Does Lactobacillus Exert a Protective Effect on the Development of Cervical and Endometrial Cancer in Women?
Abstract
Simple Summary
Abstract
1. Introduction
2. Origins of the Microbiome
3. The Microbial Environment of the Vagina and Upper Reproductive Tract
4. The Role of Lactobacillus in the Female Reproductive Tract
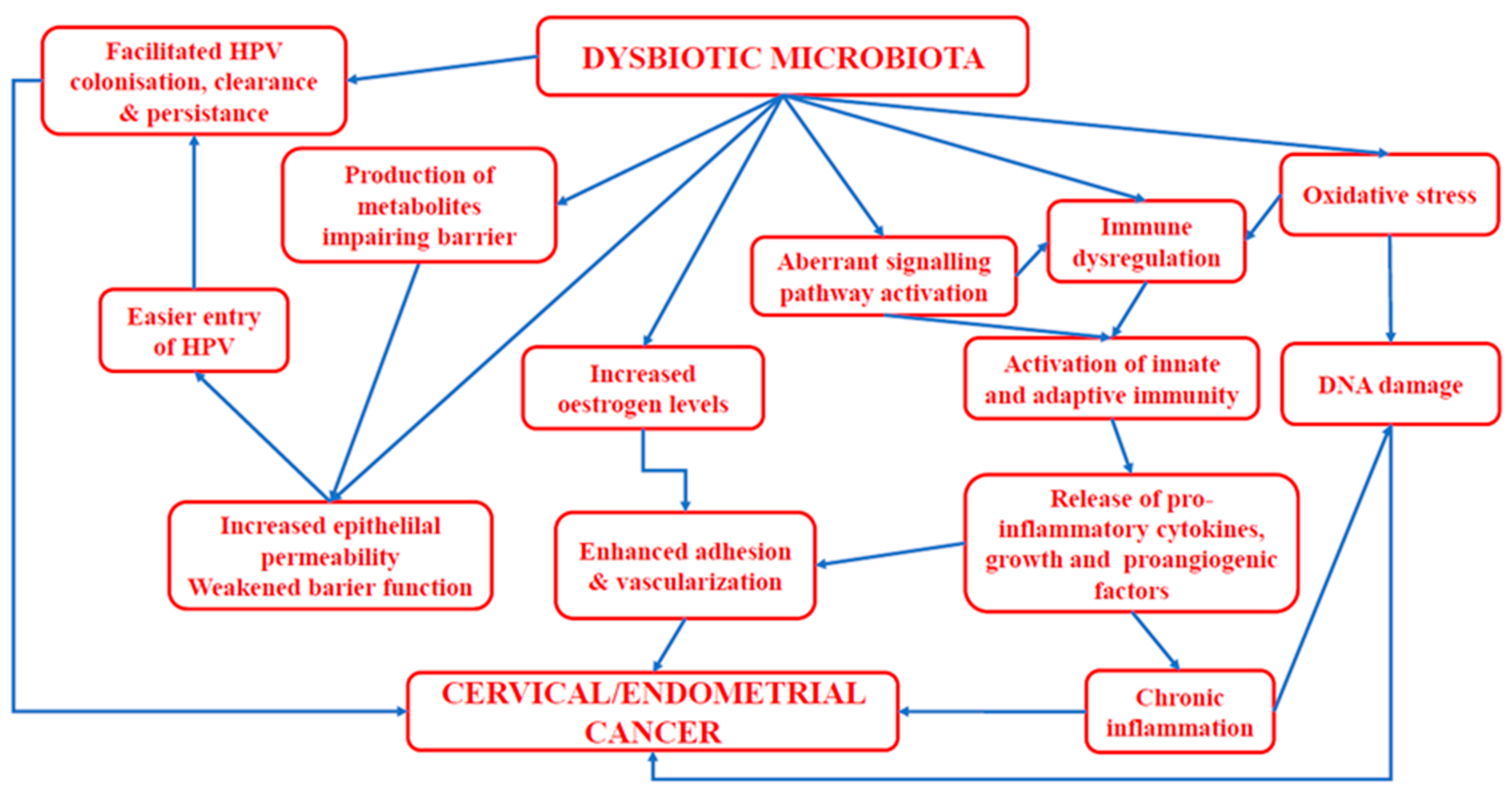
5. The Impact of Human Papillomavirus and Vaginal Microbiota on the Development of Cervical Cancer
6. Endometrial Cancer
7. Treatment
7.1. Vaccines
7.2. Probiotics and Prebiotics
8. Future Perspectives and Limitations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Z.-W.; Long, H.-Z.; Cheng, Y.; Luo, H.-Y.; Wen, D.-D.; Gao, L.-C. From Microbiome to Inflammation: The Key Drivers of Cervical Cancer. Front. Microbiol. 2021, 12, 767931. [Google Scholar] [CrossRef]
- Olusola, P.; Banerjee, H.N.; Philley, J.V.; Dasgupta, S. Human Papilloma Virus-Associated Cervical Cancer and Health Disparities. Cells 2019, 8, 622. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Marchesi, J.R.; Lee, Y.S.; Bennett, P.R.; Kyrgiou, M. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: What do we know and where are we going next? Microbiome 2016, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Muñoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Plummer, M.; Schiffman, M.; Castle, P.E.; Maucort-Boulch, D.; Wheeler, C.M.; ALTS Group. A 2-Year Prospective Study of Human Papillomavirus Persistence among Women with a Cytological Diagnosis of Atypical Squamous Cells of Undetermined Significance or Low-Grade Squamous Intraepithelial Lesion. J. Infect. Dis. 2007, 195, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M.A. Epithelial Cell Responses to Infection with Human Papillomavirus. Clin. Microbiol. Rev. 2012, 25, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Audirac-Chalifour, A.; Torres-Poveda, K.; Bahena-Román, M.; Téllez-Sosa, J.; Martinez-Barnetche, J.; Cortina-Ceballos, B.; López-Estrada, G.; Delgado-Romero, K.; Burguete-García, A.I.; Cantú, D.; et al. Cervical Microbiome and Cytokine Profile at Various Stages of Cervical Cancer: A Pilot Study. PLoS ONE 2016, 11, e0153274. [Google Scholar] [CrossRef]
- Curty, G.; de Carvalho, P.S.; Soares, M.A. The Role of the Cervicovaginal Microbiome on the Genesis and as a Biomarker of Premalignant Cervical Intraepithelial Neoplasia and Invasive Cervical Cancer. Int. J. Mol. Sci. 2019, 21, 222. [Google Scholar] [CrossRef]
- Yue, X.-A.; Chen, P.; Tang, Y.; Wu, X.; Hu, Z. The dynamic changes of vaginal microecosystem in patients with recurrent vulvovaginal candidiasis: A retrospective study of 800 patients. Arch. Gynecol. Obstet. 2015, 292, 1285–1294. [Google Scholar] [CrossRef]
- Adebamowo, S.N.; Ma, B.; Zella, D.; Famooto, A.; Ravel, J.; Adebamowo, C. Mycoplasma hominis and Mycoplasma genitalium in the Vaginal Microbiota and Persistent High-Risk Human Papillomavirus Infection. Front. Public Health 2017, 5, 140. [Google Scholar] [CrossRef]
- Mead, P.B. Cervical-vaginal flora of women with invasive cervical cancer. Obstet. Gynecol. 1978, 52, 601–604. [Google Scholar] [PubMed]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [PubMed]
- Belizário, J.E.; Faintuch, J. Microbiome and Gut Dysbiosis. Exp. Suppl. 2018, 109, 459–476. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.E.; Kahana, D.D.; Ghuman, S.; Wilson, H.P.; Wilson, J.; Kim, S.C.J.; Lagishetty, V.; Jacobs, J.P.; Sinha-Hikim, A.P.; Friedman, T.C. Unhealthy Lifestyle and Gut Dysbiosis: A Better Understanding of the Effects of Poor Diet and Nicotine on the Intestinal Microbiome. Front. Endocrinol. 2021, 12, 667066. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Lieber, A.D.; Wu, F.; Perez-Perez, G.I.; Chen, Y.; et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 2016, 8, 343ra82. [Google Scholar] [CrossRef]
- Yassour, M.; Vatanen, T.; Siljander, H.; Hämäläinen, A.-M.; Härkönen, T.; Ryhänen, S.J.; Franzosa, E.A.; Vlamakis, H.; Huttenhower, C.; Gevers, D.; et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci. Transl. Med. 2016, 8, 343ra81. [Google Scholar] [CrossRef]
- Nagpal, R.; Tsuji, H.; Takahashi, T.; Kawashima, K.; Nagata, S.; Nomoto, K.; Yamashiro, Y. Sensitive Quantitative Analysis of the Meconium Bacterial Microbiota in Healthy Term Infants Born Vaginally or by Cesarean Section. Front. Microbiol. 2016, 7, 1997. [Google Scholar] [CrossRef]
- Backhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P. Dynamics 684 and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Fouhy, F.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C.; Cotter, P.D. Composition of the early intestinal microbiota: Knowledge, knowledge gaps and the use of high-throughput sequencing to address these gaps. Gut Microbes 2012, 3, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Bidart, G.N.; Rodríguez-Díaz, J.; Yebra, M.J. The Extracellular Wall-Bound β-N-Acetylglucosaminidase from Lactobacillus casei Is Involved in the Metabolism of the Human Milk Oligosaccharide Lacto- N -Triose. Appl. Environ. Microbiol. 2016, 82, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Gritz, E.C.; Bhandari, V. Corrigendum: The Human Neonatal Gut Microbiome: A Brief Review. Front. Pediatr. 2015, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Guaraldi, F.; Salvatori, G. Effect of Breast and Formula Feeding on Gut Microbiota Shaping in Newborns. Front. Cell. Infect. Microbiol. 2012, 2, 94. [Google Scholar] [CrossRef] [PubMed]
- Bezirtzoglou, E.; Tsiotsias, A.; Welling, G.W. Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH). Anaerobe 2011, 17, 478–482. [Google Scholar] [CrossRef]
- Eggesbø, M.; Botten, G.; Stigum, H.; Nafstad, P.; Magnus, P. Is delivery by cesarean section a risk factor for food allergy? J. Allergy Clin. Immunol. 2003, 112, 420–426. [Google Scholar] [CrossRef]
- Huh, S.Y.; Rifas-Shiman, S.L.; A Zera, C.; Edwards, J.W.R.; Oken, E.; Weiss, S.T.; Gillman, M.W. Delivery by caesarean section and risk of obesity in preschool age children: A prospective cohort study. Arch. Dis. Child. 2012, 97, 610–616. [Google Scholar] [CrossRef]
- Lahtinen, S.J.; Boyle, R.J.; Kivivuori, S.; Oppedisano, F.; Smith, K.R.; Robins-Browne, R.; Salminen, S.J.; Tang, M.L. Prenatal probiotic administration can influence Bifidobacterium microbiota development in infants at high risk of allergy. J. Allergy Clin. Immunol. 2009, 123, 499–501.e8. [Google Scholar] [CrossRef]
- Gueimonde, M.; Sakata, S.; Kalliomäki, M.; Isolauri, E.; Benno, Y.; Salminen, S. Effect of maternal consumption of lactobacillus GG on transfer and establishment of fecal bifidobacterial microbiota in neonates. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 166–170. [Google Scholar]
- Stojanović, N.; Plećaš, D.; Plešinac, S. Normal vaginal flora, disorders and application of probiotics in pregnancy. Arch. Gynecol. Obstet. 2012, 286, 325–332. [Google Scholar] [CrossRef]
- Foster, K.R.; Schluter, J.; Coyte, K.Z.; Rakoff-Nahoum, S. The evolution of the host microbiome as an ecosystem on a leash. Nature 2017, 548, 43–51. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef] [PubMed]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef]
- Fredricks, D.N.; Fiedler, T.L.; Marrazzo, J.M. Molecular Identification of Bacteria Associated with Bacterial Vaginosis. N. Engl. J. Med. 2005, 353, 1899–1911. [Google Scholar] [CrossRef]
- Martin, D.H.; Marrazzo, J.M. The Vaginal Microbiome: Current Understanding and Future Directions. J. Infect. Dis. 2016, 214, S36–S41. [Google Scholar] [CrossRef] [PubMed]
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Fadrosh, D.; Chang, K.; Silver, M.; Viscidi, R.P.; Burke, A.E.; Ravel, J.; Gravitt, P.E. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause 2014, 21, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Antonio, M.A.D.; Hawes, S.E.; Hillier, S.L. The Identification of Vaginal Lactobacillus Species and the Demographic and Microbiologic Characteristics of Women Colonized by These Species. J. Infect. Dis. 1999, 180, 1950–1956. [Google Scholar] [CrossRef]
- Younes, J.A.; Lievens, E.; Hummelen, R.; van der Westen, R.; Reid, G.; Petrova, M.I. Women and Their Microbes: The Unexpected Friendship. Trends Microbiol. 2018, 26, 16–32. [Google Scholar] [CrossRef]
- Miller, E.A.; Beasley, D.E.; Dunn, R.R.; Archie, E.A. Lactobacilli dominance and vaginal pH: Why is the human vaginal microbiome unique? Front. Microbiol. 2016, 7, 1936. [Google Scholar] [CrossRef]
- Fettweis, J.M.; Brooks, J.P.; Serrano, M.G.; Sheth, N.U.; Girerd, P.H.; Edwards, D.J.; Strauss, J.F., III; Jefferson, K.K.; Buck, G.A.; Consortium, V.M. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology 2014, 160, 2272. [Google Scholar] [CrossRef]
- Borgdorff, H.; van der Veer, C.; van Houdt, R.; Alberts, C.J.; de Vries, H.J.; Bruisten, S.M.; Snijder, M.B.; Prins, M.; Geerlings, S.E.; van der Loeff, M.F.S.; et al. The association between ethnicity and vaginal microbiota composition in Amsterdam, the Netherlands. PLoS ONE 2017, 12, e0181135. [Google Scholar] [CrossRef]
- Laniewski, P.; Barnes, D.; Goulder, A.; Cui, H.; Roe, D.J.; Chase, D.M.; Herbst-Kralovetz, M.M. Linking cervicovaginal immune signatures, HPV and microbiota composition in cervical carcinogenesis in non-Hispanic and Hispanic women. Sci. Rep. 2018, 8, 7593. [Google Scholar] [CrossRef] [PubMed]
- Łaniewski, P.; Ilhan, Z.E.; Herbst-Kralovetz, M.M. The microbiome and gynaecological cancer development, prevention and therapy. Nat. Rev. Urol. 2020, 17, 232–250. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Kim, B.-S.; Seo, S.-S.; Kong, J.-S.; Lee, J.-K.; Park, S.-Y.; Hong, K.-M.; Kim, H.-K.; Kim, M. The association of uterine cervical microbiota with an increased risk for cervical intraepithelial neoplasia in Korea. Clin. Microbiol. Infect. 2015, 21, 674.e1–674.e9. [Google Scholar] [CrossRef] [PubMed]
- Van De Wijgert, J.H.H.M.; Borgdorff, H.; Verhelst, R.; Crucitti, T.; Francis, S.C.; Verstraelen, H.; Jespers, V. The Vaginal Microbiota: What Have We Learned after a Decade of Molecular Characterization? PLoS ONE 2014, 9, e105998. [Google Scholar] [CrossRef]
- Kyrgiou, M.; Mitra, A.; Moscicki, A.-B. Does the vaginal microbiota play a role in the development of cervical cancer? Transl. Res. 2017, 179, 168–182. [Google Scholar] [CrossRef]
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schütte, U.M.E.; Zhong, X.; Koenig, S.S.K.; Fu, L.; Ma, Z.; Zhou, X.; et al. Temporal Dynamics of the Human Vaginal Microbiota. Sci. Transl. Med. 2012, 4, 132ra52. [Google Scholar] [CrossRef]
- Hickey, R.J.; Zhou, X.; Pierson, J.D.; Ravel, J.; Forney, L.J. Understanding vaginal microbiome complexity from an ecological perspective. Transl. Res. 2012, 160, 267–282. [Google Scholar] [CrossRef]
- Lewis, F.M.T.; Bernstein, K.T.; Aral, S.O. Vaginal Microbiome and Its Relationship to Behavior, Sexual Health, and Sexually Transmitted Diseases. Obstet. Gynecol. 2017, 129, 643–654. [Google Scholar] [CrossRef]
- Serrano, M.G.; Parikh, H.I.; Brooks, J.P.; Edwards, D.J.; Arodz, T.J.; Edupuganti, L.; Huang, B.; Girerd, P.H.; Bokhari, Y.A.; Bradley, S.P.; et al. Racioethnic diversity in the dynamics of the vaginal microbiome during pregnancy. Nat. Med. 2019, 25, 1001–1011. [Google Scholar] [CrossRef]
- Jespers, V.; Kyongo, J.; Joseph, S.; Hardy, L.; Cools, P.; Crucitti, T.; Mwaura, M.; Ndayisaba, G.; Delany-Moretlwe, S.; Buyze, J.; et al. A longitudinal analysis of the vaginal microbiota and vaginal immune mediators in women from sub-Saharan Africa. Sci. Rep. 2017, 7, 11974. [Google Scholar] [CrossRef]
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Fadrosh, D.W.; Nikita, L.; Galuppi, M.; Lamont, R.F.; Chaemsaithong, P.; Miranda, J.; et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2014, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen–gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Plottel, C.S.; Blaser, M.J. Microbiome and Malignancy. Cell Host Microbe 2011, 10, 324–335. [Google Scholar] [CrossRef]
- Flores, R.; Shi, J.; Fuhrman, B.; Xu, X.; Veenstra, T.D.; Gail, M.H.; Gajer, P.; Ravel, J.; Goedert, J.J. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: A cross-sectional study. J. Transl. Med. 2012, 10, 253. [Google Scholar] [CrossRef]
- Cruickshank, R.; Sharman, A. The biology of the vagina in the human subject. BJOG Int. J. Obstet. Gynaecol. 1934, 41, 208–226. [Google Scholar] [CrossRef]
- Hickey, R.J.; Zhou, X.; Settles, M.L.; Erb, J.; Malone, K.; Hansmann, M.A.; Shew, M.L.; Van Der Pol, B.; Fortenberry, J.D.; Forney, L.J. Vaginal Microbiota of Adolescent Girls Prior to the Onset of Menarche Resemble Those of Reproductive-Age Women. mBio 2015, 6, e00097-15. [Google Scholar] [CrossRef] [PubMed]
- Mirmonsef, P.; Hotton, A.L.; Gilbert, D.; Gioia, C.J.; Maric, D.; Hope, T.J.; Landay, A.L.; Spear, G.T. Glycogen Levels in Undiluted Genital Fluid and Their Relationship to Vaginal pH, Estrogen, and Progesterone. PLoS ONE 2016, 11, e0153553. [Google Scholar] [CrossRef]
- Muhleisen, A.L.; Herbst-Kralovetz, M.M. Menopause and the vaginal microbiome. Maturitas 2016, 91, 42–50. [Google Scholar] [CrossRef]
- Boskey, E.; Cone, R.; Whaley, K.; Moench, T. Origins of vaginal acidity: High d/l lactate ratio is consistent with bacteria being the primary source. Hum. Reprod. 2001, 16, 1809–1813. [Google Scholar] [CrossRef]
- Spear, G.T.; French, A.L.; Gilbert, D.; Zariffard, M.R.; Mirmonsef, P.; Sullivan, T.H.; Spear, W.W.; Landay, A.; Micci, S.; Lee, B.-H.; et al. Human α-amylase Present in Lower-Genital-Tract Mucosal Fluid Processes Glycogen to Support Vaginal Colonization by Lactobacillus. J. Infect. Dis. 2014, 210, 1019–1028. [Google Scholar] [CrossRef]
- Vodstrcil, L.A.; Hocking, J.S.; Law, M.; Walker, S.; Tabrizi, S.N.; Fairley, C.K.; Bradshaw, C.S. Hormonal Contraception Is Associated with a Reduced Risk of Bacterial Vaginosis: A Systematic Review and Meta-Analysis. PLoS ONE 2013, 8, e73055. [Google Scholar] [CrossRef]
- Brotman, R.M.; He, X.; Gajer, P.; Fadrosh, D.; Sharma, E.; Mongodin, E.F.; Ravel, J.; Glover, E.D.; Rath, J.M. Association between cigarette smoking and the vaginal microbiota: A pilot study. BMC Infect. Dis. 2014, 14, 471. [Google Scholar] [CrossRef]
- Mändar, R.; Punab, M.; Borovkova, N.; Lapp, E.; Kiiker, R.; Korrovits, P.; Metspalu, A.; Krjutškov, K.; Nõlvak, H.; Preem, J.-K.; et al. Complementary seminovaginal microbiome in couples. Res. Microbiol. 2015, 166, 440–447. [Google Scholar] [CrossRef]
- Schwebke, J.R.; Desmond, R.A.; Oh, M.K. Predictors of Bacterial Vaginosis in Adolescent Women Who Douche. Sex Transm Dis 2004, 31, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; Chase, D.M.; Herbst-Kralovetz, M.M. Uterine microbiota: Residents, tourists, or invaders? Front. Immunol. 2018, 9, 208. [Google Scholar] [CrossRef]
- Chen, C.; Song, X.; Chunwei, Z.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef]
- E Fouts, D.; Pieper, R.; Szpakowski, S.; Pohl, H.; Knoblach, S.; Suh, M.-J.; Huang, S.-T.; Ljungberg, I.; Sprague, B.M.; Lucas, S.K.; et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J. Transl. Med. 2012, 10, 174. [Google Scholar] [CrossRef]
- Hilt, E.E.; McKinley, K.; Pearce, M.M.; Rosenfeld, A.B.; Zilliox, M.J.; Mueller, E.R.; Brubaker, L.; Gai, X.; Wolfe, A.J.; Schreckenberger, P.C. Urine Is Not Sterile: Use of Enhanced Urine Culture Techniques to Detect Resident Bacterial Flora in the Adult Female Bladder. J. Clin. Microbiol. 2014, 52, 871–876. [Google Scholar] [CrossRef]
- Thomas-White, K.; Forster, S.C.; Kumar, N.; Van Kuiken, M.; Putonti, C.; Stares, M.D.; Hilt, E.E.; Price, T.K.; Wolfe, A.J.; Lawley, T.D. Culturing of female bladder bacteria reveals an interconnected urogenital microbiota. Nat. Commun. 2018, 9, 1557. [Google Scholar] [CrossRef]
- Antonio, M.A.D.; Rabe, L.K.; Hillier, S.L. Colonization of the Rectum by Lactobacillus Species and Decreased Risk of Bacterial Vaginosis. J. Infect. Dis. 2005, 192, 394–398. [Google Scholar] [CrossRef]
- El Aila, N.A.; Tency, I.; Claeys, G.; Verstraelen, H.; Saerens, B.; Santiago, G.L.D.S.; De Backer, E.; Cools, P.; Temmerman, M.; Verhelst, R.; et al. Identification and genotyping of bacteria from paired vaginal and rectal samples from pregnant women indicates similarity between vaginal and rectal microflora. BMC Infect. Dis. 2009, 9, 167. [Google Scholar] [CrossRef]
- Dong, Q.; Nelson, D.E.; Toh, E.; Diao, L.; Gao, X.; Fortenberry, J.D.; Van Der Pol, B. The Microbial Communities in Male First Catch Urine Are Highly Similar to Those in Paired Urethral Swab Specimens. PLoS ONE 2011, 6, e19709. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A.; et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef]
- Robertson, S.A.; Chin, P.Y.; Glynn, D.J.; Thompson, J.G. Peri-conceptual cytokines–setting the trajectory for embryo implantation, pregnancy and beyond. Am. J. Reprod. Immunol. 2011, 66, 2–10. [Google Scholar] [CrossRef]
- Dominguez, F.; Gadea, B.; Mercader, A.; Esteban, F.J.; Pellicer, A.; Simón, C. Embryologic outcome and secretome profile of implanted blastocysts obtained after coculture in human endometrial epithelial cells versus the sequential system. Fertil. Steril. 2010, 93, 774–782.e1. [Google Scholar] [CrossRef] [PubMed]
- Medina-Colorado, A.A.; Vincent, K.L.; Miller, A.L.; Maxwell, C.A.; Dawson, L.N.; Olive, T.; Kozlova, E.V.; Baum, M.M.; Pyles, R.B. Vaginal ecosystem modeling of growth patterns of anaerobic bacteria in microaerophilic conditions. Anaerobe 2017, 45, 10–18. [Google Scholar] [CrossRef]
- Graver, M.A.; Wade, J.J. The role of acidification in the inhibition of Neisseria gonorrhoeae by vaginal lactobacilli during anaerobic growth. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 8. [Google Scholar] [CrossRef]
- Gong, Z.; Luna, Y.; Yu, P.; Fan, H. Lactobacilli Inactivate Chlamydia trachomatis through Lactic Acid but Not H2O2. PLoS ONE 2014, 9, e107758. [Google Scholar] [CrossRef]
- Breshears, L.M.; Edwards, V.L.; Ravel, J.; Peterson, M.L. Lactobacillus crispatus inhibits growth of Gardnerella vaginalis and Neisseria gonorrhoeae on a porcine vaginal mucosa model. BMC Microbiol. 2015, 15, 276. [Google Scholar] [CrossRef]
- A Clarke, M.; Rodriguez, A.C.; Gage, J.C.; Herrero, R.; Hildesheim, A.; Wacholder, S.; Burk, R.; Schiffman, M. A large, population-based study of age-related associations between vaginal pH and human papillomavirus infection. BMC Infect. Dis. 2012, 12, 33. [Google Scholar] [CrossRef]
- Straight, S.W.; Herman, B.; McCance, D.J. The E5 oncoprotein of human papillomavirus type 16 inhibits the acidification of endosomes in human keratinocytes. J. Virol. 1995, 69, 3185–3192. [Google Scholar] [CrossRef]
- Linhares, I.M.; Summers, P.R.; Larsen, B.; Giraldo, P.C.; Witkin, S.S. Contemporary perspectives on vaginal pH and lactobacilli. Am. J. Obstet. Gynecol. 2011, 204, 120.e1–120.e5. [Google Scholar] [CrossRef] [PubMed]
- Witkin, S.S.; Mendes-Soares, H.; Linhares, I.; Jayaram, A.; Ledger, W.J.; Forney, L.J. Influence of Vaginal Bacteria and d- and l-Lactic Acid Isomers on Vaginal Extracellular Matrix Metalloproteinase Inducer: Implications for Protection against Upper Genital Tract Infections. mBio 2013, 4, e00460-13. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Olmos, M.I.; Barousse, M.M.; Rajan, L.; Van Der Pol, B.J.; Fortenberry, D.; Orr, D.; Fidel Jr, P.L. Vaginal lactobacilli in adolescents: Presence and relationship to local and systemic immunity, and to bacterial vaginosis. Sex. Transm. Dis. 2004, 31, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Nunn, K.L.; Wang, Y.-Y.; Harit, D.; Humphrys, M.S.; Ma, B.; Cone, R.; Ravel, J.; Lai, S.K. Enhanced Trapping of HIV-1 by Human Cervicovaginal Mucus Is Associated with Lactobacillus crispatus-Dominant Microbiota. mBio 2015, 6, e01084-15. [Google Scholar] [CrossRef]
- Sun, S.; Li, H.; Chen, J.; Qian, Q. Lactic Acid: No Longer an Inert and End-Product of Glycolysis. Physiology 2017, 32, 453–463. [Google Scholar] [CrossRef]
- Yang, X.; Da, M.; Zhang, W.; Qi, Q.; Zhang, C.; Han, S. Role of Lactobacillus in cervical cancer. Cancer Manag. Res. 2018, 10, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Selle, K.; Klaenhammer, T.R. Genomic and phenotypic evidence for probiotic influences of Lactobacillus gasseri on human health. FEMS Microbiol. Rev. 2013, 37, 915–935. [Google Scholar] [CrossRef]
- Aroutcheva, A.; Gariti, D.; Simon, M.; Shott, S.; Faro, J.; Simoes, J.A.; Gurguis, A.; Faro, S. Defense factors of vaginal lactobacilli. Am. J. Obstet. Gynecol. 2001, 185, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Malik, R.K.; Kaushik, J.K.; Singroha, G. Gassericin A: A circular bacteriocin produced by Lactic acid bacteria Lactobacillus gasseri. World J. Microbiol. Biotechnol. 2013, 29, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Stoyancheva, G.; Marzotto, M.; Dellaglio, F.; Torriani, S. Bacteriocin production and gene sequencing analysis from vaginal Lactobacillus strains. Arch. Microbiol. 2014, 196, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Heinemann, C.; Velraeds, M.; van der Mei, H.C.; Busscher, H.J. [31] Biosurfactants produced by Lactobacillus. In Methods in enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 310, pp. 426–433. [Google Scholar]
- Ojala, T.; Kankainen, M.; Castro, J.; Cerca, N.; Edelman, S.; Westerlund-Wikström, B.; Paulin, L.; Holm, L.; Auvinen, P. Comparative genomics of Lactobacillus crispatus suggests novel mechanisms for the competitive exclusion of Gardnerella vaginalis. BMC Genom. 2014, 15, 1070. [Google Scholar] [CrossRef] [PubMed]
- AL Kassaa, I.; Hober, D.; Hamze, M.; Chihib, N.E.; Drider, D. Antiviral Potential of Lactic Acid Bacteria and Their Bacteriocins. Probiotics Antimicrob. Proteins 2014, 6, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Q.; Yang, E.; Yan, L.; Li, T.; Zhuang, H. Antimicrobial Compounds Produced by Vaginal Lactobacillus crispatus Are Able to Strongly Inhibit Candida albicans Growth, Hyphal Formation and Regulate Virulence-related Gene Expressions. Front. Microbiol. 2017, 8, 564. [Google Scholar] [CrossRef] [PubMed]
- Sgibnev, A.V.; Kremleva, E.A. Vaginal Protection by H2O2-Producing Lactobacilli. Jundishapur J. Microbiol. 2015, 8, e22913. [Google Scholar] [CrossRef]
- Hancock, R.E.; Rozek, A. Role of membranes in the activities of antimicrobial cationic peptides. FEMS Microbiol. Lett. 2002, 206, 143–149. [Google Scholar] [CrossRef]
- Frew, L.; Stock, S.J. Antimicrobial peptides and pregnancy. Reproduction 2011, 141, 725–735. [Google Scholar] [CrossRef]
- O’Hanlon, D.E.; Lanier, B.R.; Moench, T.R.; Cone, R.A. Cervicovaginal fluid and semen block the microbicidal activity of hydrogen peroxide produced by vaginal lactobacilli. BMC Infect. Dis. 2010, 10, 120. [Google Scholar] [CrossRef]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect. Dis. 2011, 11, 200. [Google Scholar] [CrossRef]
- Al-Nasiry, S.; Ambrosino, E.; Schlaepfer, M.; Morré, S.A.; Wieten, L.; Voncken, J.W.; Spinelli, M.; Mueller, M.; Kramer, B.W. The Interplay Between Reproductive Tract Microbiota and Immunological System in Human Reproduction. Front. Immunol. 2020, 11, 378. [Google Scholar] [CrossRef]
- Maldonado-Barragán, A.; Caballero-Guerrero, B.; Martín, V.; Ruiz-Barba, J.L.; Rodríguez, J.M. Purification and genetic characterization of gassericin E, a novel co-culture inducible bacteriocin from Lactobacillus gasseri EV1461 isolated from the vagina of a healthy woman. BMC Microbiol. 2016, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Nasioudis, D.; Forney, L.J.; Schneider, G.M.; Gliniewicz, K.; France, M.T.; Boester, A.; Sawai, M.; Scholl, J.; Witkin, S.S. The composition of the vaginal microbiome in first trimester pregnant women influences the level of autophagy and stress in vaginal epithelial cells. J. Reprod. Immunol. 2017, 123, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.-X.; Li, T.; Zhang, X.; Wang, S.-X.; Liu, Z.-H. Lactobacillus crispatus Modulates Vaginal Epithelial Cell Innate Response to Candida albicans. Chin. Med. J. 2017, 130, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Zadravec, P.; Štrukelj, B.; Berlec, A. Improvement of LysM-Mediated Surface Display of Designed Ankyrin Repeat Proteins (DARPins) in Recombinant and Nonrecombinant Strains of Lactococcus lactis and Lactobacillus Species. Appl. Environ. Microbiol. 2015, 81, 2098–2106. [Google Scholar] [CrossRef][Green Version]
- Fichera, G.A.; Fichera, M.; Milone, G. Antitumoural activity of a cytotoxic peptide of Lactobacillus casei peptidoglycan and its interaction with mitochondrial-bound hexokinase. Anti-Cancer Drugs 2016, 27, 609–619. [Google Scholar] [CrossRef][Green Version]
- Radtke, A.L.; Quayle, A.J.; Herbst-Kralovetz, M.M. Microbial Products Alter the Expression of Membrane-Associated Mucin and Antimicrobial Peptides in a Three-Dimensional Human Endocervical Epithelial Cell Model1. Biol. Reprod. 2012, 87, 132. [Google Scholar] [CrossRef]
- Yao, X.-Y.; Yuan, M.-M.; Li, D.-J. Molecular adjuvant C3d3 improved the anti-hCGβ humoral immune response in vaginal inoculation with live recombinant Lactobacillus expressing hCGβ-C3d3 fusion protein. Vaccine 2007, 25, 6129–6139. [Google Scholar] [CrossRef]
- Lee, T.-Y.; Kim, Y.-H.; Lee, K.-S.; Kim, J.-K.; Lee, I.-H.; Yang, J.-M.; Sung, M.-H.; Park, J.-S.; Poo, H. Human papillomavirus type 16 E6-specific antitumor immunity is induced by oral administration of HPV16 E6-expressing Lactobacillus casei in C57BL/6 mice. Cancer Immunol. Immunother. 2010, 59, 1727–1737. [Google Scholar] [CrossRef]
- Chase, D.; Goulder, A.; Zenhausern, F.; Monk, B.; Herbst-Kralovetz, M. The vaginal and gastrointestinal microbiomes in gynecologic cancers: A review of applications in etiology, symptoms and treatment. Gynecol. Oncol. 2015, 138, 190–200. [Google Scholar] [CrossRef]
- Motevaseli, E.; Shirzad, M.; Akrami, S.M.; Mousavi, A.-S.; Mirsalehian, A.; Modarressi, M.H. Normal and tumour cervical cells respond differently to vaginal lactobacilli, independent of pH and lactate. J. Med. Microbiol. 2013, 62, 1065–1072. [Google Scholar] [CrossRef]
- Borgdorff, H.; Tsivtsivadze, E.; Verhelst, R.; Marzorati, M.; Jurriaans, S.; Ndayisaba, G.F.; Schuren, F.H.; Van De Wijgert, J.H.H.M. Lactobacillus-dominated cervicovaginal microbiota associated with reduced HIV/STI prevalence and genital HIV viral load in African women. ISME J. 2014, 8, 1781–1793. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Balkus, J.E.; Fredricks, D.; Liu, C.; McKernan-Mullin, J.; Frenkel, L.M.; Mwachari, C.; Luque, A.; Cohn, S.E.; Cohen, C.R. Interaction between lactobacilli, bacterial vaginosis-associated bacteria, and HIV Type 1 RNA and DNA Genital shedding in US and Kenyan women. AIDS Res. Hum. Retrovir. 2013, 29, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, M.; Sani, C.; Clemente, A.M.; Iossa, A.; Perissi, E.; Castronovo, G.; Tanturli, M.; Rivero, D.; Cozzolino, F.; Cavalieri, D.; et al. Characterization of cervico-vaginal microbiota in women developing persistent high-risk Human Papillomavirus infection. Sci. Rep. 2017, 7, 10200. [Google Scholar] [CrossRef] [PubMed]
- Drago, F.; Herzum, A.; Ciccarese, G.; Dezzana, M.; Casazza, S.; Pastorino, A.; Bandelloni, R.; Parodi, A. Ureaplasma parvum as a possible enhancer agent of HPV-induced cervical intraepithelial neoplasia: Preliminary results. J. Med. Virol. 2016, 88, 2023–2024. [Google Scholar] [CrossRef] [PubMed]
- Borgdorff, H.; Gautam, R.; Armstrong, S.D.; Xia, D.; Ndayisaba, G.F.; van Teijlingen, N.H.; Geijtenbeek, T.B.H.; Wastling, J.M.; van de Wijgert, J.H.H.M. Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier. Mucosal Immunol. 2016, 9, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, Z.E.; Łaniewski, P.; Thomas, N.; Roe, D.J.; Chase, D.M.; Herbst-Kralovetz, M.M. Deciphering the complex interplay between microbiota, HPV, inflammation and cancer through cervicovaginal metabolic profiling. eBioMedicine 2019, 44, 675–690. [Google Scholar] [CrossRef]
- Nelson, T.M.; Borgogna, J.-L.C.; Brotman, R.M.; Ravel, J.; Walk, S.T.; Yeoman, C.J. Vaginal biogenic amines: Biomarkers of bacterial vaginosis or precursors to vaginal dysbiosis? Front. Physiol. 2015, 6, 253. [Google Scholar] [CrossRef]
- Srinivasan, S.; Morgan, M.T.; Fiedler, T.L.; Djukovic, D.; Hoffman, N.G.; Raftery, D.; Marrazzo, J.M.; Fredricks, D. Metabolic Signatures of Bacterial Vaginosis. mBio 2015, 6, e00204-15. [Google Scholar] [CrossRef]
- Kovachev, S.M. Cervical cancer and vaginal microbiota changes. Arch. Microbiol. 2020, 202, 323–327. [Google Scholar] [CrossRef]
- Hedges, S.; Barrientes, F.; Desmond, R.A.; Schwebke, J.R. Local and Systemic Cytokine Levels in Relation to Changes in Vaginal Flora. J. Infect. Dis. 2006, 193, 556–562. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Caselli, E.; D’Accolti, M.; Santi, E.; Soffritti, I.; Conzadori, S.; Mazzacane, S.; Greco, P.; Contini, C.; Bonaccorsi, G. Vaginal Microbiota and Cytokine Microenvironment in HPV Clearance/Persistence in Women Surgically Treated for Cervical Intraepithelial Neoplasia: An Observational Prospective Study. Front. Cell. Infect. Microbiol. 2020, 10, 540900. [Google Scholar] [CrossRef] [PubMed]
- Tilborghs, S.; Corthouts, J.; Verhoeven, Y.; Arias, D.; Rolfo, C.; Trinh, X.B.; van Dam, P.A. The role of Nuclear Factor-kappa B signaling in human cervical cancer. Crit. Rev. Oncol. 2017, 120, 141–150. [Google Scholar] [CrossRef]
- Kim, D.-E.; Kim, J.-K.; Han, S.-K.; Jang, S.-E.; Han, M.J.; Kim, D.-H. Lactobacillus plantarum NK3 and Bifidobacterium longum NK49 Alleviate Bacterial Vaginosis and Osteoporosis in Mice by Suppressing NF-κB-Linked TNF-α Expression. J. Med. Food 2019, 22, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-G.; Kim, H.I.; Kwon, E.K.; Han, M.J.; Kim, D.-H. Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 mitigate alcoholic steatosis in mice by inhibiting LPS-mediated NF-κB activation through restoration of the disturbed gut microbiota. Food Funct. 2018, 9, 4255–4265. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-F.; Yuan, S.-S.F.; Yeh, Y.-T.; Wu, M.-T.; Su, J.-H.; Hung, S.-C.; Chai, C.-Y. The role of p-STAT3 (ser727) revealed by its association with Ki-67 in cervical intraepithelial neoplasia. Gynecol. Oncol. 2005, 98, 446–452. [Google Scholar] [CrossRef]
- Chen, C.-L.; Hsieh, F.-C.; Lieblein, J.C.; Brown, J.; Chan, C.; A Wallace, J.; Cheng, G.; Hall, B.M.; Lin, J. Stat3 activation in human endometrial and cervical cancers. Br. J. Cancer 2007, 96, 591–599. [Google Scholar] [CrossRef]
- Shukla, S.; Shishodia, G.; Mahata, S.; Hedau, S.; Pandey, A.; Bhambhani, S.; Batra, S.; Basir, S.F.; Das, B.C.; Bharti, A.C. Aberrant expression and constitutive activation of STAT3 in cervical carcinogenesis: Implications in high-risk human papillomavirus infection. Mol. Cancer 2010, 9, 282. [Google Scholar] [CrossRef]
- Gracia-Sancho, J.; Salvadó, M.J. Gastrointestinal Tissue: Oxidative Stress and Dietary Antioxidants; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Lee, J.E.; Lee, S.; Lee, H.; Song, Y.-M.; Lee, K.; Han, M.J.; Sung, J.; Ko, G. Association of the Vaginal Microbiota with Human Papillomavirus Infection in a Korean Twin Cohort. PLoS ONE 2013, 8, e63514. [Google Scholar] [CrossRef]
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Tracy, J.K.; Zenilman, J.M.; Ravel, J.; Gravitt, P.E. Interplay Between the Temporal Dynamics of the Vaginal Microbiota and Human Papillomavirus Detection. J. Infect. Dis. 2014, 210, 1723–1733. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Lee, Y.S.; Smith, A.; Marchesi, J.R.; Lehne, B.; Bhatia, R.; Lyons, D.; Paraskevaidis, E.; Li, J.V.; et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci. Rep. 2015, 5, 16865. [Google Scholar] [CrossRef] [PubMed]
- Üren, A.; Fallen, S.; Yuan, H.; Usubütün, A.; Küçükali, T.; Schlegel, R.; Toretsky, J.A. Activation of the Canonical Wnt Pathway during Genital Keratinocyte Transformation: A Model for Cervical Cancer Progression. Cancer Res. 2005, 65, 6199–6206. [Google Scholar] [CrossRef] [PubMed]
- Norenhag, J.; Du, J.; Olovsson, M.; Verstraelen, H.; Engstrand, L.; Brusselaers, N. The vaginal microbiota, human papillomavirus and cervical dysplasia: A systematic review and network meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Garcea, G.; Dennison, A.; Steward, W.; Berry, D. Role of inflammation in pancreatic carcinogenesis and the implications for future therapy. Pancreatology 2005, 5, 514–529. [Google Scholar] [CrossRef] [PubMed]
- So, K.A.; Yang, E.J.; Kim, N.R.; Hong, S.R.; Lee, J.-H.; Hwang, C.-S.; Shim, S.-H.; Lee, S.J.; Kim, T.J. Changes of vaginal microbiota during cervical carcinogenesis in women with human papillomavirus infection. PLoS ONE 2020, 15, e0238705. [Google Scholar] [CrossRef]
- Fernandes, J.V.; Fernandes, T.A.A.D.M.; DE Azevedo, J.C.V.; Cobucci, R.N.O.; DE Carvalho, M.G.F.; Andrade, V.S.; DE Araújo, J.M.G. Link between chronic inflammation and human papillomavirus-induced carcinogenesis (Review). Oncol. Lett. 2015, 9, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Hwang, L.Y.; Ma, Y.; Shiboski, S.C.; Farhat, S.; Jonte, J.; Moscicki, A.-B. Active Squamous Metaplasia of the Cervical Epithelium Is Associated with Subsequent Acquisition of Human Papillomavirus 16 Infection Among Healthy Young Women. J. Infect. Dis. 2012, 206, 504–511. [Google Scholar] [CrossRef]
- Vaneechoutte, M. Lactobacillus Iners, the Unusual Suspect. Res. Microbiol. 2017, 168, 826–836. [Google Scholar] [CrossRef]
- Jouhi, L.; Renkonen, S.; Atula, T.; Mã¤Kitie, A.; Haglund, C.; Hagström, J.; Hagström, J. Different Toll-Like Receptor Expression Patterns in Progression toward Cancer. Front. Immunol. 2014, 5, 638. [Google Scholar] [CrossRef]
- Liu, C.; Li, J.; Shi, W.; Zhang, L.; Liu, S.; Lian, Y.; Liang, S.; Wang, H. Progranulin regulates inflammation and tumor. Anti-Inflamm. Anti-Allergy Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Inflamm. Anti-Allergy Agents) 2020, 19, 88–102. [Google Scholar] [CrossRef]
- Schäfer, G.; Kabanda, S.; Van Rooyen, B.; Marušič, M.B.; Banks, L.; Parker, M.I. The role of inflammation in HPV infection of the Oesophagus. BMC Cancer 2013, 13, 185. [Google Scholar] [CrossRef]
- Yu, H.; Kortylewski, M.; Pardoll, D. Crosstalk between cancer and immune cells: Role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. 2007, 7, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Basukala, O.; Mittal, S.; Massimi, P.; Bestagno, M.; Banks, L. The HPV-18 E7 CKII phospho acceptor site is required for maintaining the transformed phenotype of cervical tumour-derived cells. PLOS Pathog. 2019, 15, e1007769. [Google Scholar] [CrossRef] [PubMed]
- Bonab, F.R.; Baghbanzadeh, A.; Ghaseminia, M.; Bolandi, N.; Mokhtarzadeh, A.; Amini, M.; Dadashzadeh, K.; Hajiasgharzadeh, K.; Baradaran, B.; Baghi, H.B. Molecular pathways in the development of HPV-induced cervical cancer. EXCLI J. 2021, 20, 320–337. [Google Scholar]
- Kipanyula, M.J.; Etet, P.F.S.; Vecchio, L.; Farahna, M.; Nukenine, E.N.; Kamdje, A.H.N. Signaling pathways bridging microbial-triggered inflammation and cancer. Cell. Signal. 2013, 25, 403–416. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, F.; Mao, X.; Huang, J.; Yang, J.; Yin, X.; Wu, L.; Zheng, L.; Wang, Q. Elevation of miR-27b by HPV16 E7 inhibits PPARγ expression and promotes proliferation and invasion in cervical carcinoma cells. Int. J. Oncol. 2015, 47, 1759–1766. [Google Scholar] [CrossRef]
- Allouch, S.; Malki, A.; Allouch, A.; Gupta, I.; Vranic, S.; Al Moustafa, A.-E. High-Risk HPV Oncoproteins and PD-1/PD-L1 Interplay in Human Cervical Cancer: Recent Evidence and Future Directions. Front. Oncol. 2020, 10, 914. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, I.; Bergsten, E.; Couffin, S.; Amiot, A.; Nebbad, B.; Barau, C.; De’Angelis, N.; Rabot, S.; Canoui-Poitrine, F.; Mestivier, D.; et al. Colorectal cancer-associated microbiota contributes to oncogenic epigenetic signatures. Proc. Natl. Acad. Sci. USA 2019, 116, 24285–24295. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Domingue, J.C.; Sears, C.L. Microbiota dysbiosis in select human cancers: Evidence of association and causality. Semin. Immunol. 2017, 32, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, J.; Su, H.; Shi, L.; Chen, B.; Zhang, S. Endometrial microbiota from endometrial cancer and paired pericancer tissues in postmenopausal women: Differences and clinical relevance. Menopause 2022, 29, 1168–1175. [Google Scholar] [CrossRef]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Lu, K.H.; Broaddus, R.R. Endometrial cancer. N. Engl. J. Med. 2020, 383, 2053–2064. [Google Scholar] [CrossRef] [PubMed]
- Gjorgoska, M.; Rizner, T.L. Integration of androgen hormones in endometrial cancer biology. Trends Endocrinol. Metab. 2022, 33, 639–651. [Google Scholar] [CrossRef]
- Moch, H. Female genital tumours: WHO Classification of Tumours. In WHO Classification of Tumours; WHO: Geneva, Switzerland, 2020; Volume 4, p. 4. [Google Scholar]
- Hampel, H.; Frankel, W.; Panescu, J.; Lockman, J.; Sotamaa, K.; Fix, D.; Comeras, I.; La Jeunesse, J.; Nakagawa, H.; Westman, J.A.; et al. Screening for Lynch Syndrome (Hereditary Nonpolyposis Colorectal Cancer) among Endometrial Cancer Patients. Cancer Res. 2006, 66, 7810–7817. [Google Scholar] [CrossRef]
- Zanssen, S.; A Schon, E. Mitochondrial DNA Mutations in Cancer. PLOS Med. 2005, 2, e401. [Google Scholar] [CrossRef]
- Winters, A.D.; Romero, R.; Gervasi, M.T.; Gomez-Lopez, N.; Tran, M.R.; Garcia-Flores, V.; Pacora, P.; Jung, E.; Hassan, S.S.; Hsu, C.-D. Does the endometrial cavity have a molecular microbial signature? Sci. Rep. 2019, 9, 9905. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Fujishita, A.; Kitajima, M.; Hiraki, K.; Nakashima, M.; Masuzaki, H. Intra-uterine microbial colonization and occurrence of endometritis in women with endometriosis†. Hum. Reprod. 2014, 29, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.-L.; Chen, L.-X.; Shu, W.-S.; Yao, S.-Z.; Wang, S.-W.; Chen, Y.-Q. Barcoded sequencing reveals diverse intrauterine microbiomes in patients suffering with endometrial polyps. Am. J. Transl. Res. 2016, 8, 1581–1592. [Google Scholar]
- Walther-António, M.R.S.; Chen, J.; Multinu, F.; Hokenstad, A.; Distad, T.J.; Cheek, E.H.; Keeney, G.L.; Creedon, D.J.; Nelson, H.; Mariani, A.; et al. Potential contribution of the uterine microbiome in the development of endometrial cancer. Genome Med. 2016, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gu, Y.; He, Q.; Huang, J.; Song, Y.; Wan, X.; Li, Y. Integrated Analysis of Microbiome and Transcriptome Data Reveals the Interplay Between Commensal Bacteria and Fibrin Degradation in Endometrial Cancer. Front. Cell. Infect. Microbiol. 2021, 11, 748558. [Google Scholar] [CrossRef] [PubMed]
- Dossus, L.; Rinaldi, S.; Becker, S.; Lukanova, A.; Tjonneland, A.; Olsen, A.; Stegger, J.; Overvad, K.; Chabbert-Buffet, N.; Jimenez-Corona, A.; et al. Obesity, inflammatory markers, and endometrial cancer risk: A prospective case–control study. Endocr.-Relat. Cancer 2010, 17, 1007–1019. [Google Scholar] [CrossRef]
- Yang, T.-K.; Chung, S.-D.; Muo, C.-H.; Chang, C.-H.; Huang, C.-Y.; Chung, C.-J. Risk of endometrial cancer in women with pelvic inflammatory disease: A nationwide population-based retrospective cohort study. Medicine 2015, 94, e1278. [Google Scholar] [CrossRef]
- Chen, P.; Guo, Y.; Jia, L.; Wan, J.; He, T.; Fang, C.; Li, T. Interaction Between Functionally Activate Endometrial Microbiota and Host Gene Regulation in Endometrial Cancer. Front. Cell Dev. Biol. 2021, 9, 727286. [Google Scholar] [CrossRef]
- Dossus, L.; Lukanova, A.; Rinaldi, S.; Allen, N.; Cust, A.E.; Becker, S.; Tjonneland, A.; Hansen, L.; Overvad, K.; Chabbert-Buffet, N. Hormonal, metabolic, and inflammatory profiles and endometrial cancer risk within the EPIC cohort—A factor analysis. Am. J. Epidemiol. 2013, 177, 787–799. [Google Scholar] [CrossRef]
- Francescone, R.; Hou, V.; Grivennikov, S.I. Microbiome, inflammation and cancer. Cancer J. 2014, 20, 181. [Google Scholar] [CrossRef]
- Ganz, T. Defensins: Antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, Q.; Celestino, J.; Milam, M.R.; Westin, S.N.; Lacour, R.A.; Meyer, L.A.; Shipley, G.L.; Davies, P.J.; Deng, L.; et al. Enhanced estrogen-induced proliferation in obese rat endometrium. Am. J. Obstet. Gynecol. 2009, 200, 186.e1–186.e8. [Google Scholar] [CrossRef][Green Version]
- Van Weelden, W.J.; Massuger, L.F.A.G.; Pijnenborg, J.M.A.; Romano, A. Enitec Anti-estrogen Treatment in Endometrial Cancer: A Systematic Review. Front. Oncol. 2019, 9, 359. [Google Scholar] [CrossRef]
- Rodriguez, A.C.; Blanchard, Z.; Maurer, K.A.; Gertz, J. Estrogen Signaling in Endometrial Cancer: A Key Oncogenic Pathway with Several Open Questions. Horm. Cancer 2019, 10, 51–63. [Google Scholar] [CrossRef]
- Knoester, M.; Lashley, L.E.E.L.O.; Wessels, E.; Oepkes, D.; Kuijper, E.J. First Report of Atopobium vaginae Bacteremia with Fetal Loss after Chorionic Villus Sampling. J. Clin. Microbiol. 2011, 49, 1684–1686. [Google Scholar] [CrossRef]
- Marconi, C.; Cruciani, F.; Vitali, B.; Donders, G. Correlation of Atopobium vaginae Amount with Bacterial Vaginosis Markers. J. Low. Genit. Tract Dis. 2012, 16, 127–132. [Google Scholar] [CrossRef]
- Chan, J.F.W.; Lau, S.K.P.; Curreem, S.O.T.; To, K.K.W.; Leung, S.S.M.; Cheng, V.C.C.; Yuen, K.-Y.; Woo, P.C.Y. First Report of Spontaneous Intrapartum Atopobium vaginae Bacteremia. J. Clin. Microbiol. 2012, 50, 2525–2528. [Google Scholar] [CrossRef]
- Walsh, D.M.; Hokenstad, A.N.; Chen, J.; Sung, J.; Jenkins, G.D.; Chia, N.; Nelson, H.; Mariani, A.; Walther-Antonio, M.R.S. Postmenopause as a key factor in the composition of the Endometrial Cancer Microbiome (ECbiome). Sci. Rep. 2019, 9, 19213–19216. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; He, F.; Lin, Z.; Liu, S.; Tang, L.; Huang, Y.; Hu, Z. Dysbiosis of the endometrial microbiota and its association with inflammatory cytokines in endometrial cancer. Int. J. Cancer 2021, 148, 1708–1716. [Google Scholar] [CrossRef]
- Taghinezhad-S, S.; Keyvani, H.; Bermúdez-Humarán, L.G.; Donders, G.G.G.; Fu, X.; Mohseni, A.H. Twenty years of research on HPV vaccines based on genetically modified lactic acid bacteria: An overview on the gut-vagina axis. Experientia 2021, 78, 1191–1206. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.T.; Scalia, G.; Cassaro, N.; Boemi, S. Multiple HPV 16 infection with two strains: A possible marker of neoplastic progression. BMC Cancer 2020, 20, 444. [Google Scholar] [CrossRef]
- Moscicki, A.-B.; Schiffman, M.; Franceschi, S. The natural history of human papillomavirus infection in relation to cervical cancer. In Human Papillomavirus; Elsevier: Amsterdam, The Netherlands, 2020; pp. 149–160. [Google Scholar]
- Ribelles, P.; Benbouziane, B.; Langella, P.; Suárez, J.E.; Bermúdez-Humarán, L.G.; Riazi, A. Protection against human papillomavirus type 16-induced tumors in mice using non-genetically modified lactic acid bacteria displaying E7 antigen at its surface. Appl. Microbiol. Biotechnol. 2013, 97, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Pasetti, M.F.; Simon, J.K.; Sztein, M.B.; Levine, M.M. Immunology of gut mucosal vaccines. Immunol. Rev. 2011, 239, 125–148. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Kawana, K.; Yokoyama, T.; Fujii, T.; Tomio, A.; Miura, S.; Tomio, K.; Kojima, S.; Oda, K.; Sewaki, T.; et al. Oral immunization with a Lactobacillus casei vaccine expressing human papillomavirus (HPV) type 16 E7 is an effective strategy to induce mucosal cytotoxic lymphocytes against HPV16 E7. Vaccine 2010, 28, 2810–2817. [Google Scholar] [CrossRef]
- Taguchi, A.; Kawana, K.; Yokoyama, T.; Adachi, K.; Yamashita, A.; Tomio, K.; Kojima, S.; Oda, K.; Fujii, T.; Kozuma, S. Adjuvant effect of Japanese herbal medicines on the mucosal type 1 immune responses to human papillomavirus (HPV) E7 in mice immunized orally with Lactobacillus-based therapeutic HPV vaccine in a synergistic manner. Vaccine 2012, 30, 5368–5372. [Google Scholar] [CrossRef] [PubMed]
- Cyriac, J.M.; James, E. Switch over from intravenous to oral therapy: A concise overview. J. Pharmacol. Pharmacother. 2014, 5, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Guzmán, C.A. Use of live bacterial vaccine vectors for antigen delivery: Potential and limitations. Vaccine 2001, 19, 1573–1580. [Google Scholar] [CrossRef]
- Wells, J.M.; Mercenier, A. Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat. Rev. Genet. 2008, 6, 349–362. [Google Scholar] [CrossRef]
- Yam, K.K.; Pouliot, P.; N’Diaye, M.M.; Fournier, S.; Olivier, M.; Cousineau, B. Innate inflammatory responses to the Gram-positive bacterium Lactococcus lactis. Vaccine 2008, 26, 2689–2699. [Google Scholar] [CrossRef]
- Taghinezhad-S, S.; Mohseni, A.H.; Keyvani, H.; Razavi, M.R. Phase 1 Safety and Immunogenicity Trial of Recombinant Lactococcus lactis Expressing Human Papillomavirus Type 16 E6 Oncoprotein Vaccine. Mol. Ther.-Methods Clin. Dev. 2019, 15, 40–51. [Google Scholar] [CrossRef]
- Kawana, K.; Adachi, K.; Kojima, S.; Taguchi, A.; Tomio, K.; Yamashita, A.; Nishida, H.; Nagasaka, K.; Arimoto, T.; Yokoyama, T.; et al. Oral vaccination against HPV E7 for treatment of cervical intraepithelial neoplasia grade 3 (CIN3) elicits E7-specific mucosal immunity in the cervix of CIN3 patients. Vaccine 2014, 32, 6233–6239. [Google Scholar] [CrossRef]
- Wang, M.; Gao, Z.; Zhang, Y.; Pan, L. Lactic acid bacteria as mucosal delivery vehicles: A realistic therapeutic option. Appl. Microbiol. Biotechnol. 2016, 100, 5691–5701. [Google Scholar] [CrossRef]
- Mohseni, A.H.; Razavilar, V.; Keyvani, H.; Razavi, M.R.; Khavari-Nejad, R.A. Codon Usage Optimization and Construction of Plasmid Encoding Iranian Human Papillomavirus Type 16 E7 Oncogene for Lactococcus Lactis Subsp. Cremoris MG1363. Asian Pac. J. Cancer Prev. 2017, 18, 783–788. [Google Scholar] [CrossRef]
- Del Rio, B.; Redruello, B.; Fernandez, M.; Martin, M.C.; Ladero, V.; Alvarez, M.A. Lactic Acid Bacteria as a Live Delivery System for the in situ Production of Nanobodies in the Human Gastrointestinal Tract. Front. Microbiol. 2019, 9, 3179. [Google Scholar] [CrossRef]
- Humaran, L.G.B.; Cortes-Perez, N.G.; Lefèvre, F.; Guimarães, V.; Rabot, S.; Alcocer-Gonzalez, J.M.; Gratadoux, J.-J.; Rodriguez-Padilla, C.; Tamez-Guerra, R.S.; Corthier, G.; et al. A Novel Mucosal Vaccine Based on Live Lactococci Expressing E7 Antigen and IL-12 Induces Systemic and Mucosal Immune Responses and Protects Mice against Human Papillomavirus Type 16-Induced Tumors. J. Immunol. 2005, 175, 7297–7302. [Google Scholar] [CrossRef]
- Cho, H.-J.; Shin, H.-J.; Han, I.-K.; Jung, W.-W.; Kim, Y.B.; Sul, D.; Oh, Y.-K. Induction of mucosal and systemic immune responses following oral immunization of mice with Lactococcus lactis expressing human papillomavirus type 16 L1. Vaccine 2007, 25, 8049–8057. [Google Scholar] [CrossRef] [PubMed]
- Aires, K.A.; Cianciarullo, A.M.; Carneiro, S.M.; Villa, L.L.; Boccardo, E.; Pérez-Martinez, G.; Perez-Arellano, I.; Oliveira, M.L.S.; Ho, P.L. Production of Human Papillomavirus Type 16 L1 Virus-Like Particles by Recombinant Lactobacillus casei Cells. Appl. Environ. Microbiol. 2006, 72, 745–752. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cortes-Perez, N.G.; Kharrat, P.; Langella, P.; Humaran, L.G.B. Heterologous production of human papillomavirus type-16 L1 protein by a lactic acid bacterium. BMC Res. Notes 2009, 2, 167. [Google Scholar] [CrossRef] [PubMed]
- Gambhira, R.; Jagu, S.; Karanam, B.; Gravitt, P.E.; Culp, T.D.; Christensen, N.D.; Roden, R.B.S. Protection of Rabbits against Challenge with Rabbit Papillomaviruses by Immunization with the N Terminus of Human Papillomavirus Type 16 Minor Capsid Antigen L2. J. Virol. 2007, 81, 11585–11592. [Google Scholar] [CrossRef]
- Yoon, S.-W.; Lee, T.-Y.; Kim, S.-J.; Lee, I.-H.; Sung, M.-H.; Park, J.-S.; Poo, H. Oral administration of HPV-16 L2 displayed on Lactobacillus casei induces systematic and mucosal cross-neutralizing effects in Balb/c mice. Vaccine 2012, 30, 3286–3294. [Google Scholar] [CrossRef]
- Mohseni, A.H.; Razavilar, V.; Keyvani, H.; Razavi, M.R.; Khavari-Nejad, R.A. Oral immunization with recombinant Lactococcus lactis NZ9000 expressing human papillomavirus type 16 E7 antigen and evaluation of its immune effects in female C57BL/6 mice. J. Med. Virol. 2019, 91, 296–307. [Google Scholar] [CrossRef]
- Taghinezhad-S, S.; Mohseni, A.H.; Keyvani, H.; Razavilar, V. Protection against human papillomavirus type 16-induced tumors in C57BL/6 mice by mucosal vaccination with Lactococcus lactis NZ9000 expressing E6 oncoprotein. Microb. Pathog. 2019, 126, 149–156. [Google Scholar] [CrossRef]
- Poo, H.; Pyo, H.-M.; Lee, T.-Y.; Yoon, S.-W.; Lee, J.-S.; Kim, C.-J.; Sung, M.-H.; Lee, S.-H. Oral administration of human papillomavirus type 16 E7 displayed on Lactobacillus casei induces E7-specific antitumor effects in C57/BL6 mice. Int. J. Cancer 2006, 119, 1702–1709. [Google Scholar] [CrossRef]
- Mohseni, A.H.; Taghinezhad-S, S.; Keyvani, H. The First Clinical Use of a Recombinant Lactococcus lactis Expressing Human Papillomavirus Type 16 E7 Oncogene Oral Vaccine: A Phase I Safety and Immunogenicity Trial in Healthy Women Volunteers. Mol. Cancer Ther. 2020, 19, 717–727. [Google Scholar] [CrossRef]
- Park, Y.-C.; Ouh, Y.-T.; Sung, M.-H.; Park, H.-G.; Kim, T.-J.; Cho, C.-H.; Park, J.S.; Lee, J.-K. A phase 1/2a, dose-escalation, safety and preliminary efficacy study of oral therapeutic vaccine in subjects with cervical intraepithelial neoplasia 3. J. Gynecol. Oncol. 2019, 30, e88. [Google Scholar] [CrossRef]
- Bermúdez-Humarán, L.G.; Langella, P.; Miyoshi, A.; Gruss, A.; Guerra, R.T.; de Oca-Luna, R.M.; Le Loir, Y. Production of Human Papillomavirus Type 16 E7 Protein in Lactococcus lactis. Appl. Environ. Microbiol. 2002, 68, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Taghinezhad-S, S.; Razavilar, V.; Keyvani, H.; Razavi, M.R.; Nejadsattari, T. Extracellular overproduction of recombinant Iranian HPV-16 E6 oncoprotein in Lactococcus lactis using the NICE system. Futur. Virol. 2018, 13, 697–710. [Google Scholar] [CrossRef]
- Humaran, L.G.B.; Cortes-Perez, N.G.; Le Loir, Y.; Alcocer-González, J.M.; Tamez-Guerra, R.S.; De Oca-Luna, R.M.; Langella, P. An inducible surface presentation system improves cellular immunity against human papillomavirus type 16 E7 antigen in mice after nasal administration with recombinant lactococci. J. Med. Microbiol. 2004, 53, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, A.H.; Taghinezhad-S, S.; Keyvani, H.; Razavilar, V. Extracellular overproduction of E7 oncoprotein of Iranian human papillomavirus type 16 by genetically engineered Lactococcus lactis. BMC Biotechnol. 2019, 19, 8. [Google Scholar] [CrossRef]
- Kim, E.; Yang, J.; Sung, M.-H.; Poo, H. Oral Administration of Poly-Gamma-Glutamic Acid Significantly Enhances the Antitumor Effect of HPV16 E7-Expressing Lactobacillus casei in a TC-1 Mouse Model. J. Microbiol. Biotechnol. 2019, 29, 1444–1452. [Google Scholar] [CrossRef]
- Rangel-Colmenero, B.R.; Gomez-Gutierrez, J.G.; Villatoro-Hernández, J.; Zavala-Flores, L.M.; Quistián-Martínez, D.; Rojas-Martínez, A.; Arce-Mendoza, A.Y.; Guzmán-López, S.; Montes-De-Oca-Luna, R.; Saucedo-Cárdenas, O. Enhancement of Ad-CRT/E7-Mediated Antitumor Effect by Preimmunization with L. lactis Expressing HPV-16 E7. Viral Immunol. 2014, 27, 463–467. [Google Scholar] [CrossRef] [PubMed]
- García-Fruitós, E. Lactic acid bacteria: A promising alternative for recombinant protein production. Microb. Cell Factories 2012, 11, 157. [Google Scholar] [CrossRef]
- Bahey-El-Din, M. Lactococcus lactis-based vaccines from laboratory bench to human use: An overview. Vaccine 2012, 30, 685–690. [Google Scholar] [CrossRef]
- Del Rio, B.; Dattwyler, R.J.; Aroso, M.; Neves, V.; Meirelles, L.; Seegers, J.F.M.L.; Gomes-Solecki, M. Oral Immunization with Recombinant Lactobacillus plantarum Induces a Protective Immune Response in Mice with Lyme Disease. Clin. Vaccine Immunol. 2008, 15, 1429–1435. [Google Scholar] [CrossRef][Green Version]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi, M.; Dana, P.M.; Badehnoosh, B.; Asemi, Z.; Hallajzadeh, J.; Mansournia, M.A.; Yousefi, B.; Moazzami, B.; Chaichian, S. Anti-tumor activities of probiotics in cervical cancer. J. Ovarian Res. 2020, 13, 68. [Google Scholar] [CrossRef]
- Sanders, M.E. Probiotics in 2015. J. Clin. Gastroenterol. 2015, 49, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Saber, A.; Alipour, B.; Faghfoori, Z.; Khosroushahi, A.Y. Cellular and molecular effects of yeast probiotics on cancer. Crit. Rev. Microbiol. 2017, 43, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Chen, C.-C.; Lin, W.-C.; Kong, M.-S.; Shi, H.N.; Walker, W.A.; Lin, C.-Y.; Huang, C.-T.; Lin, Y.-C.; Jung, S.-M.; Lin, T.-Y. Oral inoculation of probiotics Lactobacillus acidophilus NCFM suppresses tumour growth both in segmental orthotopic colon cancer and extra-intestinal tissue. Br. J. Nutr. 2012, 107, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, F.; Tajadadi-Ebrahimi, M.; Kolahdooz, F.; Mazouchi, M.; Hadaegh, H.; Jamal, A.-S.; Mazroii, N.; Asemi, S.; Asemi, Z. The Consumption of Synbiotic Bread Containing Lactobacillus sporogenes and Inulin Affects Nitric Oxide and Malondialdehyde in Patients with Type 2 Diabetes Mellitus: Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Coll. Nutr. 2016, 35, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Kerry, R.G.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.-S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef]
- Bodera, P.; Chcialowski, A. Immunomodulatory effect of probiotic bacteria. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 58–64. [Google Scholar] [CrossRef]
- Klimek, R.; Klimek, M.; Jasiczek, D. Immunotherapy of cervical cancer as a biological dissipative structure. Neuro Endocrinol. Lett. 2011, 32, 380–388. [Google Scholar] [PubMed]
- Mohamadzadeh, M.; Klaenhammer, T.R. Specific Lactobacillus species differentially activate Toll-like receptors and downstream signals in dendritic cells. Expert Rev. Vaccines 2008, 7, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, H.; Watanabe, H.; Shimosato, T.; Kawai, Y.; Itoh, T.; Saito, T. Immunostimulatory oligonucleotide, CpG-like motif exists in Lactobacillus delbrueckii ssp. bulgaricus NIAI B6. Int. J. Food Microbiol. 2003, 85, 11–21. [Google Scholar] [CrossRef]
- Esfandiary, A.; Taherian-Esfahani, Z.; Abedin-Do, A.; Mirfakhraie, R.; Shirzad, M.; Ghafouri-Fard, S.; Motevaseli, E. Lactobacilli Modulate Hypoxia-Inducible Factor (HIF)-1 Regulatory Pathway in Triple Negative Breast Cancer Cell Line. Cell J. 2016, 18, 237–244. [Google Scholar] [CrossRef]
- Wang, K.-D.; Xu, D.-J.; Wang, B.-Y.; Yan, D.-H.; Lv, Z.; Su, J.-R. Inhibitory Effect of Vaginal Lactobacillus Supernatants on Cervical Cancer Cells. Probiotics Antimicrob. Proteins 2018, 10, 236–242. [Google Scholar] [CrossRef]
- Yim, E.-K.; Park, J.-S. The Role of HPV E6 and E7 Oncoproteins in HPV-associated Cervical Carcinogenesis. Cancer Res. Treat. 2005, 37, 319–324. [Google Scholar] [CrossRef]
- Nami, Y.; Abdullah, N.; Haghshenas, B.; Radiah, D.; Rosli, R.; Khosroushahi, A.Y. Assessment of probiotic potential and anticancer activity of newly isolated vaginal bacterium Lactobacillus plantarum 5BL. Microbiol. Immunol. 2014, 58, 492–502. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Zhao, H.; Lu, Y.; Lian, Z.; Li, N.; Hussain, N.; Shao, D.; Jin, M.; Li, Q.; Shi, J. Anticancer potential against cervix cancer (HeLa) cell line of probiotic Lactobacillus casei and Lactobacillus paracasei strains isolated from human breast milk. Food Funct. 2018, 9, 2705–2715. [Google Scholar] [CrossRef] [PubMed]
- Sungur, T.; Aslim, B.; Karaaslan, C.; Aktas, B. Impact of Exopolysaccharides (EPSs) of Lactobacillus gasseri strains isolated from human vagina on cervical tumor cells (HeLa). Anaerobe 2017, 47, 137–144. [Google Scholar] [CrossRef]
- Nouri, Z.; Karami, F.; Neyazi, N.; Modarressi, M.H.; Karimi, R.; Khorramizadeh, M.R.; Taheri, B.; Motevaseli, E. Dual Anti-Metastatic and Anti-Proliferative Activity Assessment of Two Probiotics on HeLa and HT-29 Cell Lines. Cell J. 2016, 18, 127–134. [Google Scholar] [CrossRef]
- Li, C.; Jia, L.; Yu, Y.; Jin, L. Lactic acid induced microRNA-744 enhances motility of SiHa cervical cancer cells through targeting ARHGAP5. Chem. Interact. 2019, 298, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, N.; Watari, H.; Ushijima, K. Chemotherapy and molecular targeting therapy for recurrent cervical cancer. Chin. J. Cancer Res. 2016, 28, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Gui, Q.-F.; Lu, H.-F.; Zhang, C.-X.; Xu, Z.-R.; Yang, Y.-H. Well-balanced commensal microbiota contributes to anti-cancer response in a lung cancer mouse model. Genet. Mol. Res. 2015, 14, 5642–5651. [Google Scholar] [CrossRef] [PubMed]
- Hummelen, R.; Changalucha, J.; Butamanya, N.L.; Cook, A.; Habbema, J.D.F.; Reid, G. Lactobacillus rhamnosus GR-1 and L. reuteri RC-14 to prevent or cure bacterial vaginosis among women with HIV. Int. J. Gynecol. Obstet. 2010, 111, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Kabuki, T.; Saito, T.; Kawai, Y.; Uemura, J.; Itoh, T. Production, purification and characterization of reutericin 6, a bacteriocin with lytic activity produced by Lactobacillus reuteri LA6. Int. J. Food Microbiol. 1997, 34, 145–156. [Google Scholar] [CrossRef]
- Okawa, T.; Kita, M.; Arai, T.; Iida, K.; Dokiya, T.; Takegawa, Y.; Hirokawa, Y.; Yamazaki, K.; Hashimoto, S. Phase II randomized clinical trial of LC9018 concurrently used with radiation in the treatment of carcinoma of the uterine cervix. Its effect on tumor reduction and histology. Cancer 1989, 64, 1769–1776. [Google Scholar] [CrossRef]
- Pardini, B.; De Maria, D.; Francavilla, A.; Di Gaetano, C.; Ronco, G.; Naccarati, A. MicroRNAs as markers of progression in cervical cancer: A systematic review. BMC Cancer 2018, 18, 696. [Google Scholar] [CrossRef]
- Saadat, Y.R.; Pourseif, M.M.; Vahed, S.Z.; Barzegari, A.; Omidi, Y.; Barar, J. Modulatory Role of Vaginal-Isolated Lactococcus lactis on the Expression of miR-21, miR-200b, and TLR-4 in CAOV-4 Cells and In Silico Revalidation. Probiotics Antimicrob. Proteins 2020, 12, 1083–1096. [Google Scholar] [CrossRef] [PubMed]

| Type of Study | Studied Population | Main Results | Ref |
|---|---|---|---|
| Open, single-site study | 32 women aged 38–55 years with established cervical cancer (FIGO I stage) | - Disturbances of vaginal microbiota occurred in 71% of patients with FIGO I stage cervical cancer. | [120] |
| Oriented observational, prospective, cohort study | 85 women with CIN2/CIN3 diagnosis, candidates for LEEP | - CIN2: microbiome dominated by Lactobacillus spp., but a high presence of anaerobic Gram-negative BV-associated bacteria (especially A. vaginae, G. vaginalis, and Ureaplasma parvum) and less widespread microbes, including Candida albicans, Finegoldia magna, Peptoniphilus asaccharolyticus, P. anaerobius, Prevotella bivia, and Streptococci, was observed. - CIN3: reduction in lactobacilli, except for L. iners, and high prevalence of A. vaginae, G. vaginalis, and U. parvum, as well as Aerococcus christensenii, Anaerococcus prevotii, Leptotrichia amnionii, M. hominis, Parvimonas micra, Peptoniphilus asaccharolyticus, Porphyromonas asaccharolitica, P. bivia, Prevotella buccalis, and S. sanguinegens. - High concentration of pro-inflammatory cytokines in the vaginal environment of CIN patients, including IL1α, IL1β, IL6, IL8, and TNFα, confirming that BV-like vaginal microbiomes are associated with increased local inflammation. - Surgical removal of hrHPV-related CIN lesions per se triggered microbiome remodulation. | [123] |
| In vitro study of cervical cancer cell lines C33a (HPV-), SiHa and CaSki (HPV16+), and HeLa (HPV18+) cells | 120 fresh cervical tissue biopsies (70 malignant, 30 premalignant, and 20 normal (control) cervical tissues) | - Aberrantly expressed and constitutively active STAT3 was found both in cervical cancer cell lines and in cervical precancer and cancer lesions. - Increased expression of STAT3 was regulated at transcription level. - Concurrent raise in phosphorylation at Tyr705 and Ser727 responsible for the regulation of STAT3 dimerization, nuclear transport, and DNA-binding and transactivation. Dually phosphorylated STAT3 present in cervical precancer and cancer lesions was found to localise to the nuclei and possessed a functional DNA-binding activity. - STAT3 expression and activation correlated well with HPV16 positivity in cervical precancer and cancer lesions. - Activation of STAT3 in cervical cancer cases increased along with disease severity. | [129] |
| Prospective study | 23 HPV-positive and 45 HPV-negative women who participated in the Healthy Twin Study | - The percentage of Lactobacillus spp. was considerably decreased in the HPV-infected group. - Higher diversity of vaginal microbiota of the HPV-positive group compared with the HPV-negative group. - HPV infection strongly correlated with the abundance of various vaginal microbiota species, e.g., Prevotella, Sneathia, Dialister, and Bacillus. - Sneathia spp. was a microbiological marker of high-risk HPV infection. - 17% of HPV-positive premenopausal women had CIN (a potential precursor of cervical cancer). | [131] |
| Prospective cohort study | 169 women: healthy (n = 20), low-grade squamous intraepithelial lesion (LSIL) (n = 52), high-grade squamous intraepithelial lesion (HSIL) (n = 92), and ICC (n = 5). | - 2-fold increase in the rate of a CST IV vaginal microbiome in women with LSIL; 3-fold increase in women with HSIL; 4-fold increase in women with invasive cancer compared to controls. - Presence of HSIL markers P. anaerobius and A. tetradius. - Presence and predominance of specific vaginal microbiome CSTs may be involved in the pathogenesis of CIN and cervical cancer. | [133] |
| A cross-sectional study | 32 cases: non-cervical lesions (NCL: n = 10 HPV-negative; n = 10 HPV-positive), SILs (n = 4 HPV-positive), and CC (n = 8 HPV-positive) | - Cervical microbiome is notably different in all stages of the natural history of cervical cancer. - Higher median cervical levels of IL-4 and TGF-β1 mRNA in CST VIII, dominated by Fusobacterium spp. - Sneathia spp., Megasphaera elsdenii, and S. satelles were most representative in the SIL cases. | [7] |
| A systematic review and network meta-analysis | Analysis of 11 included studies | - Vaginal microbiota dominated by non-lactobacilli species or L. iners were associated with 3–5-times higher odds of any prevalent HPV and 2–3-times higher for hrHPV and dysplasia/cervical cancer compared with L. crispatus. | [135] |
| Prospective study | 50 cervicovaginal swab specimens obtained from women aged 20 to 50 (40 positive for hrHPV and 10 negative for hrHPV) | - Abundance of Lactobacillus species was decreased in women with cervical disease; the amount of L. crispatus was significantly reduced in women with CIN and cervical cancer. - Markedly increased abundance in anaerobic bacteria: G. vaginalis, P. anaerobius, and Porphyromonas uenonis in women with CIN and cervical cancer. - Presence of G. vaginalis is associated with a high risk for developing CIN 2 or 3 and cervical cancer. | [137] |
| In vitro study | Clinical samples obtained from six HPV16-positive cervical cancer patients, HPV16-positive human cervical carcinoma cell lines CaSki and SiHa, and HPV-negative cervical cancer cell line C33A | - Increased miR-27b expression levels in cervical cancer tissues compared to adjacent normal tissues. - miR-27b-enhanced proliferation and invasion of cervical cancer cell lines, confirming that miR-27b serves as an oncogene in cervical cancer. - Inhibition of PPARγ-promoted proliferation and invasion of cervical cancer cells, both antitumour roles of PPARγ in cervical cancer. - miR-27b was positively regulated by HPV16 E7. - miR-27b inhibited the expression of PPARγ. - Overexpression of HPV16 E7 suppressed the expression of PPARγ depending on the existence of miR-27b; HPV16 E7 is able to repress the expression of PPARγ through the stimulation of miR-27b. | [148] |
| Vaccines | ||||
|---|---|---|---|---|
| Animal Studies | ||||
| Studied Agent | Route of Administration | Type of Study | Observed Effects | Ref |
| Recombinant Lactobacillus casei expressing HPV16 E7 (LacE7) | Mucosal (oral) | Animal study | - Elicit E7-specific IFN gamma-producing cells (T cells with E7-type 1 immune responses) - Greater induction of T cells compared to subcutaneous or intramuscular antigen delivery. - Trigger mucosal cytotoxic cellular immune responses | [185] |
| L. lactis MG1363 was transformed with two types of HPV16 L1-encoding plasmids for intracellular expression or secretion. | Oral | Animal study | - Serum IgG responses after immunizations with L. lactis secreting HPV16 L1. - Vaginal IgA immune responses after oral immunization with L. lactis expressing HPV16 L1, but secreting HPV - HPV16 L1-specific mucosal immune responses affected by immunization frequency. | [197] |
| N-terminal L2 polypeptides comprising residues 11 to 200 derived from HPV16 produced in bacteria (HPV16 L2 11–200) | Vaccination | Animal study | - Effective protection of rabbits against cutaneous and mucosal challenge with CRPV and ROPV - Generation of broadly cross-neutralizing serum antibody - potential of L2 as a second-generation preventive HPV vaccine antigen. | [200] |
| A partial HPV-16 L2 protein (N-terminal 1–224 amino acid) on the surface of L. casei. | Mucosal (oral) | Animal study | - Production of L2-specific serum IgG and vaginal IgG and IgA in Balb/c mice - Trigger systemic and mucosal cross-neutralizing effects in mice | [201] |
| L. lactis NZ9000 expressing human papillomavirus type 16 E7 antigen | Mucosal (oral) | Animal study | - Elicit the highest levels of E7-specific antibody and numbers of E7-specific CD4+ T helper and CD8+ T cell precursors. - Potent protective effects against challenge with the E7-expressing tumour cell line (TC-1) - pNZ8123-HPV16-optiE7 containing L. lactis showed strong therapeutic antitumour effects against established tumours in vivo. - Trigger humoral and cellular immune responses in mice | [202] |
| Recombinant strains of L. lactis NZ9000 expressing native and codon-optimized E6 protein (fused to the SPusp45 secretion signal) | Mucosal (oral) | Animal study | - Improved inhibitory effect on tumour growth, improved treatment effects on progression of tumour size, and improved survival rates in comparison with L. lactis having native E6 oncogene - Induce humoral and cellular immunity | [203] |
| HPV16 E7 antigen expressed on the surface of L. casei | Mucosal (oral) vaccine | Animal study | - Enhanced E7-specific serum IgG and mucosal IgA production. - Reduced tumour size and increased survival rate in E7-based mouse tumour model compared to versus mice receiving control (L. casei-PgsA) immunization. | [204] |
| HPV16 E7-expressing L. casei (L. casei-E7) combined with γ-PGA secreted by Bacillus subtilis | Mucosal (oral) vaccine | Animal study (TC-1 mouse model) | - Enhanced innate immune response including activation of dendritic cells - Significantly suppressed growth of TC-1 tumour cells and an increased survival rate compared to mice vaccinated with L. casei-E7 alone. - Markedly enhanced activation of natural killer (NK) cells, no impact on E7-specific cytolytic activity of CD8+ T lymphocytes. | [211] |
| Combination of adenovirus expressing calreticulin-E7 (Ad-CRT-E7) and L. lactis encoding HPV-16 E7 (Ll-E7) anchored to its surface | Intranasal preimmunization of Ll-E7, followed by a single Ad-CRT/E7 application | Animal study (mouse model) | - ∼80% of tumour suppression compared to controls. - 70% survival rate 300 days post-treatment (100% of controls died by 50 days). - Significant CD8+ cytotoxic T-lymphocytes infiltration in tumours of mice treated with Ll-E7+Ad-CRT/E7. | [212] |
| Clinical Studies and Trials | ||||
| Attenuated L. casei expressing modified full-length HPV16 E7 protein | Oral (during dose optimization studies (1, 2, 4, or 6 capsules/day) at weeks 1, 2, 4, and 8 (n = 10) or optimized vaccine formulation (n = 7) | Patients with HPV16-associated CIN3 | - Most patients (70%) receiving the optimized dose experienced a pathological down-grade to CIN2 at week 9 of treatment - E7-specific mucosal immunity was elicited in the uterine cervical lesions. | [192] |
| NZ8123-HPV16-optiE7 vaccine involving recombinant L. lactis expressing the codon-optimized human papillomavirus (HPV)-16 E7 oncogene | Oral vaccine or placebo | A dose-escalation, randomized, double-blind, placebo-controlled phase I clinical trial was performed in healthy Iranian volunteer women | - Vaccination was well tolerated, and no serious adverse effects were reported - Dose-dependent response to NZ8123-HPV16-optiE7 vaccine following oral administration - Safety and immunogenicity profile achieved in this study encourages further phase II trials with the 5 × 109 CFU/mL dose vaccine | [205]. |
| BLS-M07 (HPV 16 E7 antigen expressed on the surface of L. casei) | Oral administration Phase 1: 5 times a week, on weeks 1, 2, 4, and 8 with dosages of 500 mg, 1000 mg, and 1500 mg Phase 2a: 1000 mg dose. | A phase 1/2a, dose-escalation, safety, and preliminary efficacy study performed in patients with CIN 3 | - No dose limiting toxicity. - No grade 3 or 4 treatment-related adverse events or deaths - Improved RCI grading (16 weeks after treatment) - Increased serum HPV16 E7 specific antibody production. | [206] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frąszczak, K.; Barczyński, B.; Kondracka, A. Does Lactobacillus Exert a Protective Effect on the Development of Cervical and Endometrial Cancer in Women? Cancers 2022, 14, 4909. https://doi.org/10.3390/cancers14194909
Frąszczak K, Barczyński B, Kondracka A. Does Lactobacillus Exert a Protective Effect on the Development of Cervical and Endometrial Cancer in Women? Cancers. 2022; 14(19):4909. https://doi.org/10.3390/cancers14194909
Chicago/Turabian StyleFrąszczak, Karolina, Bartłomiej Barczyński, and Adrianna Kondracka. 2022. "Does Lactobacillus Exert a Protective Effect on the Development of Cervical and Endometrial Cancer in Women?" Cancers 14, no. 19: 4909. https://doi.org/10.3390/cancers14194909
APA StyleFrąszczak, K., Barczyński, B., & Kondracka, A. (2022). Does Lactobacillus Exert a Protective Effect on the Development of Cervical and Endometrial Cancer in Women? Cancers, 14(19), 4909. https://doi.org/10.3390/cancers14194909






