Neoadjuvant Chemotherapy before Nephroureterectomy in High-Risk Upper Tract Urothelial Cancer: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
2.4. Study Records
2.5. Data Items
2.6. Risk of Bias Assessment
2.7. Data Synthesis
3. Results
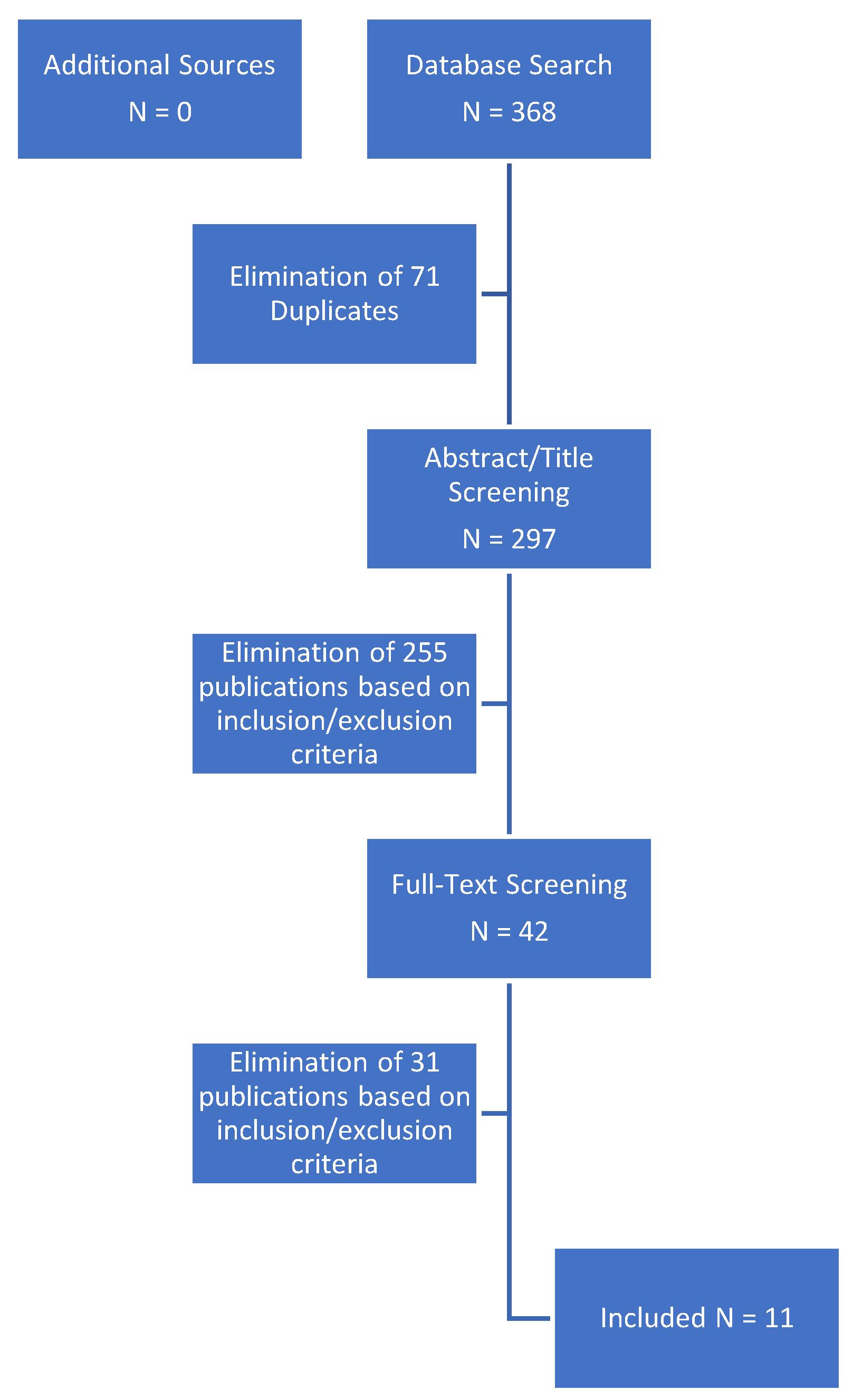
3.1. Search Results
3.2. Baseline Parameters
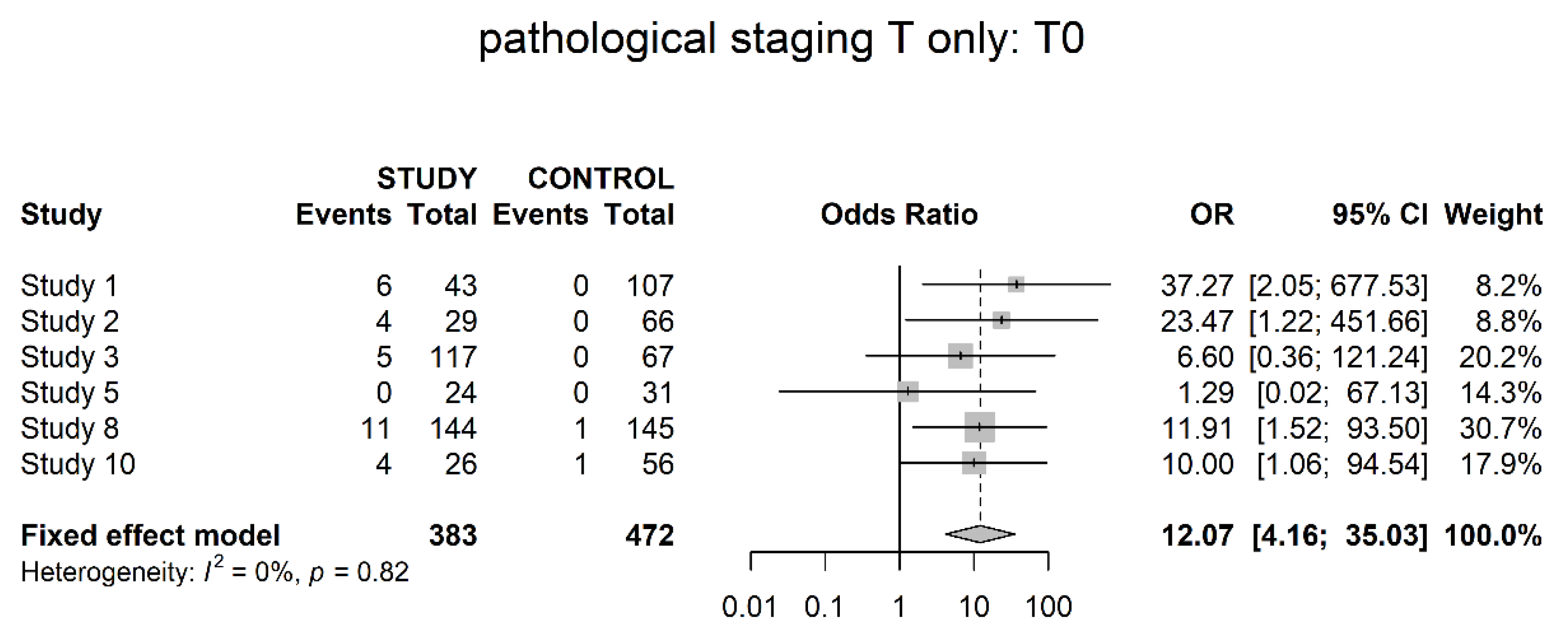
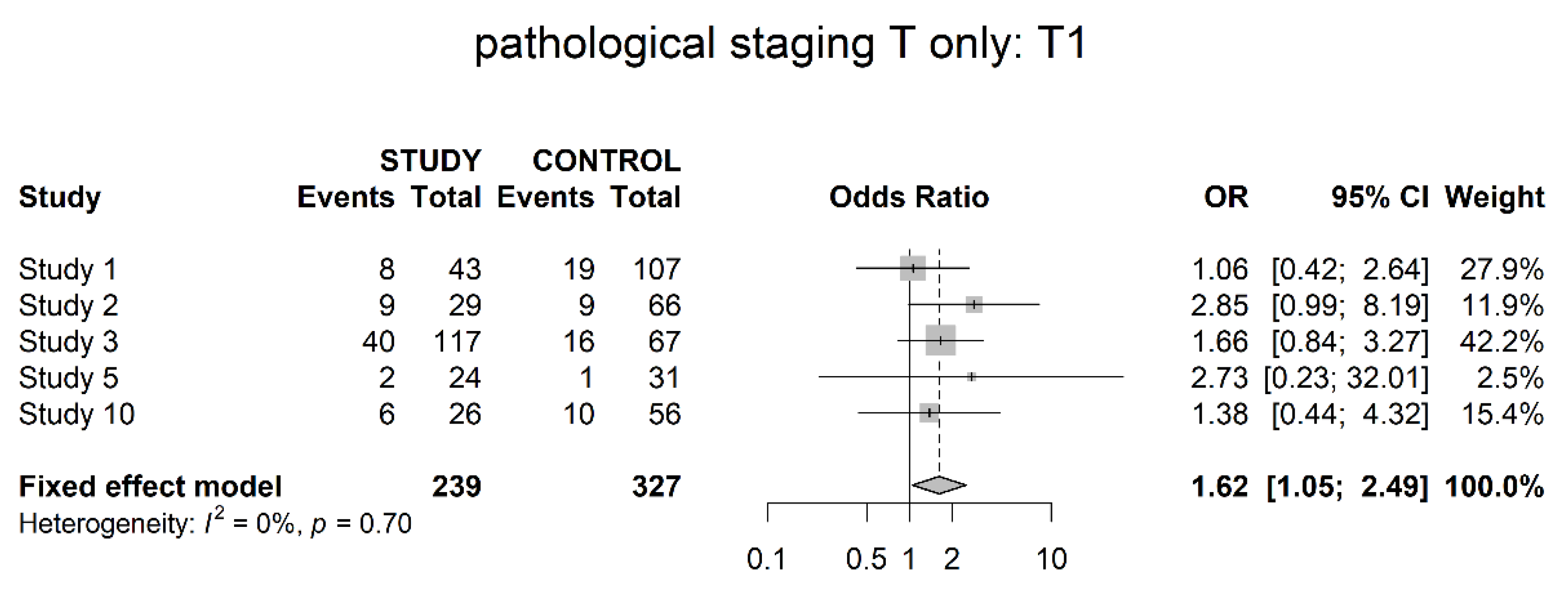
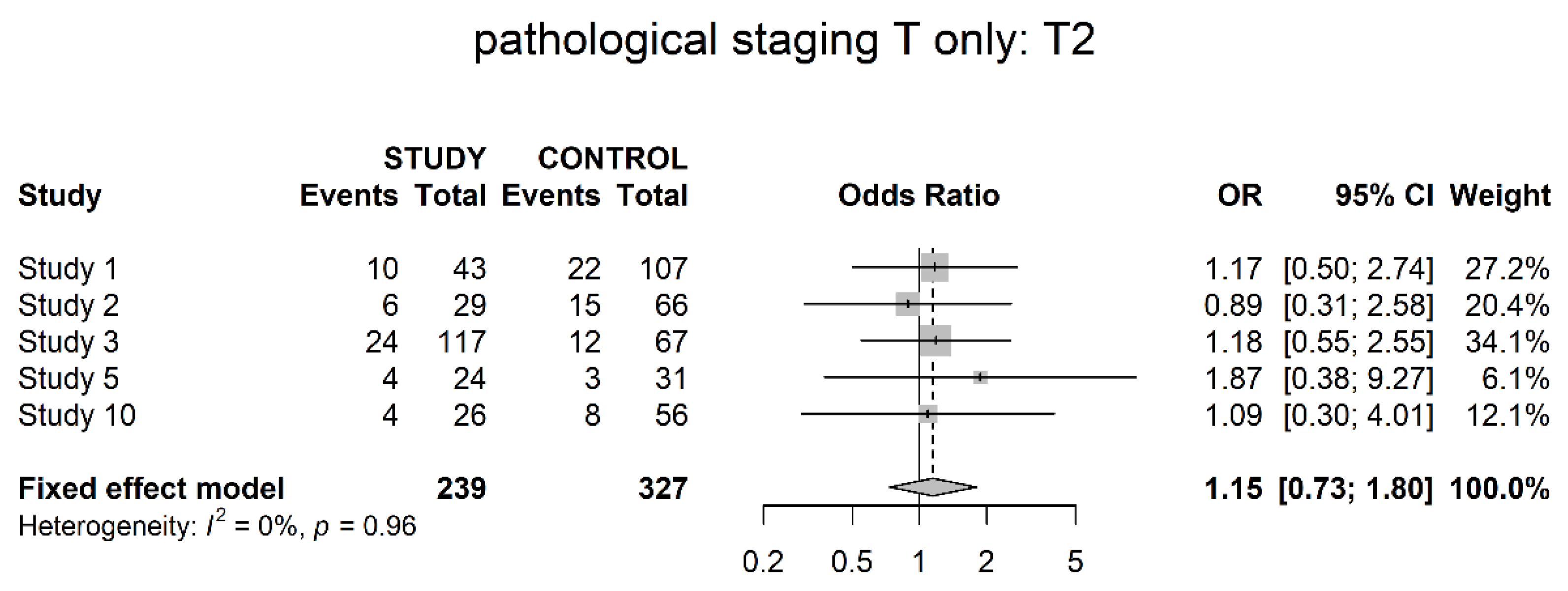
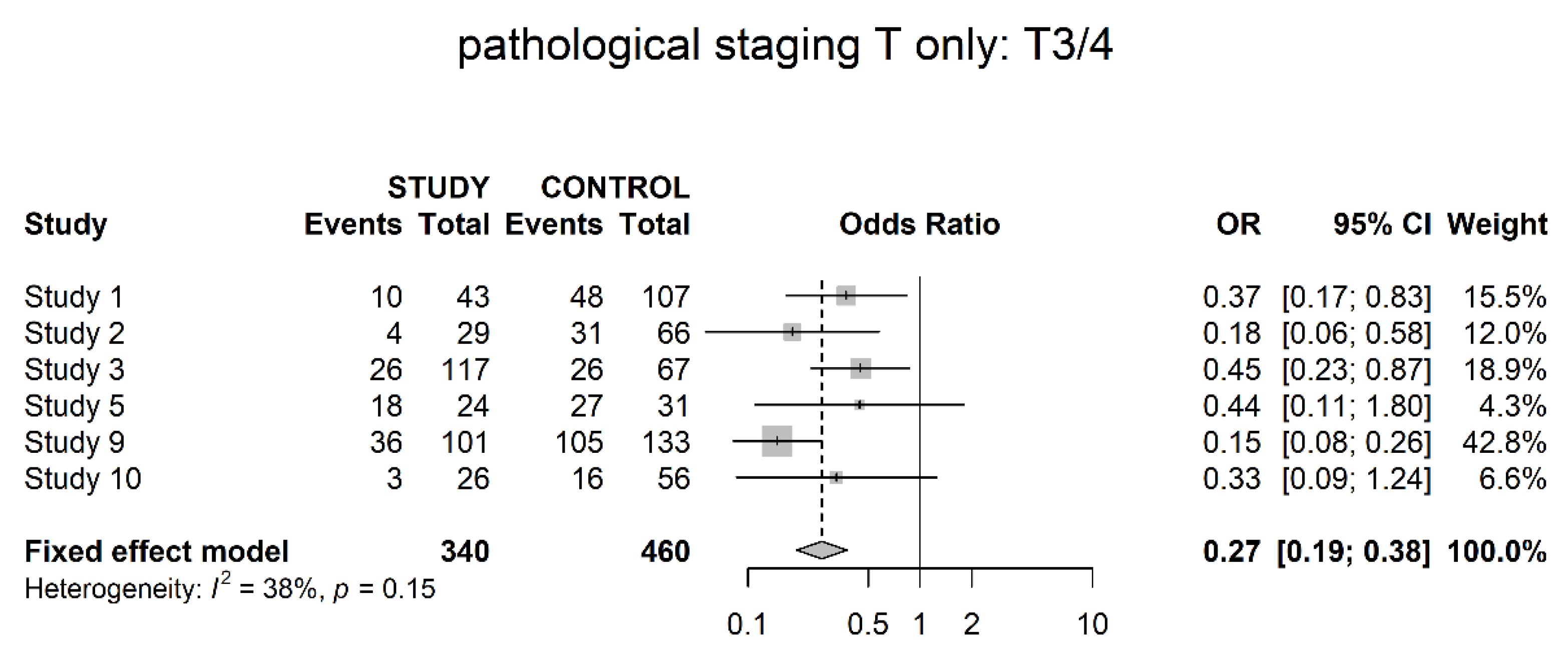
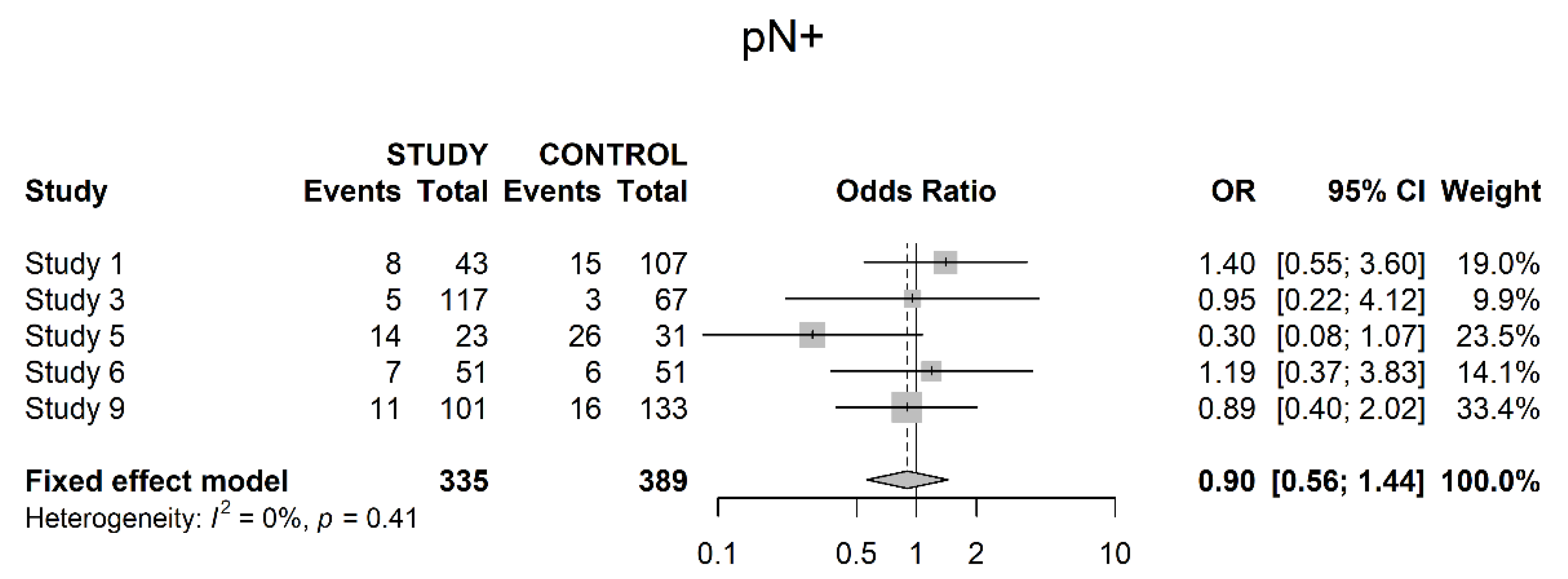
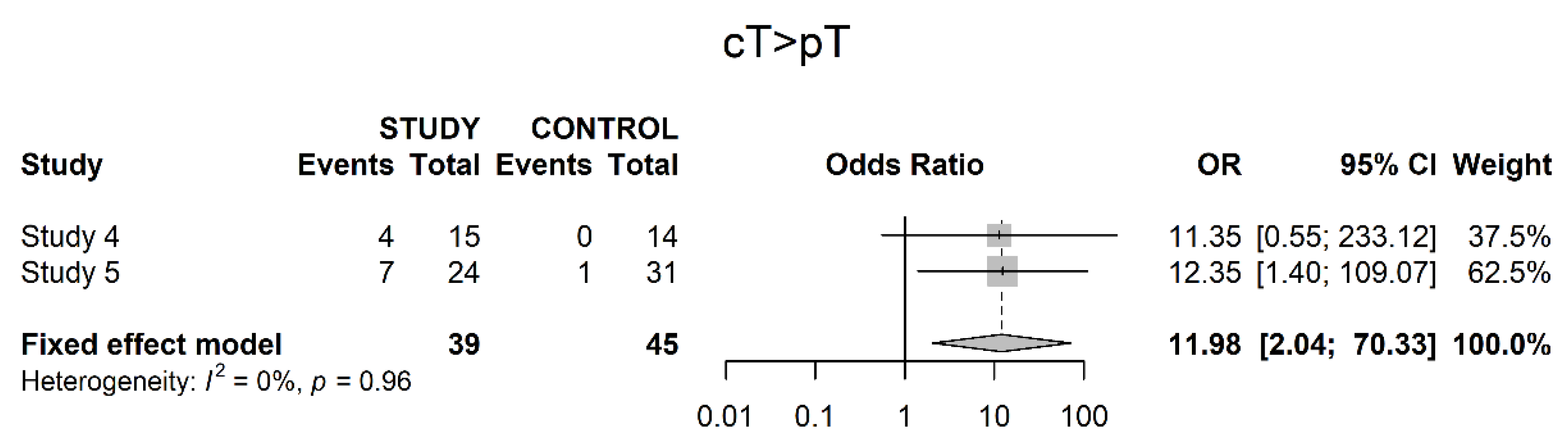
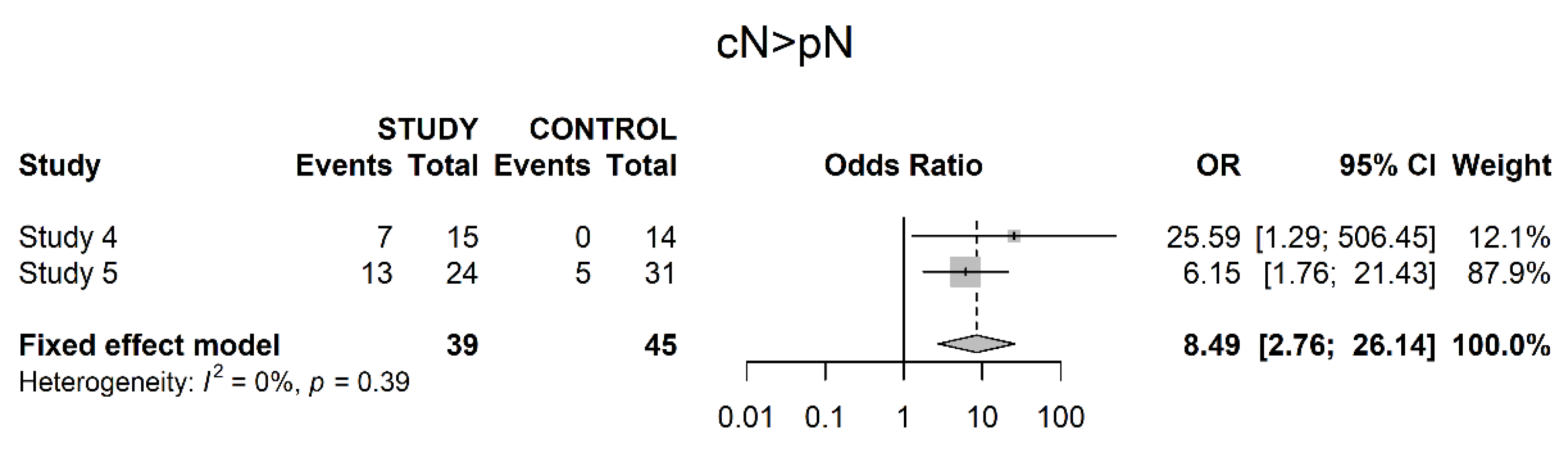
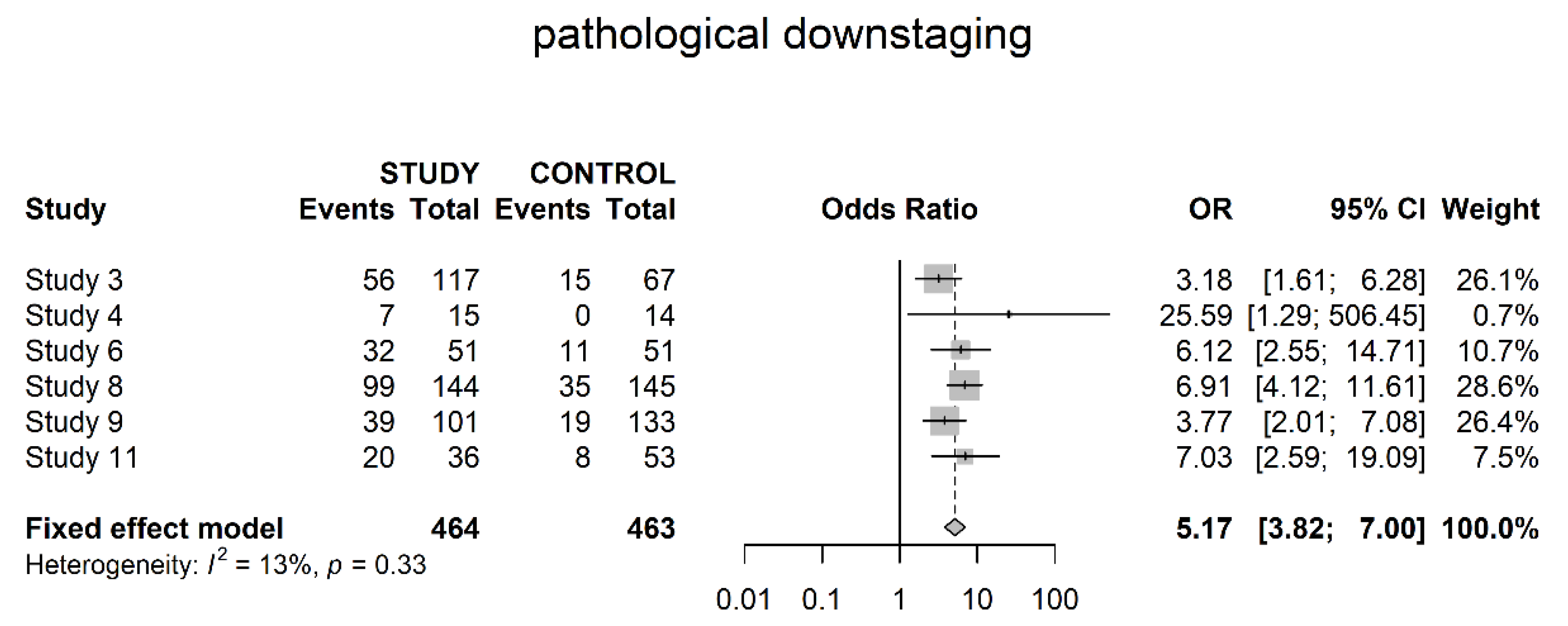
3.3. Pathological Downstaging
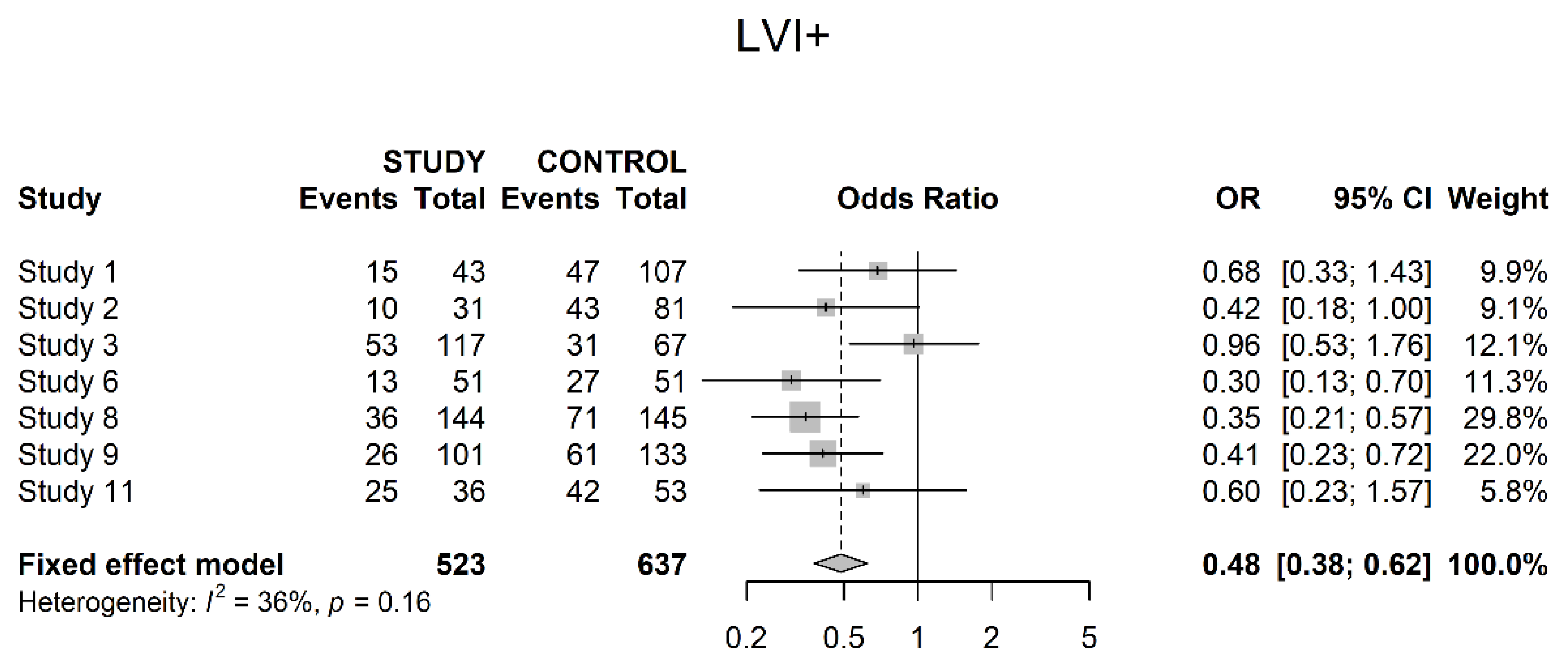
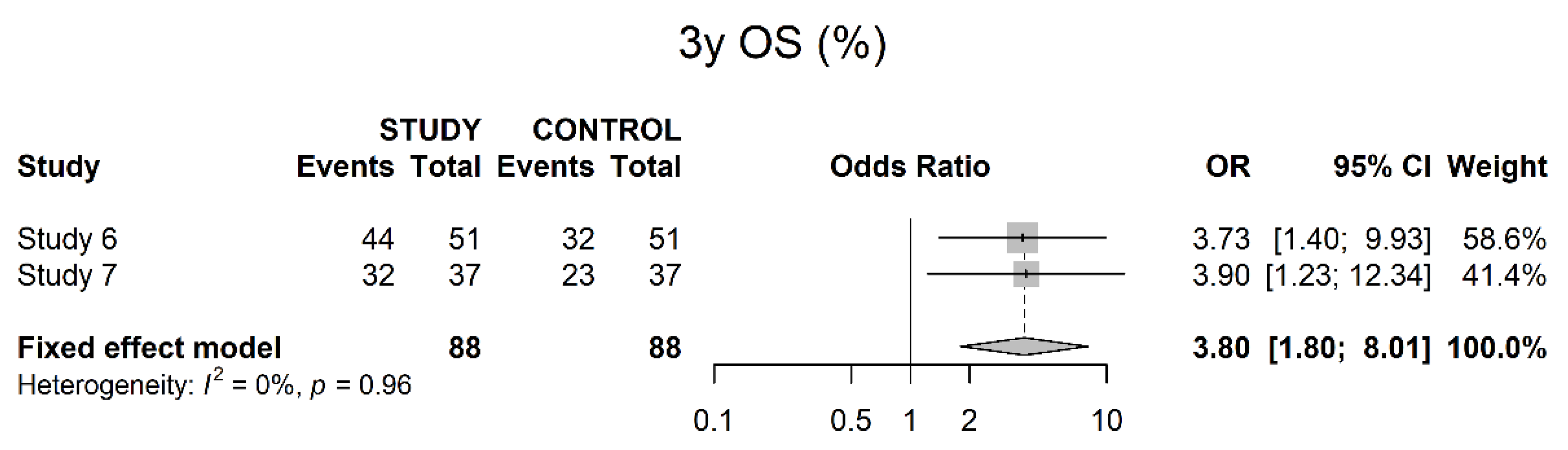
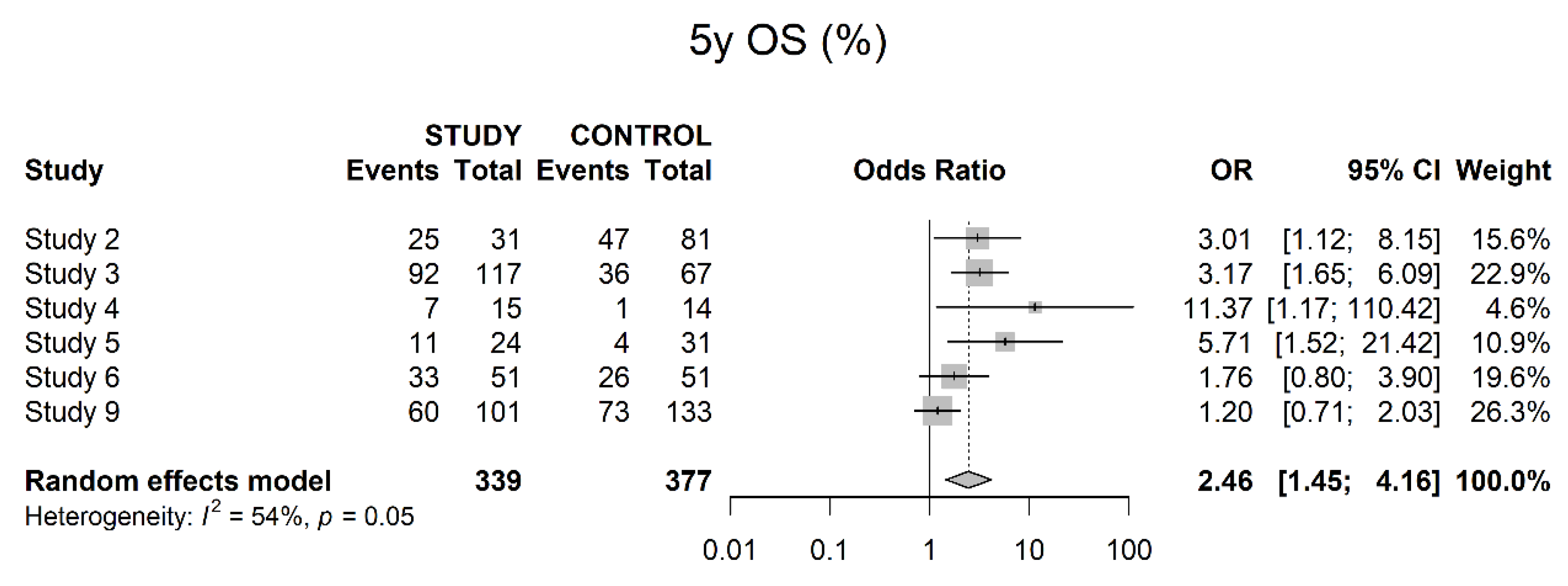
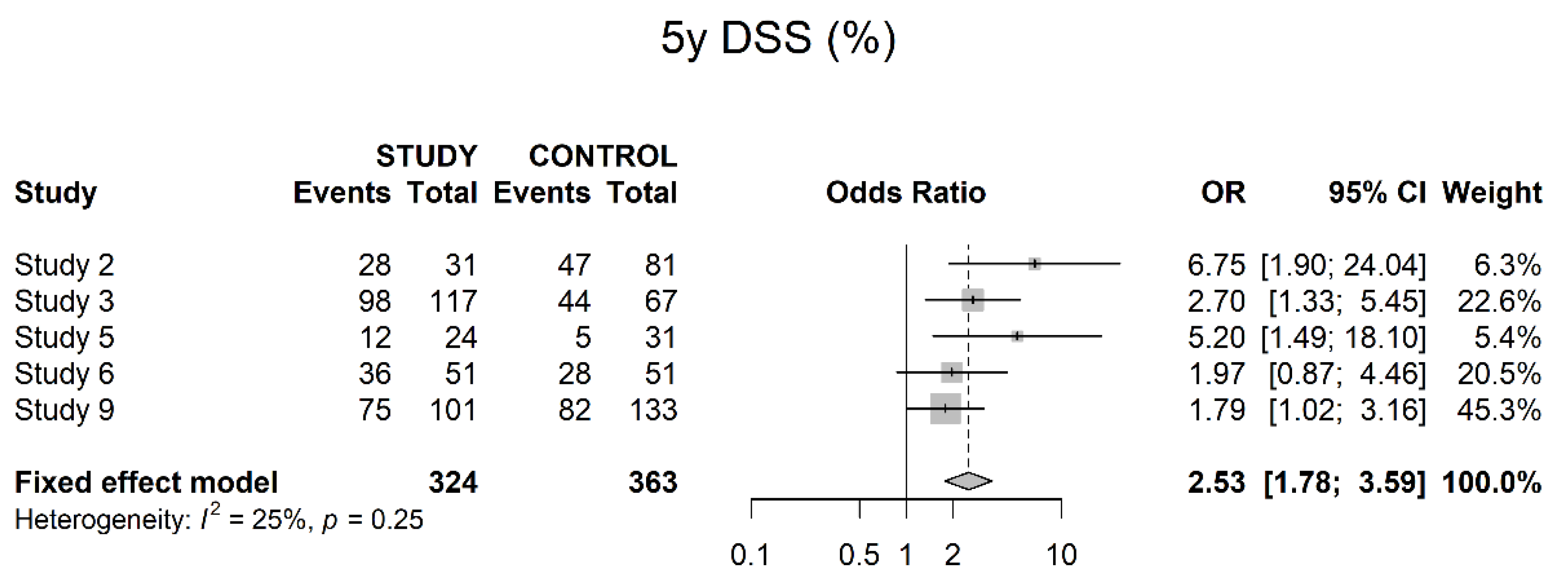
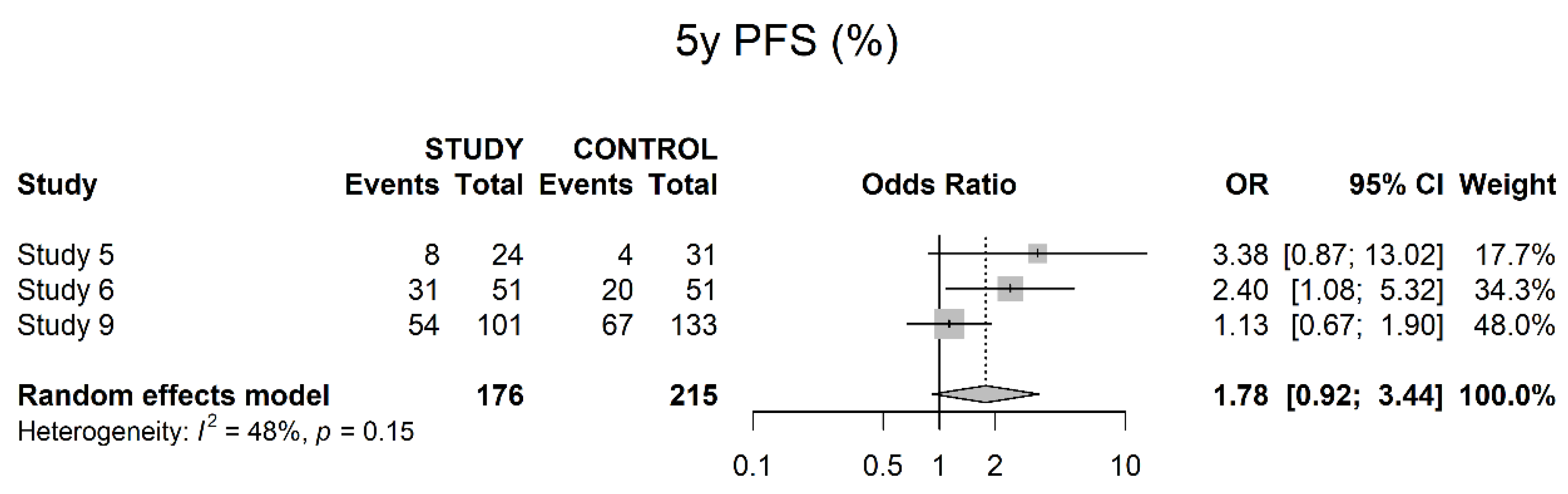
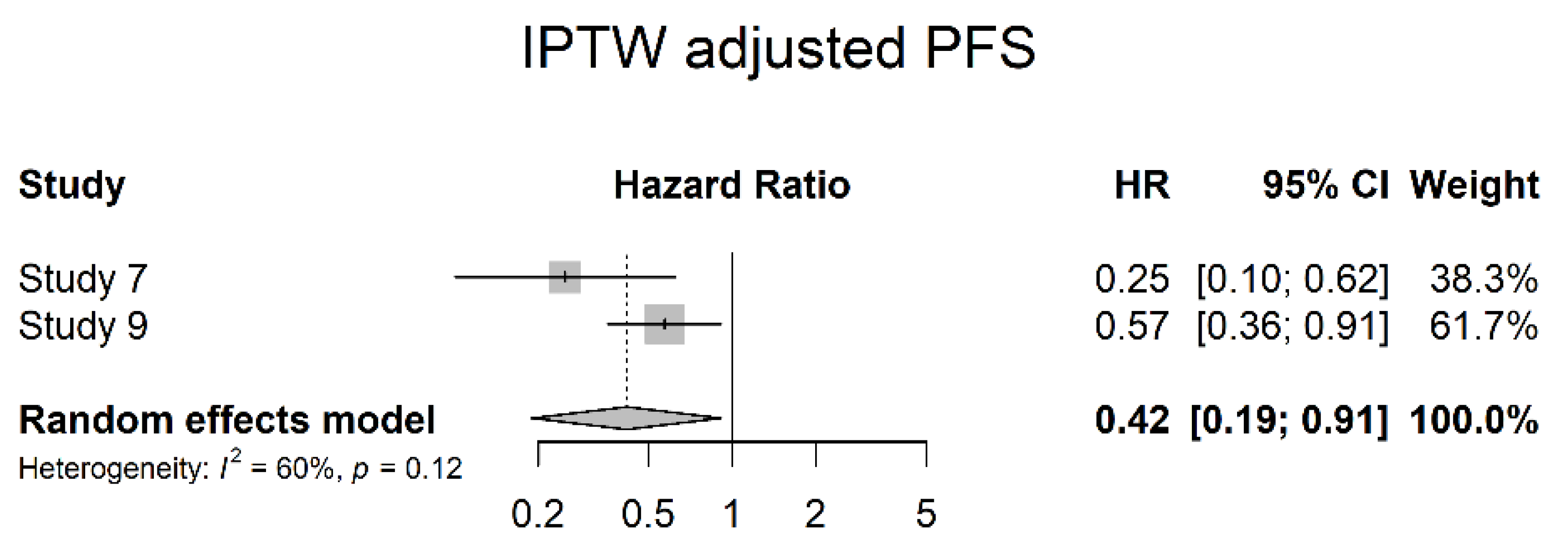
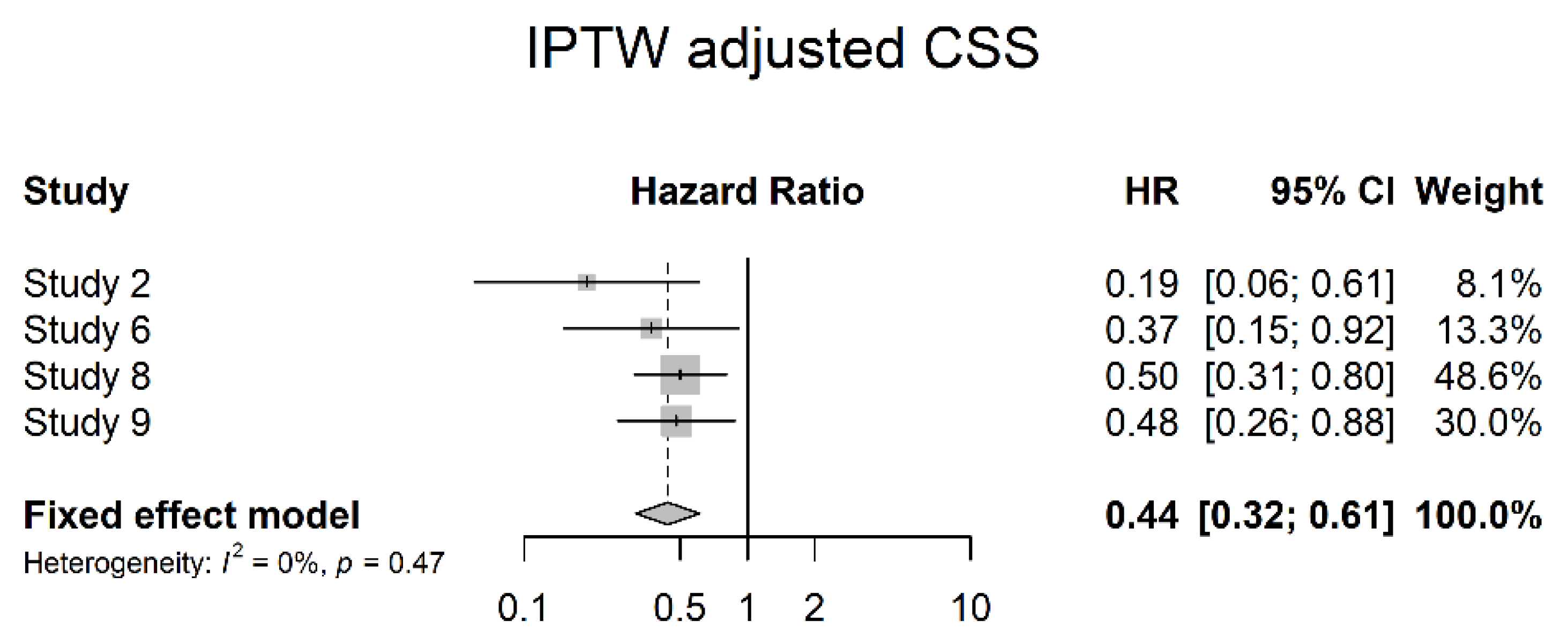
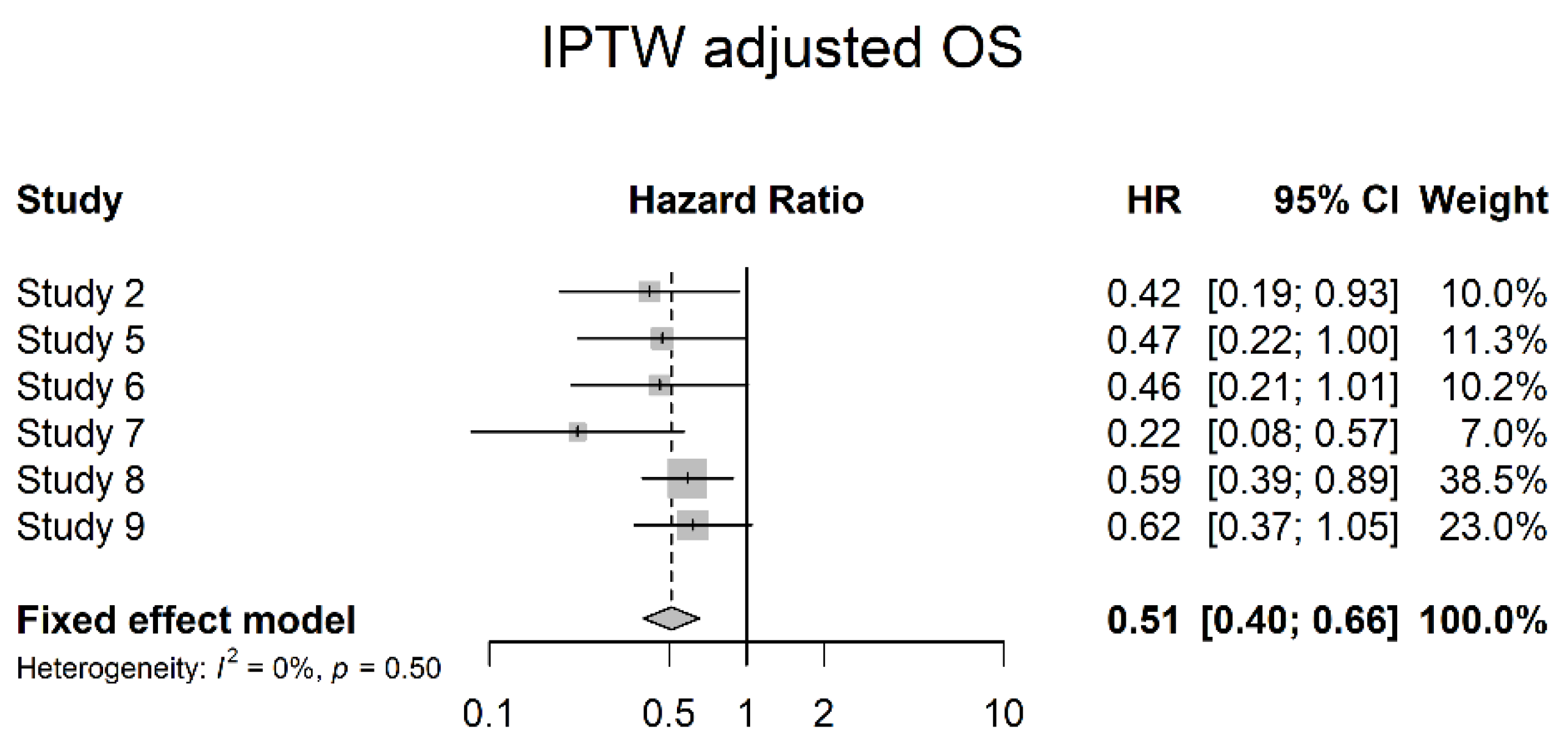
3.4. Oncological Outcomes
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| UC | urothelial carcinoma |
| UTUC | upper urinary tract urothelial carcinoma |
| BC | bladder cancer |
| DSS | disease-specific survival |
| CT | computed tomography |
| URS | ureterorenoscopy |
| RNU | radical nephroureterectomy |
| EAU | European Association of Urology |
| MIBC | muscle-invasive bladder cancer |
| OS | overall survival |
| CPI | checkpoint-Inhibitor |
| CR | complete remission |
| NAC | neoadjuvant chemotherapy |
| MVAC/MVEC | methotrexate, vinblastine, adriamycin/epirubicin and cisplatin |
| PICO | participants, interventions, comparators, outcomes |
| PR | partial remission |
| SD | stable disease |
| PD | progressive disease |
| PFS | progression-free survival |
| GFR | glomerular filtration rate |
| ECOG | Eastern Cooperative Oncology Group |
| LVI | lymphovascular invasion |
| OR | odds ratio |
| HR | hazard ratio |
| CI | confidence interval |
| IPTW | intuition for inverse probability of treatment weighting |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Rouprêt, M.; Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Cowan, N.C.; Dominguez-Escrig, J.L.; Gontero, P.; Hugh Mostafid, A.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur. Urol. 2021, 79, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Margulis, V.; Shariat, S.F.; Matin, S.F.; Kamat, A.M.; Zigeuner, R.; Kikuchi, E.; Lotan, Y.; Weizer, A.; Raman, J.D.; Wood, C.G.; et al. Outcomes of radical nephroureterectomy: A series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 2009, 115, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Lughezzani, G.; Jeldres, C.; Isbarn, H.; Sun, M.; Shariat, S.F.; Widmer, H.; Arjane, P.; Graefen, M.; Perrotte, P.; Montorsi, F.; et al. Temporal stage and grade migration in surgically treated patients with upper tract urothelial carcinoma. BJU Int. 2010, 105, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Compérat, E.; Larré, S.; Roupret, M.; Neuzillet, Y.; Pignot, G.; Quintens, H.; Houéde, N.; Roy, C.; Durand, X.; Varinot, J.; et al. Clinicopathological characteristics of urothelial bladder cancer in patients less than 40 years old. Virchows Arch. 2015, 466, 589–594. [Google Scholar] [CrossRef]
- Rosiello, G.; Palumbo, C.; Knipper, S.; Pecoraro, A.; Luzzago, S.; Deuker, M.; Mistretta, F.A.; Tian, Z.; Fossati, N.; Gallina, A.; et al. Contemporary conditional cancer-specific survival after radical nephroureterectomy in patients with nonmetastatic urothelial carcinoma of upper urinary tract. J. Surg. Oncol. 2020, 121, 1154–1161. [Google Scholar] [CrossRef]
- van Doeveren, T.; van der Mark, M.; van Leeuwen, P.J.; Boormans, J.L.; Aben, K.K.H. Rising incidence rates and unaltered survival rates for primary upper urinary tract urothelial carcinoma: A Dutch population-based study from 1993 to 2017. BJU Int. 2021, 128, 343–351. [Google Scholar] [CrossRef]
- Cohen, A.; Kuchta, K.; Park, S. Neoadjuvant and adjuvant chemotherapy use in upper tract urothelial carcinoma. Urol. Oncol. 2017, 35, 322–327. [Google Scholar] [CrossRef]
- Leow, J.J.; Orsola, A.; Chang, S.L.; Bellmunt, J. A contemporary review of management and prognostic factors of upper tract urothelial carcinoma. Cancer Treat. Rev. 2015, 41, 310–319. [Google Scholar] [CrossRef]
- Soria, F.; Shariat, S.F.; Lerner, S.P.; Fritsche, H.M.; Rink, M.; Kassouf, W.; Spiess, P.E.; Lotan, Y.; Ye, D.; Fernández, M.I.; et al. Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of upper-tract urothelial carcinoma (UTUC). World J. Urol. 2017, 35, 379–387. [Google Scholar] [CrossRef]
- Seisen, T.; Granger, B.; Colin, P.; Léon, P.; Utard, G.; Renard-Penna, R.; Compérat, E.; Mozer, P.; Cussenot, O.; Shariat, S.F.; et al. A Systematic Review and Meta-analysis of Clinicopathologic Factors Linked to Intravesical Recurrence After Radical Nephroureterectomy to Treat Upper Tract Urothelial Carcinoma. Eur. Urol. 2015, 67, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Cha, E.K.; Shariat, S.F.; Kormaksson, M.; Novara, G.; Chromecki, T.F.; Scherr, D.S.; Lotan, Y.; Raman, J.D.; Kassouf, W.; Zigeuner, R.; et al. Predicting clinical outcomes after radical nephroureterectomy for upper tract urothelial carcinoma. Eur. Urol. 2012, 61, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Lughezzani, G.; Burger, M.; Margulis, V.; Matin, S.F.; Novara, G.; Roupret, M.; Shariat, S.F.; Wood, C.G.; Zigeuner, R. Prognostic factors in upper urinary tract urothelial carcinomas: A comprehensive review of the current literature. Eur. Urol. 2012, 62, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Ruvolo, C.C.; Nocera, L.; Stolzenbach, L.F.; Wenzel, M.; Cucchiara, V.; Tian, Z.; Shariat, S.F.; Saad, F.; Longo, N.; Montorsi, F.; et al. Incidence and Survival Rates of Contemporary Patients with Invasive Upper Tract Urothelial Carcinoma. Eur. Urol. Oncol. 2021, 4, 792–801. [Google Scholar] [CrossRef]
- Colin, P.; Koenig, P.; Ouzzane, A.; Berthon, N.; Villers, A.; Biserte, J.; Rouprêt, M. Environmental factors involved in carcinogenesis of urothelial cell carcinomas of the upper urinary tract. BJU Int. 2009, 104, 1436–1440. [Google Scholar] [CrossRef]
- Birtle, A.; Johnson, M.; Chester, J.; Jones, R.; Dolling, D.; Bryan, R.T.; Harris, C.; Winterbottom, A.; Blacker, A.; Catto, J.W.F.; et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): A phase 3, open-label, randomised controlled trial. Lancet 2020, 395, 1268–1277. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Espinós, E.L.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2020, 79, 82–104. [Google Scholar] [CrossRef]
- Grossman, H.B.; Natale, R.B.; Tangen, C.M.; Speights, V.O.; Vogelzang, N.J.; Trump, D.L.; de Vere White, R.W.; Sarosdy, M.F.; Wood, D.P., Jr.; Raghavan, D.; et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N. Engl. J. Med. 2003, 349, 859–866. [Google Scholar] [CrossRef]
- Vale, C.L. Neoadjuvant chemotherapy in invasive bladder cancer: Update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur. Urol. 2005, 48, 202–206. [Google Scholar] [CrossRef]
- Powles, T.; Kockx, M.; Rodriguez-Vida, A.; Duran, I.; Crabb, S.J.; Van Der Heijden, M.S.; Szabados, B.; Pous, A.F.; Gravis, G.; Herranz, U.A.; et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat. Med. 2019, 25, 1706–1714. [Google Scholar] [CrossRef]
- Necchi, A.; Anichini, A.; Raggi, D.; Briganti, A.; Massa, S.; Lucianò, R.; Colecchia, M.; Giannatempo, P.; Mortarini, R.; Bianchi, M.; et al. Pembrolizumab as Neoadjuvant Therapy Before Radical Cystectomy in Patients With Muscle-Invasive Urothelial Bladder Carcinoma (PURE-01): An Open-Label, Single-Arm, Phase II Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 3353–3360. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.T.; Shah, A.Y.; Matin, S.F.; Siefker-Radtke, A.O. Optimizing management of upper tract urothelial carcinoma. Urol. Oncol. 2017, 35, 492–498. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, P.H.; Stadler, W.M. The role of chemotherapy in upper tract urothelial carcinoma. Adv. Urol. 2009, 2009, 419028. [Google Scholar] [CrossRef] [PubMed]
- Raman, J.D.; Scherr, D.S. Management of patients with upper urinary tract transitional cell carcinoma. Nat. Clin. Pract. Urol. 2007, 4, 432–443. [Google Scholar] [CrossRef]
- Green, D.A.; Rink, M.; Xylinas, E.; Matin, S.F.; Stenzl, A.; Roupret, M.; Karakiewicz, P.I.; Scherr, D.S.; Shariat, S.F. Urothelial carcinoma of the bladder and the upper tract: Disparate twins. J. Urol. 2013, 189, 1214–1221. [Google Scholar] [CrossRef]
- Leow, J.J.; Chong, K.T.; Chang, S.L.; Bellmunt, J. Upper tract urothelial carcinoma: A different disease entity in terms of management. ESMO open 2017, 1, e000126. [Google Scholar] [CrossRef]
- Compérat, E.; Egevad, L.; Lopez-Beltran, A.; Camparo, P.; Algaba, F.; Amin, M.; Epstein, J.I.; Hamberg, H.; Hulsbergen-van de Kaa, C.; Kristiansen, G.; et al. An interobserver reproducibility study on invasiveness of bladder cancer using virtual microscopy and heatmaps. Histopathology 2013, 63, 756–766. [Google Scholar] [CrossRef]
- Sfakianos, J.P.; Cha, E.K.; Iyer, G.; Scott, S.N.; Zabor, E.C.; Shah, R.H.; Ren, Q.; Bagrodia, A.; Kim, P.H.; Hakimi, A.A.; et al. Genomic Characterization of Upper Tract Urothelial Carcinoma. Eur. Urol. 2015, 68, 970–977. [Google Scholar] [CrossRef]
- Moss, T.J.; Qi, Y.; Xi, L.; Peng, B.; Kim, T.B.; Ezzedine, N.E.; Mosqueda, M.E.; Guo, C.C.; Czerniak, B.A.; Ittmann, M.; et al. Comprehensive Genomic Characterization of Upper Tract Urothelial Carcinoma. Eur. Urol. 2017, 72, 641–649. [Google Scholar] [CrossRef]
- Audenet, F.; Isharwal, S.; Cha, E.K.; Donoghue, M.T.A.; Drill, E.N.; Ostrovnaya, I.; Pietzak, E.J.; Sfakianos, J.P.; Bagrodia, A.; Murugan, P.; et al. Clonal Relatedness and Mutational Differences between Upper Tract and Bladder Urothelial Carcinoma. Clin. Cancer Res. 2019, 25, 967–976. [Google Scholar] [CrossRef]
- Necchi, A.; Raggi, D.; Gallina, A.; Ross, J.S.; Fare, E.; Giannatempo, P.; Marandino, L.; Colecchia, M.; Luciano, R.; Bianchi, M.; et al. Impact of Molecular Subtyping and Immune Infiltration on Pathological Response and Outcome Following Neoadjuvant Pembrolizumab in Muscle-invasive Bladder Cancer. Eur. Urol. 2020, 77, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef]
- D’Andrea, D.; Matin, S.; Black, P.C.; Petros, F.G.; Zargar, H.; Dinney, C.P.; Cookson, M.S.; Kassouf, W.; Dall’Era, M.A.; McGrath, J.S.; et al. Comparative effectiveness of neoadjuvant chemotherapy in bladder and upper urinary tract urothelial carcinoma. BJU Int. 2021, 127, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Audenet, F.; Yates, D.R.; Cussenot, O.; Rouprêt, M. The role of chemotherapy in the treatment of urothelial cell carcinoma of the upper urinary tract (UUT-UCC). Urol. Oncol. 2013, 31, 407–413. [Google Scholar] [CrossRef]
- Siefker-Radtke, A.O.; Dinney, C.P.; Shen, Y.; Williams, D.L.; Kamat, A.M.; Grossman, H.B.; Millikan, R.E. A phase 2 clinical trial of sequential neoadjuvant chemotherapy with ifosfamide, doxorubicin, and gemcitabine followed by cisplatin, gemcitabine, and ifosfamide in locally advanced urothelial cancer: Final results. Cancer 2013, 119, 540–547. [Google Scholar] [CrossRef]
- Peyton, C.C.; Tang, D.; Reich, R.R.; Azizi, M.; Chipollini, J.; Pow-Sang, J.M.; Manley, B.; Spiess, P.E.; Poch, M.A.; Sexton, W.J.; et al. Downstaging and Survival Outcomes Associated With Neoadjuvant Chemotherapy Regimens Among Patients Treated With Cystectomy for Muscle-Invasive Bladder Cancer. JAMA Oncol. 2018, 4, 1535–1542. [Google Scholar] [CrossRef]
- Kaag, M.G.; O’Malley, R.L.; O’Malley, P.; Godoy, G.; Chen, M.; Smaldone, M.C.; Hrebinko, R.L.; Raman, J.D.; Bochner, B.; Dalbagni, G.; et al. Changes in renal function following nephroureterectomy may affect the use of perioperative chemotherapy. Eur. Urol. 2010, 58, 581–587. [Google Scholar] [CrossRef]
- Xylinas, E.; Rink, M.; Margulis, V.; Clozel, T.; Lee, R.K.; Comploj, E.; Novara, G.; Raman, J.D.; Lotan, Y.; Weizer, A.; et al. Impact of renal function on eligibility for chemotherapy and survival in patients who have undergone radical nephro-ureterectomy. BJU Int. 2013, 112, 453–461. [Google Scholar] [CrossRef]
- Lane, B.R.; Smith, A.K.; Larson, B.T.; Gong, M.C.; Campbell, S.C.; Raghavan, D.; Dreicer, R.; Hansel, D.E.; Stephenson, A.J. Chronic kidney disease after nephroureterectomy for upper tract urothelial carcinoma and implications for the administration of perioperative chemotherapy. Cancer 2010, 116, 2967–2973. [Google Scholar] [CrossRef]
- Raman, J.D.; Lin, Y.K.; Kaag, M.; Atkinson, T.; Crispen, P.; Wille, M.; Smith, N.; Hockenberry, M.; Guzzo, T.; Peyronnet, B.; et al. High rates of advanced disease, complications, and decline of renal function after radical nephroureterectomy. Urol. Oncol. 2014, 32, 47.e9–47.e14. [Google Scholar] [CrossRef]
- Gayed, B.A.; Thoreson, G.R.; Margulis, V. The role of systemic chemotherapy in management of upper tract urothelial cancer. Curr. Urol. Rep. 2013, 14, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Waldert, M.; Karakiewicz, P.I.; Raman, J.D.; Remzi, M.; Isbarn, H.; Lotan, Y.; Capitanio, U.; Bensalah, K.; Marberger, M.J.; Shariat, S.F. A delay in radical nephroureterectomy can lead to upstaging. BJU Int. 2010, 105, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Subiela, J.D.; Territo, A.; Mercadé, A.; Balañà, J.; Aumatell, J.; Calderon, J.; Gallioli, A.; González-Padilla, D.A.; Gaya, J.M.; Palou, J.; et al. Diagnostic accuracy of ureteroscopic biopsy in predicting stage and grade at final pathology in upper tract urothelial carcinoma: Systematic review and meta-analysis. Eur. J. Surg. Oncol. 2020, 46, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Leow, J.J.; Liu, Z.; Tan, T.W.; Lee, Y.M.; Yeo, E.K.; Chong, Y.L. Optimal Management of Upper Tract Urothelial Carcinoma: Current Perspectives. Onco Targets Ther. 2020, 13, 1–15. [Google Scholar] [CrossRef]
- Tabayoyong, W.; Li, R.; Gao, J.; Kamat, A. Optimal Timing of Chemotherapy and Surgery in Patients with Muscle-Invasive Bladder Cancer and Upper Urinary Tract Urothelial Carcinoma. Urol. Clin. North Am. 2018, 45, 155–167. [Google Scholar] [CrossRef]
- Igawa, M.; Urakami, S.; Shiina, H.; Kishi, H.; Himeno, Y.; Ishibe, T.; Kadena, H.; Usui, T. Neoadjuvant chemotherapy for locally advanced urothelial cancer of the upper urinary tract. Urol. Int. 1995, 55, 74–77. [Google Scholar] [CrossRef]
- Browne, B.M.; Stensland, K.D.; Moynihan, M.J.; Canes, D. An Analysis of Staging and Treatment Trends for Upper Tract Urothelial Carcinoma in the National Cancer Database. Clin. Genitourin. Cancer 2018, 16, e743–e750. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- P.R.O.S.P.E.R.O. Available online: https://www.crd.york.ac.uk/prospero/ (accessed on 10 May 2022).
- Mendeley Ltd. Mendeley. 2021. Available online: https://www.mendeley.com (accessed on 1 March 2022).
- Microsoft Corporation. Microsoft Excel. 2018. Available online: https://office.microsoft.com/excel (accessed on 1 March 2022).
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumors. UICC International Union Against Cancer, 85th ed.; Wiley-Blackwell and UICC: New York, NY, USA, 2017. [Google Scholar]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Matin, S.F.; Margulis, V.; Kamat, A.; Wood, C.G.; Grossman, H.B.; Brown, G.A.; Dinney, C.P.N.; Millikan, R.; Siefker-Radtke, A.O. Incidence of downstaging and complete remission after neoadjuvant chemotherapy for high-risk upper tract transitional cell carcinoma. Cancer 2010, 116, 3127–3134. [Google Scholar] [CrossRef] [PubMed]
- Porten, S.; Siefker-Radtke, A.O.; Xiao, L.; Margulis, V.; Kamat, A.M.; Wood, C.G.; Jonasch, E.; Dinney, C.P.N.; Matin, S.F. Neoadjuvant chemotherapy improves survival of patients with upper tract urothelial carcinoma. Cancer 2014, 120, 1794–1799. [Google Scholar] [CrossRef] [PubMed]
- Zennami, K.; Sumitomo, M.; Takahara, K.; Nukaya, T.; Takenaka, M.; Fukaya, K.; Ichino, M.; Fukami, N.; Sasaki, H.; Kusaka, M.; et al. Two cycles of neoadjuvant chemotherapy improves survival in patients with high-risk upper tract urothelial carcinoma. BJU Int. 2021, 127, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H.; Igarashi, M.; Tanaka, T.; Shindo, T.; Masumori, N.; Tamakawa, M.; Kawaai, Y.; Tsukamoto, T. A role for preoperative systemic chemotherapy in node-positive upper tract urothelial carcinoma treated with radical nephroureterectomy. Jpn. J. Clin. Oncol. 2012, 42, 1192–1196. [Google Scholar] [CrossRef]
- Kobayashi, K.; Saito, T.; Kitamura, Y.; Bilim, V.; Toba, T.; Kawasaki, T.; Hara, N.; Tanikawa, T.; Tomita, Y. Effect of preoperative chemotherapy on survival of patients with upper urinary tract urothelial carcinoma clinically involving regional lymph nodes. Int. J. Urol. 2016, 23, 153–158. [Google Scholar] [CrossRef]
- Hosogoe, S.; Hatakeyama, S.; Kusaka, A.; Hamano, I.; Iwamura, H.; Fujita, N.; Yamamoto, H.; Tobisawa, Y.; Yoneyama, T.; Yoneyama, T.; et al. Platinum-based Neoadjuvant Chemotherapy Improves Oncological Outcomes in Patients with Locally Advanced Upper Tract Urothelial Carcinoma. Eur. Urol. Focus 2018, 4, 946–953. [Google Scholar] [CrossRef]
- Chen, L.; Ou, Z.; Wang, R.; Zhang, M.; He, W.; Zhang, J.; Zu, X.; Yi, L.; Xu, R.; Jiang, S.; et al. Neoadjuvant Chemotherapy Benefits Survival in High-Grade Upper Tract Urothelial Carcinoma: A Propensity Score-Based Analysis. Ann. Surg. Oncol. 2020, 27, 1297–1303. [Google Scholar] [CrossRef]
- Hamaya, T.; Hatakeyama, S.; Tanaka, T.; Kubota, Y.; Togashi, K.; Hosogoe, S.; Fujita, N.; Kusaka, A.; Tokui, N.; Okamoto, T.; et al. Trends in the use of neoadjuvant chemotherapy and oncological outcomes for high-risk upper tract urothelial carcinoma: A multicentre retrospective study. BJU Int. 2021, 128, 468–476. [Google Scholar] [CrossRef]
- Kubota, Y.; Hatakeyama, S.; Tanaka, T.; Fujita, N.; Iwamura, H.; Mikami, J.; Yamamoto, H.; Tobisawa, Y.; Yoneyama, T.; Yoneyama, T.; et al. Oncological outcomes of neoadjuvant chemotherapy in patients with locally advanced upper tract urothelial carcinoma: A multicenter study. Oncotarget 2017, 8, 101500–101508. [Google Scholar] [CrossRef]
- Rajput, M.Z.; Kamat, A.M.; Clavell-Hernandez, J.; Siefker-Radtke, A.O.; Grossman, H.B.; Dinney, C.P.N.; Matin, S.F. Perioperative outcomes of laparoscopic radical nephroureterectomy and regional lymphadenectomy in patients with upper urinary tract urothelial carcinoma after neoadjuvant chemotherapy. Urology 2011, 78, 61–67. [Google Scholar] [CrossRef]
- Shigeta, K.; Matsumoto, K.; Ogihara, K.; Murakami, T.; Anno, T.; Umeda, K.; Izawa, M.; Baba, Y.; Sanjo, T.; Shojo, K.; et al. Does neoadjuvant chemotherapy have therapeutic benefit for node-positive upper tract urothelial carcinoma? Results of a multi-center cohort study. Urol. Oncol. 2022, 40, 105.e19–105.e26. [Google Scholar] [CrossRef] [PubMed]
- Leow, J.J.; Chong, Y.L.; Chang, S.L.; Valderrama, B.P.; Powles, T.; Bellmunt, J. Neoadjuvant and Adjuvant Chemotherapy for Upper Tract Urothelial Carcinoma: A 2020 Systematic Review and Meta-analysis, and Future Perspectives on Systemic Therapy. Eur. Urol. 2021, 79, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Shariat, S.F.; Favaretto, R.L.; Gupta, A.; Fritsche, H.M.; Matsumoto, K.; Kassouf, W.; Walton, T.J.; Tritschler, S.; Baba, S.; Matsushita, K.; et al. Gender differences in radical nephroureterectomy for upper tract urothelial carcinoma. World J. Urol. 2011, 29, 481–486. [Google Scholar] [CrossRef]
- Favaretto, R.L.; Shariat, S.F.; Chade, D.C.; Godoy, G.; Adamy, A.; Kaag, M.; Bochner, B.H.; Coleman, J.; Dalbagni, G. The effect of tumor location on prognosis in patients treated with radical nephroureterectomy at Memorial Sloan-Kettering Cancer Center. Eur. Urol. 2010, 58, 574–580. [Google Scholar] [CrossRef]
- van Dijk, N.; Gil-Jimenez, A.; Silina, K.; Hendricksen, K.; Smit, L.A.; de Feijter, J.M.; van Montfoort, M.L.; van Rooijen, C.; Peters, D.; Broeks, A.; et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: The NABUCCO trial. Nat. Med. 2020, 26, 1839–1844. [Google Scholar] [CrossRef]
- Gao, J.; Navai, N.; Alhalabi, O.; Siefker-Radtke, A.; Campbell, M.T.; Tidwell, R.S.; Guo, C.C.; Kamat, A.M.; Matin, S.F.; Araujo, J.C.; et al. Neoadjuvant PD-L1 plus CTLA-4 blockade in patients with cisplatin-ineligible operable high-risk urothelial carcinoma. Nat. Med. 2020, 26, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114. [Google Scholar] [CrossRef]
- Necchi, A.; Martini, A.; Raggi, D.; Cucchiara, V.; Colecchia, M.; Lucianò, R.; Villa, L.; Mazzone, E.; Basile, G.; Scuderi, S.; et al. A feasibility study of preoperative pembrolizumab before radical nephroureterectomy in patients with high-risk, upper tract urothelial carcinoma: PURE-02. Urol. Oncol. 2022, 40, 10.e1–10.e6. [Google Scholar] [CrossRef]
- Iacovino, M.L.; Miceli, C.C.; De Felice, M.; Barone, B.; Pompella, L.; Chiancone, F.; Di Zazzo, E.; Tirino, G.; Della Corte, C.M.; Imbimbo, C.; et al. Novel Therapeutic Opportunities in Neoadjuvant Setting in Urothelial Cancers: A New Horizon Opened by Molecular Classification and Immune Checkpoint Inhibitors. Int. J. Mol. Sci. 2022, 23, 1133. [Google Scholar] [CrossRef]
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazadeh, A.; Mellado, B.; Varlamov, S.; et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2019, 381, 338–348. [Google Scholar] [CrossRef]
- Kim, D.K.; Lee, J.Y.; Kim, J.W.; Hah, Y.S.; Cho, K.S. Effect of neoadjuvant chemotherapy on locally advanced upper tract urothelial carcinoma: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2019, 135, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Gregg, R.W.; Vera-Badillo, F.E.; Booth, C.M.; Mahmud, A.; Brundage, M.; Leveridge, M.J.; Hanna, T.P. Perioperative chemotherapy for urothelial carcinoma of the upper urinary tract: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2018, 128, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, P.; Deng, X.; Dong, H.; Cheng, Y.; Zhang, X.; Yang, C.; Tang, J.; Yuan, W.; Xu, X.; et al. Perioperative treatments for resected upper tract urothelial carcinoma: A network meta-analysis. Oncotarget 2017, 8, 3568–3580. [Google Scholar] [CrossRef] [PubMed]
- Clements, T.; Messer, J.C.; Terrell, J.D.; Herman, M.P.; Ng, C.K.; Scherr, D.S.; Scoll, B.; Boorjian, S.A.; Uzzo, R.G.; Wille, M.; et al. High-grade ureteroscopic biopsy is associated with advanced pathology of upper-tract urothelial carcinoma tumors at definitive surgical resection. J. Endourol. 2012, 26, 398–402. [Google Scholar] [CrossRef]
- Rojas, C.P.; Castle, S.M.; Llanos, C.A.; Cortes, J.A.S.; Bird, V.; Rodriguez, S.; Reis, I.M.; Zhao, W.; Gomez-Fernandez, C.; Leveillee, R.J.; et al. Low biopsy volume in ureteroscopy does not affect tumor biopsy grading in upper tract urothelial carcinoma. Urol. Oncol. 2013, 31, 1696–1700. [Google Scholar] [CrossRef]
- Brown, G.A.; Matin, S.F.; Busby, J.E.; Dinney, C.P.N.; Grossman, H.B.; Pettaway, C.A.; Munsell, M.F.; Kamat, A.M. Ability of clinical grade to predict final pathologic stage in upper urinary tract transitional cell carcinoma: Implications for therapy. Urology 2007, 70, 252–256. [Google Scholar] [CrossRef]
- Seisen, T.; Colin, P.; Rouprêt, M. Risk-adapted strategy for the kidney-sparing management of upper tract tumours. Nat. Rev. Urol. 2015, 12, 155–166. [Google Scholar] [CrossRef]
- Honda, Y.; Nakamura, Y.; Teishima, J.; Goto, K.; Higaki, T.; Narita, K.; Akagi, M.; Terada, H.; Kaichi, Y.; Fujii, S.; et al. Clinical staging of upper urinary tract urothelial carcinoma for T staging: Review and pictorial essay. Int. J. Urol. 2019, 26, 1024–1032. [Google Scholar] [CrossRef]
- Millan-Rodriguez, F.; Palou, J.; De la Torre-Holguera, P.; Vayreda-Martija, J.M.; Villavicencio-Mavrich, H.; Vicente-Rodriguez, J. Conventional CT signs in staging transitional cell tumors of the upper urinary tract. Eur. Urol. 1999, 35, 318–322. [Google Scholar] [CrossRef]
- Fritz, G.A.; Schoellnast, H.; Deutschmann, H.A.; Quehenberger, F.; Tillich, M. Multiphasic multidetector-row CT (MDCT) in detection and staging of transitional cell carcinomas of the upper urinary tract. Eur. Radiol. 2006, 16, 1244–1252. [Google Scholar] [CrossRef]
| Study | Authors | Journal | Year | Study Type | Timeline | Pair-Matching | Agent | Cycles | N | Staging Modalities | cN+ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 [55] | Matin et al. | Cancer | 2010 | retrospective single-center | 2004–2008 | no | MVAC (19), CGI (9), GTA (6), GC (5), other (4) | 2–9 (median 4) | 43 | CT (Recist Criteria) | no |
| 1993–2004 | 107 | ||||||||||
| 2 [56] | Porten et al. | Cancer | 2014 | retrospective single-center | 2004–2008 | yes | Cisplatin containing (MVAC, Gem/Cis, Cis/Gem/Ifos; 21), high dose ifosf/doxo/gem (3), kidney sparing (primarily gem/pac/doxo; 7) | 2–6 (median 4) | 31 | no | |
| 1993–2003 | 81 | ||||||||||
| 3 [57] | Zennamni et al. | BJU Int. | 2021 | retrospective single-center | 2005–2019 | yes | Gem/Cis, MVAC | 2 | 117 | CT | no |
| 67 | |||||||||||
| 4 [58] | Kitamura et al. | Jpn J Clin Oncol | 2012 | retrospective single-center | 1995–2010 | no | MVAC (14), Gem/Cis (1) | 2–3 (median 2) | 15 | CT (Recist Criteria) | yes (only) |
| 14 | |||||||||||
| 5 [59] | Kobayashi et al. | Int J Urol | 2016 | retrospective single-center | 1991–2013 | no | 1991–1995 MEP (3), 1996–2009 MVAC (9), 2010- Gem/Cis (14) | 2–4 (median 3) | 24 | CT (Recist Criteria) | yes (only) |
| 31 | |||||||||||
| 6 [60] | Hosogoe et al. | Eur Urol Focus | 2018 | retrospective single-center | 1995–2016 | yes | Gem/Cis (16), Gem/Carbo (35) | 2–4 q3w | 51 | CT (Recist Criteria) | yes |
| 51 | |||||||||||
| 7 [61] | Chen et al. | Ann Surg Oncol | 2020 | retrospective multi-center | 2012–2015 | yes | Gem/Cis | 2–4 q3w | 37 | CT (Recist Criteria) | no |
| 37 | |||||||||||
| 8 [62] | Hamaya et al. | BJU Int | 2021 | retrospective multi-center | 2000–2020 | no | Gem/Cis, Gem/Carbo, MVAC, docetaxel-based regimen | 2–4 | 144 | CT (Recist Criteria) | yes |
| 145 | |||||||||||
| 9 [63] | Kubota et al. | Onco-target | 2017 | retrospective multi-center | 1995–2017 | no | Gem/Cis (21), Gem/Carbo (76), MVAC (4) | 2–4 q3w | 101 | CT (Recist Criteria) | yes |
| 133 | |||||||||||
| 10 [64] | Rajput et al. | Urology | 2011 | retrospective single-center | 2003–2010 | no | variable (MVAC, CGI, MVAC + Bevacizumab, GTA, IAG, CG, GT) | 1–7 (median 4) | 26 | CT or MRI | no |
| 56 | |||||||||||
| 11 [65] | Shigeta et al. | Urol Oncol | 2021 | retrospective single-center | 1990–2016 | no | GC (25), MVAC (11) | 36 | CT (Recist Criteria) or MRI | yes (only) | |
| 53 |
| Study | Control | ||
|---|---|---|---|
| cT3/T4 | ntotal | 229 | 246 |
| naverage | 38 | 41 | |
| # of studies | 6 | 6 | |
| %weighted | 84.90% | 87.22% | |
| cT2 | ntotal | 74 | 57 |
| naverage | 12 | 10 | |
| # of studies | 6 | 6 | |
| %weighted | 40.84% | 37.59% | |
| c T1 | ntotal | 40 | 27 |
| naverage | 10 | 7 | |
| # of studies | 4 | 4 | |
| %weighted | 31.13% | 32.92% | |
| cN+ | ntotal | 132 | 134 |
| naverage | 22 | 22 | |
| # of studies | 6 | 6 | |
| %weighted | 65.65% | 76.12% | |
| surgery: open | ntotal | 188 | 336 |
| naverage | 38 | 67 | |
| # of studies | 5 | 5 | |
| %weighted | 76.76% | 89.96% | |
| surgery: laparoscopic/robotic | ntotal | 67 | 39 |
| naverage | 13 | 8 | |
| # of studies | 5 | 5 | |
| %weighted | 34.79% | 13.49% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oswald, D.; Pallauf, M.; Deininger, S.; Törzsök, P.; Sieberer, M.; Eiben, C. Neoadjuvant Chemotherapy before Nephroureterectomy in High-Risk Upper Tract Urothelial Cancer: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 4841. https://doi.org/10.3390/cancers14194841
Oswald D, Pallauf M, Deininger S, Törzsök P, Sieberer M, Eiben C. Neoadjuvant Chemotherapy before Nephroureterectomy in High-Risk Upper Tract Urothelial Cancer: A Systematic Review and Meta-Analysis. Cancers. 2022; 14(19):4841. https://doi.org/10.3390/cancers14194841
Chicago/Turabian StyleOswald, David, Maximilian Pallauf, Susanne Deininger, Peter Törzsök, Manuela Sieberer, and Christian Eiben. 2022. "Neoadjuvant Chemotherapy before Nephroureterectomy in High-Risk Upper Tract Urothelial Cancer: A Systematic Review and Meta-Analysis" Cancers 14, no. 19: 4841. https://doi.org/10.3390/cancers14194841
APA StyleOswald, D., Pallauf, M., Deininger, S., Törzsök, P., Sieberer, M., & Eiben, C. (2022). Neoadjuvant Chemotherapy before Nephroureterectomy in High-Risk Upper Tract Urothelial Cancer: A Systematic Review and Meta-Analysis. Cancers, 14(19), 4841. https://doi.org/10.3390/cancers14194841





