Application of Magnetic Nanoparticles for Rapid Detection and In Situ Diagnosis in Clinical Oncology
Abstract
:Simple Summary
Abstract
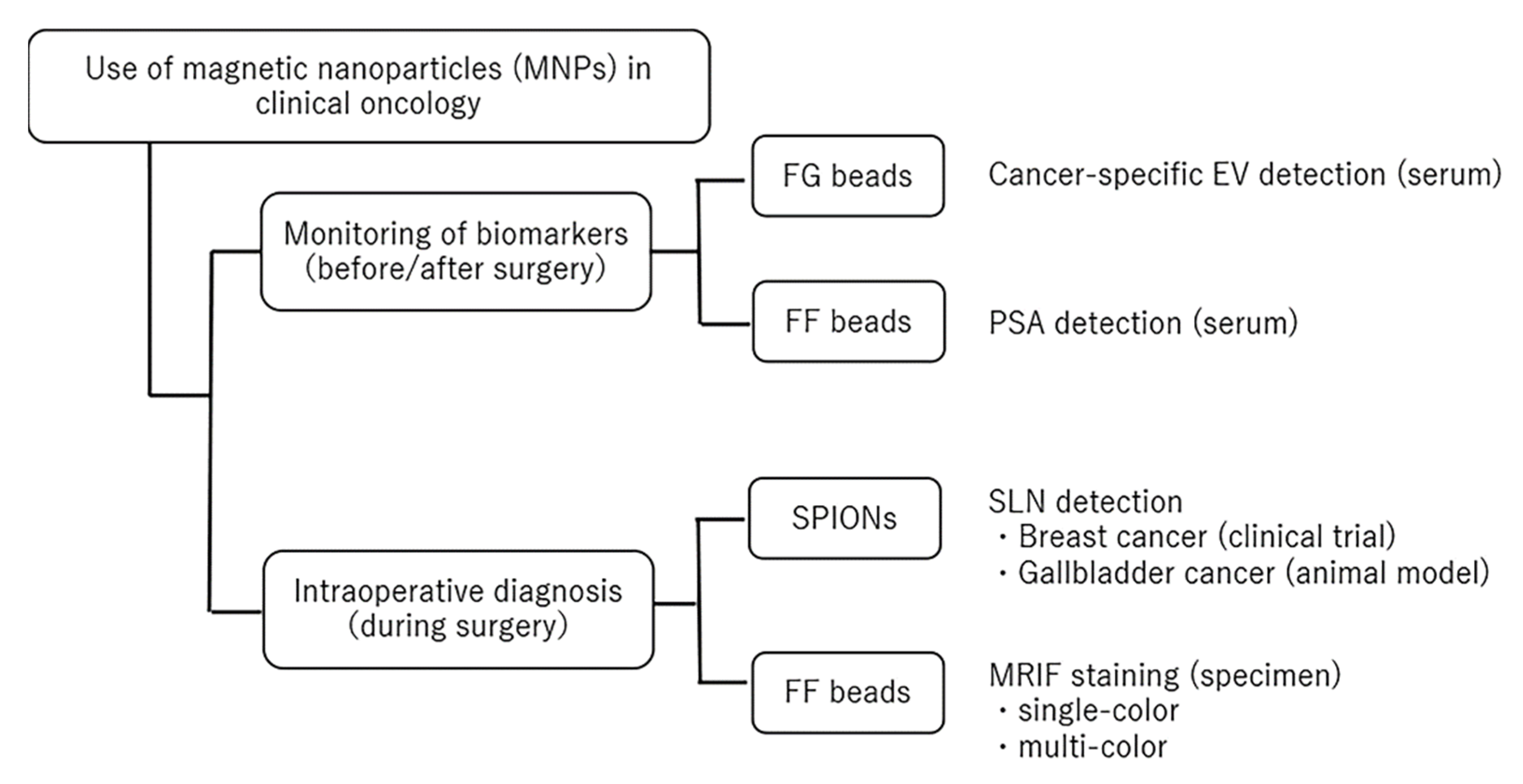
1. Introduction
2. Monitoring Biomarkers before and after Surgery
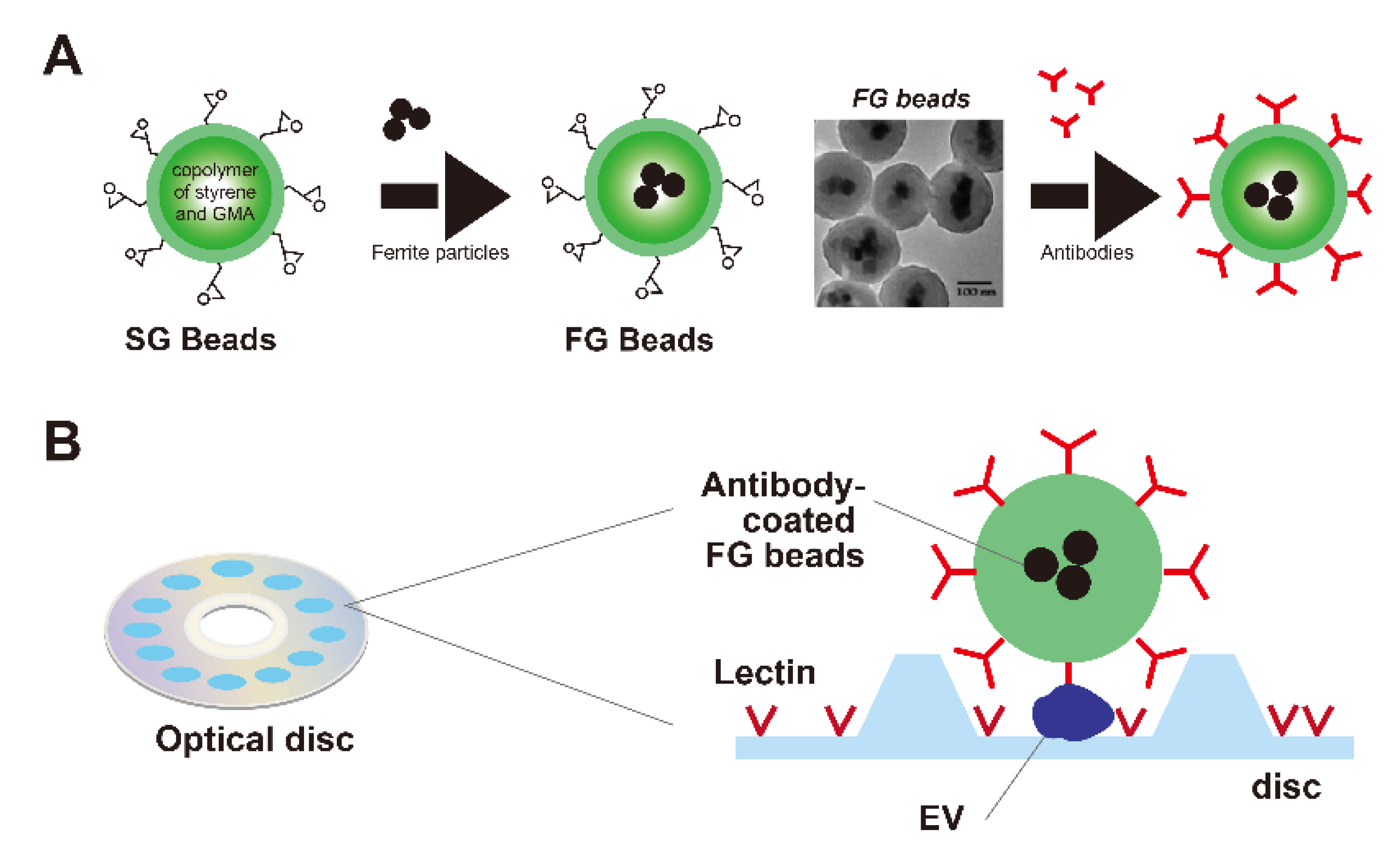
2.1. Measuring a Biomarker in Serum Using FG Beads
2.1.1. FG Beads
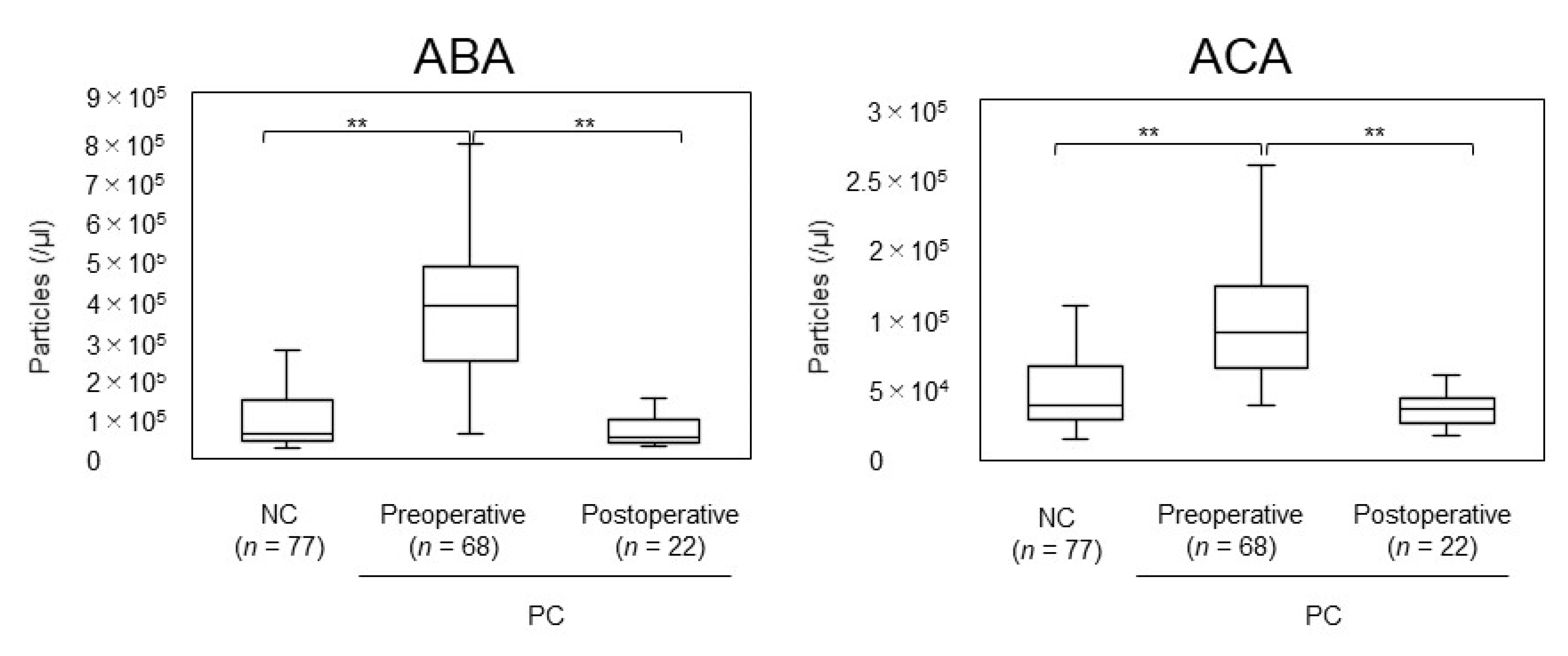
2.1.2. Screening or Monitoring of EVs with FG Beads
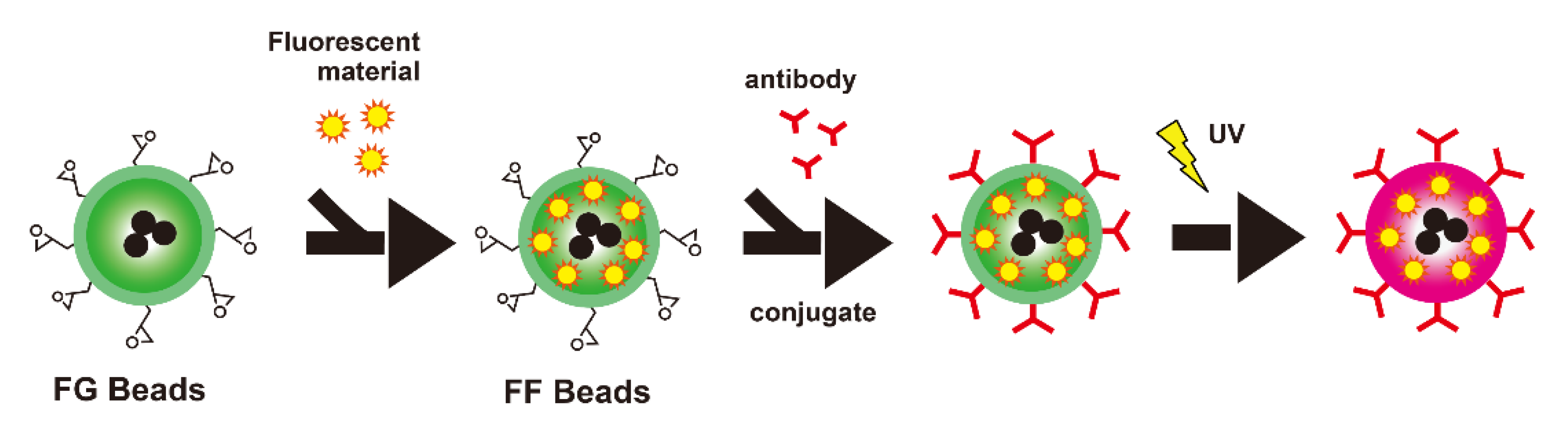
2.2. Measuring a Biomarker in Serum Using FF Beads
2.2.1. FF Beads
2.2.2. Screening or Monitoring of Cancer Biomarkers with FF Beads
3. Intraoperative Diagnosis during Surgery
3.1. Detecting Sentinel Node during Surgery Using SPIONs
3.1.1. SPIONs
3.1.2. Magnetic Probes
Magnetic Probes for Breast Cancer
Magnetic Probe for Laparoscopic Study
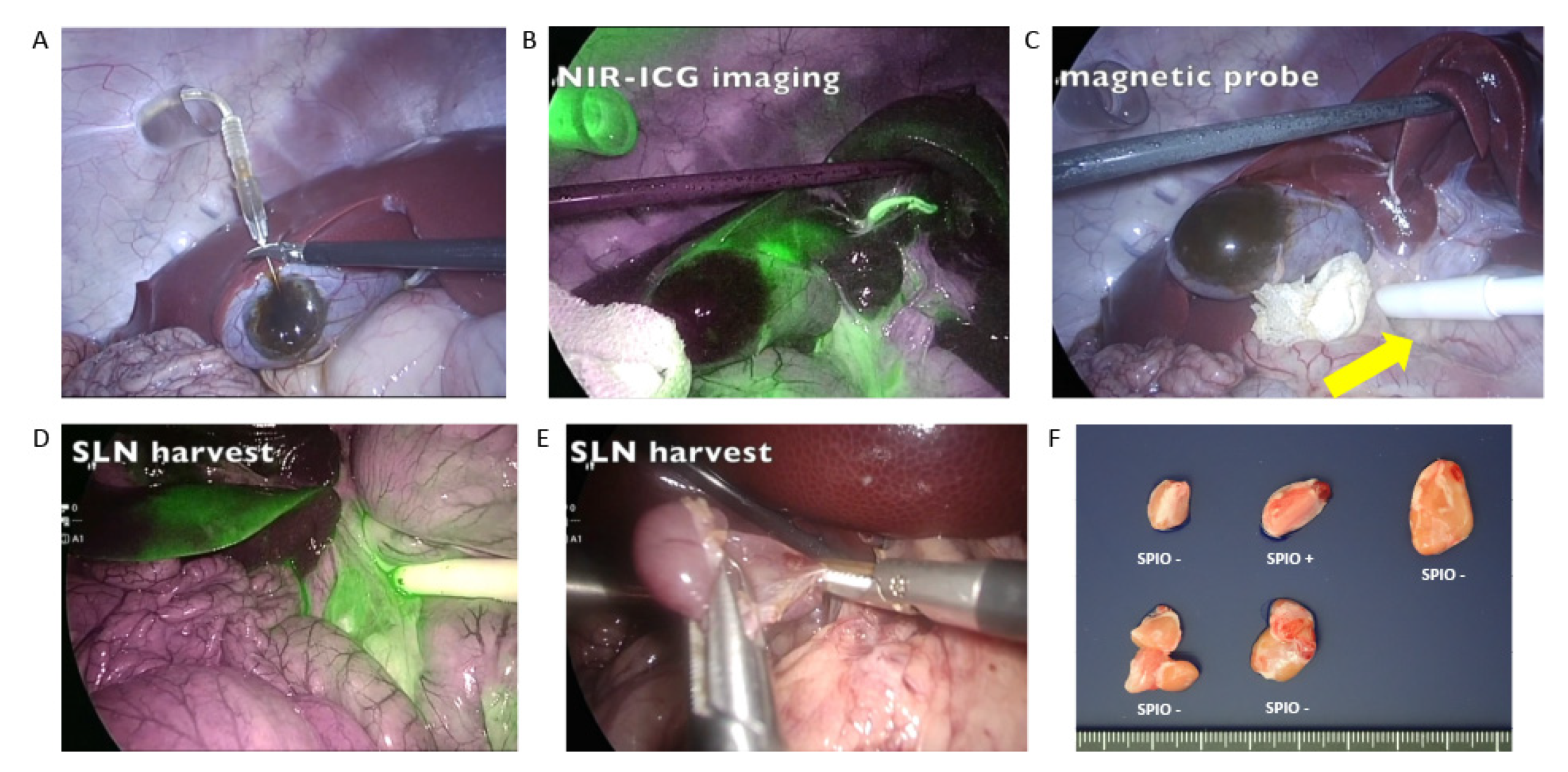
3.1.3. SLN Detection during Surgery
Breast Cancer
Gallbladder Cancer
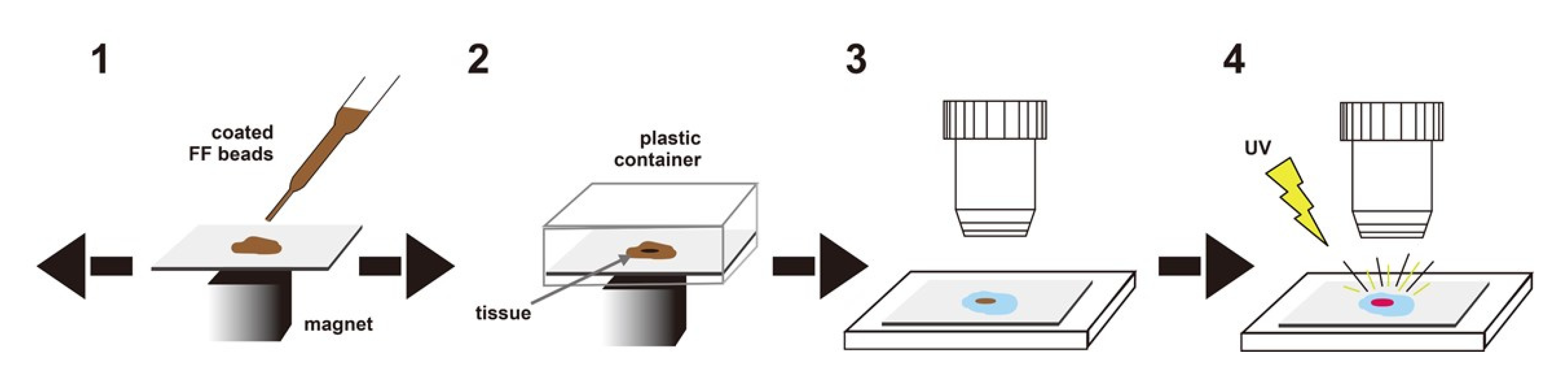
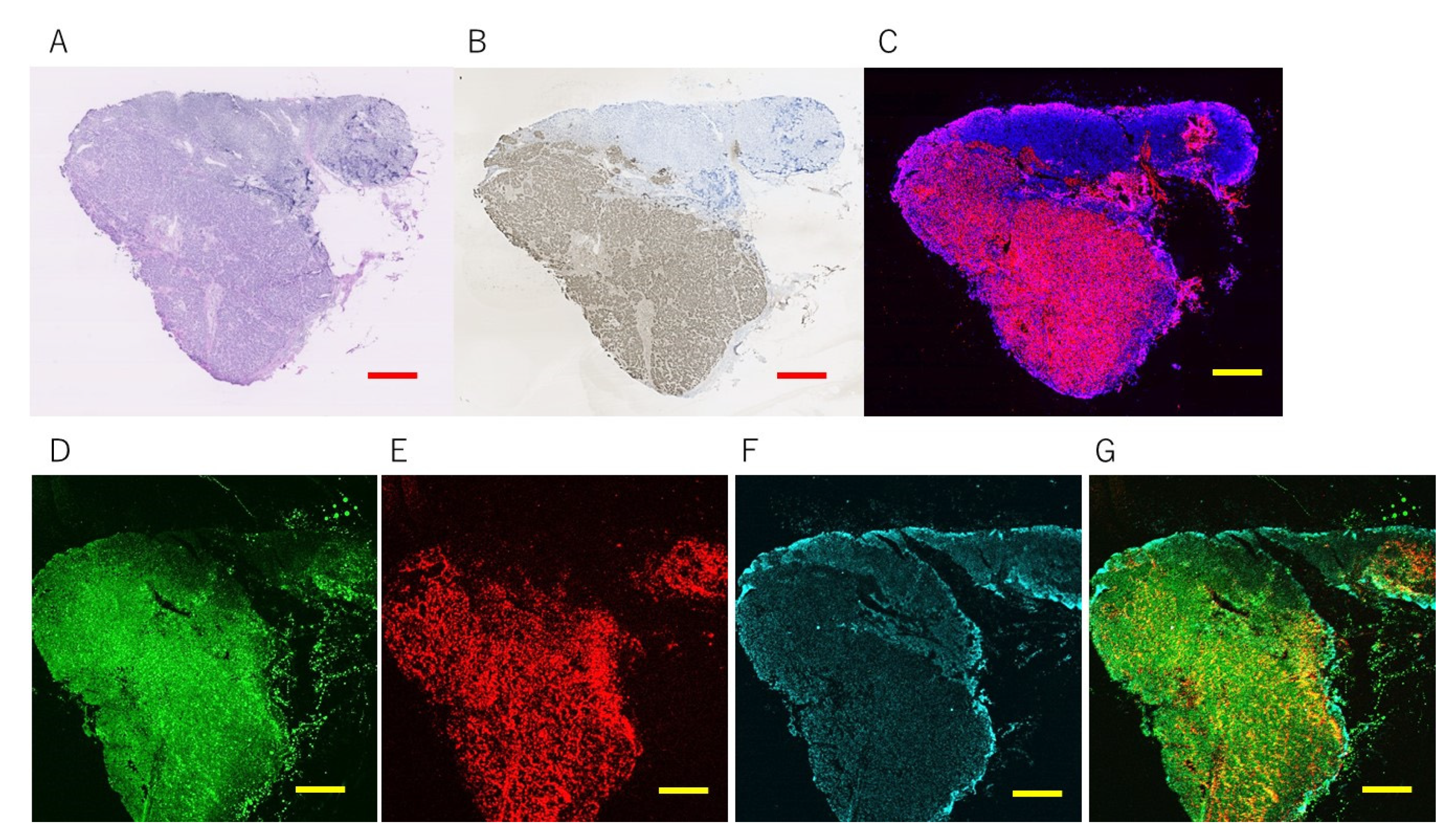
3.2. Magnetically Promoted Rapid Immunofluorescence (MRIF) Staining Using FF Beads
3.2.1. Europium Single Staining
3.2.2. Multi-Colored Staining
4. Discussion and Future Perspectives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dadfar, S.M.; Roemhild, K.; Drude, N.; Von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef] [PubMed]
- Plouffe, B.; Murthy, S.K.; Lewis, L.H. Fundamentals and application of magnetic particles in cell isolation and enrichment: A review. Rep. Prog. Phys. 2015, 78, 016601. [Google Scholar] [CrossRef] [PubMed]
- Schwerdt, J.I.; Goya, G.F.; Calatayud, M.P.; Herenu, C.B.; Reggiani, P.C.; Goya, R.G. Magnetic field-assisted gene delivery: Achievements and therapeutic potential. Curr. Gene Ther. 2012, 12, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Reimer, P.; Tombach, B. Hepatic MRI with SPIO: Detection and characterization of focal liver lesions. Eur. Radiol. 1998, 8, 1198–1204. [Google Scholar] [CrossRef]
- Engvall, E.; Jonsson, K.; Perlmann, P. Enzyme-linked immunosorbent assay. II. Quantitative assay of protein antigen, immunoglobulin g, by means of enzyme-labelled antigen and antibody-coated tubes. Biochim. Biophys. Acta (BBA)-Protein Struct. 1971, 251, 427–434. [Google Scholar] [CrossRef]
- Engvall, E.; Perlmann, P. Enzyme-linked immunosorbent assay (ELISA) quantitative assay of immunoglobulin G. Immunochemistry 1971, 8, 871–874. [Google Scholar] [CrossRef]
- Van Weemen, B.K.; Schuurs, A.H. The influence of heterologous combinations of antiserum and enzyme-labeled estrogen on the characteristics of estrogen enzyme-immunoassays. Immunochemistry 1975, 12, 667–670. [Google Scholar] [CrossRef]
- Inomata, Y.; Wada, T.; Handa, H.; Fujimoto, K.; Kawaguchi, H. Preparation of DNA-carrying affinity latex and purification of transcription factors with the latex. J. Biomater. Sci. Polym. Ed. 1994, 5, 293–302. [Google Scholar] [CrossRef]
- Nishio, K.; Gokon, N.; Hasegawa, M.; Ogura, Y.; Ikeda, M.; Narimatsu, H.; Tada, M.; Yamaguchi, Y.; Sakamoto, S.; Abe, M.; et al. Identification of a chemical substructure that is immobilized to ferrite nanoparticles (FP). Colloids Surf. B Biointerfaces 2007, 54, 249–253. [Google Scholar] [CrossRef]
- Nishio, K.; Masaike, Y.; Ikeda, M.; Narimatsu, H.; Gokon, N.; Tsubouchi, S.; Hatakeyama, M.; Sakamoto, S.; Hanyu, N.; Sandhu, A.; et al. Development of novel magnetic nano-carriers for high-performance affinity purification. Colloids Surf. B Biointerfaces 2008, 64, 162–169. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Kalra, H.; Drummen, G.P.C.; Mathivanan, S. Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int. J. Mol. Sci. 2016, 17, 170. [Google Scholar] [CrossRef] [Green Version]
- Belov, L.; Matic, K.J.; Hallal, S.; Best, G.; Mulligan, S.P.; Christopherson, R.I. Extensive surface protein profiles of extracellular vesicles from cancer cells may provide diagnostic signatures from blood samples. J. Extracell. Vesicles 2016, 5, 25355. [Google Scholar] [CrossRef]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-’t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef]
- Millimaggi, D.; Mari, M.; D’Ascenzo, S.; Carosa, E.; Jannini, E.A.; Zucker, S.; Carta, G.; Pavan, A.; Dolo, V. Tumor Vesicle—Associated CD147 Modulates the Angiogenic Capability of Endothelial Cells. Neoplasia 2007, 9, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Yoshioka, Y.; Kosaka, N.; Konishi, Y.; Ohta, H.; Okamoto, H.; Sonoda, H.; Nonaka, R.; Yamamoto, H.; Ishii, H.; Mori, M.; et al. Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat. Commun. 2014, 5, 3591. [Google Scholar] [CrossRef]
- Marleau, A.M.; Chen, C.-S.; Joyce, J.A.; Tullis, R.H. Exosome removal as a therapeutic adjuvant in cancer. J. Transl. Med. 2012, 10, 134. [Google Scholar] [CrossRef] [Green Version]
- Ueda, K.; Ishikawa, N.; Tatsuguchi, A.; Saichi, N.; Fujii, R.; Nakagawa, H. Antibody-coupled monolithic silica microtips for highthroughput molecular profiling of circulating exosomes. Sci. Rep. 2014, 4, srep06232. [Google Scholar] [CrossRef]
- Gardiner, C.; Ferreira, Y.J.; Dragovic, R.A.; Redman, C.W.G.; Sargent, I.L. Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis. J. Extracell. Vesicles 2013, 2, 19671. [Google Scholar] [CrossRef]
- Tatischeff, I.; Larquet, E.; Falcon-Perez, J.M.; Turpin, P.-Y.; Kruglik, S.G. Fast characterisation of cell-derived extracellular vesicles by nanoparticles tracking analysis, cryo-electron microscopy, and Raman tweezers microspectroscopy. J. Extracell. Vesicles 2012, 1, 19179. [Google Scholar] [CrossRef]
- Coumans, F.A.W.; van der Pol, E.; Böing, A.N.; Hajji, N.; Sturk, G.; van Leeuwen, T.; Nieuwland, R. Reproducible extracellular vesicle size and concentration determination with tunable resistive pulse sensing. J. Extracell. Vesicles 2014, 3, 25922. [Google Scholar] [CrossRef]
- Maas, S.L.N.; De Vrij, J.; Broekman, M.L.D. Quantification and Size-profiling of Extracellular Vesicles Using Tunable Resistive Pulse Sensing. J. Vis. Exp. 2014, 8, 1443–1458. [Google Scholar] [CrossRef]
- Logozzi, M.; De Milito, A.; Lugini, L.; Borghi, M.; Calabro’, L.; Spada, M.; Perdicchio, M.; Marino, M.L.; Federici, C.; Iessi, E.; et al. High Levels of Exosomes Expressing CD63 and Caveolin-1 in Plasma of Melanoma Patients. PLoS ONE 2009, 4, e5219. [Google Scholar] [CrossRef] [Green Version]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. In Current Protocols in Cell Biology; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Yokose, T.; Kabe, Y.; Matsuda, A.; Kitago, M.; Matsuda, S.; Hirai, M.; Nakagawa, T.; Masugi, Y.; Hishiki, T.; Nakamura, Y.; et al. O-Glycan-Altered Extracellular Vesicles: A Specific Serum Marker Elevated in Pancreatic Cancer. Cancers 2020, 12, 2469. [Google Scholar] [CrossRef]
- Sakamoto, S.; Omagari, K.; Kita, Y.; Mochizuki, Y.; Naito, Y.; Kawata, S.; Matsuda, S.; Itano, O.; Jinno, H.; Takeuchi, H.; et al. Magnetically Promoted Rapid Immunoreactions Using Functionalized Fluorescent Magnetic Beads: A Proof of Principle. Clin. Chem. 2014, 60, 610–620. [Google Scholar] [CrossRef] [Green Version]
- Hatakeyama, M.; Mochizuki, Y.; Kita, Y.; Kishi, H.; Nishio, K.; Sakamoto, S.; Abe, M.; Handa, H. Characterization of a magnetic carrier encapsulating europium and ferrite nanoparticles for biomolecular recognition and imaging. J. Magn. Magn. Mater. 2009, 321, 1364–1367. [Google Scholar] [CrossRef]
- Kabe, Y.; Sakamoto, S.; Hatakeyama, M.; Yamaguchi, Y.; Suematsu, M.; Itonaga, M.; Handa, H. Application of high-performance magnetic nanobeads to biological sensing devices. Anal. Bioanal. Chem. 2019, 411, 1825–1837. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.K.; Zetter, B.R. Cancer biomarkers: Knowing the present and predicting the future. Future Oncol. 2005, 1, 37–50. [Google Scholar] [CrossRef]
- Finne, P.; Auvinen, A.; Määttänen, L.; Tammela, T.L.; Ruutu, M.; Juusela, H.; Martikainen, P.; Hakama, M.; Stenman, U.-H. Diagnostic value of free prostate-specific antigen among men with a prostate-specific antigen level of <3.0 microg per liter. Eur Urol. 2008, 54, 362–370. [Google Scholar] [CrossRef]
- Krag, D.N.; Weaver, D.L.; Alex, J.C.; Fairbank, J.T. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg. Oncol. 1993, 2, 335–340. [Google Scholar] [CrossRef]
- Schneebaum, S.; Stadler, J.; Cohen, M.; Yaniv, D.; Baron, J.; Skornick, Y. Gamma probe-guided sentinel node biopsy—optimal timing for injection. Eur. J. Surg. Oncol. 1998, 24, 515–519. [Google Scholar] [CrossRef]
- Vander, A.J.; Sherman, J.H.; Luciano, D.S. Human Physiology: The Mechanisms of Body Function; Mcgraw-Hill: New York, NY, USA, 1994. [Google Scholar]
- Morton, D.L.; Thompson, J.F.; Cochran, A.J.; Mozzillo, N.; Elashoff, R.; Essner, R.; Nieweg, O.E.; Roses, D.F.; Hoekstra, H.J.; Karakousis, C.P.; et al. Sentinel-Node Biopsy or Nodal Observation in Melanoma. N. Engl. J. Med. 2006, 355, 1307–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, C.E.; Kiluk, J.V.; Riker, A.I.; Cox, J.M.; Allred, N.; Ramos, D.C.; Dupont, E.L.; Vrcel, V.; Diaz, N.; Boulware, D. Significance of Sentinel Lymph Node Micrometastases in Human Breast Cancer. J. Am. Coll. Surg. 2008, 206, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.L.; Wen, D.-R.; Wong, J.H.; Economou, J.S.; Cagle, L.A.; Storm, F.K.; Foshag, L.J.; Cochran, A.J. Technical Details of Intraoperative Lymphatic Mapping for Early Stage Melanoma. Arch. Surg. 1992, 127, 392–399. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Kirgan, D.M.; Guenther, J.M.; Morton, D.L. Lymphatic Mapping and Sentinel Lymphadenectomy for Breast Cancer. Ann. Surg. 1994, 220, 391–401. [Google Scholar] [CrossRef]
- Tsioulias, G.J.; Wood, T.F.; Morton, N.L.; Bilchik, A.J. Lymphatic mapping and focused analysis of sentinel lymph nodes upstage gastrointestinal neoplasms. Arch. Surg. 2000, 135, 926–932. [Google Scholar] [CrossRef] [Green Version]
- Gasparri, M.L.; Caserta, D.; Panici, P.B.; Papadia, A.; Mueller, M.D. Surgical staging in endometrial cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 213–221. [Google Scholar] [CrossRef]
- Tajima, Y.; Murakami, M.; Yamazaki, K.; Masuda, Y.; Kato, M.; Sato, A.; Goto, S.; Otsuka, K.; Kato, T.; Kusano, M. Sentinel Node Mapping Guided by Indocyanine Green Fluorescence Imaging During Laparoscopic Surgery in Gastric Cancer. Ann. Surg. Oncol. 2010, 17, 1787–1793. [Google Scholar] [CrossRef]
- McMasters, K.M.; Tuttle, T.; Carlson, D.J.; Brown, C.M.; Noyes, R.D.; Glaser, R.L.; Vennekotter, D.J.; Turk, P.S.; Tate, P.S.; Sardi, A.; et al. Sentinel Lymph Node Biopsy for Breast Cancer: A Suitable Alternative to Routine Axillary Dissection in Multi-Institutional Practice When Optimal Technique Is Used. J. Clin. Oncol. 2000, 18, 2560–2566. [Google Scholar] [CrossRef]
- Kato, N.; Takahashi, M.; Tsuji, T.; Ihara, S.; Brautigam, M.; Miyazawa, T. Dose-Dependency and Rate of Decay of Efficacy of Resovist on MR Images in a Rat Cirrhotic Liver Model. Investig. Radiol. 1999, 34, 551–557. [Google Scholar] [CrossRef]
- Douek, M.; On behalf of the SentiMAG Trialists Group; Klaase, J.; Monypenny, I.; Kothari, A.; Zechmeister, K.; Brown, D.; Wyld, L.; Drew, P.; Garmo, H.; et al. Sentinel Node Biopsy Using a Magnetic Tracer Versus Standard Technique: The SentiMAG Multicentre Trial. Ann. Surg. Oncol. 2014, 21, 1237–1245. [Google Scholar] [CrossRef]
- Ghilli, M.; Carretta, E.; Di Filippo, F.; Battaglia, C.; Fustaino, L.; Galanou, I.; Rucci, P.; Fantini, M.; Roncella, M. The superparamagnetic iron oxide tracer: A valid alternative in sentinel node biopsy for breast cancer treatment. Eur. J. Cancer Care 2015, 26, e12385. [Google Scholar] [CrossRef]
- Houpeau, J.-L.; Chauvet, M.-P.; Guillemin, F.; Bendavid-Athias, C.; Charitansky, H.; Kramar, A.; Giard, S. Sentinel lymph node identification using superparamagnetic iron oxide particles versus radioisotope: The French Sentimag feasibility trial. J. Surg. Oncol. 2016, 113, 501–507. [Google Scholar] [CrossRef]
- Karakatsanis, A.; Christiansen, P.M.; Fischer, L.; Hedin, C.; Pistioli, L.; Sund, M.; Rasmussen, N.R.; Jørnsgård, H.; Tegnelius, D.; Eriksson, S.; et al. The Nordic SentiMag trial: A comparison of super paramagnetic iron oxide (SPIO) nanoparticles versus Tc(99) and patent blue in the detection of sentinel node (SN) in patients with breast cancer and a meta-analysis of earlier studies. Breast Cancer Res. Treat. 2016, 157, 281–294. [Google Scholar] [CrossRef] [Green Version]
- Piñero-Madrona, A.; Torró-Richart, J.; de León-Carrillo, J.; de Castro-Parga, G.; Navarro-Cecilia, J.; Domínguez-Cunchillos, F.; Román-Santamaría, J.; Fuster-Diana, C.; Pardo-García, R. Superparamagnetic iron oxide as a tracer for sentinel node biopsy in breast cancer: A comparative non-inferiority study. Eur. J. Surg. Oncol. 2015, 41, 991–997. [Google Scholar] [CrossRef]
- Rubio, I.; Diaz-Botero, S.; Esgueva, A.; Rodriguez, R.; Cortadellas, T.; Cordoba, O.; Espinosa-Bravo, M. The superparamagnetic iron oxide is equivalent to the Tc99 radiotracer method for identifying the sentinel lymph node in breast cancer. Eur. J. Surg. Oncol. 2015, 41, 46–51. [Google Scholar] [CrossRef]
- Thill, M.; Kurylcio, A.; Welter, R.; van Haasteren, V.; Grosse, B.; Berclaz, G.; Polkowski, W.; Hauser, N. The Central-European SentiMag study: Sentinel lymph node biopsy with superparamagnetic iron oxide (SPIO) vs. radioisotope. Breast 2014, 23, 175–179. [Google Scholar] [CrossRef] [Green Version]
- Elrefai, A.L.; Sasada, I. Magnetic particle detection in unshielded environment using orthogonal fluxgate gradiometer. J. Appl. Phys. 2015, 117, 17C114. [Google Scholar] [CrossRef]
- Karo, H.; Sasada, I. Superparamagnetic nanoparticle detection system by using a fundamental mode orthogonal fluxgate (FM-OFG) gradiometer. AIP Adv. 2017, 7, 56716. [Google Scholar] [CrossRef] [Green Version]
- Paperno, E. Suppression of magnetic noise in the fundamental-mode orthogonal fluxgate. Sens. Actuators A Phys. 2004, 116, 405–409. [Google Scholar] [CrossRef]
- Sasada, I. Orthogonal fluxgate mechanism operated with dc biased excitation. J. Appl. Phys. 2002, 91, 7789. [Google Scholar] [CrossRef]
- Cousins, A.; Balalis, G.L.; Thompson, S.K.; Morales, D.F.; Mohtar, A.; Wedding, A.B.; Thierry, B. Novel Handheld Magnetometer Probe Based on Magnetic Tunnelling Junction Sensors for Intraoperative Sentinel Lymph Node Identification. Sci. Rep. 2015, 5, 10842. [Google Scholar] [CrossRef] [Green Version]
- Visscher, M.; Waanders, S.; Krooshoop, H.; Haken, B.T. Selective detection of magnetic nanoparticles in biomedical applications using differential magnetometry. J. Magn. Magn. Mater. 2014, 365, 31–39. [Google Scholar] [CrossRef]
- Waanders, S.; Visscher, M.; Wildeboer, R.R.; Oderkerk, T.O.B.; Krooshoop, H.J.G.; Haken, B.T. A handheld SPIO-based sentinel lymph node mapping device using differential magnetometry. Phys. Med. Biol. 2016, 61, 8120–8134. [Google Scholar] [CrossRef]
- Sekino, M.; Kuwahata, A.; Ookubo, T.; Shiozawa, M.; Ohashi, K.; Kaneko, M.; Saito, I.; Inoue, Y.; Ohsaki, H.; Takei, H.; et al. Handheld magnetic probe with permanent magnet and Hall sensor for identifying sentinel lymph nodes in breast cancer patients. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mellor, K.L.; Powell, A.; Lewis, W.G. Laparoscopic Surgery’s 100 Most Influential Manuscripts: A Bibliometric Analysis. Surg. Laparosc. Endosc. Percutaneous Tech. 2018, 28, 13–19. [Google Scholar] [CrossRef]
- Mihara, K.; Matsuda, S.; Nakamura, Y.; Aiura, K.; Kuwahata, A.; Chikaki, S.; Sekino, M.; Kusakabe, M.; Suzuki, S.; Fuchimoto, D.; et al. Intraoperative laparoscopic detection of sentinel lymph nodes with indocyanine green and superparamagnetic iron oxide in a swine gallbladder cancer model. PLoS ONE 2021, 16, e0248531. [Google Scholar] [CrossRef]
- Johnson, L.; Pinder, S.E.; Douek, M. Deposition of superparamagnetic iron-oxide nanoparticles in axillary sentinel lymph nodes following subcutaneous injection. Histopathology 2012, 62, 481–486. [Google Scholar] [CrossRef]
- Takeuchi, H.; Oyama, T.; Kamiya, S.; Nakamura, R.; Takahashi, T.; Wada, N.; Saikawa, Y.; Kitagawa, Y. Laparoscopy-assisted Proximal Gastrectomy with Sentinel Node Mapping for Early Gastric Cancer. World J. Surg. 2011, 35, 2463–2471. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Takeuchi, H.; Takagi, Y.; Natsugoe, S.; Terashima, M.; Murakami, N.; Fujimura, T.; Tsujimoto, H.; Hayashi, H.; Yoshimizu, N.; et al. Sentinel Node Mapping for Gastric Cancer: A Prospective Multicenter Trial in Japan. J. Clin. Oncol. 2013, 31, 3704–3710. [Google Scholar] [CrossRef]
- Kuwahata, A.; Tanaka, R.; Matsuda, S.; Amada, E.; Irino, T.; Mayanagi, S.; Chikaki, S.; Saito, I.; Tanabe, N.; Kawakubo, H.; et al. Development of Magnetic Probe for Sentinel Lymph Node Detection in Laparoscopic Navigation for Gastric Cancer Patients. Sci. Rep. 2020, 10, 1798. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Kawaharada, Y.; Ogawa, J.-I.; Saito, H.; Kudo, S.; Takashima, S.; Saito, Y.; Atari, M.; Ito, A.; Terata, K.; et al. Development of a New Magnetometer for Sentinel Lymph Node Mapping Designed for Video-Assisted Thoracic Surgery in Non–Small Cell Lung Cancer. Surg. Innov. 2015, 22, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Zada, A.; Peek, M.C.L.; Ahmed, M.; Anninga, B.; Baker, R.; Kusakabe, M.; Sekino, M.; Klaase, J.M.; Haken, B.T.; Douek, M. Meta-analysis of sentinel lymph node biopsy in breast cancer using the magnetic technique. Br. J. Surg. 2016, 103, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Taruno, K.; Kurita, T.; Kuwahata, A.; Yanagihara, K.; Enokido, K.; Katayose, Y.; Nakamura, S.; Takei, H.; Sekino, M.; Kusakabe, M. Multicenter clinical trial on sentinel lymph node biopsy using superparamagnetic iron oxide nanoparticles and a novel handheld magnetic probe. J. Surg. Oncol. 2019, 120, 1391–1396. [Google Scholar] [CrossRef]
- He, M.; Jiang, Z.; Wang, C.; Hao, Z.; An, J.; Shen, J. Diagnostic value of near-infrared or fluorescent indocyanine green guided sentinel lymph node mapping in gastric cancer: A systematic review and meta-analysis. J. Surg. Oncol. 2018, 118, 1243–1256. [Google Scholar] [CrossRef]
- Liberale, G.; Bohlok, A.; Bormans, A.; Bouazza, F.; Galdon, M.G.; El Nakadi, I.; Bourgeois, P.; Donckier, V. Indocyanine green fluorescence imaging for sentinel lymph node detection in colorectal cancer: A systematic review. Eur. J. Surg. Oncol. 2018, 44, 1301–1306. [Google Scholar] [CrossRef]
- Currie, A.; Brigic, A.; Thomas-Gibson, S.; Suzuki, N.; Moorghen, M.; Jenkins, J.; Faiz, O.; Kennedy, R. A pilot study to assess near infrared laparoscopy with indocyanine green (ICG) for intraoperative sentinel lymph node mapping in early colon cancer. Eur. J. Surg. Oncol. 2017, 43, 2044–2051. [Google Scholar] [CrossRef]
- Black, C.; Marotti, J.; Zarovnaya, E.; Paydarfar, J. Critical evaluation of frozen section margins in head and neck cancer resections. Cancer 2006, 107, 2792–2800. [Google Scholar] [CrossRef]
- Esbona, K.; Li, Z.; Wilke, L.G. Intraoperative Imprint Cytology and Frozen Section Pathology for Margin Assessment in Breast Conservation Surgery: A Systematic Review. Ann. Surg. Oncol. 2012, 19, 3236–3245. [Google Scholar] [CrossRef] [Green Version]
- Hatta, H.; Tsuneyama, K.; Kondo, T.; Takano, Y. Development of an Ultrasound-emitting Device for Performing Rapid Immunostaining Procedures. J. Histochem. Cytochem. 2010, 58, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Leong, A.S.-Y.; Daymon, M.E.; Milios, J. Microwave irradiation as a form of fixation for light and electron microscopy. J. Pathol. 1985, 146, 313–321. [Google Scholar] [CrossRef]
- Onishi, T.; Matsuda, S.; Nakamura, Y.; Kuramoto, J.; Tsuruma, A.; Sakamoto, S.; Suzuki, S.; Fuchimoto, D.; Onishi, A.; Chikaki, S.; et al. Magnetically Promoted Rapid Immunofluorescence Staining for Frozen Tissue Sections. J. Histochem. Cytochem. 2019, 67, 575–587. [Google Scholar] [CrossRef]
- Sekino, M.; Kuwahata, A.; Fujita, S.; Matsuda, S.; Kaneko, M.; Chikaki, S.; Sakamoto, S.; Saito, I.; Handa, H.; Kusakabe, M. Development of an optimized dome-shaped magnet for rapid magnetic immunostaining. AIP Adv. 2020, 10, 025317. [Google Scholar] [CrossRef]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef]
- Novis, D.A.; Zarbo, R.J. Interinstitutional comparison of frozen section turnaround time. A College of American Pathologists Q-Probes study of 32868 frozen sections in 700 hospitals. Arch. Pathol. Lab. Med. 1997, 121, 559–567. [Google Scholar]
- Dadfar, S.M.; Camozzi, D.; Darguzyte, M.; Roemhild, K.; Varvarà, P.; Metselaar, J.; Banala, S.; Straub, M.; Güvener, N.; Engelmann, U.; et al. Size-isolation of superparamagnetic iron oxide nanoparticles improves MRI, MPI and hyperthermia performance. J. Nanobiotechnol. 2020, 18, 1–13. [Google Scholar] [CrossRef]

| Measure | Probe for Breast Cancer | Probe for Laparoscopic Surgery |
|---|---|---|
| Appearance |  |  |
| Total length | 24.5 cm | 58.5 cm |
| Handle length | 16 cm | 17 cm |
| Shaft length | 7 cm | 37 cm |
| Head size (diameter) | 18 mm | 10 mm |
| Weight | 100 g | 150 g |
| Detection range (140 μg Resovist) | 8 mm | 6mm |
| Immunostaining Method | MRIF | |||
|---|---|---|---|---|
| Result | + | − | Total | |
| Conventional Immunostaining | + | 263 | 13 | 276 |
| − | 2 | 8 | 10 | |
| Total | 265 | 21 | 286 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onishi, T.; Mihara, K.; Matsuda, S.; Sakamoto, S.; Kuwahata, A.; Sekino, M.; Kusakabe, M.; Handa, H.; Kitagawa, Y. Application of Magnetic Nanoparticles for Rapid Detection and In Situ Diagnosis in Clinical Oncology. Cancers 2022, 14, 364. https://doi.org/10.3390/cancers14020364
Onishi T, Mihara K, Matsuda S, Sakamoto S, Kuwahata A, Sekino M, Kusakabe M, Handa H, Kitagawa Y. Application of Magnetic Nanoparticles for Rapid Detection and In Situ Diagnosis in Clinical Oncology. Cancers. 2022; 14(2):364. https://doi.org/10.3390/cancers14020364
Chicago/Turabian StyleOnishi, Tatsuya, Kisyo Mihara, Sachiko Matsuda, Satoshi Sakamoto, Akihiro Kuwahata, Masaki Sekino, Moriaki Kusakabe, Hiroshi Handa, and Yuko Kitagawa. 2022. "Application of Magnetic Nanoparticles for Rapid Detection and In Situ Diagnosis in Clinical Oncology" Cancers 14, no. 2: 364. https://doi.org/10.3390/cancers14020364






