterogeneity in Lymphomas: A Different Breed. Pathobiology 2017, 85, 130–145. [Google Scholar] [CrossRef] [PubMed]
- McGranahan, N.; Swanton, C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell 2017, 168, 613–628. [Google Scholar] [CrossRef]
- Burrell, R.A.; McGranahan, N.; Bartek, J.; Swanton, C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature 2013, 501, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Alic, L.; Niessen, W.J.; Veenland, J.F. Quantification of Heterogeneity as a Biomarker in Tumor Imaging: A Systematic Review. PLoS ONE 2014, 9, e110300. [Google Scholar] [CrossRef]
- Sun, R.; Limkin, E.J.; Vakalopoulou, M.; Dercle, L.; Champiat, S.; Han, S.R.; Verlingue, L.; Brandao, D.; Lancia, A.; Ammari, S.; et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: An imaging biomarker, retrospective multicohort study. Lancet Oncol. 2018, 19, 1180–1191. [Google Scholar] [CrossRef]
- Ganeshan, B.; Miles, K.A.; Babikir, S.; Shortman, R.; Afaq, A.; Ardeshna, K.M.; Groves, A.M.; Kayani, I. CT-based texture analysis potentially provides prognostic information complementary to interim fdg-pet for patients with hodgkin’s and aggressive non-hodgkin’s lymphomas. Eur. Radiol. 2017, 27, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Han, S.-A.; Won, K.Y.; Hong, I.K.; Kim, D.Y. Early Prediction of Tumor Response to Neoadjuvant Chemotherapy and Clinical Outcome in Breast Cancer Using a Novel FDG-PET Parameter for Cancer Stem Cell Metabolism. J. Pers. Med. 2020, 10, 132. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Riedl, C.C.; Kumar, A.; Gibbs, P.; Weber, M.; Tal, I.; Schilksy, J.; Schöder, H. Radiomic features of glucose metabolism enable prediction of outcome in mantle cell lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2760–2769. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-Y.; Franc, B.L.; Harnish, R.J.; Liu, G.; Mitra, D.; Copeland, T.P.; Arasu, V.A.; Kornak, J.; Jones, E.F.; Behr, S.C.; et al. Exploration of PET and MRI radiomic features for decoding breast cancer phenotypes and prognosis. npj Breast Cancer 2018, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Parvez, A.; Tau, N.; Hussey, D.; Maganti, M.; Metser, U. 18F-FDG PET/CT metabolic tumor parameters and radiomics features in aggressive non-Hodgkin’s lymphoma as predictors of treatment outcome and survival. Ann. Nucl. Med. 2018, 32, 410–416. [Google Scholar] [CrossRef]
- Park, S.; Ha, S.; Lee, S.-H.; Paeng, J.C.; Keam, B.; Kim, T.M.; Kim, D.-W.; Heo, D.S. Intratumoral heterogeneity characterized by pretreatment PET in non-small cell lung cancer patients predicts progression-free survival on EGFR tyrosine kinase inhibitor. PLoS ONE 2018, 13, e0189766. [Google Scholar] [CrossRef]
- Geisler, C.H.; Kolstad, A.; Laurell, A.; Andersen, N.S.; Pedersen, L.B.; Jerkeman, M.; Eriksson, M.; Nordström, M.; Kimby, E.; Boesen, A.M. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo–purged stem cell rescue: A nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood J. Am. Soc. Hematol. 2008, 112, 2687–2693. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.J.R.; Goh, V. What can artificial intelligence teach us about the molecular mechanisms underlying disease? Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2715–2721. [Google Scholar] [CrossRef]
- Rogasch, J.M.M.; Hundsdoerfer, P.; Hofheinz, F.; Wedel, F.; Schatka, I.; Amthauer, H.; Furth, C. Pretherapeutic FDG-PET total metabolic tumor volume predicts response to induction therapy in pediatric Hodgkin’s lymphoma. BMC Cancer 2018, 18, 521. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Pfistner, B.; Juweid, M.E.; Gascoyne, R.D.; Specht, L.; Horning, S.J.; Coiffier, B.; Fisher, R.I.; Hagenbeek, A.; Zucca, E.; et al. Revised Response Criteria for Malignant Lymphoma. J. Clin. Oncol. 2007, 25, 579–586. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Chalkidou, A.; O’Doherty, M.J.; Marsden, P.K. False Discovery Rates in PET and CT Studies with Texture Features: A Systematic Review. PLoS ONE 2015, 10, e0124165. [Google Scholar] [CrossRef]
- IBM Corporation. IBM SPSS Neural Networks 26. Available online: https://www.ibm.com/support/pages/node/874712 (accessed on 7 October 2021).
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; Tarpey, P.; et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.; Viswanath, S.; Rusu, M.; Valls, L.; Hoimes, C.; Avril, N.; Madabhushi, A. Radiomics Analysis on FLT-PET/MRI for Characterization of Early Treatment Response in Renal Cell Carcinoma: A Proof-of-Concept Study. Transl. Oncol. 2016, 9, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Coroller, T.P.; Agrawal, V.; Narayan, V.; Hou, Y.; Grossmann, P.; Lee, S.W.; Mak, R.H.; Aerts, H.J. Radiomic phenotype features predict pathological response in non-small cell lung cancer. Radiother. Oncol. 2016, 119, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.K.; Jamshidi, N.; Felker, E.R.; Raman, S.S.; Lu, D.S. Radiomics and radiogenomics of primary liver cancers. Clin. Mol. Hepatol. 2019, 25, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Park, J.E.; Kim, Y.-H.; Kim, J.H.; Oh, J.Y.; Kim, J.; Kim, Y.; Kim, S.T.; Kim, H.S. Diffusion radiomics as a diagnostic model for atypical manifestation of primary central nervous system lymphoma: Development and multicenter external validation. Neuro-Oncology 2018, 20, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Milgrom, S.A.; Elhalawani, H.; Lee, J.; Wang, Q.; Mohamed, A.S.R.; Dabaja, B.S.; Pinnix, C.C.; Gunther, J.R.; Court, L.; Rao, A.; et al. A PET Radiomics Model to Predict Refractory Mediastinal Hodgkin Lymphoma. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Li, L.; Hou, W.; Ma, X.; Tian, R. Current status and quality of radiomics studies in lymphoma: A systematic review. Eur. Radiol. 2020, 30, 6228–6240. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Xu, H.; Shen, C.; Wang, Y.; Xu, W.; Duan, S.; Chen, H.; Ou, X.; Chen, L.; Ma, X. Differential diagnostic ability of 18F-FDG PET/CT radiomics features between renal cell carcinoma and renal lymphoma. Q. J. Nucl. Med. Mol. Imaging 2019, 65, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Sollini, M.; Antunovic, L.; Chiti, A.; Kirienko, M. Towards clinical application of image mining: A systematic review on artificial intelligence and radiomics. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2656–2672. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Kim, J.; Joung, J.-G.; Cha, H.; Park, W.-Y.; Ahn, J.S.; Ahn, M.-J.; Park, K.; Choi, J.Y.; Lee, K.-H. Correlations between metabolic texture features, genetic heterogeneity, and mutation burden in patients with lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.-R.; Lee, H.Y.; Jeong, J.Y.; Choi, Y.-L.; Kim, J.; Bae, J.; Lee, K.S.; Shim, Y.M. Quantitative image variables reflect the intratumoral pathologic heterogeneity of lung adenocarcinoma. Oncotarget 2016, 7, 67302–67313. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.-M.; Fang, Y.-H.D.; Tsan, D.-L.; Lee, L.-Y.; Chang, J.T.-C.; Wang, H.-M.; Ng, S.-H.; Liao, C.-T.; Yang, L.-Y.; Yen, T.-C. Heterogeneity and irregularity of pretreatment 18F-fluorodeoxyglucose positron emission tomography improved prognostic stratification of p16-negative high-risk squamous cell carcinoma of the oropharynx. Oral Oncol. 2018, 78, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Van Helden, E.J.; Vacher, Y.J.L.; van Wieringen, W.; Van Velden, F.H.P.; Verheul, H.; Hoekstra, O.S.; Boellaard, R.; Menke-van der Houven van Oordt, C.W. Radiomics analysis of pre-treatment [18F]FDG PET/CT for patients with metastatic colorectal cancer undergoing palliative systemic treatment. Eur. J. Pediatr. 2018, 45, 2307–2317. [Google Scholar] [CrossRef] [PubMed]
- Bea, S.; Mas, R.M.V.; Navarro, A.; Salaverria, I.; Martín-Garcia, D.; Jares, P.; Giné, E.; Pinyol, M.; Royo, C.; Nadeu, F.; et al. Landscape of somatic mutations and clonal evolution in mantle cell lymphoma. Proc. Natl. Acad. Sci. USA 2013, 110, 18250–18255. [Google Scholar] [CrossRef] [PubMed]
- Götz, M.; Maier-Hein, K.H. Optimal Statistical Incorporation of Independent Feature Stability Information into Radiomics Studies. Sci. Rep. 2020, 10, 737. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | MCL Cohort (n = 31) | Reference Group (n = 39) |
|---|---|---|
| Age, median (range) | 61.5 ± 9.7 years, (42–76 years) | 64.9 ± 8.5 years, (47–81 years) |
| Male | 26 | 21 |
| Tissue Sample for histopathological Diagnosis | Lymph node: 12 patients Bone marrow: 11 patients Blood (liquid biopsy): 4 patients GI tract: 4 patients Nasal mucosa: 1 patient | PET/CT, clinical, and imaging follow-up over the next 5 years to confirm normal lymph node tissue and exclude hematological disease |
| Ann Arbor | not applicable | |
| Stage I | 0 patients | |
| Stage II | 2 patients | |
| Stage III | 5 patients | |
| Stage IV | 25 patients | |
| MIPI (obtained/range) | 7 patients (range 3–5.9) | not applicable |
| Radiotherapy | 9 patients (29%) | not applicable |
| Therapy Regimen | ||
| CHOP ± R alternatingwith DHAP ± R | 19 patients (61.3%) | not applicable |
| CHOP ± R alone | 10 patients (32.2%) | |
| FC + R | 2 patients (6.5%) |
| Radiomic Feature | AUC | Standard Error | p-Value | 95% CI |
|---|---|---|---|---|
| Uniformity | 0.788 | 0.063 | 0.001 | 0.655/0.902 |
| Entropy | 0.777 | 0.061 | 0.001 | 0.658/0.896 |
| Skewness | 0.738 | 0.066 | 0.004 | 0.608/0.867 |
| Difference Entropy | 0.734 | 0.071 | 0.004 | 0.595/0.874 |
| SAD | 0.620 | 0.072 | 0.145 | 0.479/0.760 |
| LAD | 0.532 | 0.083 | 0.695 | 0.369/0.695 |
| Volume | 0.500 | 0.084 | 1.000 | 0.226/0.664 |
| Radiomic Feature | Sensitivity | Specificity | Cut-Off for Relapse | Accuracy |
|---|---|---|---|---|
| Uniformity | 87 | 65 | ≤0.0159 | 69 |
| Entropy | 80 | 72 | ≥6.2920 | 73 |
| Skewness | 67 | 77 | ≥−0.1890 | 76 |
| Difference Entropy | 80 | 67 | ≥5.2850 | 69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisson, C.S.; Lisson, C.G.; Achilles, S.; Mezger, M.F.; Wolf, D.; Schmidt, S.A.; Thaiss, W.M.; Bloehdorn, J.; Beer, A.J.; Stilgenbauer, S.; et al. Longitudinal CT Imaging to Explore the Predictive Power of 3D Radiomic Tumour Heterogeneity in Precise Imaging of Mantle Cell Lymphoma (MCL). Cancers 2022, 14, 393. https://doi.org/10.3390/cancers14020393
Lisson CS, Lisson CG, Achilles S, Mezger MF, Wolf D, Schmidt SA, Thaiss WM, Bloehdorn J, Beer AJ, Stilgenbauer S, et al. Longitudinal CT Imaging to Explore the Predictive Power of 3D Radiomic Tumour Heterogeneity in Precise Imaging of Mantle Cell Lymphoma (MCL). Cancers. 2022; 14(2):393. https://doi.org/10.3390/cancers14020393
Chicago/Turabian StyleLisson, Catharina Silvia, Christoph Gerhard Lisson, Sherin Achilles, Marc Fabian Mezger, Daniel Wolf, Stefan Andreas Schmidt, Wolfgang M. Thaiss, Johannes Bloehdorn, Ambros J. Beer, Stephan Stilgenbauer, and et al. 2022. "Longitudinal CT Imaging to Explore the Predictive Power of 3D Radiomic Tumour Heterogeneity in Precise Imaging of Mantle Cell Lymphoma (MCL)" Cancers 14, no. 2: 393. https://doi.org/10.3390/cancers14020393
APA StyleLisson, C. S., Lisson, C. G., Achilles, S., Mezger, M. F., Wolf, D., Schmidt, S. A., Thaiss, W. M., Bloehdorn, J., Beer, A. J., Stilgenbauer, S., Beer, M., & Götz, M. (2022). Longitudinal CT Imaging to Explore the Predictive Power of 3D Radiomic Tumour Heterogeneity in Precise Imaging of Mantle Cell Lymphoma (MCL). Cancers, 14(2), 393. https://doi.org/10.3390/cancers14020393







