AKT Isoforms Interplay in High-Grade Serous Ovarian Cancer Prognosis and Characterization
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cases Selection
2.2. Histological Review
2.3. RNA Extraction and Reverse Transcription
2.4. Droplet Digital PCR
2.5. BRCA1 Immunohistochemistry and Homologous Recombination (HR) Status
2.6. Data validation
2.7. Statistical Analysis
3. Results
3.1. Patients’ Features
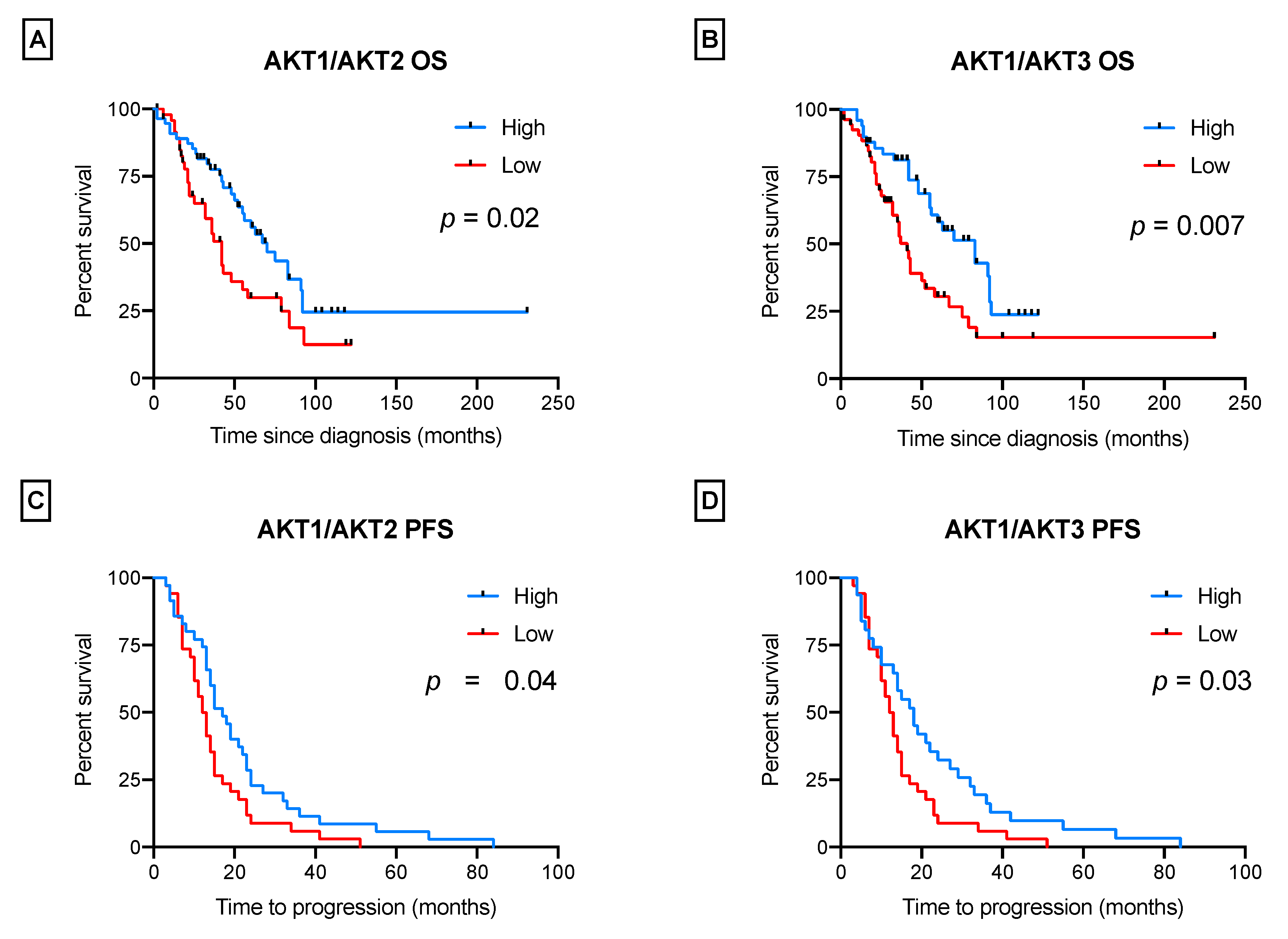
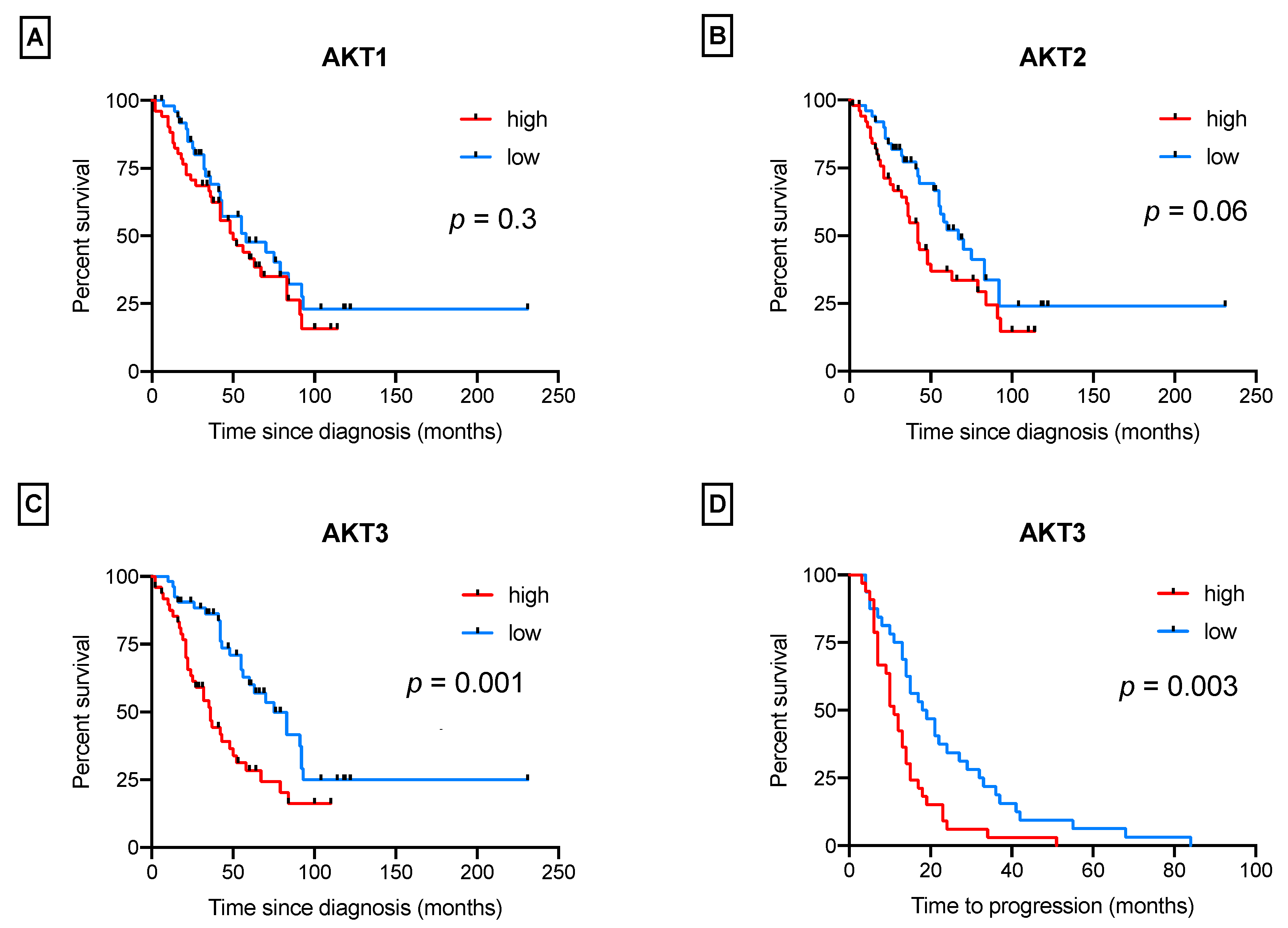
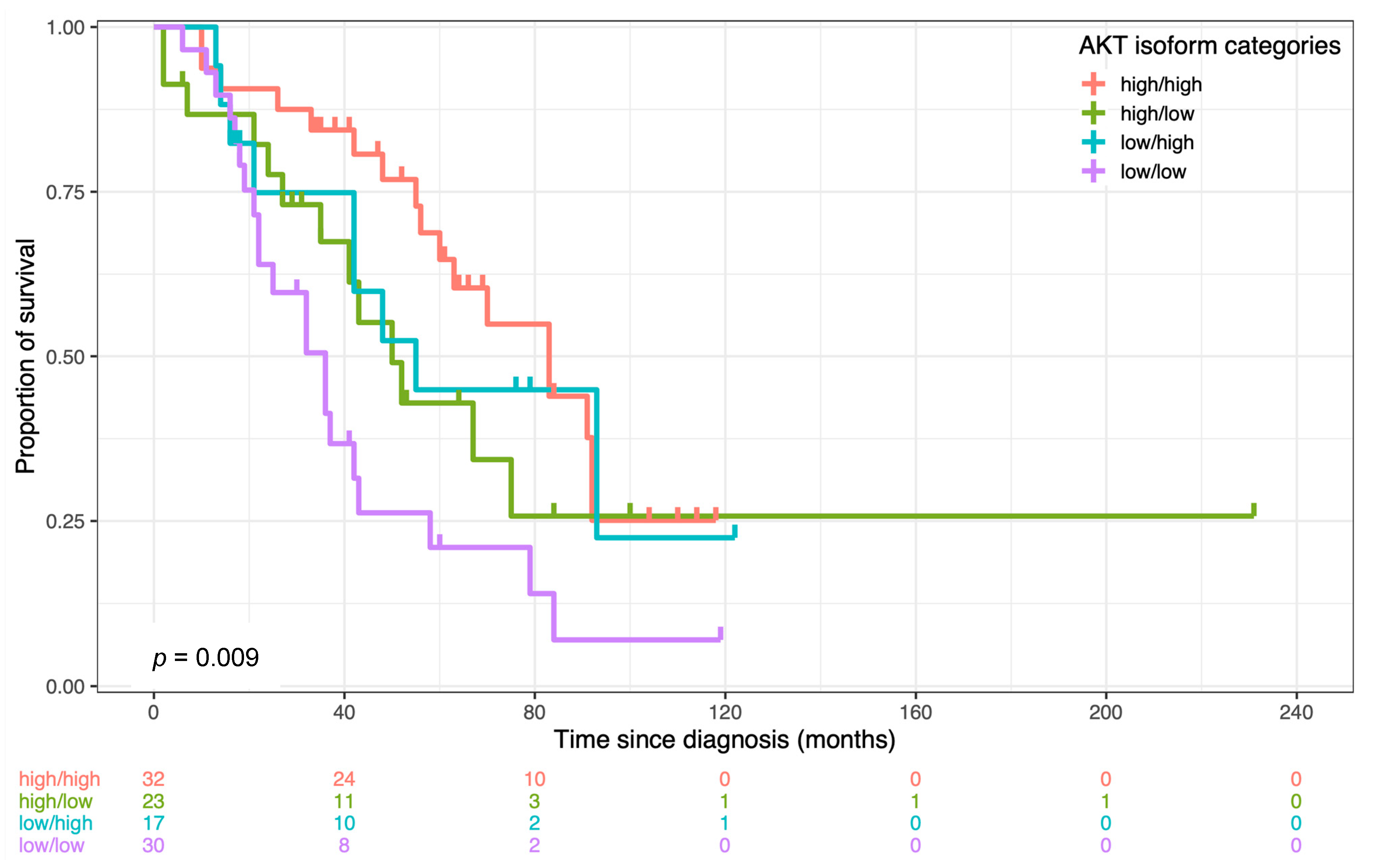
3.2. AKT Isoforms Are Predictive of Patients’ Survival
- “high/high”: AKT1 was prevalent over AKT2 and AKT3 (ratios ≥ 1.6 and ≥ 5.8);
- “high/low”: AKT1 was over AKT2, but not over AKT3 (ratios ≥ 1.6 and < 5.8);
- “low/high”: AKT1 was over AKT3, but not over AKT2 (ratios < 1.6 and ≥ 5.8);
- “low/low”: AKT1 was not prevalent over any isoform (AKT2 and AKT3) (ratios < 1.6 and < 5.8).
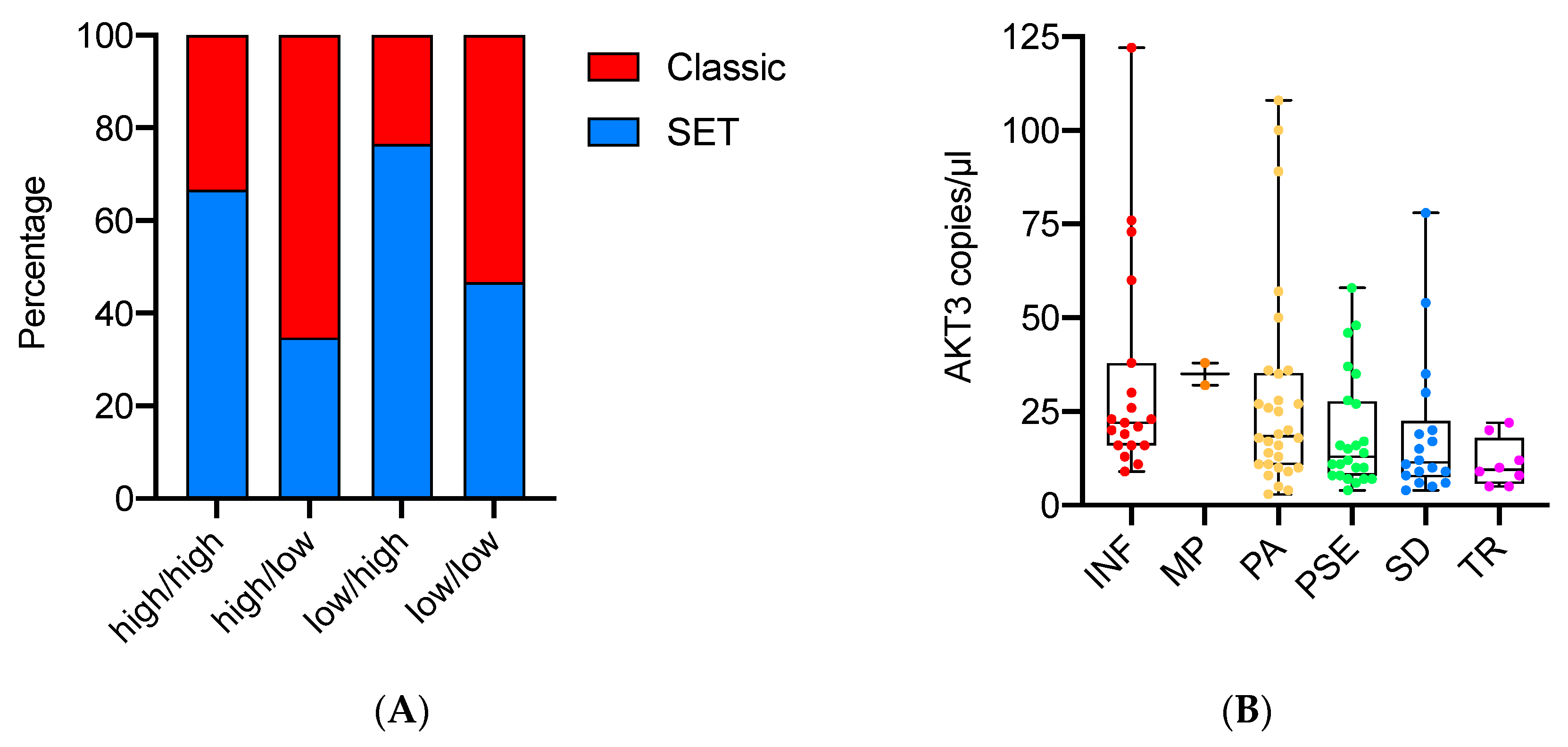
3.3. AKT Isoform Ratios Are Related to HGSOC Morphology and Proliferation
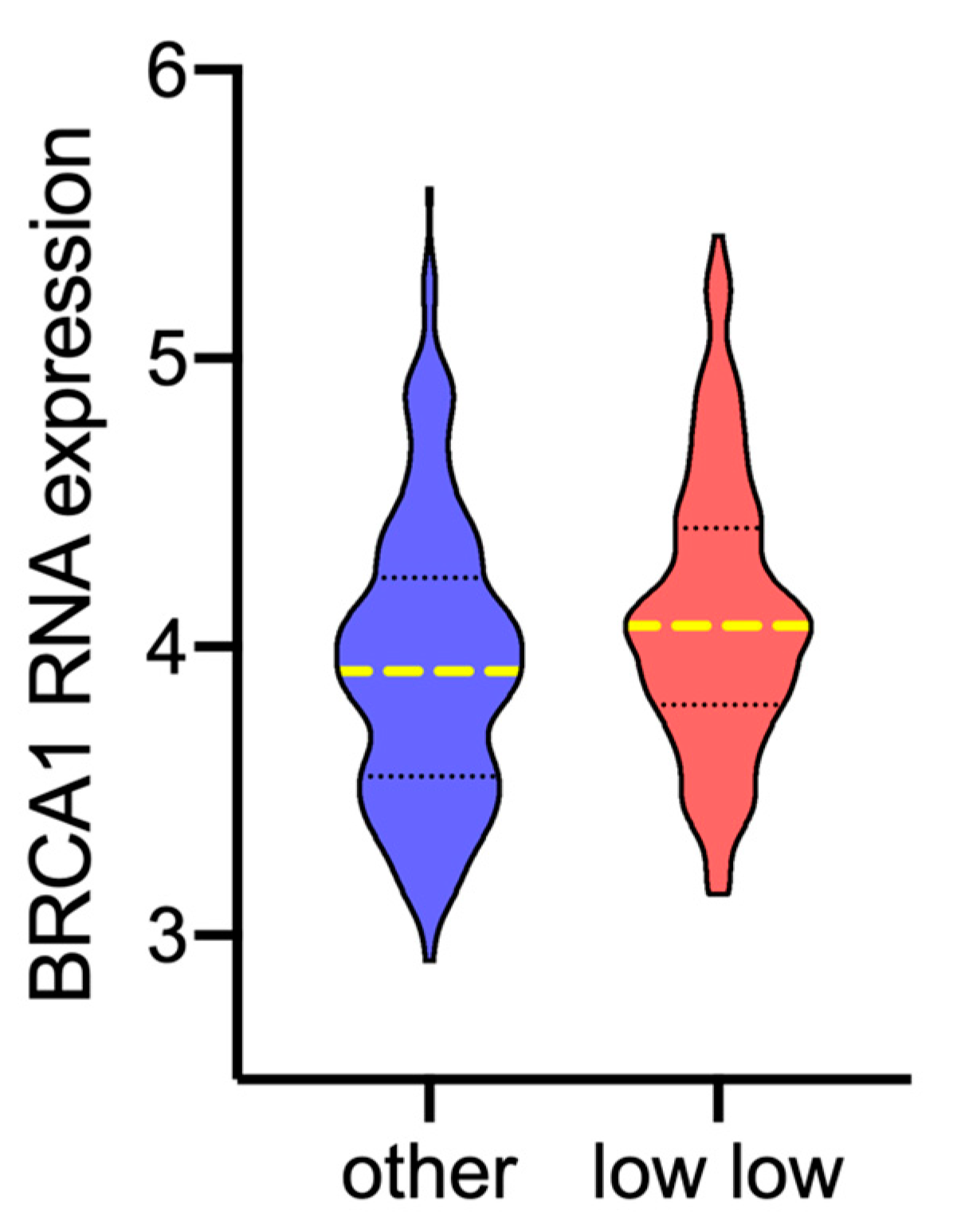
3.4. AKT Isoforms Are Related to BRCA1 Expression and Homologous Recombination (HR) Status
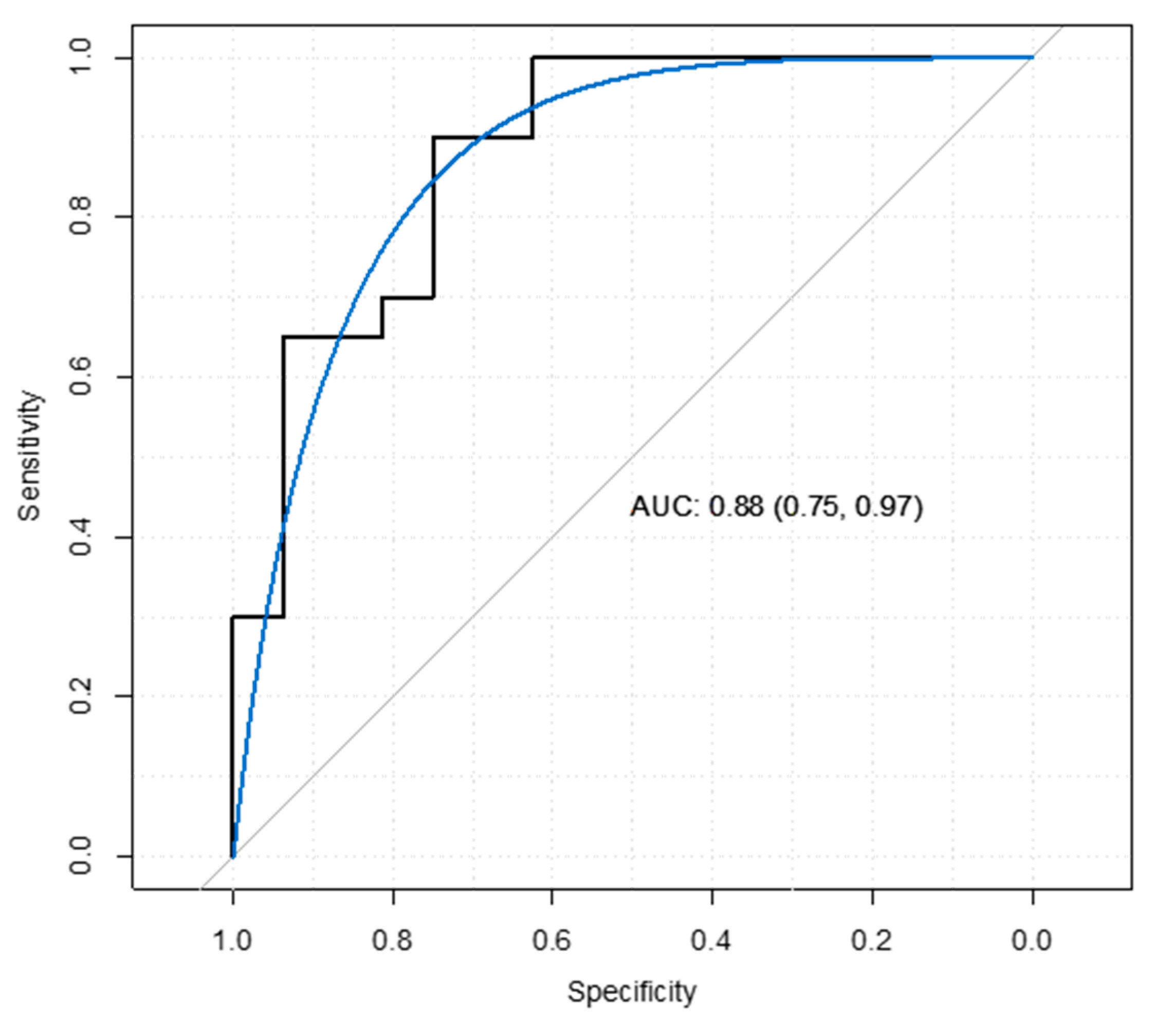
3.5. Data Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Gene | Primers and Probe | Length | Annealing Temp. |
|---|---|---|---|
| AKT1 | Forward 5′- CCA CTG TCA TCG AACGCA CCT - 3′ | 77 bp | 56 °C |
| Reverse 5′ - CAC AGT CTG GAT GGC GGT TGT - 3′ | |||
| Probe 5′ - CTG AGG AGC GGG AGG AG - 3′ |
| Progression-Free Survival (N = 62) | |||
|---|---|---|---|
| HR | 95% CI | p-Value | |
| Age at diagnosis | 1.02 | 1.00–1.05 | 0.1 |
| FIGO stage (III/IV) | 1.14 | 0.63–2.03 | 0.7 |
| Residual tumor after surgery | 2.00 | 1.11–3.61 | 0.02 * |
| AKT categories | |||
| High/high1 | 1 | ||
| High/low | 1.21 | 0.57–2.57 | 0.6 |
| Low/high | 1.37 | 0.61–3.07 | 0.4 |
| Low/low | 1.90 | 0.95–3.79 | 0.07 |
| Ph Test: p = 0.5 | |||
| Logistic Regression Model | ||||
|---|---|---|---|---|
| Estimate | Std. Error | z-Value | p-Value | |
| Intercept | 5.42 | 2.08 | 2.61 | 0.009 * |
| AKT1/AKT2 | −0.58 | 0.39 | −1.48 | 0.14 |
| AKT1/AKT3 | −0.30 | 0.15 | −1.98 | 0.04 * |
| SET% | −0.02 | 0.01 | −2.00 | 0.04 * |
| BRCA1 H-score | −0.01 | 0.01 | −1.05 | 0.3 |
| Hosmer and Lemeshow test: Chi-square = 11.51; df = 8; p = 0.2 | ||||
References
- Giusti, I.; Bianchi, S.; Nottola, S.A.; Macchiarelli, G.; Dolo, V. Clinical electron microscopy in the study of human ovarian tissues. EuroMediterranean Biomed. J. 2019, 14, 145–151. [Google Scholar] [CrossRef]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMullen, M.; Madariaga, A.; Lheureux, S. New approaches for targeting platinum-resistant ovarian cancer. Semin. Cancer Biol. 2021, 77, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.N.; Lord, C.J. First-line PARP inhibition in ovarian cancer–standard of care for all? Nat. Rev. Clin. Oncol. 2020, 17, 136–137. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Vanacker, H.; Le Saux, O.; Tredan, O. Overcoming resistance to PARP inhibitor in epithelial ovarian cancer, are we ready? EBioMedicine 2020, 61, 103046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, T.; Lin, J. Synergistic Effect of Bazedoxifene and PARP Inhibitor in the Treatment of Ovarian Cancer Regardless of BRCA Mutation. Anticancer Res. 2021, 41, 2277–2286. [Google Scholar] [CrossRef]
- Yap, T.A.; Kristeleit, R.; Michalarea, V.; Pettitt, S.J.; Lim, J.S.J.; Carreira, S.; Roda, D.; Miller, R.; Riisnaes, R.; Miranda, S.; et al. Phase I Trial of the PARP Inhibitor Olaparib and AKT Inhibitor Capivasertib in Patients with BRCA1/2- and Non-BRCA1/2-Mutant Cancers. Cancer Discov. 2020, 10, 1528–1543. [Google Scholar] [CrossRef]
- Li, H.; Zeng, J.; Shen, K. PI3K/AKT/mTOR signaling pathway as a therapeutic target for ovarian cancer. Arch. Gynecol. Obstet. 2014, 290, 1067–1078. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Alzahrani, A.S. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Semin. Cancer Biol. 2019, 59, 125–132. [Google Scholar] [CrossRef]
- Linnerth-Petrik, N.M.; Santry, L.A.; Petrik, J.J.; Wootton, S.K. Opposing functions of Akt isoforms in lung tumor initiation and progression. PLoS ONE 2014, 9, e94595. [Google Scholar] [CrossRef] [Green Version]
- Chin, Y.R.; Toker, A. Function of Akt/PKB signaling to cell motility, invasion and the tumor stroma in cancer. Cell Signal. 2009, 21, 470–476. [Google Scholar] [CrossRef] [Green Version]
- Basu, A.; Lambring, C.B. Akt Isoforms: A Family Affair in Breast Cancer. Cancers 2021, 13, 3445. [Google Scholar] [CrossRef]
- Hanrahan, A.J.; Schultz, N.; Westfal, M.L.; Sakr, R.A.; Giri, D.D.; Scarperi, S.; Janakiraman, M.; Olvera, N.; Stevens, E.V.; She, Q.B.; et al. Genomic complexity and AKT dependence in serous ovarian cancer. Cancer Discov. 2012, 2, 56–67. [Google Scholar] [CrossRef] [Green Version]
- Van der Ploeg, P.; Uittenboogaard, A.; Thijs, A.M.J.; Westgeest, H.M.; Boere, I.A.; Lambrechts, S.; van de Stolpe, A.; Bekkers, R.L.M.; Piek, J.M.J. The effectiveness of monotherapy with PI3K/AKT/mTOR pathway inhibitors in ovarian cancer: A meta-analysis. Gynecol. Oncol. 2021, 163, 433–444. [Google Scholar] [CrossRef]
- Azzalini, E.; Abdurakhmanova, N.; Parisse, P.; Bartoletti, M.; Canzonieri, V.; Stanta, G.; Casalis, L.; Bonin, S. Cell-stiffness and morphological architectural patterns in clinical samples of high grade serous ovarian cancers. Nanomedicine 2021, 37, 102452. [Google Scholar] [CrossRef]
- Azzalini, E.; Barbazza, R.; Stanta, G.; Giorda, G.; Bortot, L.; Bartoletti, M.; Puglisi, F.; Canzonieri, V.; Bonin, S. Histological patterns and intra-tumor heterogeneity as prognostication tools in high grade serous ovarian cancers. Gynecol. Oncol. 2021, 163, 498–505. [Google Scholar] [CrossRef]
- Soslow, R.A.; Han, G.; Park, K.J.; Garg, K.; Olvera, N.; Spriggs, D.R.; Kauff, N.D.; Levine, D.A. Morphologic patterns associated with BRCA1 and BRCA2 genotype in ovarian carcinoma. Mod. Pathol. 2012, 25, 625–636. [Google Scholar] [CrossRef] [PubMed]
- De Martino, E.; Brunetti, D.; Canzonieri, V.; Conforti, C.; Eisendle, K.; Mazzoleni, G.; Nobile, C.; Rao, F.; Zschocke, J.; Jukic, E.; et al. The Association of Residential Altitude on the Molecular Profile and Survival of Melanoma: Results of an Interreg Study. Cancers 2020, 12, 2796. [Google Scholar] [CrossRef] [PubMed]
- Nardon, E.; Donada, M.; Bonin, S.; Dotti, I.; Stanta, G. Higher random oligo concentration improves reverse transcription yield of cDNA from bioptic tissues and quantitative RT-PCR reliability. Exp. Mol. Pathol. 2009, 87, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Bonin, S.; Pracella, D.; Barbazza, R.; Dotti, I.; Boffo, S.; Stanta, G. PI3K/AKT Signaling in Breast Cancer Molecular Subtyping and Lymph Node Involvement. Dis. Markers 2019, 2019, 7832376. [Google Scholar] [CrossRef] [Green Version]
- Linnerth-Petrik, N.M.; Santry, L.A.; Moorehead, R.; Jucker, M.; Wootton, S.K.; Petrik, J. Akt isoform specific effects in ovarian cancer progression. Oncotarget 2016, 7, 74820–74833. [Google Scholar] [CrossRef] [Green Version]
- Bayraktar, S.; Gluck, S. Systemic therapy options in BRCA mutation-associated breast cancer. Breast Cancer Res. Treat. 2012, 135, 355–366. [Google Scholar] [CrossRef]
- Plo, I.; Laulier, C.; Gauthier, L.; Lebrun, F.; Calvo, F.; Lopez, B.S. AKT1 inhibits homologous recombination by inducing cytoplasmic retention of BRCA1 and RAD51. Cancer Res. 2008, 68, 9404–9412. [Google Scholar] [CrossRef] [Green Version]
- Nojima, A.; Yamashita, M.; Yoshida, Y.; Shimizu, I.; Ichimiya, H.; Kamimura, N.; Kobayashi, Y.; Ohta, S.; Ishii, N.; Minamino, T. Haploinsufficiency of akt1 prolongs the lifespan of mice. PLoS ONE 2013, 8, e69178. [Google Scholar] [CrossRef] [Green Version]
- Kolora, S.R.R.; Owens, G.L.; Vazquez, J.M.; Stubbs, A.; Chatla, K.; Jainese, C.; Seeto, K.; McCrea, M.; Sandel, M.W.; Vianna, J.A.; et al. Origins and evolution of extreme life span in Pacific Ocean rockfishes. Science 2021, 374, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Barry, W.T.; Birrer, M.; Westin, S.N.; Cadoo, K.A.; Shapiro, G.I.; Mayer, E.L.; O’Cearbhaill, R.E.; Coleman, R.L.; Kochupurakkal, B.; et al. Olaparib and alpha-specific PI3K inhibitor alpelisib for patients with epithelial ovarian cancer: A dose-escalation and dose-expansion phase 1b trial. Lancet Oncol. 2019, 20, 570–580. [Google Scholar] [CrossRef]
- Li, L.Y.; Kim, H.J.; Park, S.A.; Lee, S.H.; Kim, L.K.; Lee, J.Y.; Kim, S.; Kim, Y.T.; Kim, S.W.; Nam, E.J. Genetic Profiles Associated with Chemoresistance in Patient-Derived Xenograft Models of Ovarian Cancer. Cancer Res. Treat. 2019, 51, 1117–1127. [Google Scholar] [CrossRef]
- Liby, T.A.; Spyropoulos, P.; Buff Lindner, H.; Eldridge, J.; Beeson, C.; Hsu, T.; Muise-Helmericks, R.C. Akt3 controls vascular endothelial growth factor secretion and angiogenesis in ovarian cancer cells. Int. J. Cancer 2012, 130, 532–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, Y.R.; Yoshida, T.; Marusyk, A.; Beck, A.H.; Polyak, K.; Toker, A. Targeting Akt3 signaling in triple-negative breast cancer. Cancer Res. 2014, 74, 964–973. [Google Scholar] [CrossRef] [Green Version]
- Cristiano, B.E.; Chan, J.C.; Hannan, K.M.; Lundie, N.A.; Marmy-Conus, N.J.; Campbell, I.G.; Phillips, W.A.; Robbie, M.; Hannan, R.D.; Pearson, R.B. A specific role for AKT3 in the genesis of ovarian cancer through modulation of G(2)-M phase transition. Cancer Res. 2006, 66, 11718–11725. [Google Scholar] [CrossRef] [Green Version]
- You, Y.; Bi, F.F.; Jiang, Y.; Xu, Y.T.; An, Y.Y.; Li, D.; Yang, Q. BRCA1 affects the resistance and stemness of SKOV3-derived ovarian cancer stem cells by regulating autophagy. Cancer Med. 2019, 8, 656–668. [Google Scholar] [CrossRef]
- Wagener-Ryczek, S.; Merkelbach-Bruse, S.; Siemanowski, J. Biomarkers for Homologous Recombination Deficiency in Cancer. J. Pers. Med. 2021, 11, 612. [Google Scholar] [CrossRef] [PubMed]

| Features | N = 103, n (%) |
|---|---|
| Age at diagnosis mean | 62 (range 30–79) |
| FIGO stage | |
| III | 68 (68) |
| IV | 32 (32) |
| NA | 3 |
| Progression after first-line CT | |
| No | 24 (26) |
| Yes | 70 (74) |
| NA | 9 |
| Residual tumor after surgery | |
| No | 34 (36) |
| Yes | 61 (64) |
| NA | 8 |
| Primary platinum response | |
| Never progressed | 24 (27) |
| Resistant | 25 (28) |
| Sensitive | 39 (44) |
| NA | 15 |
| Anatomical sites | |
| Ovaries | 85 (83) |
| Implants | 18 (17) |
| BMI categories | |
| Normal | 50 (56) |
| Obese | 10 (11) |
| Overweight | 19 (21) |
| Underweight | 10(11) |
| NA | 14 |
| Bevacizumab | |
| No | 64 (71) |
| Yes | 26 (29) |
| NA | 13 |
| Survival status | |
| Alive | 42 (41) |
| Dead | 60 (59) |
| NA | 1 |
| Overall Survival (N = 93) | |||
|---|---|---|---|
| HR | 95% CI | P-Value | |
| Age at diagnosis | 1.03 | 1.00–1.06 | 0.07 |
| FIGO stage (III/IV) | 2.65 | 1.50–4.68 | 0.0008 * |
| Residual tumor after surgery | 0.81 | 0.47–1.41 | 0.5 |
| AKT categories | |||
| high/high1 | 1 | ||
| high/low | 2.43 | 1.13–5.19 | 0.02 * |
| low/high | 2.01 | 0.87–4.68 | 0.1 |
| low/low | 3.46 | 1.71–7.03 | 0.0006 * |
| Ph Test: p = 0.3 | |||
| AKT Isoform Categories | |||||
|---|---|---|---|---|---|
| Clinicopathological Variables | High/High (33) | High/Low (23) | Low/High (17) | Low/Low (30) | p-Value |
| Tumor pattern INF MP PA PSE SD TR | |||||
| 5 (15) | 9 (39) | 0 (0) | 6(20) | ||
| 0 (0) | 2 (9) | 0 (0) | 0 (0) | ||
| 7 (21) | 6 (26) | 6 (35) | 12 (40) | 0.01 * | |
| 7 (21) | 4 (17) | 8 (47) | 5 (17) | ||
| 9 (28) | 2 (9) | 2 (12) | 5 (17) | ||
| 5 (15) | 0 (0) | 1 (6) | 2 (7) | ||
| SET/Classic Classic SET | |||||
| 11 (33) | 15 (65) | 4 (23) | 16 (53) | 0.02 * | |
| 22 (67) | 8 (35) | 13 (77) | 14 (47) | ||
| Mitoses (x10 HPF) Median (range) | |||||
| 23 (10–110) | 16 (5–42) | 22 (5–62) | 17 (5–92) | 0.03 * | |
| Psammoma bodies Absent Present | |||||
| 31 (94) | 17 (74) | 17 (100) | 23 (77) | 0.03 * | |
| 2 (6) | 6 (26) | 0 (0) | 7 (23) | ||
| N° lymphocytes (x 1HPF) | |||||
| Median (range) | 10 (5–60) | 10 (0–70) | 10 (5–80) | 20 (0–60) | 0.5 |
| Age at diagnosis | |||||
| Median (range) | 65 (30–79) | 64 (32–73) | 61 (38–74) | 65 (48–79) | 0.5 |
| FIGO stage | |||||
| III | 22 (67) | 17 (74) | 12 (71) | 18 (67) | 0.9 |
| IV | 11 (33) | 6 (26) | 5 (29) | 9 (33) | |
| NA | 0 | 0 | 0 | 3 | |
| Residual tumor | |||||
| No | 11 (34) | 7 (33) | 6 (35) | 10 (40) | 1 |
| Yes | 21 (66) | 14 (67) | 11 (65) | 15 (60) | |
| NA | 1 | 2 | 0 | 5 | |
| Primary platinum response | |||||
| Never progressed | 8 (29) | 4 (22) | 6 (37) | 6 (23) | |
| Resistant | 6 (21) | 2 (11) | 4 (25) | 13 (50) | 0.07 |
| Sensitive | 14 (50) | 12 (67) | 6 (38) | 7 (27) | |
| NA | 5 | 5 | 1 | 4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzalini, E.; Tierno, D.; Bartoletti, M.; Barbazza, R.; Giorda, G.; Puglisi, F.; Cecere, S.C.; Losito, N.S.; Russo, D.; Stanta, G.; et al. AKT Isoforms Interplay in High-Grade Serous Ovarian Cancer Prognosis and Characterization. Cancers 2022, 14, 304. https://doi.org/10.3390/cancers14020304
Azzalini E, Tierno D, Bartoletti M, Barbazza R, Giorda G, Puglisi F, Cecere SC, Losito NS, Russo D, Stanta G, et al. AKT Isoforms Interplay in High-Grade Serous Ovarian Cancer Prognosis and Characterization. Cancers. 2022; 14(2):304. https://doi.org/10.3390/cancers14020304
Chicago/Turabian StyleAzzalini, Eros, Domenico Tierno, Michele Bartoletti, Renzo Barbazza, Giorgio Giorda, Fabio Puglisi, Sabrina Chiara Cecere, Nunzia Simona Losito, Daniela Russo, Giorgio Stanta, and et al. 2022. "AKT Isoforms Interplay in High-Grade Serous Ovarian Cancer Prognosis and Characterization" Cancers 14, no. 2: 304. https://doi.org/10.3390/cancers14020304
APA StyleAzzalini, E., Tierno, D., Bartoletti, M., Barbazza, R., Giorda, G., Puglisi, F., Cecere, S. C., Losito, N. S., Russo, D., Stanta, G., Canzonieri, V., & Bonin, S. (2022). AKT Isoforms Interplay in High-Grade Serous Ovarian Cancer Prognosis and Characterization. Cancers, 14(2), 304. https://doi.org/10.3390/cancers14020304









