Circulating Cytokines Reflect the Etiology-Specific Immune Environment in Cirrhosis and HCC
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Participants
Cirrhotic Patients
2.2. Sample Collection and Processing
2.3. Ethics
2.4. Assay Methods
2.5. Statistical Analysis
3. Results
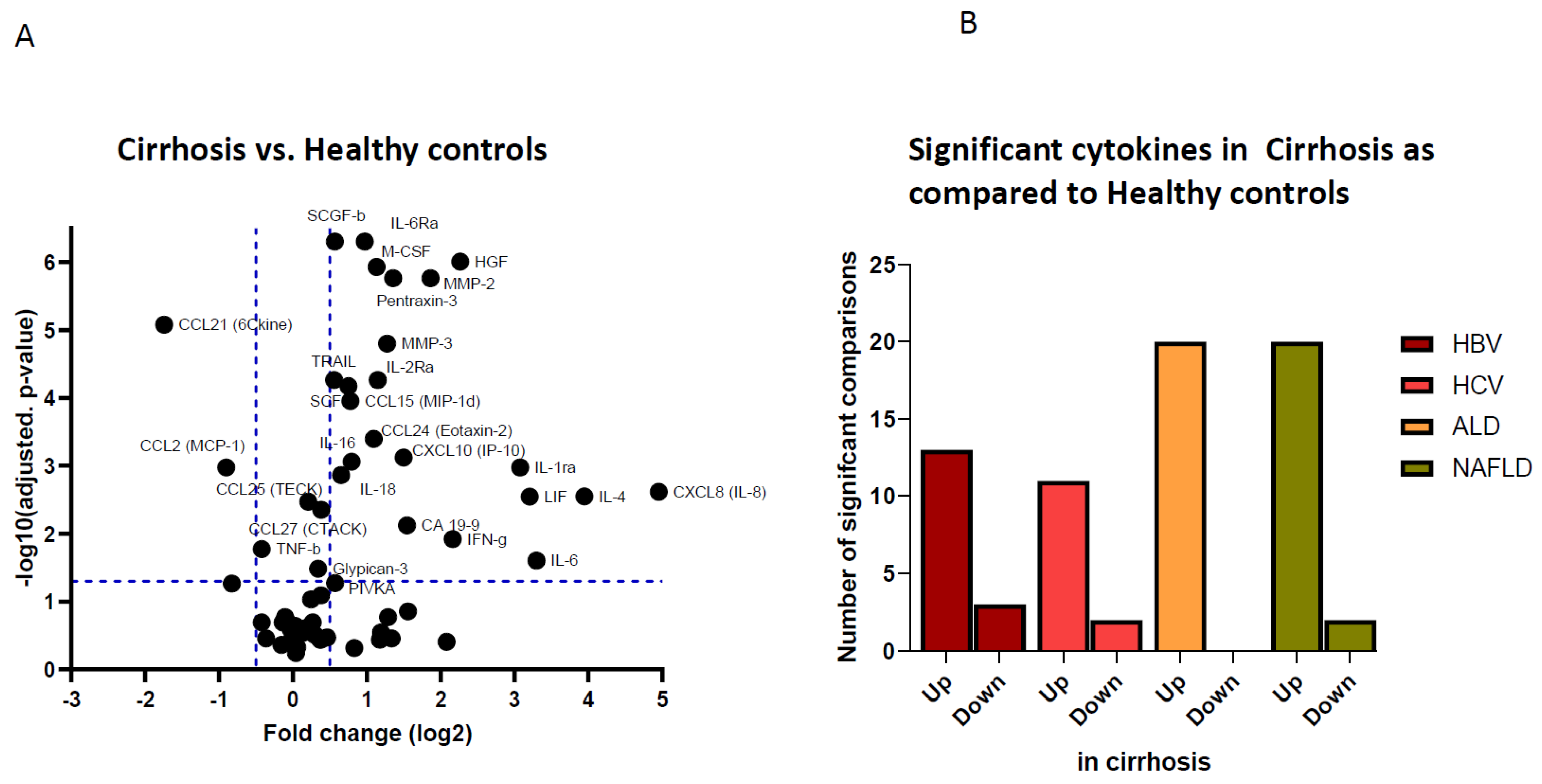
3.1. Pro-Inflammatory and Pro-Oncogenic Cytokine Patterns in Patients with Compensated Liver Cirrhosis
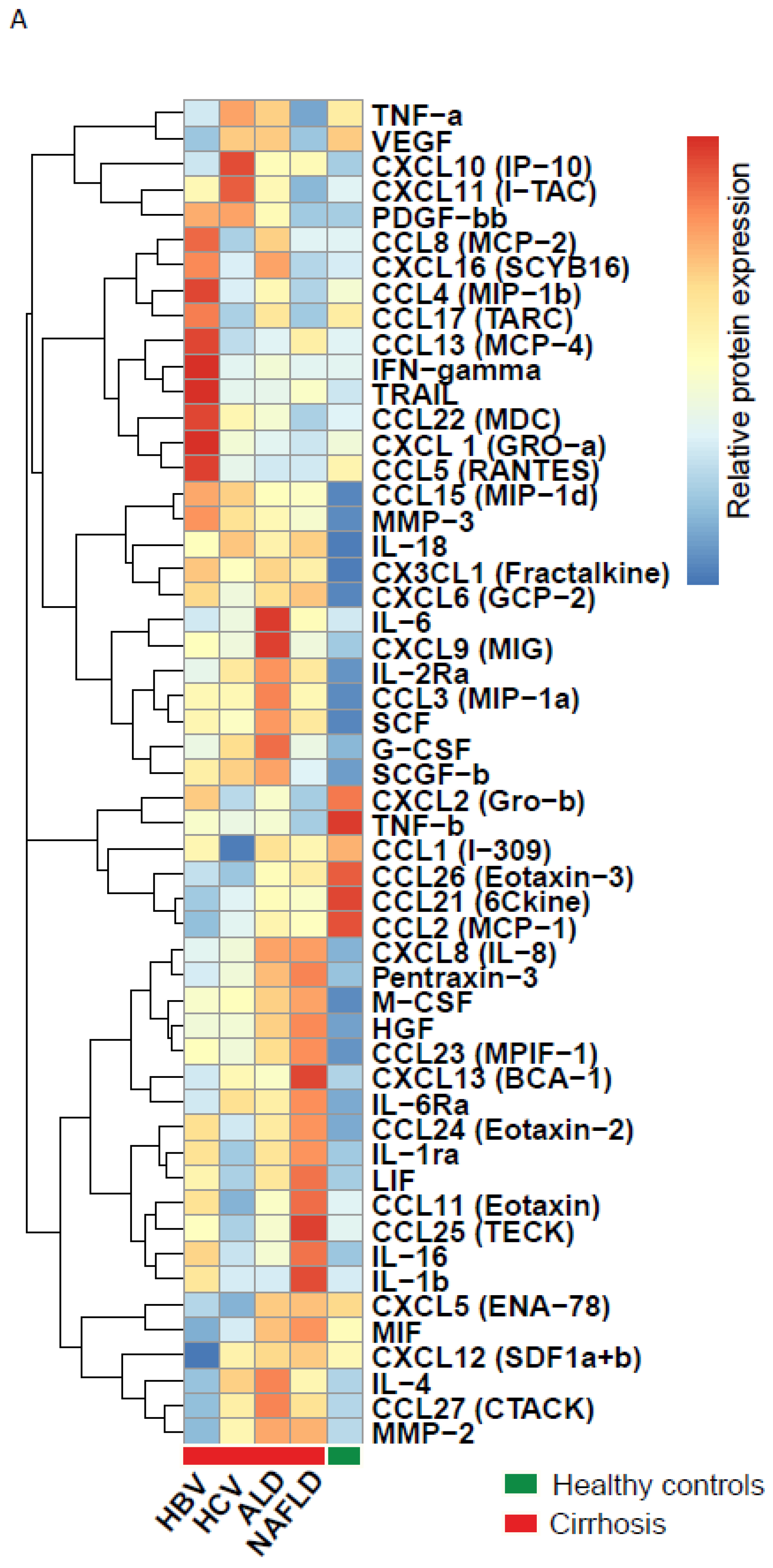
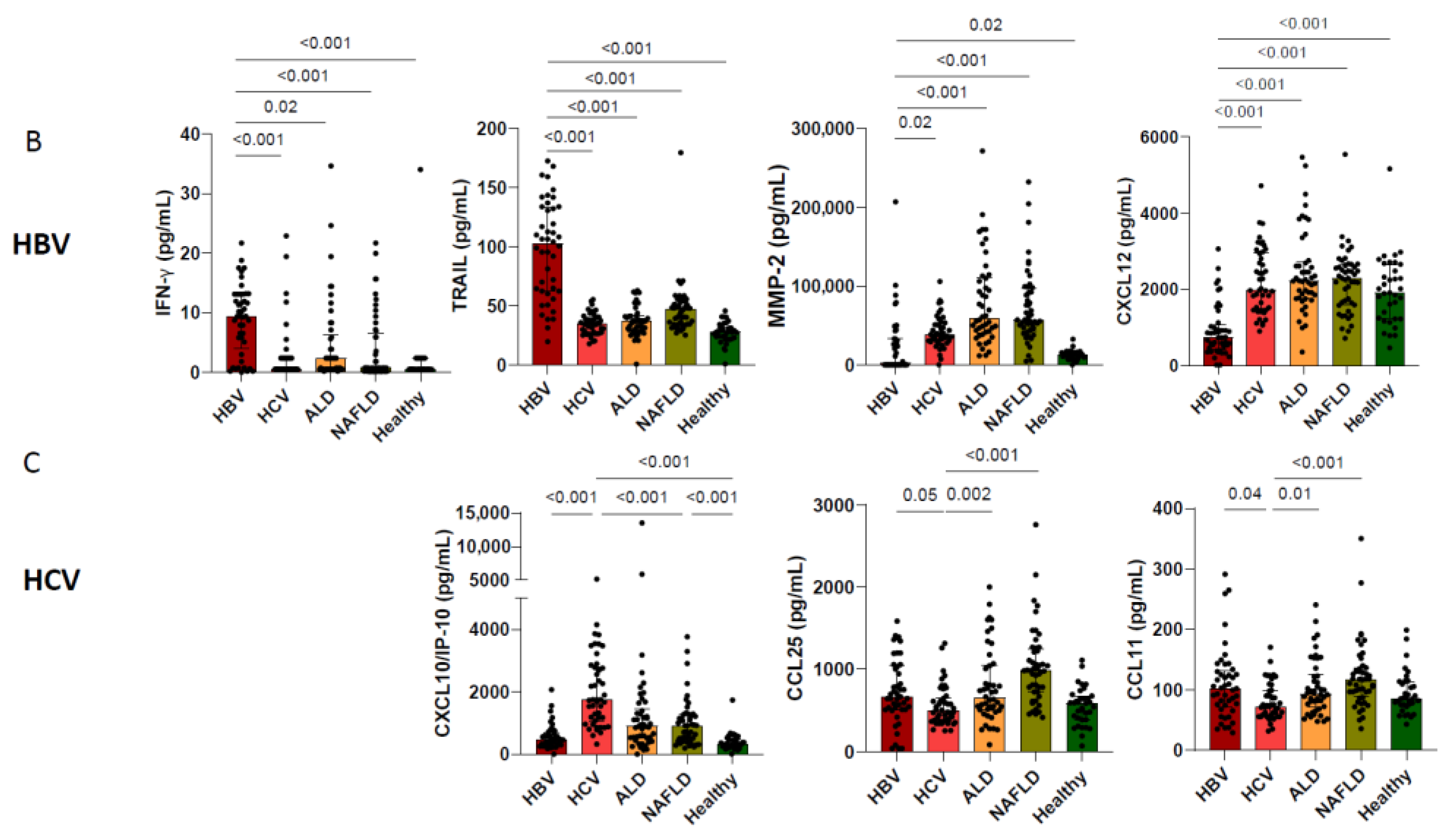
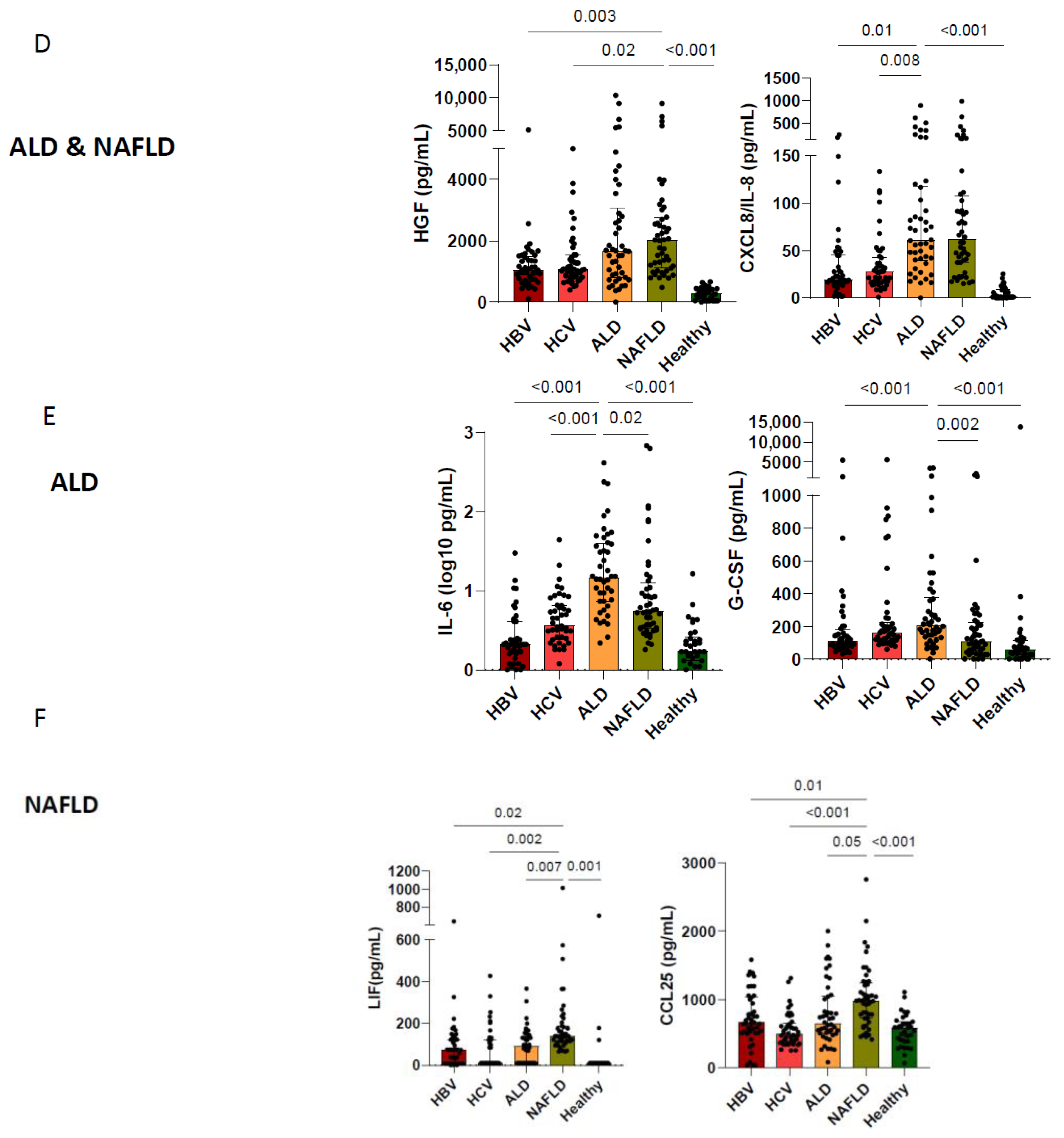
3.2. Etiological Cause of Cirrhosis Associates with Unique Immune Profiles in Blood
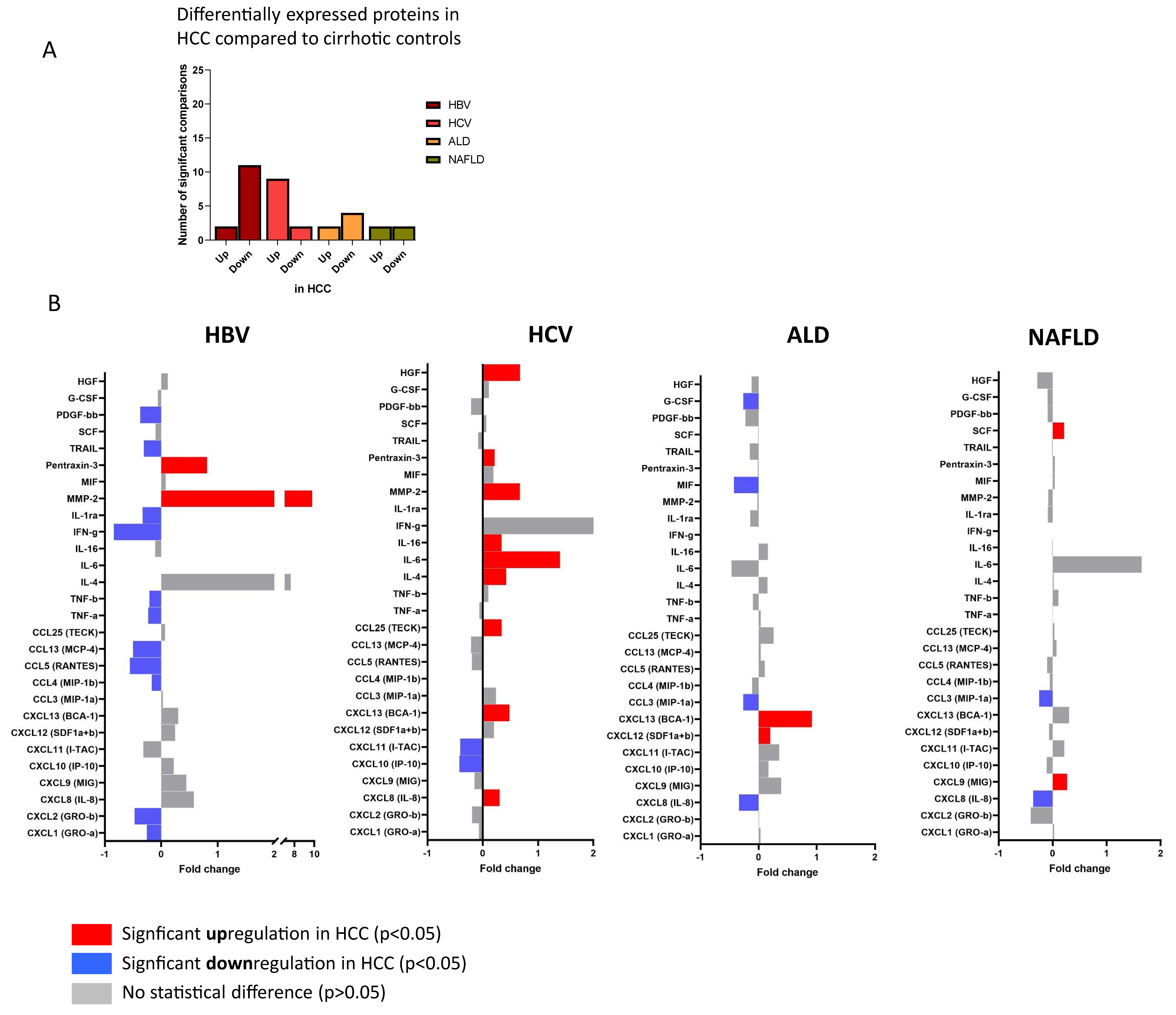
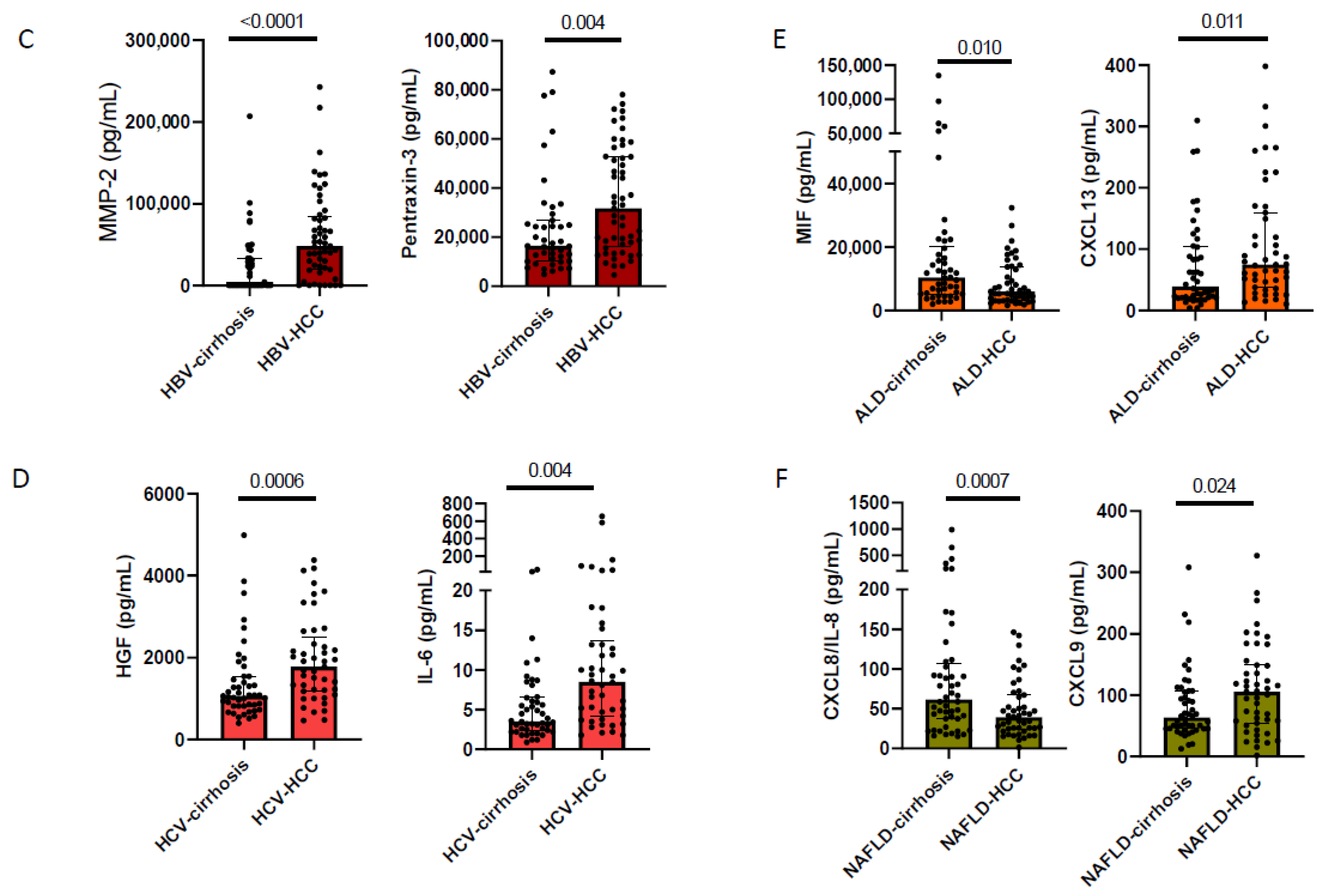
3.3. Cirrhosis Etiology Impacts Peripheral Immune Patterns of Early-Stage HCC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef]
- Debes, J.D.; van Tilborg, M.; Groothuismink, Z.M.A.; Hansen, B.E.; Schulze Zur Wiesch, J.; von Felden, J.; de Knegt, R.J.; Boonstra, A. Levels of Cytokines in Serum Associate With Development of Hepatocellular Carcinoma in Patients with HCV Infection Treated With Direct-Acting Antivirals. Gastroenterology 2018, 154, 515–517.e513. [Google Scholar] [CrossRef]
- Wilson, C.L.; Jurk, D.; Fullard, N.; Banks, P.; Page, A.; Luli, S.; Elsharkawy, A.M.; Gieling, R.G.; Chakraborty, J.B.; Fox, C.; et al. NFκB1 is a suppressor of neutrophil-driven hepatocellular carcinoma. Nat. Commun. 2015, 6, 6818. [Google Scholar] [CrossRef]
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer 2020, 6, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Z.; Zhang, Z.; Zheng, B.H.; Shi, Y.; Duan, M.; Ma, L.J.; Wang, Z.C.; Dong, L.Q.; Dong, P.P.; Shi, J.Y.; et al. CCL15 Recruits Suppressive Monocytes to Facilitate Immune Escape and Disease Progression in Hepatocellular Carcinoma. Hepatology 2019, 69, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Pfister, D.; Núñez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021, 592, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.; Pfister, D.; Donakonda, S.; Filpe, P.; Schneider, A.; Laschinger, M.; Hartmann, D.; Hüser, N.; Meiser, P.; Bayerl, F.; et al. Auto-aggressive CXCR6(+) CD8 T cells cause liver immune pathology in NASH. Nature 2021, 592, 444–449. [Google Scholar] [CrossRef]
- Monvoisin, A.; Bisson, C.; Si-Tayeb, K.; Balabaud, C.; Desmoulière, A.; Rosenbaum, J. Involvement of matrix metalloproteinase type-3 in hepatocyte growth factor-induced invasion of human hepatocellular carcinoma cells. Int. J. Cancer 2002, 97, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.M.; Fusenig, N.E. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat. Rev. Cancer 2004, 4, 839–849. [Google Scholar] [CrossRef]
- Estevez, J.; Chen, V.L.; Podlaha, O.; Li, B.; Le, A.; Vutien, P.; Chang, E.T.; Rosenberg-Hasson, Y.; Jiang, Z.; Pflanz, S.; et al. Differential Serum Cytokine Profiles in Patients with Chronic Hepatitis B, C, and Hepatocellular Carcinoma. Sci. Rep. 2017, 7, 11867. [Google Scholar] [CrossRef]
- Koshiol, J.; Argirion, I.; Liu, Z.; Kim Lam, T.; O’Brien, T.R.; Yu, K.; McGlynn, K.A.; Petrick, J.L.; Pinto, L.; Chen, C.-J.; et al. Immunologic markers and risk of hepatocellular carcinoma in hepatitis B virus- and hepatitis C virus-infected individuals. Aliment. Pharmacol. Ther. 2021, 54, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Inada, Y.; Mizukoshi, E.; Seike, T.; Tamai, T.; Iida, N.; Kitahara, M.; Yamashita, T.; Arai, K.; Terashima, T.; Fushimi, K.; et al. Characteristics of Immune Response to Tumor-Associated Antigens and Immune Cell Profile in Patients With Hepatocellular Carcinoma. Hepatology 2019, 69, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Nischalke, H.D.; Fischer, J.; Klüners, A.; Matz-Soja, M.; Krämer, B.; Langhans, B.; Goeser, F.; Soyka, M.; Stickel, F.; Spengler, U.; et al. A genetic variant in toll-like receptor 5 is linked to chemokine levels and hepatocellular carcinoma in steatohepatitis. Liver Int. 2021, 41, 2139–2148. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, H.W.; Seidler, S.; Gassler, N.; Nattermann, J.; Luedde, T.; Trautwein, C.; Tacke, F. Interleukin-8 Is Activated in Patients with Chronic Liver Diseases and Associated with Hepatic Macrophage Accumulation in Human Liver Fibrosis. PLoS ONE 2011, 6, e21381. [Google Scholar] [CrossRef]
- Glass, O.; Henao, R.; Patel, K.; Guy, C.D.; Gruss, H.J.; Syn, W.-K.; Moylan, C.A.; Streilein, R.; Hall, R.; Mae Diehl, A.; et al. Serum Interleukin-8, Osteopontin, and Monocyte Chemoattractant Protein 1 Are Associated With Hepatic Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Hepatol. Commun. 2018, 2, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Huang, J.; Ma, T.; Li, D.; Wu, Z.; Hou, B.; Jian, Z. Macrophages activating chemokine (C-X-C motif) ligand 8/miR-17 cluster modulate hepatocellular carcinoma cell growth and metastasis. Am. J. Transl Res. 2017, 9, 2403–2411. [Google Scholar] [PubMed]
- Sukowati, C.H.C.; Patti, R.; Pascut, D.; Ladju, R.B.; Tarchi, P.; Zanotta, N.; Comar, M.; Tiribelli, C.; Crocè, L.S. Serum Stem Cell Growth Factor Beta for the Prediction of Therapy Response in Hepatocellular Carcinoma. BioMed Res. Int. 2018, 2018, 6435482. [Google Scholar] [CrossRef] [PubMed]
- Straussman, R.; Morikawa, T.; Shee, K.; Barzily-Rokni, M.; Qian, Z.R.; Du, J.; Davis, A.; Mongare, M.M.; Gould, J.; Frederick, D.T. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 2012, 487, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.-C.; Wang, T.-T.; Liu, W.; Fu, B.-S.; Hua, X.; Wang, G.-Y.; Li, T.-J.; Li, X.; Wu, X.-Y.; Tai, Y. Cancer-associated fibroblasts from hepatocellular carcinoma promote malignant cell proliferation by HGF secretion. PLoS ONE 2013, 8, e63243. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ren, H.; Zhao, T.; Chen, J.; Sun, W.; Sun, Y.; Ma, W.; Wang, J.; Gao, C.; Gao, S.; et al. Stem cell factor is a novel independent prognostic biomarker for hepatocellular carcinoma after curative resection. Carcinogenesis 2014, 35, 2283–2290. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, O.; Cacho, B.; Morales-Espinosa, D.; Ruelas-Villavicencio, A.; Flores-Estrada, D.; Hernández-Pedro, N. The progressive elevation of alpha fetoprotein for the diagnosis of hepatocellular carcinoma in patients with liver cirrhosis. BMC Cancer 2007, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Girbl, T.; Lenn, T.; Perez, L.; Rolas, L.; Barkaway, A.; Thiriot, A.; Del Fresno, C.; Lynam, E.; Hub, E.; Thelen, M. Distinct compartmentalization of the chemokines CXCL1 and CXCL2 and the atypical receptor ACKR1 determine discrete stages of neutrophil diapedesis. Immunity 2018, 49, 1062–1076.e1066. [Google Scholar] [CrossRef] [PubMed]
- Scapini, P.; Morini, M.; Tecchio, C.; Minghelli, S.; Di Carlo, E.; Tanghetti, E.; Albini, A.; Lowell, C.; Berton, G.; Noonan, D.M. CXCL1/macrophage inflammatory protein-2-induced angiogenesis in vivo is mediated by neutrophil-derived vascular endothelial growth factor-A. J. Immunol. 2004, 172, 5034–5040. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed]
- Argirion, I.; Pfeiffer, R.M.; Lam, T.K.; O’Brien, T.R.; Yu, K.; McGlynn, K.A.; Petrick, J.L.; Pinto, L.; Chen, C.J.; Lee, M.H.; et al. Association between immunologic markers and cirrhosis in individuals with chronic hepatitis B. Sci. Rep. 2021, 11, 21194. [Google Scholar] [CrossRef]
- Piratvisuth, T.; Tanwandee, T.; Thongsawat, S.; Sukeepaisarnjaroen, W.; Esteban, J.I.; Bes, M.; Köhler, B.; He, Y.; Swiatek-de Lange, M.; Morgenstern, D.; et al. Multimarker Panels for Detection of Early Stage Hepatocellular Carcinoma: A Prospective, Multicenter, Case-Control Study. Hepatol. Commun. 2022, 6, 679–691. [Google Scholar] [CrossRef]
- Beudeker, B.J.B.; Boonstra, A. Circulating biomarkers for early detection of hepatocellular carcinoma. Ther. Adv. Gastroenterol. 2020, 13, 1756284820931734. [Google Scholar] [CrossRef]
| HBV-Associated | HCV-Associated | Alcohol-Associated | NAFLD-Associated | Healthy | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HCC | Cirrhosis | HCC | Cirrhosis | HCC | Cirrhosis | HCC | Cirrhosis | Control | ||
| N= | 54 | 47 | 47 | 47 | 47 | 46 | 48 | 48 | 35 | |
| Age (years) | Median (IQR) | 62 (15) | 54 (15) | 60 (11) | 53 (12) | 68 (11) | 58 (12) | 72 (12) | 56 (18) | 58 (5) |
| Gender | ||||||||||
| Female | 12 (22%) | 9 (20%) | 6 (13%) | 13 (28%) | 12 (16%) | 21 (46%) | 18 (37%) | 24 (50%) | 26 (74%) | |
| Male | 42 (78%) | 37 (80%) | 40 (87%) | 34 (72%) | 35 (74%) | 25 (54%) | 30 (63%) | 24 (50%) | 9 (26%) | |
| Ethnicity | ||||||||||
| African | 7 (13.0%) | 8 (17.4%) | 6 (13.4 %) | 2 (4.3%) | 0 (0.0%) | 1 (2.2%) | 2 (4.3%) | 5 (10.4%) | 0 (0%) | |
| Asian | 13 (24.1%) | 11 (23.9%) | 3 (6.5%) | 0 (0.0%) | 1 (2.1%) | 0 (0.0%) | 1 (2.1%) | 0 (0.0%) | 0 (0%) | |
| Caucasian | 29 (53.7%) | 22 (47.8%) | 32 (69.6%) | 35 (74.5%) | 45 (95.7%) | 41 (89.1%) | 41 (87.2%) | 41 (85.4%) | 35 (100%) | |
| Other | 5 (9.3%) | 5 (10.9%) | 5 (10.9%) | 10 (21.3%) | 1 (2.1%) | 4 (8.7%) | 3 (6.4%) | 2 (4.2%) | 0 (0%) | |
| Tumor stage | ||||||||||
| BCLC 0 | 11 (20.3%) | 0 (0.0%) | 11 (23.9%) | 0 (0.0%) | 3 (6.4%) | 0 (0.0%) | 5 (10.6%) | 0 (0.0%) | na | |
| BCLC A | 43 (79.7%) | 0 (0.0%) | 35 (76.1%) | 0 (0.0%) | 44 (93.6%) | 0 (0.0%) | 42 (89.4%) | 0 (0.0%) | na | |
| Antiviral therapy | 37 (67%) | 36 (78%) | 0 (0.0%) | 0 (0.0%) | na | na | na | na | na | |
| Liver cirrhosis | 54 (100%) | 46 (100%) | 46 (100%) | 47 (100%) | 47 (100%) | 46 (100%) | 48 (100%) | 48 (100%) | 0 (0%) | |
| AFP (ng/mL) | Median (IQR) | 9 (47) | 4 (2) | 22 (86) | 10 (10) | 8 (17) | 5 (3) | 7 (11) | 4 (4) | 4 (3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beudeker, B.J.B.; Groothuismink, Z.M.A.; van der Eijk, A.A.; Debes, J.D.; Boonstra, A. Circulating Cytokines Reflect the Etiology-Specific Immune Environment in Cirrhosis and HCC. Cancers 2022, 14, 4900. https://doi.org/10.3390/cancers14194900
Beudeker BJB, Groothuismink ZMA, van der Eijk AA, Debes JD, Boonstra A. Circulating Cytokines Reflect the Etiology-Specific Immune Environment in Cirrhosis and HCC. Cancers. 2022; 14(19):4900. https://doi.org/10.3390/cancers14194900
Chicago/Turabian StyleBeudeker, Boris J. B., Zwier M. A. Groothuismink, Annemiek A. van der Eijk, Jose D. Debes, and Andre Boonstra. 2022. "Circulating Cytokines Reflect the Etiology-Specific Immune Environment in Cirrhosis and HCC" Cancers 14, no. 19: 4900. https://doi.org/10.3390/cancers14194900
APA StyleBeudeker, B. J. B., Groothuismink, Z. M. A., van der Eijk, A. A., Debes, J. D., & Boonstra, A. (2022). Circulating Cytokines Reflect the Etiology-Specific Immune Environment in Cirrhosis and HCC. Cancers, 14(19), 4900. https://doi.org/10.3390/cancers14194900






