The Overall Quality of Life and Oncological Outcomes Following Radical Hysterectomy in Cervical Cancer Survivors Results from a Large Long-Term Single-Institution Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population and Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Treatments
2.4. Statistical Analysis and Data Assessment
2.5. Questionnaires
3. Results
3.1. Patient Characteristics and Follow-Up
3.2. Survey Results
3.3. QoL Results
4. Discussion
Strength and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marth, C.; Landoni, F.; Mahner, S.; McCormack, M.; Gonzalez-Martin, A.; Colombo, N. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv72–iv83. [Google Scholar] [CrossRef] [PubMed]
- Plotti, F.; Terranova, C.; Capriglione, S.; Crispino, S.; Li Pomi, A.; De Cicco Nardone, C.; Montera, R.; Panici, P.B.; Angioli, R.; Scaletta, G. Assessment of Quality of Life and Urinary and Sexual Function After Radical Hysterectomy in Long-Term Cervical Cancer Survivors. Int. J. Gynecol. Cancer 2018, 28, 818–823. [Google Scholar] [CrossRef]
- Gupta, S.; Maheshwari, A.; Parab, P.; Mahantshetty, U.; Hawaldar, R.; Sastri, S.; Kerkar, R.; Engineer, R.; Tongaonkar, H.; Ghosh, J.; et al. Neoadjuvant Chemotherapy Followed by Radical Surgery Versus Concomitant Chemotherapy and Radiotherapy in Patients With Stage IB2, IIA, or IIB Squamous Cervical Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2018, 36, 1548–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Rustum, N.R.; Yashar, C.M.; Bean, S.; Bradley, K.; Campos, S.M.; Chon, H.S.; Chu, C.; Cohn, D.; Crispens, M.A.; Damast, S.; et al. NCCN Guidelines Insights: Cervical Cancer, Version 1.2020. J. Natl. Compr. Cancer Netw. JNCCN 2020, 18, 660–666. [Google Scholar] [CrossRef]
- Yuan, L.; Guo, J.; Zhang, X.; Chen, M.; Xu, C.; Yao, L. Feasibility of radical hysterectomy in women with figo stage iib cervical cancer: An observation study of 10-year experience in a tertiary center. OncoTargets Ther. 2018, 11, 5527–5533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tierney, J. Neoadjuvant chemotherapy for locally advanced cervical cancer: A systematic review and meta-analysis of individual patient data from 21 randomised trials. Eur. J. Cancer 2003, 39, 2470–2486. [Google Scholar] [CrossRef] [Green Version]
- Ferrandina, G.; Legge, F.; Fagotti, A.; Fanfani, F.; Distefano, M.; Morganti, A.; Cellini, N.; Scambia, G. Preoperative concomitant chemoradiotherapy in locally advanced cervical cancer: Safety, outcome, and prognostic measures. Gynecol. Oncol. 2007, 107, S127–S132. [Google Scholar] [CrossRef] [PubMed]
- Kirchheiner, K.; Pötter, R.; Tanderup, K.; Lindegaard, J.C.; Haie-Meder, C.; Petrič, P.; Mahantshetty, U.; Jürgenliemk-Schulz, I.M.; Rai, B.; Cooper, R.; et al. Health-Related Quality of Life in Locally Advanced Cervical Cancer Patients After Definitive Chemoradiation Therapy Including Image Guided Adaptive Brachytherapy: An Analysis From the EMBRACE Study. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 1088–1098. [Google Scholar] [CrossRef]
- Weiderpass, E.; Ferlay, J.; Bray, F.; Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Artic. Lancet Glob. Health 2019, 8, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Health Profile Romania. Available online: https://www.worldlifeexpectancy.com/country-health-profile/romania (accessed on 29 November 2021).
- Salvo, G.; Odetto, D.; Pareja, R.; Frumovitz, M.; Ramirez, P.T. Revised 2018 International Federation of Gynecology and Obstetrics (FIGO) cervical cancer staging: A review of gaps and questions that remain. Int. J. Gynecol. Cancer 2020, 30, 873–878. [Google Scholar] [CrossRef]
- Stanca, M.; Căpîlna, M.E. Prognostic Factors Associated with 5-Year Overall Survival in Cervical Cancer Patients Treated with Radical Hysterectomy Followed by Adjuvant Concurrent Chemoradiation Therapy at a Tertiary Care Center in Eastern Europe. Diagnostics 2021, 11, 570. [Google Scholar] [CrossRef]
- Căpîlna, M.E.; Moldovan, B.; Szabo, B. Pelvic exenteration—Our initial experience in 15 cases. Eur. J. Gynaecol. Oncol. 2015, 36, 142–145. [Google Scholar]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European organization for research and treatment of cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Greimel, E.R.; Vlasic, K.K.; Waldenstrom, A.C.; Duric, V.M.; Jensen, P.T.; Singer, S.; Chie, W.; Nordin, A.; Radisic, V.B.; Wydra, D. The European Organization for Research and Treatment of Cancer (EORTC) Qualtty-of-Life questionnaire cervical cancer module: EORTC QLQ-CX24. Cancer 2006, 107, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Cǎpîlna, M.E.; Szabo, B.; Rusu, S.C.; Becsi, J.; Moldovan, B.; Neagoe, R.M.; Muhlfay, G. Anatomical variations of the obturator veins and their surgical implications. Eur. J. Gynaecol. Oncol. 2017, 38, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.T.; Klee, M.C.; Thranov, I.; Groenvold, M. Validation of a questionnaire for self-assessment of sexual function and vaginal changes after gynaecological cancer. Psycho-Oncol. 2004, 13, 577–592. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Keys, H.M.; Bundy, B.N.; Stehman, F.B.; Muderspach, L.I.; Chafe, W.E.; Suggs, C.L.; Walker, J.L.; Gersell, D. Cisplatin, Radiation, and Adjuvant Hysterectomy Compared with Radiation and Adjuvant Hysterectomy for Bulky Stage IB Cervical Carcinoma. N. Engl. J. Med. 1999, 340, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Ferrandina, G.; Mantegna, G.; Petrillo, M.; Fuoco, G.; Venditti, L.; Terzano, S.; Moruzzi, C.; Lorusso, D.; Marcellusi, A.; Scambia, G. Quality of life and emotional distress in early stage and locally advanced cervical cancer patients: A prospective, longitudinal study. Gynecol. Oncol. 2012, 124, 389–394. [Google Scholar] [CrossRef]
- Kazmierczak, K.; Nowakowski, B. Radical hysterectomy and its importance in the concept of cervical cancer treatment. Ginekol. Pol. 2021, 92, 1–4. [Google Scholar] [CrossRef]
- Koensgen, D.; Sehouli, J.; Belau, A.; Weiss, M.; Stope, M.B.; Großkopf, V.; Eichbaum, M.; Ledwon, P.; Lichtenegger, W.; Zygmunt, M.; et al. Clinical Outcome of Neoadjuvant Radiochemotherapy in Locally Advanced Cervical Cancer: Results of an Open Prospective, Multicenter Phase 2 Study of the North-Eastern German Society of Gynecological Oncology. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2017, 27, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Cosio, S. Neoadjuvant Chemotherapy in Locally Advanced Cervical Cancer: Review of the Literature and Perspectives of Clinical Research. Anticancer Res. 2020, 40, 4819–4828. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, P.; Zhao, W.; Yin, Z.; Lin, Z.; Bin, X.; Lang, J.; Chen, C. Effects of preoperative radiotherapy or chemoradiotherapy on postoperative pathological outcome of cervical cancer—From the large database of 46,313 cases of cervical cancer in China. Eur. J. Surg. Oncol. 2020, 46, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Kong, W.; Li, F.; Song, D.; Liu, T.; Han, C.; Jiao, S.; Chen, J. Effect of preoperative radiotherapy on stage IB2 and IIA2 cervical cancer: A retrospective cohort study. Int. J. Surg. 2016, 30, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Benedetti Panici, P.; Bellati, F.; Manci, N.; Pernice, M.; Plotti, F.; Di Donato, V.; Calcagno, M.; Zullo, M.A.; Muzii, L.; Angioli, R. Neoadjuvant chemotherapy followed by radical surgery in patients affected by FIGO stage IVA cervical cancer. Ann. Surg. Oncol. 2007, 14, 2643–2648. [Google Scholar] [CrossRef]
- Sedlis, A.; Bundy, B.N.; Rotman, M.Z.; Lentz, S.S.; Muderspach, L.I.; Zaino, R.J. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A gynecologic oncology group study. Gynecol. Oncol. 1999, 73, 177–183. [Google Scholar] [CrossRef]
- Morris, M.; Eifel, P.J.; Lu, J.; Grigsby, P.W.; Levenback, C.; Stevens, R.E.; Rotman, M.; Gershenson, D.M.; Mutch, D.G. Pelvic Radiation with Concurrent Chemotherapy Compared with Pelvic and Para-Aortic Radiation for High-Risk Cervical Cancer. New Engl. J. Med. 1999, 340, 1137–1143. [Google Scholar] [CrossRef]
- Peters, W.A.; Liu, P.Y.; Barrett, R.J.; Stock, R.J.; Monk, B.J.; Berek, J.S.; Souhami, L.; Grigsby, P.; Gordon, W.; Alberts, D.S. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. 2000, 18, 1606–1613. [Google Scholar] [CrossRef]
- Sood, A.K.; Nygaard, I.; Shahin, M.S.; Sorosky, J.I.; Lutgendorf, S.K.; Rao, S.S.C. Anorectal dysfunction after surgical treatment for cervical cancer. J. Am. Coll. Surg. 2002, 195, 513–519. [Google Scholar] [CrossRef]
- Jensen, P.T.; Groenvold, M.; Klee, M.C.; Thranov, I.; Petersen, M.A.; Machin, D. Early-stage cervical carcinoma, radical hysterectomy, and sexual function. A longitudinal study. Cancer 2004, 100, 97–106. [Google Scholar] [CrossRef]
- Wang, S.; Wen, H.; Gao, Y.; Lv, Q.; Cao, T.; Wang, S.; Wang, J.; Li, Y.; Wang, H.; Wang, Z.; et al. Assessment of Pelvic Floor Function and Quality of Life in Patients Treated for Cervical Cancer: A Multicenter Retrospective Study. Gynecol. Obstet. Investig. 2021, 86, 353–360. [Google Scholar] [CrossRef]
- Zullo, M.A.; Manci, N.; Angioli, R.; Muzii, L.; Panici, P.B. Vesical dysfunctions after radical hysterectomy for cervical cancer: A critical review. Crit. Rev. Oncol./Hematol. 2003, 48, 287–293. [Google Scholar] [CrossRef]
- Hazewinkel, M.H.; Sprangers, M.A.G.; van der Velden, J.; van der Vaart, C.H.; Stalpers, L.J.A.; Burger, M.P.M.; Roovers, J.P.W.R. Long-term cervical cancer survivors suffer from pelvic floor symptoms: A cross-sectional matched cohort study. Gynecol. Oncol. 2010, 117, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Derks, M.; Van Lonkhuijzen, L.R.C.W.; Bakker, R.M.; Stiggelbout, A.M.; De Kroon, C.D.; Westerveld, H.; Roovers, J.P.W.R.; Kenter, G.G.; Kuile, M.M.T. Long-Term Morbidity and Quality of Life in Cervical Cancer Survivors: A Multicenter Comparison Between Surgery and Radiotherapy as Primary Treatment. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2017, 27, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.T.; Groenvold, M.; Klee, M.C.; Thranov, I.; Petersen, M.A.; Machin, D. Longitudinal study of sexual function and vaginal changes after radiotherapy for cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 937–949. [Google Scholar] [CrossRef]
- Bergmark, K.; Åvall-Lundqvist, E.; Dickman, P.W.; Henningsohn, L.; Steineck, G. Vaginal changes and sexuality in women with a history of cervical cancer. N. Engl. J. Med. 1999, 340, 1383–1389. [Google Scholar] [CrossRef] [Green Version]
- Hofsjö, A.; Bergmark, K.; Blomgren, B.; Jahren, H.; Bohm-Starke, N. Radiotherapy for cervical cancer—Impact on the vaginal epithelium and sexual function. Acta Oncol. 2018, 57, 338–345. [Google Scholar] [CrossRef] [Green Version]
- Tax, C.; Steenbergen, M.E.; Zusterzeel, P.L.M.; Bekkers, R.L.M.; Rovers, M.M. Measuring health-related quality of life in cervical cancer patients: A systematic review of the most used questionnaires and their validity. BMC Med. Res. Methodol. 2017, 17, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mantegna, G.; Petrillo, M.; Fuoco, G.; Venditti, L.; Terzano, S.; Anchora, L.P.; Scambia, G.; Ferrandina, G. Long-term prospective longitudinal evaluation of emotional distress and quality of life in cervical cancer patients who remained disease-free 2-years from diagnosis. BMC Cancer 2013, 13, 127. [Google Scholar] [CrossRef]
- Frumovitz, M.; Obermair, A.; Coleman, R.L.; Pareja, R.; Lopez, A.; Ribero, R.; Isla, D.; Rendon, G.; Bernadini, M.Q.; Buda, A.; et al. Quality of life in patients with cervical cancer after open versus minimally invasive radical hysterectomy (LACC): A secondary outcome of a multicentre, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 2020, 21, 851–860. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef]
- Wenzel, L.; DeAlba, I.; Habbal, R.; Kluhsman, B.C.; Fairclough, D.; Krebs, L.U.; Anton-Culver, H.; Berkowitz, R.; Aziz, N. Quality of life in long-term cervical cancer survivors. Gynecol. Oncol. 2005, 97, 310–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Borgne, G.; Mercier, M.; Woronoff, A.S.; Guizard, A.V.; Abeilard, E.; Caravati-Jouvenceaux, A.; Klein, D.; Velten, M.; Joly, F. Quality of life in long-term cervical cancer survivors: A population-based study. Gynecol. Oncol. 2013, 129, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Bjelic-Radisic, V.; Jensen, P.T.; Vlasic, K.K.; Waldenstrom, A.-C.; Singer, S.; Chie, W.; Nordin, A.; Greimel, E. Quality of life characteristics inpatients with cervical cancer. Eur. J. Cancer 2012, 48, 3009–3018. [Google Scholar] [CrossRef] [PubMed]
- Plotti, F.; Nelaj, E.; Sansone, M.; Antonelli, E.; Altavilla, T.; Angioli, R.; Benedetti Panici, P. Sexual function after modified radical hysterectomy (Piver II/Type B) vs. classic radical hysterectomy (Piver III/Type C2) for early stage cervical cancer. A prospective study. J. Sex. Med. 2012, 9, 909–917. [Google Scholar] [CrossRef]
- Khalil, J.; Bellefqih, S.; Sahli, N.; Afif, M.; Elkacemi, H.; Elmajjaoui, S.; Kebdani, T.; Benjaafar, N. Impact of cervical cancer on quality of life: Beyond the short term (Results from a single institution). Gynecol. Oncol. Res. Pract. 2015, 2, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holman, L.L.; Levenback, C.F.; Frumovitz, M. Sentinel lymph node evaluation in women with cervical cancer. J. Minim. Invasive Gynecol. 2014, 21, 540–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jongpipan, J.; Charoenkwan, K. Sexual function after radical hysterectomy for early-stage cervical cancer. J. Sex. Med. 2007, 4, 1659–1665. [Google Scholar] [CrossRef]
- Korfage, I.J.; Essink-Bot, M.L.; Mols, F.; van de Poll-Franse, L.; Kruitwagen, R.; van Ballegooijen, M. Health-related quality of life in cervical cancer survivors: A population-based survey. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1501–1509. [Google Scholar] [CrossRef] [Green Version]
- Vermeer, W.M.; Bakker, R.M.; Kenter, G.G.; De Kroon, C.D.; Stiggelbout, A.M.; Ter Kuile, M.M. Sexual issues among cervical cancer survivors: How can we help women seek help? Psycho-oncology 2015, 24, 458–464. [Google Scholar] [CrossRef]
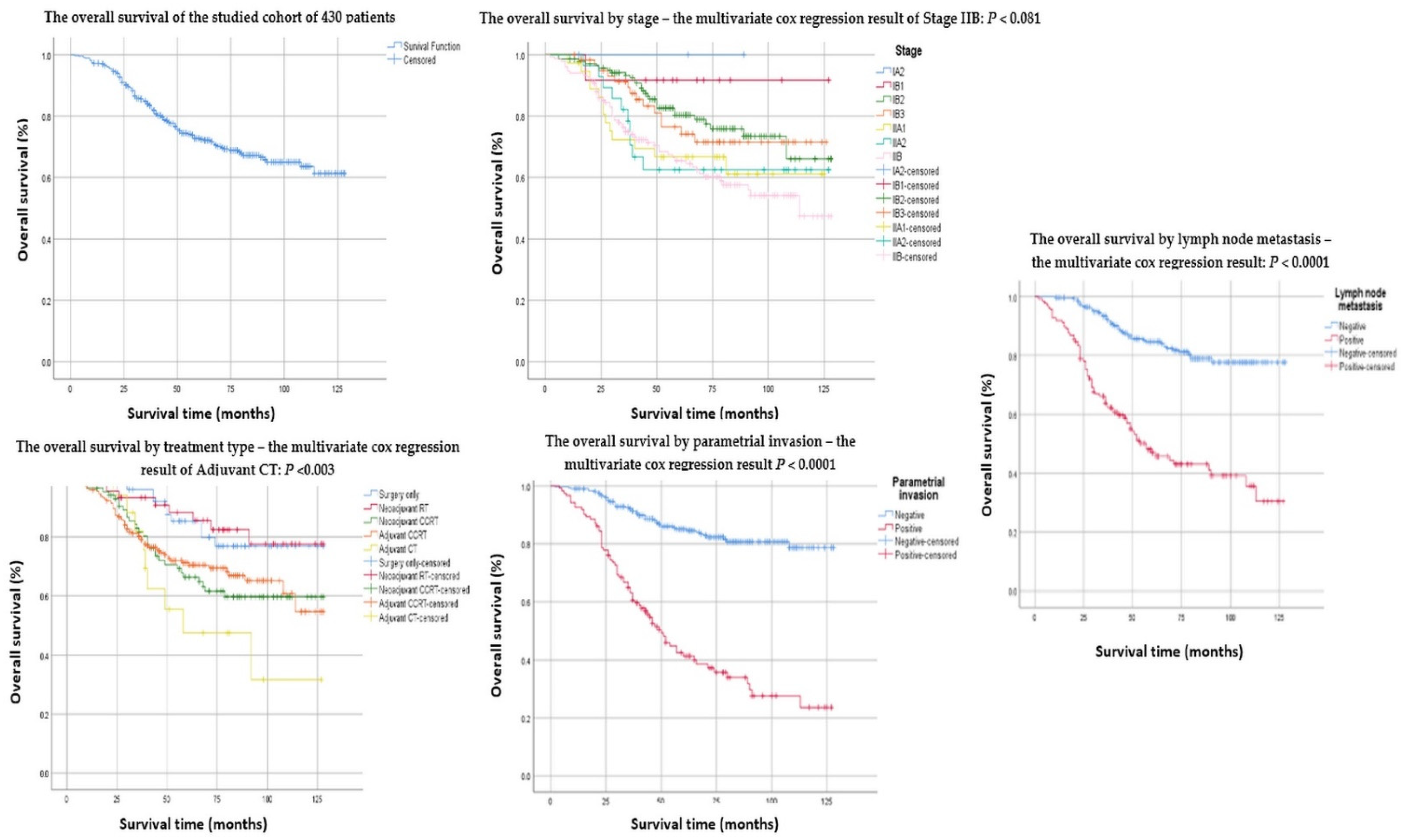
| Number (%) or Median (Range) | Overall Survival | Recurrences | |||||
|---|---|---|---|---|---|---|---|
| 5-Year Survival Rate | 95% CI | Mean Survival (Months) | p Value | Number | p Value | ||
| No. of patients | 430 | 0.792 | 22 | ||||
| Age (years) | 51 (22–76) | ||||||
| Under 30 | 3 (0.7%) | 66.7% | 69.4–63.9 | 58.6 | 0.657 | ||
| 30–40 | 71 (16.5%) | 70.8% | 71.3–69.7 | 94.5 | 0.822 | ||
| 41–50 | 121 (28.1%) | 77.0% | 77.4–76.5 | 101.8 | 0.474 | ||
| 51–60 | 152 (35.3%) | 73.3% | 73.6–72.9 | 97.9 | 0.262 | ||
| 61–70 | 79 (18.4%) | 63.2% | 63.8–62.5 | 89.8 | 0.355 | ||
| 71–80 | 4 (0.9%) | 50.0% | 52.5–47.5 | 97.4 | 0.563 | ||
| Provenance | 0.662 | ||||||
| Urban | 180 (41.9%) | 72.3% | 72.6–71.9 | 99.0 | 12 | ||
| Rural | 250 (58.1%) | 72.6% | 72.9–72.3 | 96.3 | 10 | ||
| Clinical Stage (FIGO 2018) | |||||||
| IA2 | 3 (0.7%) | 100% | 0.559 | ||||
| IB1 | 12 (2.8%) | 91.1% | 91.9% | 117.9 | 0.171 | ||
| IB2 | 140 (32.6%) | 80.3% | 80.6–79.9 | 105.3 | 0.011 | 4 | 0.060 |
| IB3 | 60 (14.0%) | 74.2% | 74.8–73.5 | 96.5 | 0.337 | 4 | 0.518 |
| IIA1 | 37 (8.6%) | 66.7% | 67.4–65.9 | 89.7 | 0.463 | 2 | 0.615 |
| IIA2 | 28 (6.5%) | 62.5% | 63.4–63.4 | 91.7 | 0.538 | 1 | 0.415 |
| IIB | 150 (34.9%) | 64.5% | 64.9–64.0 | 87.4 | 0.001 | 11 | 0.020 |
| Tumor size | 0.873 | 0.475 | |||||
| <4 cm | 328 (76.3%) | 73.0% | 73.2–72.7 | 97.0 | 16 | ||
| ≥4 cm | 102 (23.7%) | 70.8% | 71.2–70.3 | 97.5 | 6 | ||
| Histology | |||||||
| Squamous Cell Carcinoma | 358 (83.3%) | 73.0% | 73.2–72.7 | 98.2 | 0.339 | 18 | 0.572 |
| Adenocarcinoma | 43 (10.0%) | 70.1% | 70.8–69.3 | 99.6 | 0.837 | 2 | 0.537 |
| Adenosquamous | 23 (5.3%) | 67.2% | 68.2–66.1 | 85.2 | 0.279 | ||
| Other | 6 (1.4%) | 50.0% | 52.0–47.9 | 50.6 | 0.097 | 2 | 0.554 |
| Tumor differentiation grade | |||||||
| Grade 1 (well-differentiated) | 67 (15.6%) | 82.7% | 83.1–82.2 | 105.1 | 0.074 | 1 | 0.615 |
| Grade 2 (moderately-differentiated) | 178 (41.4%) | 70.7% | 71.0–70.3 | 97.1 | 0.064 | 11 | 0.135 |
| Grade 3 (poorly-differentiated) | 185 (43.0%) | 66.2% | 66.5–65.8 | 94.3 | 0.848 | 9 | 0.141 |
| Depth of cervical stromal invasion | |||||||
| Inner 1/3 | 81 (18.8%) | 84.3% | 84.7–83.8 | 113.4 | 0.091 | 1 | 0.586 |
| Middle 1/3 | 91 (21.2%) | 80.9% | 81.3–80.4 | 101.8 | 0.244 | 1 | 0.238 |
| Outer 1/3 | 258 (60.0%) | 65.8% | 66.1–65.4 | 90.7 | 0.001 | 20 | 0.200 |
| Lymphovascular space invasion | 0.0001 | 0.207 | |||||
| Positive | 263 (61.2%) | 64.4% | 64.7–64.0 | 87.7 | 19 | ||
| Negative | 167 (38.8%) | 85.4% | 85.6–85.1 | 110.7 | 3 | ||
| Parametrial involvement | 0.0001 | 0.463 | |||||
| Positive | 122 (28.4%) | 42.5% | 42.9–42.0 | 64.4 | 10 | ||
| Negative | 308 (71.6%) | 85.1% | 85.3–84.8 | 111.2 | 12 | ||
| Resection margin status | 0.0001 | 0.109 | |||||
| Positive | 79 (18.4%) | 53.1% | 53.7–52.4 | 80.5 | 8 | ||
| Negative | 351 (81.6%) | 77.2% | 77.4–76.9 | 101.2 | 14 | ||
| Pelvic lymph nodes metastases | 0.0001 | 0.162 | |||||
| Positive | 138 (32.1%) | 48.2% | 48.6–47.7 | 70.6 | 13 | ||
| Negative | 292 (67.9%) | 84.7% | 84.9–84.4 | 110.5 | 9 | ||
| Neoadjuvant/Adjuvant treatment | |||||||
| Surgery only | 57 (13.3%) | 79.9% | 80.5–79.2 | 110.4 | 0.049 | 0 | |
| Neoadjuvant RT | 46 (10.7%) | 77.6% | 78.3–76.8 | 111.5 | 0.040 | 0 | |
| Neoadjuvant CRT | 86 (20.0%) | 66.4% | 66.9–65.8 | 92.7 | 0.265 | 0 | |
| Adjuvant CCRT | 222 (34%) | 69.9% | 69.5–70.2 | 94.7 | 0.249 | 22 | |
| Adjuvant CT | 19 (4.4%) | 47.5% | 48.8–46.1 | 75.7 | 0.053 | 0 | |
| Median follow-up duration (months) | 65 (2–128) | ||||||
| Status | |||||||
| Alive | 308 (71.6%) | 72.4% | 72.6–72.1 | 97.4 | 22 | ||
| Alive free of disease | 286 (67%) | ||||||
| Alive with disease | 22 (5%) | ||||||
| Patients who answered the questionnaires | 208 | 0 | |||||
| Deceased | 122 (28.4%) | ||||||
| Variables | B | SE | Wald | p-Value | HR | 95.0% CI for OR | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Clinical Stage FIGO IIB | −0.378 | 0.217 | 3.037 | 0.081 | 0.685 | 0.389 | 0.945 |
| Adjuvant CT | −1.119 | 0.378 | 8.785 | 0.003 | 0.326 | 0.259 | 1.009 |
| Parametrial invasion | −1.109 | 0.218 | 25.866 | 0.0001 | 0.330 | 0.220 | 0.850 |
| Lymph node metastasis | −1.012 | 0.234 | 18.707 | 0.0001 | 0.363 | 0.234 | 1.001 |
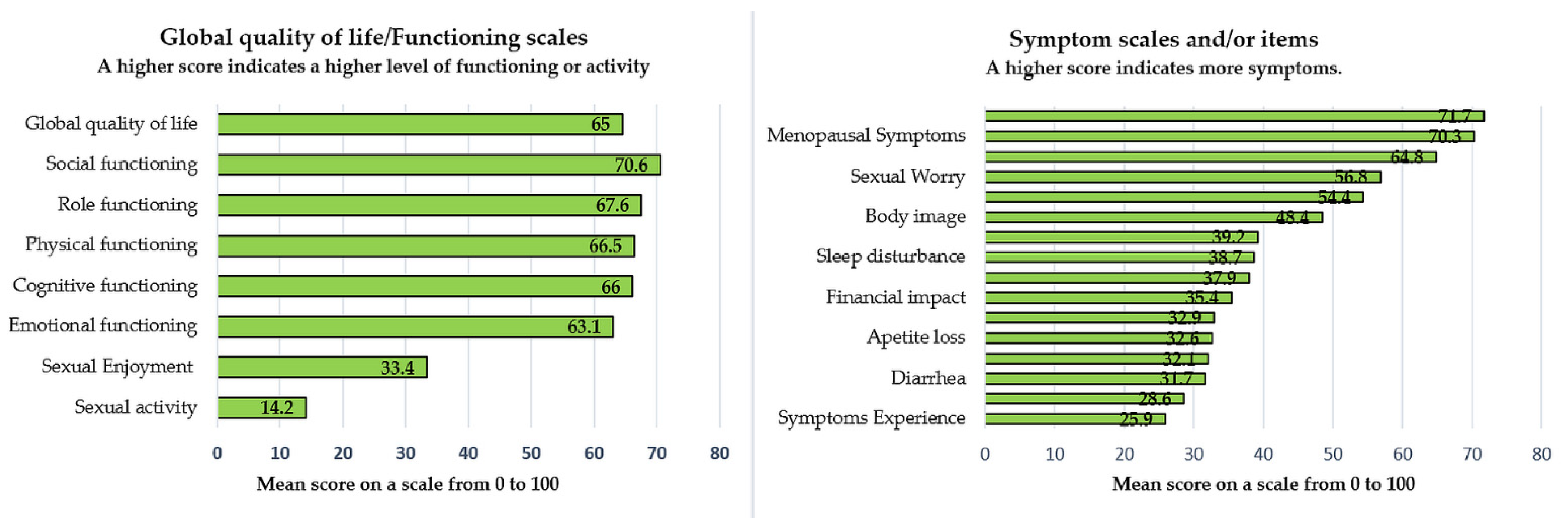
| Number of Patients = 208 | Items ~ | Mean Score | SD * | Cronbach’s Alpha Coefficient # |
|---|---|---|---|---|
| QLQ-C30 | ||||
| Functioning scales α | ||||
| Physical α | 1–5 | 66.5 | 26.1 | 0.93 |
| Role α | 6, 7 | 67.6 | 27.9 | 0.93 |
| Cognitive α | 20, 25 | 66.2 | 27.5 | 0.80 |
| Emotional α | 21–24 | 63.1 | 27.4 | 0.94 |
| Social α | 26, 27 | 70.6 | 27.7 | 0.85 |
| Global quality of life α | 29, 30 | 64.6 | 25.3 | 0.95 |
| Symptom scales and/or items γ | ||||
| Fatigue γ | 10, 12, 18 | 37.9 | 26.6 | 0.88 |
| Nausea and vomiting γ | 14, 15 | 32.1 | 28.9 | 0.86 |
| Pain γ | 9, 19 | 32.9 | 28.1 | 0.86 |
| Dyspnea γ | 8 | 28.6 | 27.7 | NA |
| Sleep disturbance γ | 11 | 38.7 | 30.2 | NA |
| Appetite loss γ | 13 | 32.6 | 30.3 | NA |
| Constipation γ | 16 | 39.2 | 33.4 | NA |
| Diarrhea γ | 17 | 31.7 | 29.9 | NA |
| Financial impact γ | 28 | 35.4 | 31.9 | NA |
| QLQ-C24 | ||||
| Symptoms Experience γ | 31–37, 39, 41–43 | 25.9 | 19.1 | 0.879 |
| Body Image γ | 45–47 | 48.4 | 31.3 | 0.946 |
| Sexual/Vaginal Functioning γ | 50–53 | 64.8 | 23.7 | 0.852 |
| Lymphoedema γ | 38 | 54.4 | 31.6 | NA |
| Peripheral Neuropathy γ | 40 | 71.7 | 30.4 | NA |
| Menopausal Symptoms γ | 44 | 70.3 | 32.3 | NA |
| Sexual Worry γ | 48 | 56.8 | 34.6 | NA |
| Sexual Activity α | 49 | 14.2 | 25.6 | NA |
| Sexual Enjoyment α | 54 | 33.4 | 28.2 | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanca, M.; Căpîlna, D.M.; Trâmbițaș, C.; Căpîlna, M.E. The Overall Quality of Life and Oncological Outcomes Following Radical Hysterectomy in Cervical Cancer Survivors Results from a Large Long-Term Single-Institution Study. Cancers 2022, 14, 317. https://doi.org/10.3390/cancers14020317
Stanca M, Căpîlna DM, Trâmbițaș C, Căpîlna ME. The Overall Quality of Life and Oncological Outcomes Following Radical Hysterectomy in Cervical Cancer Survivors Results from a Large Long-Term Single-Institution Study. Cancers. 2022; 14(2):317. https://doi.org/10.3390/cancers14020317
Chicago/Turabian StyleStanca, Mihai, Dan Mihai Căpîlna, Cristian Trâmbițaș, and Mihai Emil Căpîlna. 2022. "The Overall Quality of Life and Oncological Outcomes Following Radical Hysterectomy in Cervical Cancer Survivors Results from a Large Long-Term Single-Institution Study" Cancers 14, no. 2: 317. https://doi.org/10.3390/cancers14020317
APA StyleStanca, M., Căpîlna, D. M., Trâmbițaș, C., & Căpîlna, M. E. (2022). The Overall Quality of Life and Oncological Outcomes Following Radical Hysterectomy in Cervical Cancer Survivors Results from a Large Long-Term Single-Institution Study. Cancers, 14(2), 317. https://doi.org/10.3390/cancers14020317









