Skeletal Muscle Depletion and Major Postoperative Complications in Locally-Advanced Head and Neck Cancer: A Comparison between Ultrasound of Rectus Femoris Muscle and Neck Cross-Sectional Imaging
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection Criteria and Data Collection
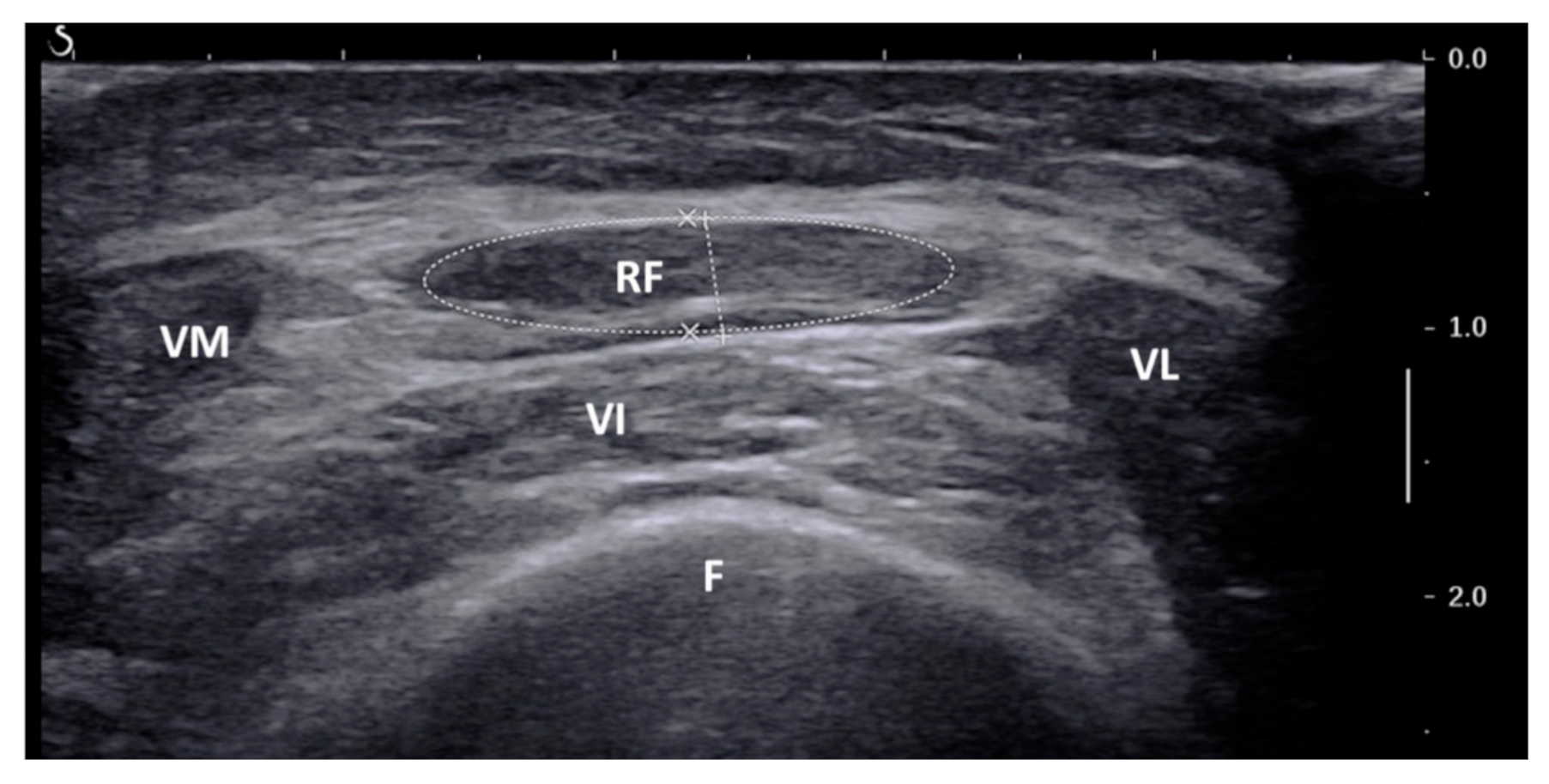
2.2. Ultrasound of Rectus Femoris
2.3. CT/MRI at C3 Level
2.4. Other Clinical Predictors
2.5. Clinical Endpoint: 30-Day Major Postoperative Complications
2.6. Statistics and Data Management
3. Results
3.1. Patients’ Characteristics
3.2. Thirty-Day Major Postoperative Complications
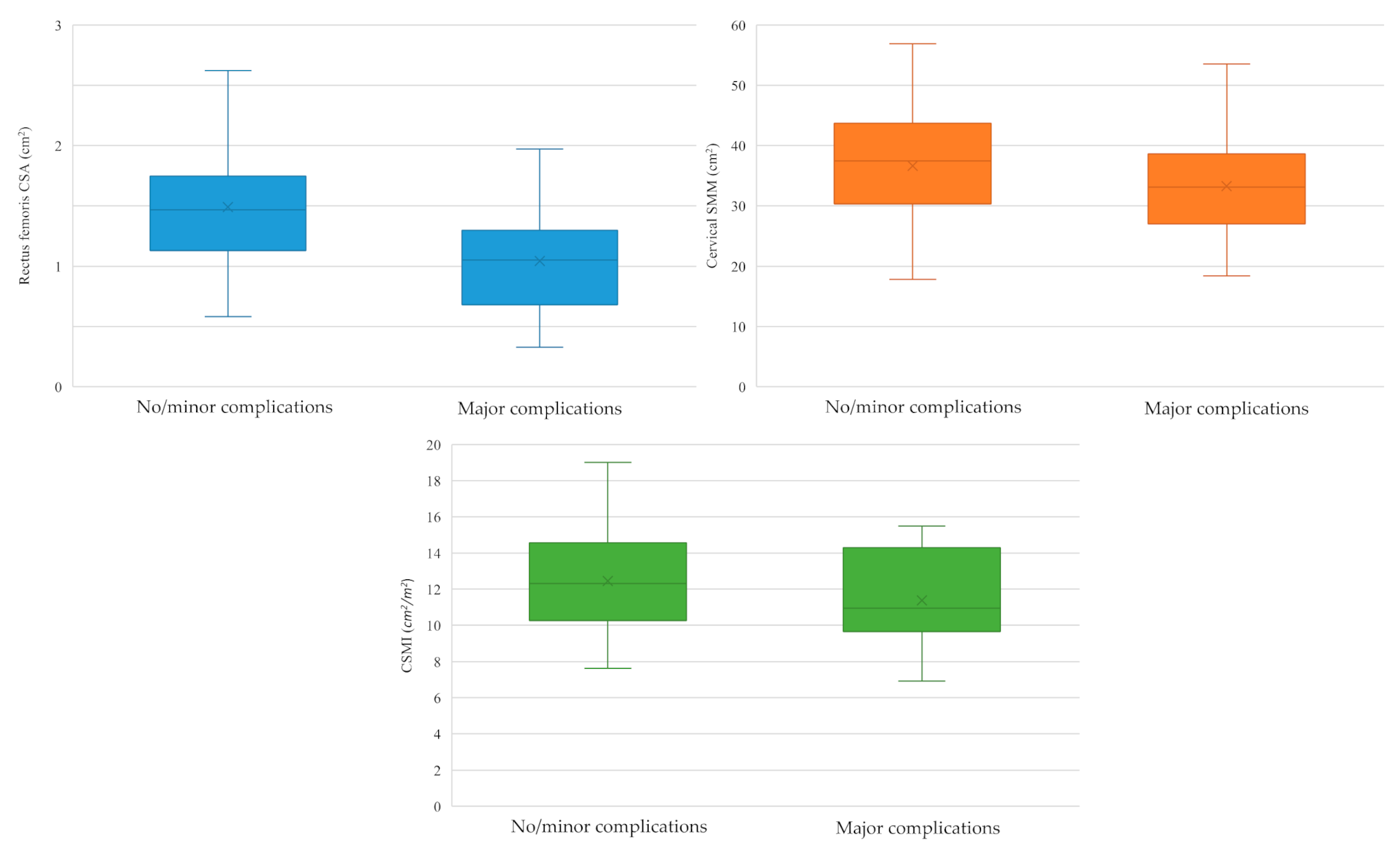
3.3. Sarcopenia and 30-Day Major Postoperative Complications
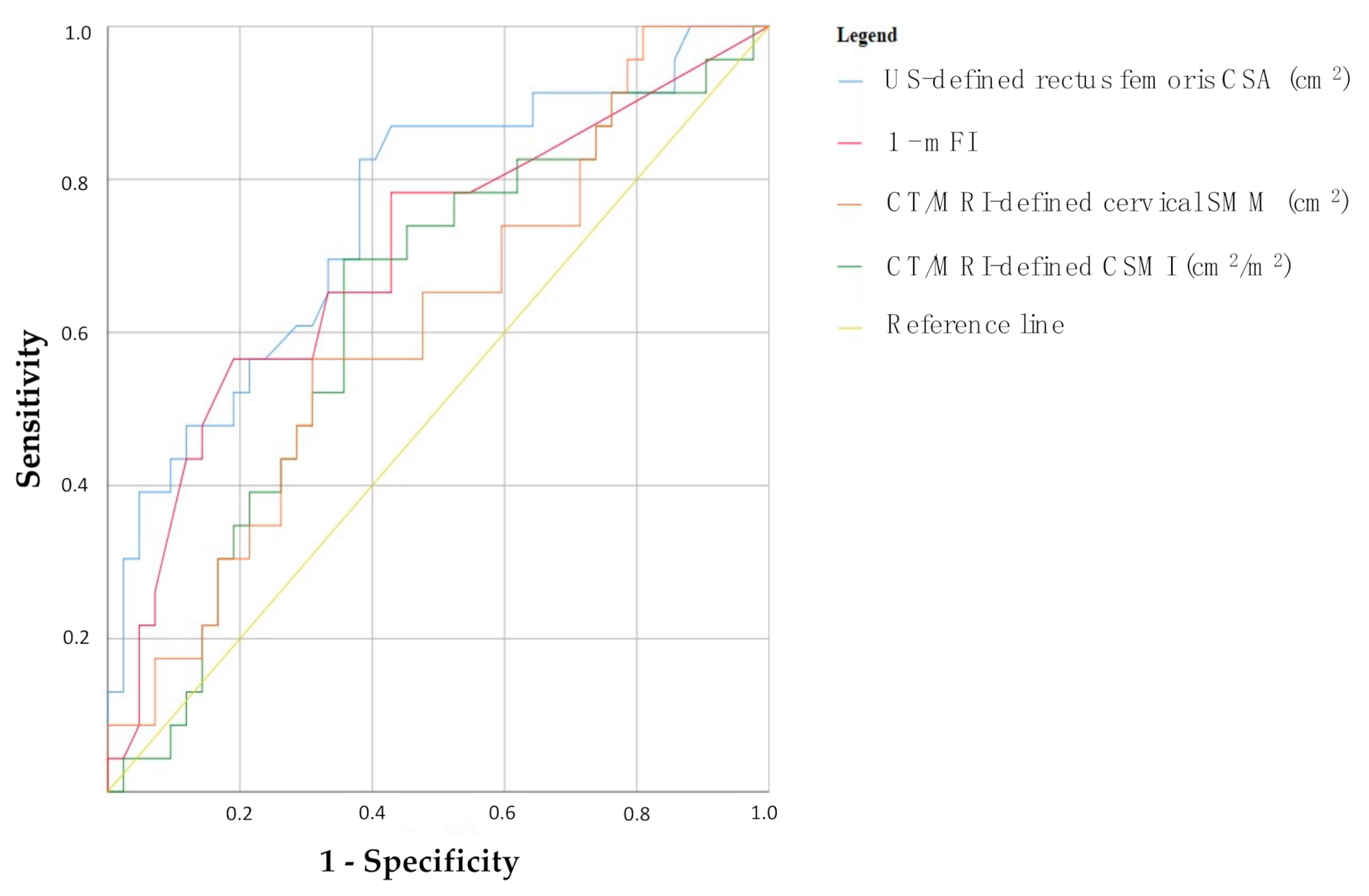
- US-defined rectus femoris CSA: AUC = 0.754, p = 0.001 (95% CI 0.63–0.88);
- 1-mFI: AUC = 0.699, p = 0.008 (95% CI 0.56–0.84);
- CT/MRI-defined cervical SMM: AUC = 0.629, p = 0.086 (95% CI 0.49–0.77);
- CT/MRI-defined CSMI: AUC = 0.612, p = 0.138 (95% CI 0.47–0.75).
- US-defined rectus femoris CSA: 1.32 cm2 (sensitivity 82%, specificity 62%, VPP 55%, VPN 87%; prevalence of low SMM: 54%, 35/65);
- CT/MRI-defined cervical SMM: 34.91 cm2 (sensitivity 70%, specificity 64%, VPP 52%, VPN 79%; prevalence of low SMM: 48%, 31/65);
- CT/MRI-defined CSMI: 11.25 cm2/m2 (sensitivity 57%, specificity 69%, VPP 50%, VPN 74%; prevalence of low SMM: 40%, 26/65).
3.4. Clinical Predictors of 30-Day Major Postoperative Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L.; et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study Global Burden. JAMA Oncol. 2017, 3, 524–548. [Google Scholar]
- Marur, S.; Forastiere, A.A. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2016, 91, 386–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccirillo, J.F.; Feinstein, A.R. Clinical symptoms and comorbidity: Significance for the prognostic classification of cancer. Cancer 1996, 77, 834–842. [Google Scholar] [CrossRef]
- Ferrier, M.B.; Spuesens, E.B.; Le Cessie, S.; Baatenburg De Jong, R.J. Comorbidity as a major risk factor for mortality and complications in head and neck surgery. Arch. Otolaryngol.-Head Neck Surg. 2005, 131, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eley, K.A.; Shah, R.; Bond, S.E.; Watt-Smith, S.R. A review of post-operative feeding in patients undergoing resection and reconstruction for oral malignancy and presentation of a pre-operative scoring system. Br. J. Oral Maxillofac. Surg. 2012, 50, 601–605. [Google Scholar] [CrossRef]
- Togni, L.; Mascitti, M.; Vignigni, A.; Alia, S.; Sartini, D.; Barlattani, A.; Emanuelli, M.; Santarelli, A. Treatment-related dysgeusia in oral and oropharyngeal cancer: A comprehensive review. Nutrients 2021, 13, 3325. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, A.H.; Tajima, T.; Kawakami, K.T.; Wang, M.B.; Tomlinson, J. The Relationship Between Measures of Nutritional Status and Masticatory Function in Untreated Patients With Head and Neck Cancer. J. Oral Maxillofac. Surg. 2008, 66, 85–92. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, A.; Li, Y.; Ding, W.; Meng, H.; Luo, P.; Zhang, J. Age and Mutations as Predictors of the Response to Immunotherapy in Head and Neck Squamous Cell Cancer. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef]
- Alshadwi, A.; Nadershah, M.; Carlson, E.R.; Young, L.S.; Burke, P.A.; Daley, B.J. Nutritional considerations for head and neck cancer patients: A review of the literature. J. Oral Maxillofac. Surg. 2013, 71, 1853–1860. [Google Scholar] [CrossRef]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. Clin. Geriatr. Med. 2011, 27, 337–339. [Google Scholar] [CrossRef]
- Ida, S.; Watanabe, M.; Yoshida, N.; Baba, Y.; Umezaki, N.; Harada, K.; Karashima, R.; Imamura, Y.; Iwagami, S.; Baba, H. Sarcopenia is a Predictor of Postoperative Respiratory Complications in Patients with Esophageal Cancer. Ann. Surg. Oncol. 2015, 22, 4432–4437. [Google Scholar] [CrossRef]
- Huang, D.D.; Wang, S.L.; Zhuang, C.L.; Zheng, B.S.; Lu, J.X.; Chen, F.F.; Zhou, C.J.; Shen, X.; Yu, Z. Sarcopenia, as defined by low muscle mass, strength and physical performance, predicts complications after surgery for colorectal cancer. Color Dis. 2015, 17, O256–O264. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Zhuang, C.L.; Huang, D.D.; Pang, W.Y.; Lou, N.; Chen, F.F.; Zhou, C.J.; Shen, X.; Yu, Z. Sarcopenia Adversely Impacts Postoperative Clinical Outcomes Following Gastrectomy in Patients with Gastric Cancer: A Prospective Study. Ann. Surg. Oncol. 2016, 23, 556–564. [Google Scholar] [CrossRef]
- Pecorelli, N.; Carrara, G.; De Cobelli, F.; Cristel, G.; Damascelli, A.; Balzano, G.; Beretta, L.; Braga, M. Effect of sarcopenia and visceral obesity on mortality and pancreatic fistula following pancreatic cancer surgery. Br. J. Surg. 2016, 103, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Harimoto, N.; Shirabe, K.; Yamashita, Y.I.; Ikegami, T.; Yoshizumi, T.; Soejima, Y.; Ikeda, T.; Maehara, Y.; Nishie, A.; Yamanaka, T. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br. J. Surg. 2013, 100, 1523–1530. [Google Scholar] [CrossRef]
- Swartz, J.E.; Pothen, A.J.; Wegner, I.; Smid, E.J.; Swart, K.M.A.; de Bree, R.; Leenen, L.P.H.; Grolman, W. Feasibility of using head and neck CT imaging to assess skeletal muscle mass in head and neck cancer patients. Oral Oncol. 2016, 62, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Galli, A.; Colombo, M.; Carrara, G.; Lira Luce, F.; Paesano, P.L.; Giordano, L.; Bondi, S.; Tulli, M.; Mirabile, A.; De Cobelli, F.; et al. Low skeletal muscle mass as predictor of postoperative complications and decreased overall survival in locally advanced head and neck squamous cell carcinoma: The role of ultrasound of rectus femoris muscle. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 3489–3502. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; American Joint Commission on Cancer, Ed.; Springer International Publishing: New York City, NY, USA, 2017. [Google Scholar]
- Mueller, N.; Murthy, S.; Tainter, C.R.; Lee, J.; Riddell, K.; Fintelmann, F.J.; Grabitz, S.D.; Timm, F.P.; Levi, B.; Kurth, T.; et al. Can sarcopenia quantified by ultrasound of the rectus femoris muscle predict adverse outcome of surgical intensive care unit patients as well as frailty? a prospective, observational cohort study. Ann. Surg. 2016, 264, 1116–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heymsfield, S.B.; Wang, Z.M.; Baumgartner, R.N.; Ross, R. Human body composition: Advances in models and methods. Annu. Rev. Nutr. 1997, 17, 527–558. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; De Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; De Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The clavien-dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [Green Version]
- van Vugt, J.L.A.; Braam, H.J.; van Oudheusden, T.R.; Vestering, A.; Bollen, T.L.; Wiezer, M.J.; de Hingh, I.H.J.T.; van Ramshorst, B.; Boerma, D. Skeletal Muscle Depletion is Associated with Severe Postoperative Complications in Patients Undergoing Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis of Colorectal Cancer. Ann. Surg. Oncol. 2015, 22, 3625–3631. [Google Scholar] [CrossRef]
- O’Neill, J.P.; Shaha, A.R. Nutrition Management of Patients with Malignancies of the Head and Neck. Surg. Clin. N. Am. 2011, 91, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Beeken, L.; Calman, F. A return to “normal eating” after curative treatment for oral cancer. What are the long-term prospects? Eur. J. Cancer. Part B Oral Oncol. 1994, 30, 387–392. [Google Scholar] [CrossRef]
- Mick, R.; Vokes, E.E.; Weichselbaum, R.R.; Panje, W.R. Prognostic factors in advanced head and neck cancer patients undergoing multimodality therapy. Otolaryngol.-Head Neck Surg. 1991, 105, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Achim, V.; Bash, J.; Mowery, A.; Guimaraes, A.R.; Li, R.; Schindler, J.; Wax, M.; Andersen, P.; Clayburgh, D. Prognostic indication of sarcopenia for wound complication after total laryngectomy. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 1159–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, A.R.; Roh, J.L.; Kim, J.S.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. The impact of skeletal muscle depletion on older adult patients with head and neck cancer undergoing primary surgery. J. Geriatr. Oncol. 2021, 12, 128–133. [Google Scholar] [CrossRef]
- Bozkurt, G.; Elhassan, H.A.; Mahmutoğlu, A.S.; Çelebi, İ.; Mcleod, R.W.J.; Soytaş, P.; Erol, Z.N.; Sözen, E. Neck Muscle Mass Index as a Predictor of Post-Laryngectomy Wound Complications. Ann. Otol. Rhinol. Laryngol. 2018, 127, 841–847. [Google Scholar] [CrossRef]
- Bril, S.I.; Pezier, T.F.; Tijink, B.M.; Janssen, L.M.; Braunius, W.W.; de Bree, R. Preoperative low skeletal muscle mass as a risk factor for pharyngocutaneous fistula and decreased overall survival in patients undergoing total laryngectomy. Head Neck 2019, 41, 1745–1755. [Google Scholar] [CrossRef] [Green Version]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [Green Version]


| Parameters | Values (%) | Means ± SD |
|---|---|---|
| Age | 65.9 ± 12.2 years | |
| <70 years ≥70 years | 38 (58) 27 (42) | - |
| Gender | ||
| Male Female | 53 (81) 12 (19) | - |
| Tumor subsite | ||
| Oral cavity/oropharynx Larynx/hypopharynx Parotid | 18 (28) 45 (69) 2 (3) | - |
| Tumor origin | ||
| Primary Persistence/relapse | 46 (71) 19 (29) | - |
| Surgical procedure | ||
| PT/TM resection OPHL TL ± PP Extended parotidectomy | 18 (28) 3 (5) 42 (64) 2 (3) | - |
| Previous radiotherapy | ||
| No Yes | 54 (83) 11 (17) | - |
| Body mass index | 25.3 ± 3.6 Kg/m2 | |
| <18.5 Kg/m2 18.5–24.9 Kg/m2 25–29.9 Kg/m2 ≥30.0 Kg/m2 | 2 (3) 29 (45) 29 (45) 5 (7) | - |
| Smoking habit | - | |
| <20 pack-year ≥20 pack-year | 21 (32) 44 (68) | - |
| Diabetes mellitus | ||
| No Yes | 53 (81) 12 (19) | - |
| Cholinesterase (ChE) | 7.4 ± 1.87 kU/L | |
| Creatine phosphokinase (CPK) | 69.25 ± 33.65 U/L | |
| Hemoglobin (Hb) | 13.41 ± 1.68 g/dL | |
| Weight loss within last 3 months | ||
| <10% ≥10% | 60 (92) 5 (8) | - |
| Surgical time | 373.3 ± 127.8 min | |
| <300 min ≥300 min | 21 (32) 44 (68) | - |
| Albumin (Alb) | 34.64 ± 6.53 g/L | |
| >35.00 g/L ≤35.00 g/L | 35 (54) 30 (46) | - |
| pTNM stage | ||
| III IVa IVb | 20 (31) 28 (43) 17 (26) | - |
| pN class | ||
| pN0 pN+ | 34 (52) 31 (48) | - |
| Free flap reconstruction | ||
| No Yes | 52 (80) 13 (20) | - |
| ASA score | ||
| I II III IV | 1 (2) 37 (57) 25 (38) 2 (3) | - |
| Charlson comorbidity index (CCI) | ||
| <5 ≥5 | 25 (39) 40 (61) | - |
| Modified frailty index (mFI) | ||
| 0–0.05 0.05–0.10 0.10–0.15 0.15–0.20 >0.20 | 19 (29) 17 (26) 14 (22) 6 (9) 9 (14) | - |
| Parameters | 30-Day Major Complications | OR (95% CI) | p * | |
|---|---|---|---|---|
| No (%) | Yes (%) | |||
| Age | ||||
| <70 years ≥70 years | 24 (63) 18 (67) | 14 (37) 9 (33) | 1 [reference] 0.86 (0.30–2.416) | 0.771 |
| Previous radiotherapy | ||||
| No Yes | 38 (70) 4 (36) | 16 (30) 7 (64) | 1 [reference] 4.16 (1.10–16.20) | 0.038 |
| BMI | ||||
| ≥18.5 Kg/m2 <18.5 Kg/m2 | 41 (65) 1 (50) | 22 (35) 1 (50) | 1 [reference] 1.87 (0.11–31.26) | 0.586 |
| Smoking habit | ||||
| <20 pack-year ≥20 pack-year | 13 (62) 29 (66) | 8 (38) 15 (34) | 1 [reference] 0.84 (0.29–2.47) | 0.752 |
| Diabetes mellitus | ||||
| No Yes | 37 (70) 5 (42) | 16 (30) 7 (58) | 1 [reference] 3.24 (0.89–11.75) | 0.068 |
| Weight loss within last 3 months | ||||
| <10% ≥10% | 39 (65) 3 (60) | 21 (35) 2 (40) | 1 [reference] 1.24 (0.19–8.00) | 0.585 |
| Surgical time | ||||
| <300 min ≥300 min | 16 (76) 26 (59) | 5 (24) 18 (41) | 1 [reference] 2.21 (0.69–7.14) | 0.178 |
| Albumin (Alb) | ||||
| >35.00 g/L ≤35.00 g/L | 24 (69) 18 (60) | 11 (31) 12 (40) | 1 [reference] 1.45 (0.52–4.04) | 0.471 |
| pTNM stage | ||||
| III IVa-IVb | 13 (65) 29 (64) | 7 (35) 16 (36) | 1 [reference] 1.03 (0.34–3.09) | 0.966 |
| Free flap | ||||
| No Yes | 34 (65) 8 (61) | 18 (35) 5 (39) | 1 [reference] 1.18 (0.33–4.14) | 0.518 |
| ASA score | ||||
| I–II III–IV | 28 (74) 14 (52) | 10 (26) 13 (48) | 1 [reference] 2.60 (0.91–7.39) | 0.061 |
| Charlson comorbidity index (CCI) | ||||
| <5 ≥5 | 18 (72) 24 (60) | 7 (28) 16 (40) | 1 [reference] 1.71 (0.58–5.04) | 0.325 |
| Modified frailty index (mFI) | ||||
| ≤0.10 >0.10 | 28 (78) 14 (48) | 8 (22) 15 (52) | 1 [reference] 3.75 (1.28–10.95) | 0.013 |
| Ultrasonographically-defined rectus femoris CSA (cm2) | ||||
| >1.32 cm2 ≤1.32 cm2 | 26 (87) 16 (46) | 4 (13) 19 (54) | 1 [reference] 7.72 (2.22–26.81) | 0.001 |
| CT/MRI-defined cervical SMM (cm2) | ||||
| >34.91 cm2 ≤34.91 cm2 | 27 (79) 15 (48) | 7 (21) 16 (52) | 1 [reference] 4.11 (1.38–12.23) | 0.009 |
| CT/MRI-defined CSMI (cm2/m2) | ||||
| >11.25 cm2/m2 ≤11.25 cm2/m2 | 29 (74) 13 (50) | 10 (26) 13 (50) | 1 [reference] 2.90 (1.01–8.31) | 0.044 |
| Parameters | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p |
|---|---|---|---|---|---|---|
| Previous radiotherapy | ||||||
| No Yes | 1 [reference] 3.22 (0.56–18.49) | 0.190 | 1 [reference] 2.35 (0.43–12.93) | 0.327 | 1 [reference] 2.32 (0.46–11.76) | 0.308 |
| ASA score | ||||||
| I–II III–IV | 1 [reference] 1.42 (0.38–5.26) | 0.599 | 1 [reference] 1.53 (0.41–5.66) | 0.525 | 1 [reference] 1.35 (0.38–4.82) | 0.648 |
| Modified frailty index (mFI) | ||||||
| ≤0.10 >0.10 | 1 [reference] 1.54 (0.36–6.63) | 0.560 | 1 [reference] 3.75 (0.79–17.77) | 0.096 | 1 [reference] 3.21 (0.73–14.19) | 0.124 |
| US-defined rectus femoris CSA (cm2) | ||||||
| >1.32 ≤1.32 | 1 [reference] 7.07 (1.87–26.67) | 0.004 | - | - | - | - |
| CT/MRI-defined cervical SMM (cm2) | ||||||
| >34.91 ≤34.91 | - | - | 1 [reference] 6.74 (1.80–25.23) | 0.005 | - | - |
| CT/MRI-defined CSMI (cm2/m2) | ||||||
| >11.25 ≤11.25 | - | - | - | - | 1 [reference] 4.02 (1.19–13.56) | 0.025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galli, A.; Colombo, M.; Prizio, C.; Carrara, G.; Lira Luce, F.; Paesano, P.L.; Della Vecchia, G.; Giordano, L.; Bondi, S.; Tulli, M.; et al. Skeletal Muscle Depletion and Major Postoperative Complications in Locally-Advanced Head and Neck Cancer: A Comparison between Ultrasound of Rectus Femoris Muscle and Neck Cross-Sectional Imaging. Cancers 2022, 14, 347. https://doi.org/10.3390/cancers14020347
Galli A, Colombo M, Prizio C, Carrara G, Lira Luce F, Paesano PL, Della Vecchia G, Giordano L, Bondi S, Tulli M, et al. Skeletal Muscle Depletion and Major Postoperative Complications in Locally-Advanced Head and Neck Cancer: A Comparison between Ultrasound of Rectus Femoris Muscle and Neck Cross-Sectional Imaging. Cancers. 2022; 14(2):347. https://doi.org/10.3390/cancers14020347
Chicago/Turabian StyleGalli, Andrea, Michele Colombo, Carmine Prizio, Giulia Carrara, Francesca Lira Luce, Pier Luigi Paesano, Giovanna Della Vecchia, Leone Giordano, Stefano Bondi, Michele Tulli, and et al. 2022. "Skeletal Muscle Depletion and Major Postoperative Complications in Locally-Advanced Head and Neck Cancer: A Comparison between Ultrasound of Rectus Femoris Muscle and Neck Cross-Sectional Imaging" Cancers 14, no. 2: 347. https://doi.org/10.3390/cancers14020347
APA StyleGalli, A., Colombo, M., Prizio, C., Carrara, G., Lira Luce, F., Paesano, P. L., Della Vecchia, G., Giordano, L., Bondi, S., Tulli, M., Di Santo, D., Mirabile, A., De Cobelli, F., & Bussi, M. (2022). Skeletal Muscle Depletion and Major Postoperative Complications in Locally-Advanced Head and Neck Cancer: A Comparison between Ultrasound of Rectus Femoris Muscle and Neck Cross-Sectional Imaging. Cancers, 14(2), 347. https://doi.org/10.3390/cancers14020347








