A Single Center Analysis of Thymic Neuroendocrine Tumors †
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics and Patient Enrollment
2.2. Clinicopathological Characteristics
2.3. Treatment
2.4. Analysis of Outcomes
3. Results
3.1. Patient Characteristics
3.2. Treatments
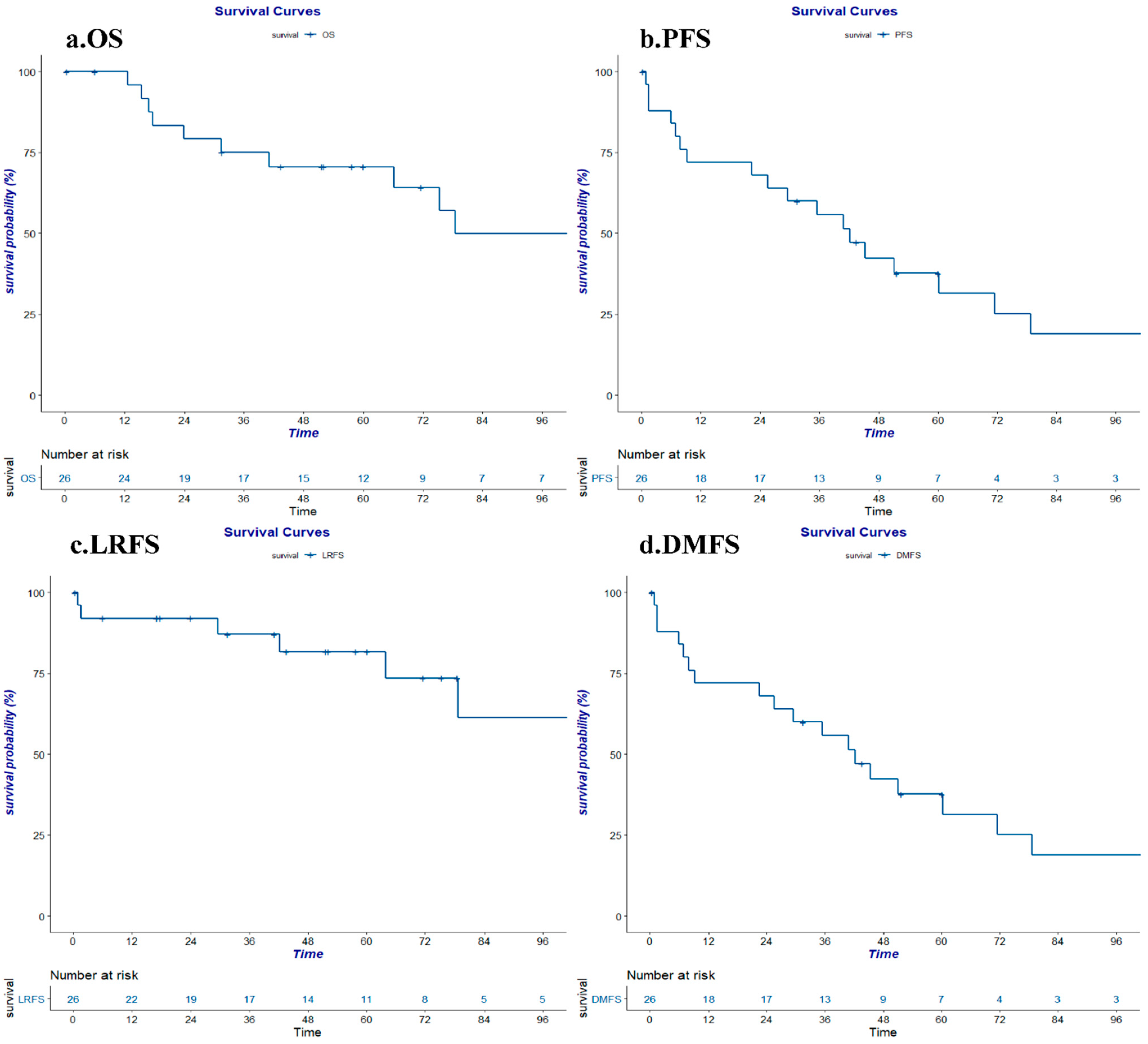
3.3. Survival
3.4. Toxicity
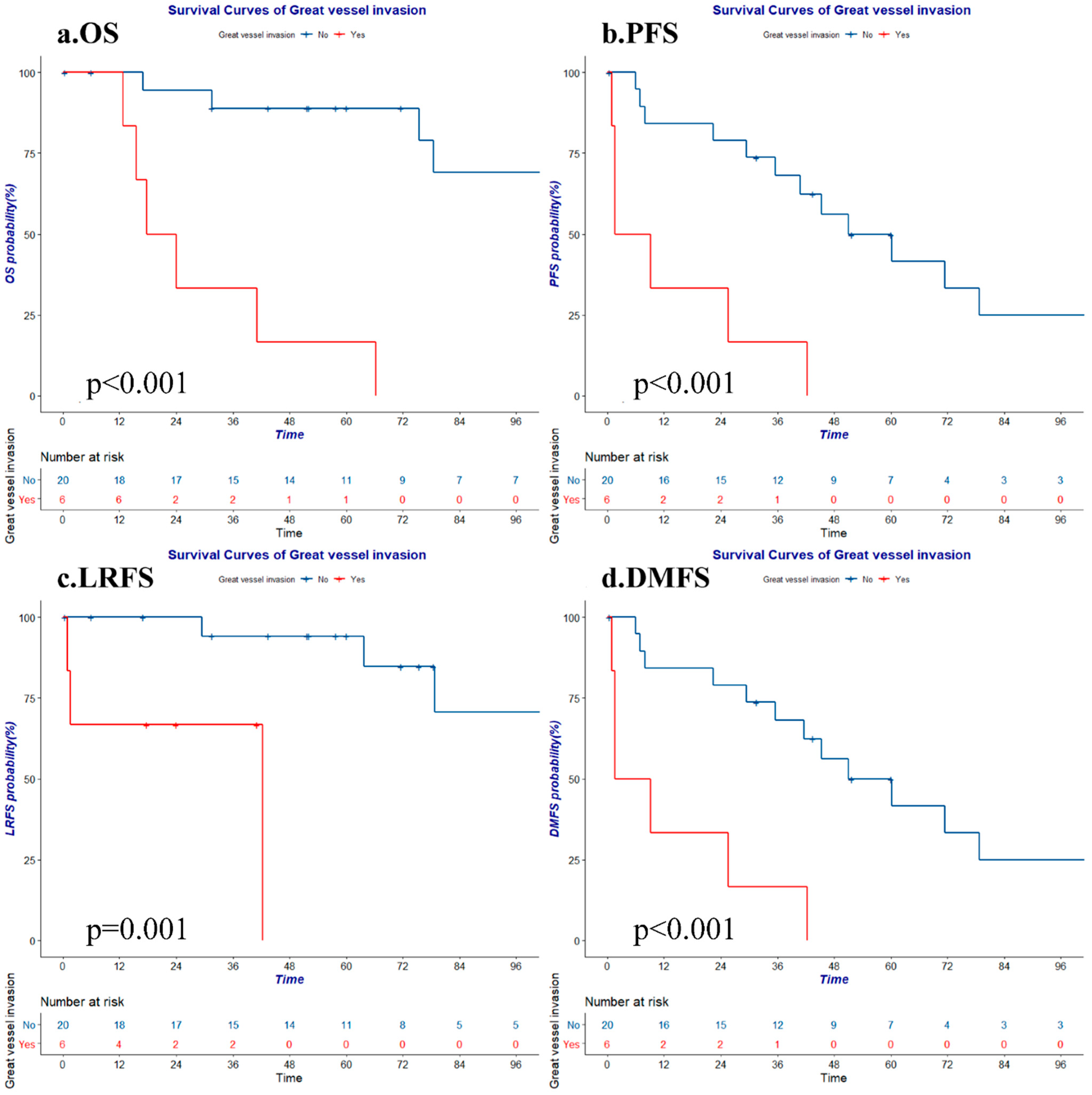
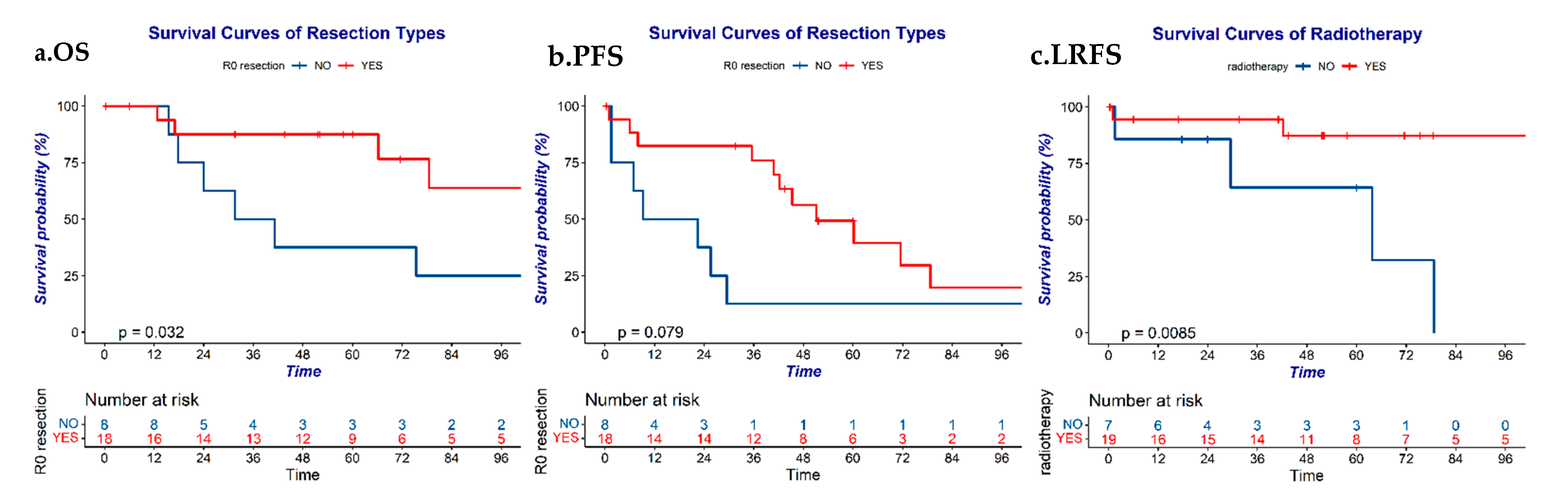
3.5. Prognostic Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Jong, W.K.; Blaauwgeers, J.L.; Schaapveld, M.; Timens, W.; Klinkenberg, T.J.; Groen, H.J. Thymic epithelial tumours: A population-based study of the incidence, diagnostic procedures and therapy. Eur. J. Cancer 2008, 44, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Phan, A.T.; Oberg, K.; Choi, J.; Harrison, L.H., Jr.; Hassan, M.M.; Strosberg, J.R.; Krenning, E.P.; Kocha, W.; Woltering, E.A.; Maples, W.J.; et al. NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: Well-differentiated neuroendocrine tumors of the thorax (includes lung and thymus). Pancreas 2010, 39, 784–798. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Chan, J.K.; Yin, C.H.; Lee, C.C.; Chern, C.U.; Liao, C.I. Trends in the incidence of thymoma, thymic carcinoma, and thymic neuroendocrine tumor in the United States. PLoS ONE 2019, 14, e0227197. [Google Scholar] [CrossRef] [PubMed]
- Oberg, K.; Jelic, S.; Group, E.G.W. Neuroendocrine bronchial and thymic tumors: ESMO clinical recommendation for diagnosis, treatment and follow-up. Ann. Oncol. 2009, 20 (Suppl. 4), 147–149. [Google Scholar] [CrossRef]
- Volante, M.; Mete, O.; Pelosi, G.; Roden, A.C.; Speel, E.J.M.; Uccella, S. Molecular pathology of well-differentiated pulmonary and thymic neuroendocrine tumors: What do pathologists need to know? Endocr. Pathol. 2021, 32, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Fukai, I.; Masaoka, A.; Fujii, Y.; Yamakawa, Y.; Yokoyama, T.; Murase, T.; Eimoto, T. Thymic neuroendocrine tumor (thymic carcinoid): A clinicopathologic study in 15 patients. Ann. Thorac. Surg. 1999, 67, 208–211. [Google Scholar] [CrossRef]
- Crona, J.; Bjorklund, P.; Welin, S.; Kozlovacki, G.; Oberg, K.; Granberg, D. Treatment, prognostic markers and survival in thymic neuroendocrine tumours. A study from a single tertiary referral centre. Lung Cancer 2013, 79, 289–293. [Google Scholar] [CrossRef]
- Strobel, P.; Zettl, A.; Shilo, K.; Chuang, W.Y.; Nicholson, A.G.; Matsuno, Y.; Gal, A.; Laeng, R.H.; Engel, P.; Capella, C.; et al. Tumor genetics and survival of thymic neuroendocrine neoplasms: A multi-institutional clinicopathologic study. Genes Chromosomes Cancer 2014, 53, 738–749. [Google Scholar] [CrossRef]
- Weissferdt, A.; Kalhor, N.; Liu, H.; Rodriguez, J.; Fujimoto, J.; Tang, X.; Wistuba, I.I.; Moran, C.A. Thymic neuroendocrine tumors (paraganglioma and carcinoid tumors): A comparative immunohistochemical study of 46 cases. Hum. Pathol. 2014, 45, 2463–2470. [Google Scholar] [CrossRef] [PubMed]
- Filosso, P.L.; Yao, X.; Ahmad, U.; Zhan, Y.; Huang, J.; Ruffini, E.; Travis, W.; Lucchi, M.; Rimner, A.; Antonicelli, A.; et al. Outcome of primary neuroendocrine tumors of the thymus: A joint analysis of the International Thymic Malignancy Interest Group and the European Society of Thoracic Surgeons databases. J. Thorac. Cardiovasc. Surg. 2015, 149, 103–109.e102. [Google Scholar] [CrossRef]
- Ma, K.; Liu, Y.; Xue, Z.; Chu, X. Treatment, prognostic markers, and survival in thymic neuroendocrine tumors: A single center experience of 41 patients. Medicine 2017, 96, e7842. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.L.; Weksler, B. Neuroendocrine tumors of the thymus: Analysis of factors affecting survival in 254 patients. Ann. Thorac. Surg. 2017, 103, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Sholl, L.M.; Hatabu, H.; Nishino, M. Radiological features and metastatic patterns of thymic neuroendocrine tumours. Clin. Radiol. 2018, 73, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Ose, N.; Maeda, H.; Inoue, M.; Morii, E.; Shintani, Y.; Matsui, H.; Tada, H.; Tokunaga, T.; Kimura, K.; Sakamaki, Y.; et al. Results of treatment for thymic neuroendocrine tumours: Multicentre clinicopathological study. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Chen, J.; Chen, D.; Liu, D.; Xu, X.; Huang, L.; Cao, J.; Zhang, J.; Gu, Y.; Fan, M.; et al. Evaluation of the prognostic value of surgery and postoperative radiotherapy for patients with thymic neuroendocrine tumors: A propensity-matched study based on the SEER database. Thorac. Cancer 2018, 9, 1603–1613. [Google Scholar] [CrossRef]
- Corsini, E.M.; Mitchell, K.G.; Sceusi, E.L.; Mehran, R.J.; Rice, D.C.; Sepesi, B.; Walsh, G.L.; Swisher, S.G.; Roth, J.A.; Vaporciyan, A.A.; et al. Multidisciplinary treatment of thymic neuroendocrine tumors: Surgery remains a key component. J. Thorac. Dis. 2019, 11, 3391–3398. [Google Scholar] [CrossRef]
- Hamaji, M.; Omasa, M.; Nakagawa, T.; Miyahara, S.; Suga, M.; Kawakami, K.; Aoyama, A.; Date, H. Survival outcomes of patients with high-grade and poorly differentiated thymic neuroendocrine carcinoma. Interact. Cardiovasc. Thorac. Surg. 2020, 31, 98–101. [Google Scholar] [CrossRef] [PubMed]
- de Montpreville, V.T.; Macchiarini, P.; Dulmet, E. Thymic neuroendocrine carcinoma (carcinoid): A clinicopathologic study of fourteen cases. J. Thorac. Cardiovasc. Surg. 1996, 111, 134–141. [Google Scholar] [CrossRef][Green Version]
- Zhai, Y.; Hui, Z.; Ji, W.; Wang, X.; Liang, J.; Mao, Y.; Luo, Y.; Zou, S.; Lv, J.; Zhou, Z.; et al. A single-center analysis of the treatment and prognosis of patients with thymic carcinoma. Ann. Thorac. Surg. 2017, 104, 1718–1724. [Google Scholar] [CrossRef] [PubMed]
- Taskin, O.C.; Clarke, C.N.; Erkan, M.; Tsai, S.; Evans, D.B.; Adsay, V. Pancreatic neuroendocrine neoplasms: Current state and ongoing controversies on terminology, classification and prognostication. J. Gastrointest. Oncol. 2020, 11, 548–558. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Zhou, M.; Guo, C.; Li, S. Real-world clinicopathological features and outcome of thymic neuroendocrine tumors: A retrospective single-institution analysis. Orphanet. J. Rare Dis. 2022, 17, 215. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.W.; Benveniste, M.F.; Madan, R.; Godoy, M.C.; Groot, P.M.; Truong, M.T.; Rosado-de-Christenson, M.L.; Marom, E.M. IASLC/ITMIG staging system and lymph node map for thymic epithelial neoplasms. Radiographics 2017, 37, 758–776. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Filosso, P.L.; Roden, A.C.; Gu, Z.; Liu, Y.; Agzarian, J.; Shen, R.K.; Ruffini, E. Clinicopathological features and current treatment outcomes of neuroendocrine thymic tumours. Eur. J. Cardiothorac. Surg. 2021, 59, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Comacchio, G.M.; Dell’Amore, A.; Marino, M.C.; Russo, M.D.; Schiavon, M.; Mammana, M.; Faccioli, E.; Lorenzoni, G.; Gregori, D.; Pasello, G.; et al. Vascular involvement in thymic epithelial tumors: Surgical and oncological outcomes. Cancers 2021, 13, 3355. [Google Scholar] [CrossRef]
- Filosso, P.L.; Yao, X.; Ruffini, E.; Ahmad, U.; Antonicelli, A.; Huang, J.; Guerrera, F.; Venuta, F.; van Raemdonck, D.; Travis, W.; et al. Comparison of outcomes between neuroendocrine thymic tumours and other subtypes of thymic carcinomas: A joint analysis of the European Society of Thoracic Surgeons and the International Thymic Malignancy Interest Group. Eur. J. Cardiothorac. Surg. 2016, 50, 766–771. [Google Scholar] [CrossRef][Green Version]
- Kundel, Y.; Yellin, A.; Popovtzer, A.; Pfeffer, R.; Symon, Z.; Simansky, D.A.; Oberman, B.; Sadezki, S.; Brenner, B.; Catane, R.; et al. Adjuvant radiotherapy for thymic epithelial tumor: Treatment results and prognostic factors. Am. J. Clin. Oncol. 2007, 30, 389–394. [Google Scholar] [CrossRef]
- Gaur, P.; Leary, C.; Yao, J.C. Thymic neuroendocrine tumors: A SEER database analysis of 160 patients. Ann. Surg. 2010, 251, 1117–1121. [Google Scholar] [CrossRef]
- Jia, R.; Sulentic, P.; Xu, J.M.; Grossman, A.B. Thymic neuroendocrine neoplasms: Biological behaviour and therapy. Neuroendocrinology 2017, 105, 105–114. [Google Scholar] [CrossRef]
- Bian, D.; Qi, M.; Hu, J.; Ning, Y.; Zhou, F.; Fei, K.; Zhang, P. The comparison of predictive factors regarding prognoses and invasion of thymic neuroendocrine tumors preoperatively and postoperatively. J. Thorac. Dis. 2018, 10, 1657–1669. [Google Scholar] [CrossRef]
- Baum, R.P.; Kulkarni, H.R.; Singh, A.; Kaemmerer, D.; Mueller, D.; Prasad, V.; Hommann, M.; Robiller, F.C.; Niepsch, K.; Franz, H.; et al. Results and adverse events of personalized peptide receptor radionuclide therapy with (90)Yttrium and (177)Lutetium in 1048 patients with neuroendocrine neoplasms. Oncotarget 2018, 9, 16932–16950. [Google Scholar] [CrossRef]
- Ambrosini, V.; Kunikowska, J.; Baudin, E.; Bodei, L.; Bouvier, C.; Capdevila, J.; Cremonesi, M.; de Herder, W.W.; Dromain, C.; Falconi, M.; et al. Consensus on molecular imaging and theranostics in neuroendocrine neoplasms. Eur. J. Cancer 2021, 146, 56–73. [Google Scholar] [CrossRef] [PubMed]
| Number of Patients n = 26 | % | ||
|---|---|---|---|
| Age | Median (range) | 45 (25–69) | |
| Sex | Male | 20 | 76.9 |
| Female | 6 | 23.1 | |
| Histological grade | Low | 17 | 65.4 |
| Intermediate | 6 | 23.1 | |
| High | 3 | 11.5 | |
| ECOG PS score | 0–1 | 23 | 88.5 |
| ≥2 | 3 | 11.5 | |
| Smoking | Yes | 12 | 46.2 |
| No | 14 | 53.8 | |
| Paraneoplastic syndrome | Yes | 3 | 11.5 |
| No | 23 | 88.5 | |
| Great vessel invasion | Yes | 6 | 23.1 |
| No | 20 | 76.9 | |
| Masaoka stage | I | 2 | 7.7 |
| II | 8 | 30.8 | |
| III | 9 | 34.6 | |
| IVa | 1 | 3.8 | |
| IVb | 6 | 23.1 | |
| T stage | T1 | 10 | 38.5 |
| T2 | 1 | 3.8 | |
| T3 | 12 | 46.2 | |
| T4 | 3 | 11.5 | |
| N stage | N0 | 24 | 92.3 |
| N1 | 2 | 7.7 | |
| M stage | M0 | 20 | 76.9 |
| M1a | 1 | 3.8 | |
| M1b | 5 | 19.2 | |
| TNM stage | I | 10 | 38.5 |
| II | 1 | 3.8 | |
| IIIa | 7 | 26.9 | |
| IIIb | 1 | 3.8 | |
| IVa | 2 | 7.7 | |
| IVb | 5 | 19.2 |
| Treatment | Number of Patients | % | |
|---|---|---|---|
| Surgery | R0 | 18 | 69.2 |
| R1/R2 | 6 | 23.1 | |
| No | 2 | 7.7 | |
| Radiation | Yes | 19 | 73.1 |
| No | 7 | 26.9 | |
| Chemotherapy | Yes | 16 | 61.5 |
| No | 10 | 38.5 | |
| Therapeutic regimens | Surgery alone | 1 | 3.8 |
| Surgery + radiotherapy | 9 | 34.6 | |
| Surgery + chemotherapy | 4 | 15.4 | |
| Surgery + chemoradiotherapy | 9 | 34.6 | |
| Chemoradiotherapy + surgery | 1 | 3.8 | |
| Chemotherapy alone | 2 | 7.7 |
| 5y OS | 5y PFS | 5y LRFS | 5y DMFS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| % | p | % | p | % | p | % | p | ||
| Sex | Male | 73.3 | 0.387 | 42.7 | 0.451 | 87.7 | 0.589 | 42.7 | 0.451 |
| Female | 60.0 | 20.0 | 60.0 | 20.0 | |||||
| Histological grade | Low | 81.3 | 0.293 | 35.7 | 0.359 | 86.5 | 0.461 | 35.7 | 0.359 |
| Medium | 60.0 | 50.0 | 83.3 | 50.0 | |||||
| High | 33.3 | 33.3 | 50.0 | 33.3 | |||||
| Age | ≤60y | 68.0 | 0.637 | 43.6 | 0.455 | 82.5 | 0.551 | 43.6 | 0.455 |
| >60y | 80.0 | 20.0 | 80.0 | 20.0 | |||||
| ECOG PS score | ≤1 | 71.4 | 0.278 | 40.0 | 0.817 | 90.9 | 0.016 | 40.0 | 0.817 |
| ≥2 | 66.7 | 0.0 | 0.0 | 0.0 | |||||
| Smoking | Yes | 62.3 | 0.836 | 42.4 | 0.753 | 75.8 | 0.814 | 42.4 | 0.753 |
| No | 76.9 | 34.3 | 84.4 | 34.3 | |||||
| Masaoka stage | I–II | 90.0 | 0.079 | 67.5 | 0.056 | 100.0 | 0.516 | 67.5 | 0.056 |
| III–IV | 57.1 | 20.0 | 68.3 | 20.0 | |||||
| TNM stage | I–II | 90.9 | 0.136 | 61.4 | 0.124 | 100.0 | 0.372 | 61.4 | 0.124 |
| III–IV | 53.8 | 21.4 | 65.3 | 21.4 | |||||
| Great vessel invasion | Yes | 16.7 | 0.000 | 0.0 | 0.000 | 0.0 | 0.001 | 0.0 | 0.000 |
| No | 88.9 | 49.9 | 94.1 | 49.9 | |||||
| R0 resection | Yes | 87.5 | 0.032 | 49.3 | 0.079 | 86.9 | 0.812 | 49.3 | 0.079 |
| No | 37.5 | 12.5 | 70.0 | 12.5 | |||||
| Radiation | Yes | 81.9 | 0.533 | 34.6 | 0.468 | 87.2 | 0.009 | 34.6 | 0.468 |
| No | 42.9 | 42.9 | 64.3 | 42.9 | |||||
| Chemotherapy | Yes | 56.3 | 0.366 | 45.7 | 0.913 | 66.9 | 0.137 | 45.7 | 0.913 |
| No | 90.0 | 30.0 | 100.0 | 30.0 | |||||
| Paraneoplastic syndrome | Yes | 100.0 | 0.409 | 35.4 | 0.612 | 100.0 | 0.496 | 35.4 | 0.612 |
| No | 68.2 | 66.7 | 100.0 | 66.7 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, Y.; Zeng, Q.; Bi, N.; Zhou, Z.; Xiao, Z.; Hui, Z.; Chen, D.; Wang, L.; Wang, J.; Liu, W.; et al. A Single Center Analysis of Thymic Neuroendocrine Tumors. Cancers 2022, 14, 4944. https://doi.org/10.3390/cancers14194944
Zhai Y, Zeng Q, Bi N, Zhou Z, Xiao Z, Hui Z, Chen D, Wang L, Wang J, Liu W, et al. A Single Center Analysis of Thymic Neuroendocrine Tumors. Cancers. 2022; 14(19):4944. https://doi.org/10.3390/cancers14194944
Chicago/Turabian StyleZhai, Yirui, Qiang Zeng, Nan Bi, Zongmei Zhou, Zefen Xiao, Zhouguang Hui, Dongfu Chen, Luhua Wang, Jianyang Wang, Wenyang Liu, and et al. 2022. "A Single Center Analysis of Thymic Neuroendocrine Tumors" Cancers 14, no. 19: 4944. https://doi.org/10.3390/cancers14194944
APA StyleZhai, Y., Zeng, Q., Bi, N., Zhou, Z., Xiao, Z., Hui, Z., Chen, D., Wang, L., Wang, J., Liu, W., Deng, L., Lv, J., Wang, W., Luo, Y., Li, J., Wang, X., Zhang, T., Gao, Y., & Feng, Q. (2022). A Single Center Analysis of Thymic Neuroendocrine Tumors. Cancers, 14(19), 4944. https://doi.org/10.3390/cancers14194944







