Impact of Lymphadenectomy on the Oncologic Outcome of Patients with Adrenocortical Carcinoma—A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
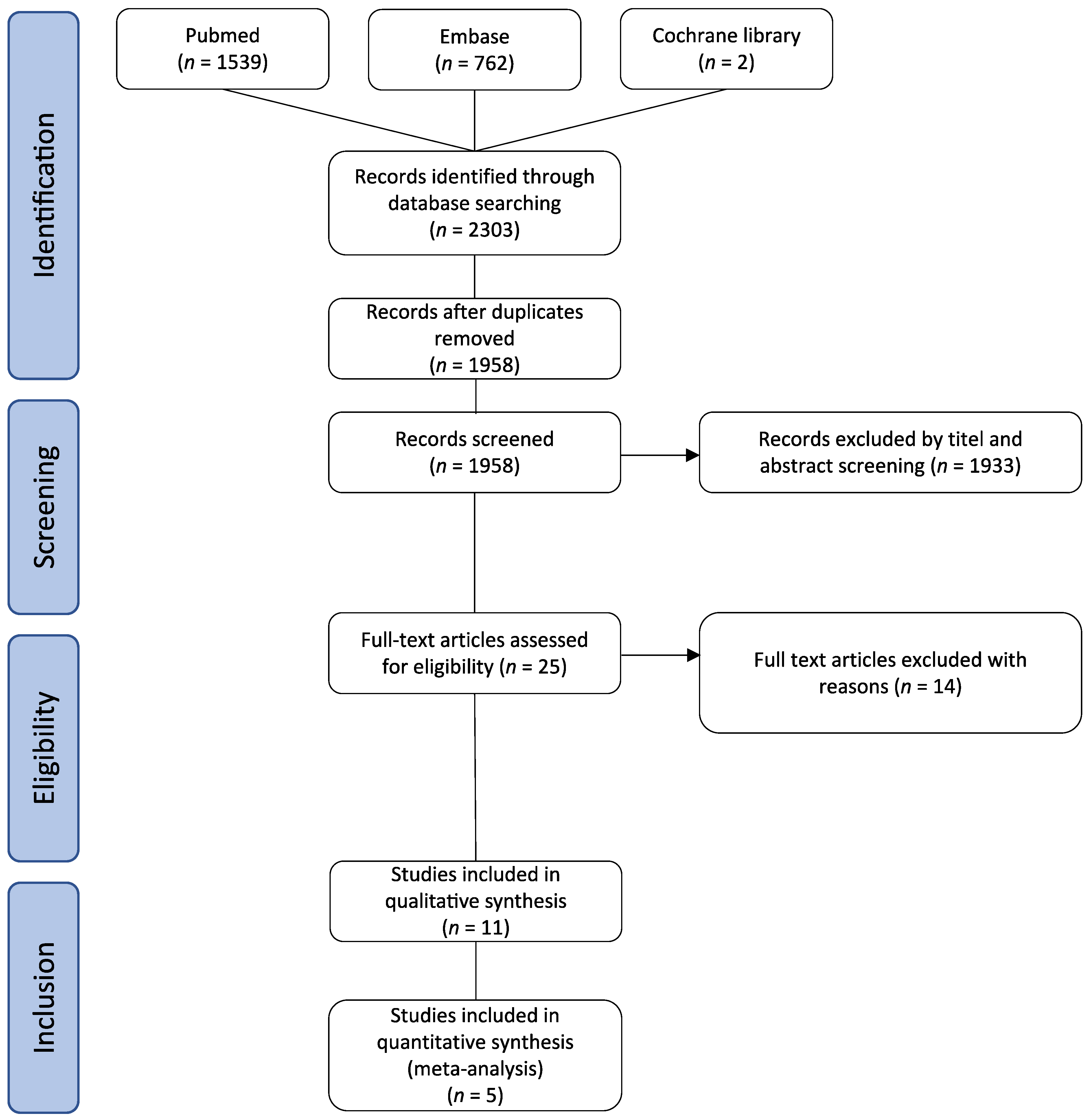
2.1. Study Design and Search Strategy
2.2. Study Selection, Inclusion Criteria and Outcome Measures
2.3. Assessment of Risk of Bias
2.4. Statistical Analysis
3. Results
3.1. Study Characteristics
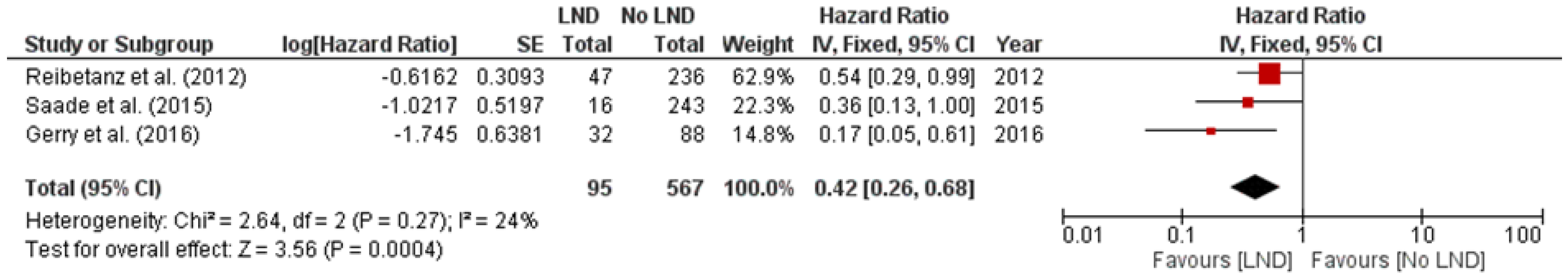
3.2. Lymphadenectomy and Survival
3.3. Postoperative Mortality
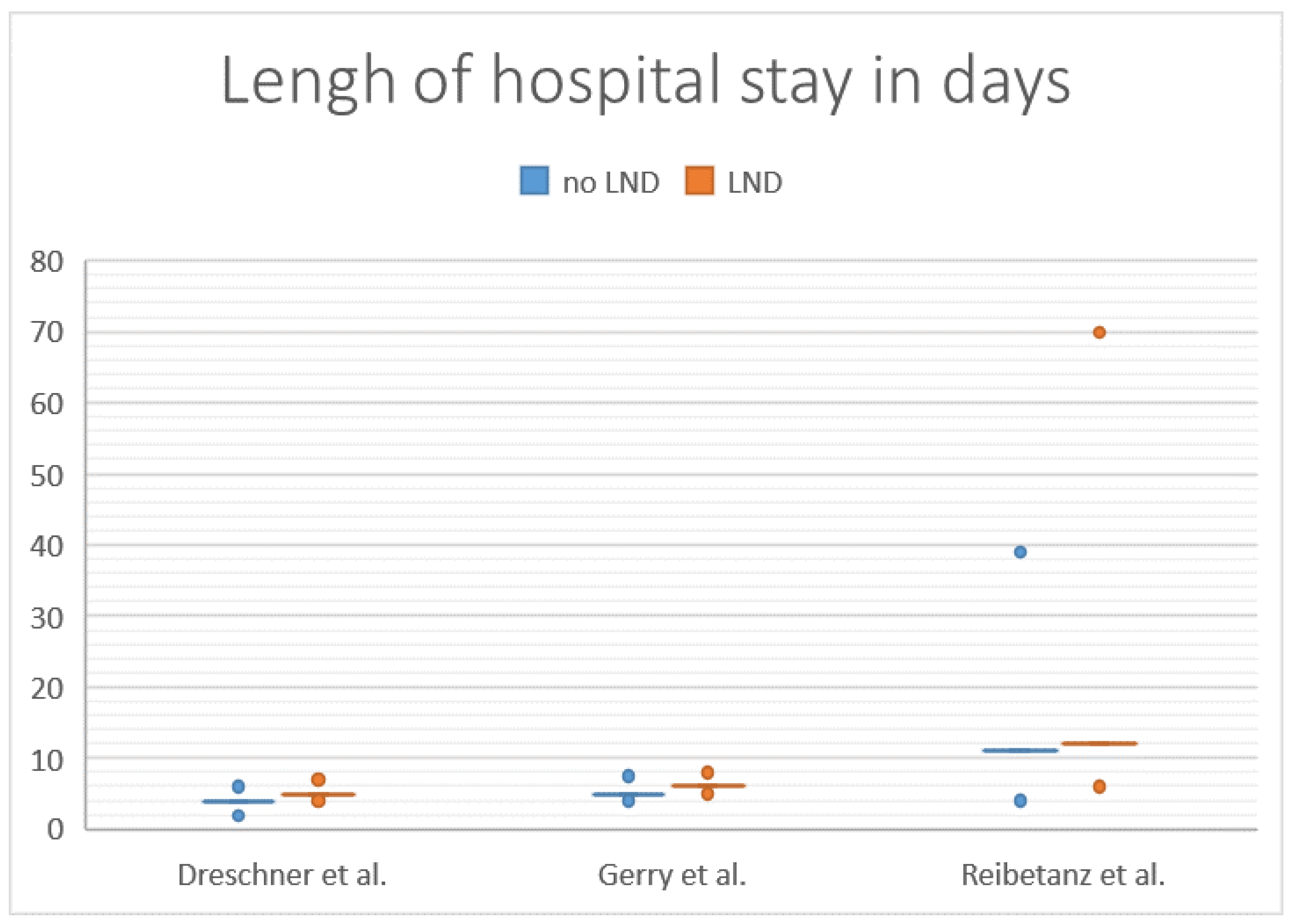
3.4. Length of Hospital Stay
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Author, Year | Baseline Confounding | Selection of Participants | Classification of Intervention | Deviation From Intended Intervention | Missing Data | Measurement of Outcomes | Selection of Reported Results | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Alanee, 2015 [18] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Dreschner, 2020 [19] | Moderate | Low | Moderate | Low | Low | Low | Low | Moderate |
| Gerry, 2016 [20] | Moderate | Low | Serious | Low | Low | Low | Low | Moderate |
| Icard, 2001 [21] | Moderate | Low | Serious | Low | Low | Low | Low | Moderate |
| Marincola, 2018 [22] | Moderate | Low | Serious | Low | Low | Low | Low | Moderate |
| Nibulol, 2016 [23] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Reibetanz, 2012 [17] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Saade, 2015 [24] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Tella, 2018 [25] | Moderate | Low | Moderate | Low | Low | Low | Low | Moderate |
| Tran, 2013 [26] | Moderate | Low | Moderate | Low | Low | Low | Low | Moderate |
| Wang, 2017 [27] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Author | Year | Country | Database | Data Collection | Population | Staging System Used | Included Tumor Stages | Surgery Performed | LND | noLND | LN-I (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alanee et al. [18] | 2015 | USA | SEER | 1991–2011 | 1732 | Not specifically mentioned | I–IV, metastatic disease included | 1037 (59.9%) | 56 (5.4%) | 981 (94.6%) | 30.1% |
| Dreschner et al. [19] | 2020 | USA | NCDB | 2004–2015 | 897 | Not specifically mentioned | Stage I–III | 897 (100%) | 46 (5.1%) | 851 (94.9%) | 16.3% |
| Gerry et al. [20] | 2016 | USA | ACCG | 1993–2014 | 265 | AJCC | Stage I–III, metastatic disease excluded | 120 (45.3%) | 32 (26.7%) | 88 (73.3%) | 8% |
| Icard et al. [21] | 2001 | France | AFCE | 1978–1997 | 253 | Modified MacFarlane | I–IV, metastatic disease included | 252 (99.6%) | 89 (35.3%) | 163 (64.7%) | nr |
| Marincola et al. [22] | 2018 | USA | ACCG | 1993–2014 | 265 | AJCC | metastatic disease excluded | 158 (59.6%) | 37 (23.4%) | 121 (76.6%) | 9.5% |
| Nilubol et al. [23] | 2015 | USA | SEER | 1973–2011 | 1525 | ‘localized, regional, and distant metastatic disease’ | I–IV, metastatic disease included | 802 (52.6%) | 67 (8.4%) | 735 (91.6%) | 12.8% |
| Reibetanz et al. [17] | 2012 | Germany | German ACC Registry | 1981–2009 | 283 | ENSAT | ENSAT I–III | 283 (100%) | 47 (16.6%) | 236 (83.4%) | 28% |
| Saade et al. [24] | 2015 | USA | SEER | 1988–2009 | 259 | ENSAT | ENSAT I–III | 259 (100%) | 16 (6.2%) | 243 (93.8%) | 43% |
| Tella et al. [25] | 2018 | USA | NCDB | 2004–2015 | 3185 | AJCC | Stage I–IV, metastatic disease included | 1559 (48.9%) I–III 824 IV 735 | I–III 125 (17.9%) IV 111 (17.8%) | I–III 574 (82.1%) IV 513 (82.2%) | 55% |
| Tran et al. [26] | 2013 | USA | SEER | 1988–2009 | 320 | AJCC | AJCC III and IV, distant metastatic disease excluded | 280 (87.5%) | 83 (29.6%) | 237 (84.6%) | 35% |
| Wang et al. [27] | 2017 | USA | SEER | 1973–2014 | 749 | AJCC and ENSAT | I–IV, metastatic disease included | 722 (96.4%) | 145 (20.1%) | 577 (79.9%) | 13.7% |
References
- Fassnacht, M.; Johanssen, S.; Quinkler, M.; Bucsky, P.; Willenberg, H.S.; Beuschlein, F.; Terzolo, M.; Mueller, H.H.; Hahner, S.; Allolio, B.; et al. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: Proposal for a Revised TNM Classification. Cancer 2009, 115, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Kroiss, M.; Allolio, B. Update in adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 2013, 98, 4551–4564. [Google Scholar] [CrossRef] [PubMed]
- Else, T.; Kim, A.C.; Sabolch, A.; Raymond, V.M.; Kandathil, A.; Caoili, E.M.; Jolly, S.; Miller, B.S.; Giordano, T.J.; Hammer, G.D. Adrenocortical carcinoma. Endocr. Rev. 2014, 35, 282–326. [Google Scholar] [CrossRef] [Green Version]
- Bilimoria, K.Y.; Shen, W.T.; Elaraj, D.; Bentrem, D.J.; Winchester, D.J.; Kebebew, E.; Sturgeon, C. Adrenocortical carcinoma in the United States: Treatment utilization and prognostic factors. Cancer 2008, 113, 3130–3136. [Google Scholar] [CrossRef] [PubMed]
- Berruti, A.; Fassnacht, M.; Haak, H.; Else, T.; Baudin, E.; Sperone, P.; Kroiss, M.; Kerkhofs, T.; Williams, A.R.; Ardito, A.; et al. Prognostic role of overt hypercortisolism in completely operated patients with adrenocortical cancer. European Urol. 2014, 65, 832–838. [Google Scholar] [CrossRef]
- Fassnacht, M.; Terzolo, M.; Allolio, B.; Baudin, E.; Haak, H.; Berruti, A.; Welin, S.; Schade-Brittinger, C.; Lacroix, A.; Jarzab, B.; et al. Combination chemotherapy in advanced adrenocortical carcinoma. N. Engl. J. Med. 2012, 366, 2189–2197. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Dekkers, O.M.; Else, T.; Baudin, E.; Berruti, A.; De Krijger, R.R.; Haak, H.R.; Mihai, R.; Assie, G.; Terzolo, M. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol./Eur. Fed. Endocr. Soc. 2018, 179, G1–G46. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Assie, G.; Baudin, E.; Eisenhofer, G.; de la Fouchardiere, C.; Haak, H.R.; de Krijger, R.; Porpiglia, F.; Terzolo, M.; Berruti, A. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol./ESMO 2020, 31, 1476–1490. [Google Scholar] [CrossRef]
- Kiseljak-Vassiliades, K.; Bancos, I.; Hamrahian, A.; Habra, M.; Vaidya, A.; Levine, A.C.; Else, T. American Association of Clinical Endocrinology Disease State Clinical Review on the Evaluation and Management of Adrenocortical Carcinoma in an Adult: A Practical Approach. Endocr. Pract. 2020, 26, 1366–1383. [Google Scholar] [CrossRef] [PubMed]
- Dackiw, A.P.; Lee, J.E.; Gagel, R.F.; Evans, D.B. Adrenal cortical carcinoma. World J. Surg. 2001, 25, 914–926. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, M.L.; Lloyd, R.; Erickson, L.; Farley, D.R.; Grant, C.S.; Thompson, G.B.; Rowland, C.; Young, W.F.; Van Heerden, J.A. Adrenocortical carcinoma: Surgical progress or status quo? Arch. Surg. 2001, 136, 543–549. [Google Scholar] [CrossRef] [Green Version]
- Schteingart, D.E.; Doherty, G.M.; Gauger, P.G.; Giordano, T.J.; Hammer, G.D.; Korobkin, M.; Worden, F.P. Management of patients with adrenal cancer: Recommendations of an international consensus conference. Endocr.-Relat. Cancer 2005, 12, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Panjwani, S.; Moore, M.D.; Gray, K.D.; Finnerty, B.M.; Beninato, T.; Brunaud, L.; Fahey, T.J.; Zarnegar, R. The Impact of Nodal Dissection on Staging in Adrenocortical Carcinoma. Ann. Surg. Oncol. 2017, 24, 3617–3623. [Google Scholar] [CrossRef]
- Fassnacht, M.; Johanssen, S.; Fenske, W.; Weismann, D.; Agha, A.; Beuschlein, F.; Fuhrer, D.; Jurowich, C.; Quinkler, M.; Petersenn, S.; et al. Improved survival in patients with stage II adrenocortical carcinoma followed up prospectively by specialized centers. J. Clin. Endocrinol. Metab. 2010, 95, 4925–4932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulick, R.D.; Brennan, M.F. Long-term survival after complete resection and repeat resection in patients with adrenocortical carcinoma. Ann. Surg. Oncol. 1999, 6, 719–726. [Google Scholar] [CrossRef]
- Gaujoux, S.; Mihai, R. European Society of Endocrine Surgeons (ESES) and European Network for the Study of Adrenal Tumours (ENSAT) recommendations for the surgical management of adrenocortical carcinoma. Br. J. Surg. 2017, 104, 358–376. [Google Scholar] [CrossRef] [Green Version]
- Reibetanz, J.; Jurowich, C.; Erdogan, I.; Nies, C.; Rayes, N.; Dralle, H.; Behrend, M.; Allolio, B.; Fassnacht, M.; German ACC Study Group. Impact of lymphadenectomy on the oncologic outcome of patients with adrenocortical carcinoma. Ann. Surg. 2012, 255, 363–369. [Google Scholar] [CrossRef]
- Alanee, S.; Dynda, D.; Holland, B. Prevalence and Prognostic Value of Lymph Node Dissection in Treating Adrenocortical Carcinoma: A National Experience. Anticancer Res. 2015, 35, 5575–5579. [Google Scholar] [PubMed]
- Deschner, B.W.; Stiles, Z.E. Critical analysis of lymph node examination in patients undergoing curative-intent resection for adrenocortical carcinoma. J. Surg. Oncol. 2020, 122, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Gerry, J.M.; Tran, T.B.; Postlewait, L.M.; Maithel, S.K.; Prescott, J.D.; Wang, T.S.; Glenn, J.A.; Phay, J.E.; Keplinger, K.; Fields, R.C.; et al. Lymphadenectomy for Adrenocortical Carcinoma: Is There a Therapeutic Benefit? Ann. Surg. Oncol. 2016, 23, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Icard, P.; Goudet, P.; Charpenay, C.; Andreassian, B.; Carnaille, B.; Chapuis, Y.; Cougard, P.; Henry, J.F.; Proye, C. Adrenocortical carcinomas: Surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J. Surg. 2001, 25, 891–897. [Google Scholar] [CrossRef]
- Smith, P.M.; Kiernan, C.M.; Tran, T.B.; Postlewait, L.M.; Maithel, S.K.; Prescott, J.; Pawlik, T.; Wang, T.S.; Glenn, J.; Hatzaras, I.; et al. Role of Additional Organ Resection in Adrenocortical Carcinoma: Analysis of 167 Patients from the U.S. Adrenocortical Carcinoma Database. Ann. Surg. Oncol. 2018, 25, 2308–2315. [Google Scholar] [CrossRef]
- Nilubol, N.; Patel, D.; Kebebew, E. Does Lymphadenectomy Improve Survival in Patients with Adrenocortical Carcinoma? A Population-Based Study. World J. Surg. 2016, 40, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Saade, N.; Sadler, C.; Goldfarb, M. Impact of Regional Lymph Node Dissection on Disease Specific Survival in Adrenal Cortical Carcinoma. Horm. Metab. Res. 2015, 47, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Tella, S.H.; Kommalapati, A.; Yaturu, S.; Kebebew, E. Predictors of Survival in Adrenocortical Carcinoma: An Analysis From the National Cancer Database. J. Clin. Endocrinol. Metab. 2018, 103, 3566–3573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, T.B.; Liou, D.; Menon, V.G.; Nissen, N.N. Surgical management of advanced adrenocortical carcinoma: A 21-year population-based analysis. Am. Surg. 2013, 79, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, S.S.; Gao, W.C.; Bai, L.; Luo, L.; Zheng, X.G.; Luo, Y. Prognostic Factors of Adrenocortical Carcinoma: An Analysis of the Surveillance Epidemiology and End Results (SEER) Database. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 2817–2823. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Open Med. 2009, 3, e123–e130. [Google Scholar]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Clin. Res. Ed.) 2016, 355, i4919. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, K.; Langer, P.; Niederle, B.; Alesina, P.; Holzer, K.; Nies, C.; Musholt, T.; Goretzki, P.E.; Rayes, N.; Quinkler, M.; et al. Surgical therapy of adrenal tumors: Guidelines from the German Association of Endocrine Surgeons (CAEK). Langenbeck’s Arch. Surg. 2019, 404, 385–401. [Google Scholar] [CrossRef]
- Stigliano, A.; Chiodini, I.; Giordano, R.; Faggiano, A.; Canu, L.; Della Casa, S.; Loli, P.; Luconi, M.; Mantero, F.; Terzolo, M. Management of adrenocortical carcinoma: A consensus statement of the Italian Society of Endocrinology (SIE). J. Endocrinol. Investig. 2016, 39, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Savoie, P.H.; Murez, T.; Flechon, A.; Sebe, P.; Rocher, L.; Camparo, P.; Morel-Journel, N.; Ferretti, L.; Méjean, A. French ccAFU guidelines—Update 2018–2020: Adrenal cancer. Prog. Urol. J. l’Association Fr. d’urologie Soc. Fr. d’urologie 2018, 28 (Suppl. 1), R177–R195. [Google Scholar] [CrossRef] [PubMed]
- Reibetanz, J.; Rinn, B.; Kunz, A.S.; Flemming, S.; Ronchi, C.L.; Kroiss, M.; Deutschbein, T.; Pulzer, A.; Hahner, S.; Kocot, A.; et al. Patterns of Lymph Node Recurrence in Adrenocortical Carcinoma: Possible Implications for Primary Surgical Treatment. Ann. Surg. Oncol. 2019, 26, 531–538. [Google Scholar] [CrossRef]
- MacFarlane, J.K.; Ryall, R.D.; Heald, R.J. Mesorectal excision for rectal cancer. Lancet 1993, 341, 457–460. [Google Scholar] [CrossRef]
- Lee, J.E.; Berger, D.H.; El-Naggar, A.K.; Hickey, R.C.; Vassilopoulou-Sellin, R.; Gagel, R.F.; Burgess, M.A.; Evans, D.B. Surgical management, DNA content, and patient survival in adrenal cortical carcinoma. Surgery 1995, 118, 1090–1098. [Google Scholar] [CrossRef]
- Sullivan, M.; Boileau, M.; Hodges, C.V. Adrenal cortical carcinoma. J. Urol. 1978, 120, 660–665. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendricks, A.; Müller, S.; Fassnacht, M.; Germer, C.-T.; Wiegering, V.A.; Wiegering, A.; Reibetanz, J. Impact of Lymphadenectomy on the Oncologic Outcome of Patients with Adrenocortical Carcinoma—A Systematic Review and Meta-Analysis. Cancers 2022, 14, 291. https://doi.org/10.3390/cancers14020291
Hendricks A, Müller S, Fassnacht M, Germer C-T, Wiegering VA, Wiegering A, Reibetanz J. Impact of Lymphadenectomy on the Oncologic Outcome of Patients with Adrenocortical Carcinoma—A Systematic Review and Meta-Analysis. Cancers. 2022; 14(2):291. https://doi.org/10.3390/cancers14020291
Chicago/Turabian StyleHendricks, Anne, Sophie Müller, Martin Fassnacht, Christoph-Thomas Germer, Verena A. Wiegering, Armin Wiegering, and Joachim Reibetanz. 2022. "Impact of Lymphadenectomy on the Oncologic Outcome of Patients with Adrenocortical Carcinoma—A Systematic Review and Meta-Analysis" Cancers 14, no. 2: 291. https://doi.org/10.3390/cancers14020291
APA StyleHendricks, A., Müller, S., Fassnacht, M., Germer, C.-T., Wiegering, V. A., Wiegering, A., & Reibetanz, J. (2022). Impact of Lymphadenectomy on the Oncologic Outcome of Patients with Adrenocortical Carcinoma—A Systematic Review and Meta-Analysis. Cancers, 14(2), 291. https://doi.org/10.3390/cancers14020291






