Simple Summary
For symptomatic stage IV gastric cancer involving major symptoms such as bleeding or obstruction, palliative surgery may be considered an option to relieve symptoms. Palliative gastrectomy or gastrojejunostomy is selected depending on the resectability of the primary tumor and/or surgical risk. However, treatment policies differ depending on the institution as to whether gastrectomy or gastrojejunostomy should be performed for symptomatic stage IV gastric cancer. We considered that gastrectomy might contribute more to prognosis than gastrojejunostomy for gastric cancer located in the middle or lower-third region where total gastrectomy can be avoided. Here, we compare the prognosis of gastrectomy and gastrojejunostomy for symptomatic stage IV gastric cancer. We demonstrate that distal gastrectomy for symptomatic stage IV gastric cancer located in the middle or lower-third regions contributes to prognosis with acceptable safety when compared to gastrojejunostomy.
Abstract
Background: The prognostic prolongation effect of reduction surgery for asymptomatic stage IV gastric cancer (GC) is unfavorable; however, its prognostic effect for symptomatic stage IV GC remains unclear. We aimed to compare the prognosis of gastrectomy and gastrojejunostomy for symptomatic stage IV GC. Methods: This multicenter retrospective study analyzed record-based data of patients undergoing palliative surgery for symptomatic stage IV GC in the middle or lower-third regions between January 2015 and December 2019. Patients were divided into distal gastrectomy and gastrojejunostomy groups. We compared clinicopathological features and outcomes after propensity score matching (PSM). Results: Among the 126 patients studied, 46 and 80 underwent distal gastrectomy and gastrojejunostomy, respectively. There was no difference in postoperative complications between the groups. Regarding prognostic factors, surgical procedures and postoperative chemotherapy were significantly different in multivariate analysis. Each group was further subdivided into groups with and without postoperative chemotherapy. After PSM, the data of 21 well-matched patients with postoperative chemotherapy and 8 without postoperative chemotherapy were evaluated. Overall survival was significantly longer in the distal gastrectomy group (p = 0.007 [group with postoperative chemotherapy], p = 0.02 [group without postoperative chemotherapy]). Conclusions: Distal gastrectomy for symptomatic stage IV GC contributes to prognosis with acceptable safety compared to gastrojejunostomy.
1. Introduction
Gastric cancer (GC) is one of the most common malignancies and the third leading cause of cancer-related deaths worldwide. There were 782,685 GC-related deaths, accounting for approximately 8.2% of the total cancer deaths among 185 countries in 2018 [1]. In the past decade, median overall survival of approximately 12 months has been reported with chemotherapy alone [2,3,4,5]. Regarding reduction surgery for asymptomatic stage IV GC, in a systematic review by Mahar et al., the prognosis-improving effect of reduction surgery was not clearly observed [6]; however, many studies have reported that the prognosis-improving effect could be achieved with limited incurable factors [7,8,9]. However, in a subsequent prospective randomized controlled trial, there was no survival benefit of additional gastrectomy over chemotherapy alone (REGATTA) [10].
On the contrary, for symptomatic stage IV GC involving major symptoms such as bleeding or obstruction, palliative surgery may be considered an option to relieve symptoms. Palliative gastrectomy or gastrojejunostomy is selected depending on the resectability of the primary tumor and/or surgical risk [11]. However, treatment policies differ depending on the institution as to whether gastrectomy or gastrojejunostomy should be performed for symptomatic stage IV GC. We considered that gastrectomy might contribute more to prognosis than gastrojejunostomy for GC located in the middle or lower-third region where total gastrectomy can be avoided. A small number of retrospective studies have reported no survival benefits of gastrectomy compared to those of gastrojejunostomy for stage IV GC with gastric outlet obstruction, but the background factors were poorly matched [12,13]. Therefore, this retrospective study elucidated whether distal gastrectomy or gastrojejunostomy for symptomatic stage IV GC provides benefits to the patients by matching in terms of not only incurable factors but also inflammation and nutritional factors. In view of this, we aimed to determine the perioperative and oncological outcomes of distal gastrectomy as a palliative surgery for symptomatic stage IV GC and compared the data with those of gastrojejunostomy through propensity score matching analysis.
2. Materials and Methods
2.1. Study Design
We conducted a retrospective cohort study wherein we reviewed data from the medical records of patients with stage IV GC who underwent R2 surgery (distal gastrectomy or gastrojejunostomy) between January 2015 and December 2019 in 13 institutions belonging to the Hiroshima Surgical Study Group of Clinical Oncology (HiSCO), Hiroshima, Japan. We selected patients who met the following inclusion criteria: stage IV GC (excluding only positive abdominal lavage cytology as an incurable factor), symptoms (hemoglobin concentration <10 g/dL or obstruction), located in the middle or lower-third regions. Patients were excluded if they met any of the following criteria: pancreatic infiltration or severe duodenal development of GC, liver dysfunction (aspartate or alanine aminotransferase concentration >100 U/L or total bilirubin concentration >2 mg/dL), and moderate or higher quantities of ascites (exceeding the pelvic cavity, etc.). The Institutional Review Board of Onomichi General Hospital approved this study (OJH-202128).
2.2. Treatment and Procedure
Each physician decided the treatment procedure, such as surgical procedure (distal gastrectomy or gastrojejunostomy), indication of chemotherapy, chemotherapy regimen, and duration of chemotherapy.
2.3. Outcomes
We compared the perioperative and oncological outcomes between the gastrectomy and gastrojejunotomy groups with or without chemotherapy. The primary endpoint was overall survival. Operative time, bleeding, and duration of hospital stay after surgery were recorded. Surgical complications were evaluated according to the Clavien–Dindo (CD) classification.
2.4. Statistical Analyses
Continuous variables were presented as medians and ranges and compared between the groups using the Mann–Whitney U test. Categorical variables were presented as numbers and percentages and compared using Fisher’s exact test. Survival curves were generated using the Kaplan–Meier method and compared between different groups using the log-rank test. Multivariate analyses for survival were performed using Cox proportional hazards regression analysis. Variables with a p-value of <0.05 in univariate analysis were entered into multivariate analysis using Cox proportional hazards regression models. Hazard ratio (HR) and 95% confidence interval (CI) were used to estimate survival predictors. Differences between the results of comparative tests were considered statistically significant at two-sided p < 0.05.
To overcome bias due to the different distributions of covariates among patients from the distal gastrectomy groups and the gastrojejunal bypass groups with and without chemotherapy, propensity score matching analysis was performed using a multiple logistic regression model to predict the probability of each patient being allocated to a distal gastrectomy group based on clinicopathological variables.
To evaluate the discrimination and calibration abilities of the propensity scores, C statistics were used. The model showed good discrimination in the chemotherapy group (C statistic, 0.822 [95% CI, 0.728–0.915]; p < 0.01) and in the non-chemotherapy group (C statistic, 0.891 [95% CI 0.790–0.993]; p < 0.01).
A one-to-one matching algorithm without replacement was used, where all treated patients were matched to the closest control within a range of 0.20 standard deviations of the logit of the estimated propensity score. This matching was successful as the C statistic was well balanced (C statistic, 0.544 [95% CI 0.368–0.721]; p = 0.624, C statistic, 0.500 [95% CI 0.208–0.792]; p = 1.000, respectively). Data analyses were performed using SPSS software (version 27; IBM Corp., Armonk, NY, USA).
3. Results
Patients’ demographic and oncological characteristics and perioperative outcomes are shown in Table 1. Overall, 126 symptomatic patients who underwent palliative surgery for stage IV GC were included in this study; 46 patients had undergone distal gastrectomy, and the remaining 80 patients underwent gastrojejunostomy. Of the 126 patients, 76 received postoperative chemotherapy, and 50 did not receive postoperative chemotherapy. Although the operative time was shorter and blood loss was less in the gastrojejunostomy group, there were no significant differences in length of stay and postoperative complications between the groups. Regarding prognostic factors, American Society of Anesthesiologists Physical Status, neutrophil-to-lymphocyte ratio (NLR), prognostic nutritional index, modified Glasgow Prognosis Score, peritoneal metastasis, number of metastasis factors, postoperative chemotherapy, surgical approach, surgical procedure, operative blood loss, and length of hospital stay were significant factors in univariate analysis. In multivariate analysis, only postoperative chemotherapy and surgical procedures were significant prognostic factors (Table 2). In all cases, distal gastrectomy had a significantly better prognosis than gastrojejunostomy (p ≤ 0.001) (Figure 1a).

Table 1.
General characteristics of 126 GC patients.

Table 2.
Univariate and multivariate analysis of overall survival.
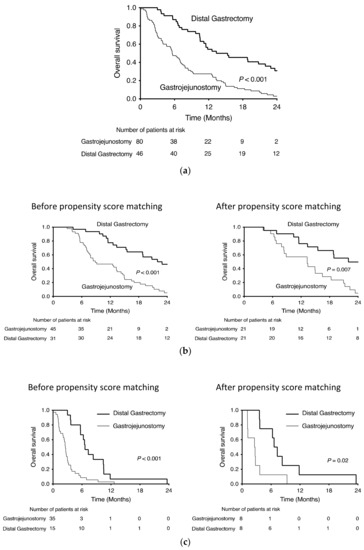
Figure 1.
Kaplan–Meier survival curves of distal gastrectomy and gastrofejunostomy for stage IV gastric cancer (a) all cases, (b) with chemotherapy and (c) without chemotherapy.
Of the 76 patients who received postoperative chemotherapy, 31 underwent distal gastrectomy, and 45 underwent gastrojejunostomy. Performance Status (PS) was better and preoperative obstruction, NLR, distant lymph node metastasis, and use of laparoscopic approach were higher in the gastrojejunostomy group (Table 3). After propensity score matching with PS, obstruction, NLR, distant lymph node metastasis and surgical approach for 76 patients who received postoperative chemotherapy, distal gastrectomy and gastrojejunostomy matched 21 cases each (Table 4). The overall survival of the two groups before and after propensity score matching is shown in Figure 1b. After matching, the median survival time was 13.3 months in the gastrojejunostomy group and 22.0 months in the distal gastrectomy group. The 24-month survival rate was 4.8% in the gastrojejunostomy group and 49.7% in the distal gastrectomy group (HR 0.406, p = 0.008).

Table 3.
General characteristics before propensity score matching.

Table 4.
General characteristics after propensity score matching.
Of the 50 patients who did not receive postoperative chemotherapy, 15 underwent distal gastrectomy, and 35 underwent gastrojejunostomy. Preoperative obstruction was higher, and NLR was higher in the gastrojejunostomy group (Table 3). After propensity score matching with obstruction and NLR for 50 patients who did not receive postoperative chemotherapy, distal gastrectomy and gastrojejunostomy required 8 matched cases for each category (Table 4). The overall survival of the two groups before and after propensity score matching is shown in Figure 1c. After matching, the median survival time was 2.6 months in the gastrojejunostomy group and 7.0 months in the distal gastrectomy group. None of the patients survived for 24 months in either group, but the prognosis was significantly prolonged in the distal gastrectomy group (HR 0.289, p = 0.026).
A similar study was conducted in 98 cases with gastric outlet obstruction (Table S1). In multivariate analysis, only postoperative chemotherapy, surgical procedures and length of hospital stays were significant prognostic factors (Table S2). In all cases with gastric outlet obstruction, distal gastrectomy had a significantly better prognosis than gastrojejunostomy (p < 0.001) (Figure S1a).
Of the 58 patients who received postoperative chemotherapy, 18 underwent distal gastrectomy, and 40 underwent gastrojejunostomy. Distant lymph node metastasis was higher in the gastrojejunostomy group (Table S3). After propensity score matching with distant lymph node metastasis for 58 patients who received postoperative chemotherapy, distal gastrectomy and gastrojejunostomy matched 13 cases each (Table S4). The overall survival of the two groups before and after propensity score matching is shown in Figure S1b. After matching, distal gastrectomy had a significantly better prognosis than gastrojejunostomy (p = 0.007) (Figure S1b).
Of the 40 patients who did not receive postoperative chemotherapy, 7 underwent distal gastrectomy, and 33 underwent gastrojejunostomy. NLR was higher in the gastrojejunostomy group (Table S3). After propensity score matching with NLR for 40 patients who did not receive postoperative chemotherapy, distal gastrectomy and gastrojejunostomy matched 6 cases each (Table S4). The overall survival of the two groups before and after propensity score matching is shown in Figure S1c. After matching, distal gastrectomy had a significantly better prognosis than gastrojejunostomy (p = 0.003) (Figure S1c).
4. Discussion
In this study, distal gastrectomy significantly prolonged overall survival compared to gastrojejunostomy in patients with symptomatic stage IV GC located in the middle or lower-third region. This result was the same with or without postoperative chemotherapy. Similar results were obtained by examining only cases with gastric outlet obstruction. There are two possible explanations for the prognosis-prolonging effect of gastrectomy. First, tumor volume reduction may have extended the prognosis, as can be inferred from the prognosis-prolonging effect obtained even in cases without chemotherapy. Second, it is suggested that chemotherapy compliance may be improved by excising the primary gastric tumor in symptomatic patients. Although not significant, gastrectomy has been reported to provide a higher rate of solid intake than gastrojejunostomy [14], which may lead to improved chemotherapy compliance. In addition, in the subgroup analysis of the REGATTA study, compliance with chemotherapy was maintained in GC located in the lower-third region, which avoided total gastrectomy, resulting in comparable overall survival [10]. Conversely, palliative total gastrectomy should be performed with caution, as it can reduce chemotherapy compliance and can worsen prognosis.
Regarding perioperative outcomes, distal gastrectomy showed significantly greater surgical time and bleeding volume than gastrojejunostomy. On the contrary, there was no significant difference in the length of hospital stay after surgery or the occurrence of complications of CD3 or higher. It can be said that distal gastrectomy can be safely performed even in patients with stage IV GC.
Similar studies conducted in the past were confounded by selection bias because patients with good PS, fewer comorbidities, better nutritional status, less inflammation, and smaller tumor burden were more likely to undergo gastrectomy [12,13]. In our study, we compared gastrectomy and gastrojejunostomy by matching nutritional and inflammatory conditions, as well as PS and tumor factors, and then proved the survival benefits of gastrectomy. This is the first study to compare distal gastrectomy with gastrojejunostomy by matching not only incurable factors but also inflammatory and nutritional factors in multiple institutions over a relatively short period.
In addition to obstruction, this study also included cases of anemia (hemoglobin concentration of <10 g/dL). It is also an important clinical question as to whether gastrectomy or gastrojejunostomy with incomplete transection should be performed in cases with tumor bleeding. Because more than half of the patients in this study were anemic, palliative gastrectomy of anemic patients was considered to have greater benefits to patients than gastrojejunostomy.
To improve oral intake for gastric outlet obstruction in GC, gastrointestinal stent placement is also a candidate, along with distal gastrectomy and gastrojejunostomy. There is no consensus on the overall survival in gastrointestinal stent placement and gastrojejunostomy [15,16]. Keranen et al. reported that palliative gastrectomy seems to provide a survival benefit in contrast to gastrointestinal stent placement and gastrojejunostomy to treat gastric outlet obstruction. Palliative resection should be considered a treatment option for patients suitable for surgery [17]. Our study suggests that gastrectomy may improve prognosis over gastrojejunostomy if the condition is tolerable to general anesthesia surgery, but there is no evidence to compare the long-term prognosis of distal gastrectomy with gastrointestinal stents. Gastrointestinal stenting, gastrojejunostomy, and distal gastrectomy are all options for improving oral intake in gastric outlet obstruction, and in clinical practice, they are selected according to the case background.
There are some limitations to our study. First, this was a retrospective study, and the number of cases after PSM was not large. Second, background matching of tumor factors may be inadequate. Stage IV GC has various oncological conditions. The degree of liver metastasis, lymph node metastasis, and peritoneal dissemination also varied. In this study, cases of massive ascites and liver dysfunction were excluded, and an attempt was made to indirectly match the oncological background with the nutrition and inflammation scores. However, this alone may not be sufficient for oncological background matching. It may be beneficial to use the stage IV GC classification proposed by Yoshida et al. [18,19] In the future, prospective studies are needed to confirm these results.
5. Conclusions
In our retrospective study, distal gastrectomy for symptomatic stage IV GC contributes to better prognosis with acceptable safety compared to gastrojejunostomy. However, it is difficult to completely align the background of stage IV GC, and randomized controlled trials are warranted to fill a gap.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14020388/s1, Figure S1: Kaplan–Meier survival curves of distal gastrectomy and gastrojejunostomy for stage IV gastric cancer with gastric outlet obstruction (a) all cases, (b) with chemotherapy and (c) without chemotherapy, Table S1: General characteristics of 98 GC patients with gastric outlet obstruction, Table S2: Univariate and multivariate analysis of overall survival of 98 GC patients with gastric outlet obstruction, Table S3: General characteristics before propensity score matching of 98 GC patients with gastric outlet obstruction, Table S4: General characteristics after propensity score matching of 98 GC patients with gastric outlet obstruction.
Author Contributions
All authors contributed to the study conception and design. Data collection were performed by N.F., Y.Y., H.T., K.T. (Kazuhiro Toyota), N.T., R.H., S.Y., Y.S. (Yoshihiro Saeki), Y.S. (Yoichi Sugiyama), M.I., M.S., T.F., K.O. and M.N. Data analysis were performed by N.F. and M.H. The first draft of the manuscript was written by N.F. and K.T. (Kazuaki Tanabe). Supervision were performed by K.T. (Kazuaki Tanabe) and H.O. All authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This manuscript is made by author’s own work without receiving any funding. There was no financial support for this research and publication.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Onomichi General Hospital approved this study (OJH-202128), Approval Date: 13 August 2021.
Informed Consent Statement
Because of the observational design of the study, informed consent from the participants was waived by the ethics committee.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data is not publicly available due to patient privacy and the General Data Protection Regulation.
Acknowledgments
This study was initiated and promoted by HiSCO. The authors thank all doctors of the participants of HiSCO for their close collaboration in providing data from their patients. The authors also thank Toshihiro Misumi (Hiroshima Prefectural Hospital, Hiroshima, Japan), Takahisa Suzuki (Kure Medical Center/Chugoku Cancer Center, Kure, Japan), Yoshihiro Sakashita (Hiroshima Memorial Hospital, Hiroshima, Japan), Jun Hihara and Mikihiro Kano (Hiroshima City Asa Citizens Hospital, Hiroshima, Japan), and Hiroshi Ota (Hiroshima University, Hiroshima, Japan).
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. C.A. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koizumi, W.; Narahara, H.; Hara, T.; Takagane, A.; Akiya, T.; Takagi, M.; Miyashita, K.; Nishizaki, T.; Kobayashi, O.; Takiyama, W.; et al. S-1 Plus Cisplatin Versus S-1 Alone for First-Line Treatment of Advanced Gastric Cancer (SPIRITS Trial): A phase III Trial. Lancet Oncol. 2008, 9, 215–221. [Google Scholar] [CrossRef]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in Combination with Chemotherapy Versus Chemotherapy Alone for Treatment of HER2-Positive Advanced Gastric or Gastro-oesophageal Junction Cancer (ToGA): A phase 3, Open-Label, Randomised Controlled Trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Ohtsu, A.; Shah, M.A.; Van Cutsem, E.; Rha, S.Y.; Sawaki, A.; Park, S.R.; Lim, H.Y.; Yamada, Y.; Wu, J.; Langer, B.; et al. Bevacizumab in Combination with Chemotherapy as First-Line Therapy in Advanced Gastric Cancer: A Randomized, Double-Blind, Placebo-Controlled phase III Study. J. Clin. Oncol. 2011, 29, 3968–3976. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Boku, N.; Mizusawa, J.; Iwasa, S.; Kadowaki, S.; Nakayama, N.; Azuma, M.; Sakamoto, T.; Shitara, K.; Tamura, T.; et al. Docetaxel Plus Cisplatin and S-1 Versus Cisplatin and S-1 in Patients with Advanced Gastric Cancer (JCOG1013): An Open-Label, phase 3, Randomised Controlled Trial. Lancet Gastroenterol. Hepatol. 2019, 4, 501–510. [Google Scholar] [CrossRef]
- Mahar, A.L.; Coburn, N.G.; Singh, S.; Law, C.; Helyer, L.K. A Systematic Review of Surgery for Non-Curative Gastric Cancer. Gastric Cancer. 2012, 15 (Suppl. 1), S125–S137. [Google Scholar] [CrossRef] [PubMed]
- He, M.M.; Zhang, D.S.; Wang, F.; Wang, Z.Q.; Luo, H.Y.; Jin, Y.; Wei, X.L.; Xu, R.H. The Role of Non-Curative Surgery in Incurable, Asymptomatic Advanced Gastric Cancer. PLoS ONE 2013, 8, e83921. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Song, Y.; Wang, Z.; Chen, X.; Gao, P.; Xu, Y.; Zhou, B.; Xu, H. Clinical Significance of Palliative Gastrectomy on the Survival of Patients with Incurable Advanced Gastric Cancer: A Systematic Review and Meta-Analysis. BMC Cancer 2013, 13, 577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warschkow, R.; Baechtold, M.; Leung, K.; Schmied, B.M.; Nussbaum, D.P.; Gloor, B.; Blazer, D.G.; Worni, M. Selective Survival Advantage Associated with Primary Tumor Resection for Metastatic Gastric Cancer in a Western Population. Gastric Cancer 2018, 21, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Fujitani, K.; Yang, H.K.; Mizusawa, J.; Kim, Y.W.; Terashima, M.; Han, S.U.; Iwasaki, Y.; Hyung, W.J.; Takagane, A.; Park, D.J.; et al. Gastrectomy Plus Chemotherapy Versus Chemotherapy Alone for Advanced Gastric Cancer with a Single Non-Curable Factor (REGATTA): A phase 3, Randomised Controlled Trial. Lancet Oncol. 2016, 17, 309–318. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2018, 5th ed. Gastric Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okumura, Y.; Yamashita, H.; Aikou, S.; Yagi, K.; Yamagata, Y.; Nishida, M.; Mori, K.; Nomura, S.; Kitayama, J.; Watanabe, T.; et al. Palliative Distal Gastrectomy Offers No Survival Benefit Over Gastrojejunostomy for Gastric Cancer with Outlet Obstruction: Retrospective Analysis of an 11-Year Experience. World J. Surg. Oncol. 2014, 12, 364. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Chen, G.M.; Wei, Y.C.; Yu, H.; Wang, X.C.; Zhao, Z.K.; Luo, T.Q.; Nie, R.C.; Zhou, Z.W. Palliative Gastrectomy Versus Gastrojejunostomy for Advanced Gastric Cancer with Outlet Obstruction: A Propensity Score Matching Analysis. BMC Cancer 2021, 21, 188. [Google Scholar] [CrossRef] [PubMed]
- Fujitani, K.; Ando, M.; Sakamaki, K.; Terashima, M.; Kawabata, R.; Ito, Y.; Yoshikawa, T.; Kondo, M.; Kodera, Y.; Yoshida, K. Multicentre Observational Study of Quality of Life After Surgical Palliation of Malignant Gastric Outlet Obstruction for Gastric Cancer. BJS Open 2017, 1, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Ly, J.; O’Grady, G.; Mittal, A.; Plank, L.; Windsor, J.A. A Systematic Review of Methods to Palliate Malignant Gastric Outlet Obstruction. Surg. Endosc. 2010, 24, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.B.; Shen, W.S.; Xi, H.Q.; Wei, B.; Chen, L. Palliative Therapy for Gastric Outlet Obstruction Caused by Unresectable Gastric Cancer: A Meta-Analysis Comparison of Gastrojejunostomy with Endoscopic Stenting. Chin. Med. J. 2016, 129, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Keranen, I.; Kylanpaa, L.; Udd, M.; Louhimo, J.; Lepisto, A.; Halttunen, J.; Kokkola, A. Gastric Outlet Obstruction in Gastric Cancer: A Comparison of Three Palliative Methods. J. Surg. Oncol. 2013, 108, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Yamaguchi, K.; Okumura, N.; Tanahashi, T.; Kodera, Y. Is Conversion Therapy Possible in stage IV Gastric Cancer: The Proposal of New Biological Categories of Classification. Gastric Cancer. 2016, 19, 329–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, K.; Yoshida, K.; Tanahashi, T.; Takahashi, T.; Matsuhashi, N.; Tanaka, Y.; Tanabe, K.; Ohdan, H. The Long-Term Survival of stage IV Gastric Cancer Patients with Conversion Therapy. Gastric Cancer 2018, 21, 315–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).