Bacteriocins as Potential Therapeutic Approaches in the Treatment of Various Cancers: A Review of In Vitro Studies
Abstract
Simple Summary
Abstract
1. Introduction
2. Cancer
2.1. Diagnosis of Cancers
2.2. Treatment Options for Cancer
2.2.1. Surgical Treatment
2.2.2. Chemotherapy
2.2.3. Radiotherapy
3. Potential Treatment of Cancer
4. Bacteriocin
4.1. Classes of Bacteriocins
4.1.1. Class I: Ribosomally-Produced and Posttranslationally-Modified Peptides (RiPPs)
4.1.2. Class II: Thermostable Unmodified Bacteriocins
4.1.3. Class III: Thermolabile Unmodified Bacteriocins
4.2. Bacteriocins for Anticancer Treatment
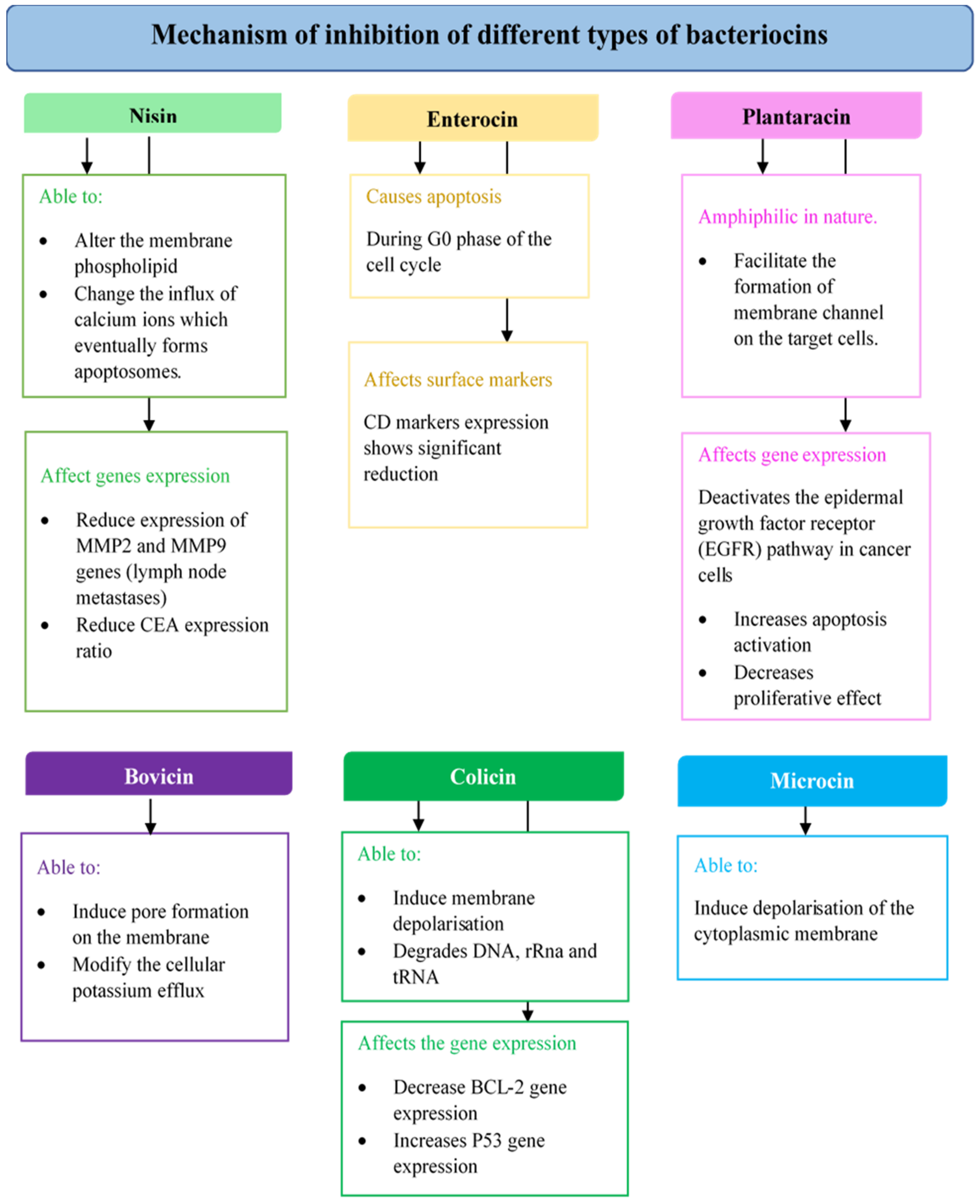
4.2.1. Nisin
4.2.2. Enterocin
4.2.3. Plantaricin
4.2.4. Pediocin
4.2.5. Bovicin
4.2.6. Colicins
4.2.7. Microcins
4.3. Bacteriocins In Vivo Study
5. Complications Regarding Bacteriocins in Medical Applications
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Norouzi, Z.; Salimi, A.; Halabian, R.; Fahimi, H. Nisin, a potent bacteriocin and anti-bacterial peptide, attenuates expression of metastatic genes in colorectal cancer cell lines. Microb. Pathog. 2018, 123, 183–189. [Google Scholar] [CrossRef]
- Fathizadeh, H.; Saffari, M.; Esmaeili, D.; Moniri, R.; Mahabadi, J.A. Anticancer effect of Enterocin A-Colicin E1 fusion peptide on gastric cancer cell. Probiotics Antimicrob. Proteins 2021, 13, 1443–1451. [Google Scholar] [CrossRef]
- Taherikalani, M.; Ghafourian, S. Anticancer properties of colicin E7 against colon cancer. Gastroenterol. Rev. 2021, 16, 364–368. [Google Scholar] [CrossRef]
- Ahmed, N.; Dawson, M.; Smith, C.; Wood, E. Biology of Disease; Taylor & Francis Group: New York, NY, USA, 2017. [Google Scholar]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef]
- Rodrigues, G.; Silva, G.G.; Buccini, D.F.; Duque, H.M.; Dias, S.C.; Franco, O.L. Bacterial proteinaceous compounds with multiple activity toward cancers and microbial infection. Front. Microbiol. 2019, 10, 1690. [Google Scholar] [CrossRef]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transductiuon Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef]
- Kohoutova, D.; Forstlova, M.; Moravkova, P.; Cyrany, J.; Bosak, J.; Smajs, D.; Rejchrt, S.; Bures, J. Bacteriocin production by mucosal bacteria in current and previous colorectal neoplasia. BMC Cancer 2020, 20, 39. [Google Scholar] [CrossRef]
- Al-Madboly, L.A.; El-Deeb, N.M.; Kabbash, A.; Nael, M.A.; Kenawy, A.M.; Ragab, A.E. Purification, characterization, identification, and anticancer activity of a circular bacteriocin from Enterococcus thailandicus. Front. Bioeng. Biotechnol. 2020, 5, 22. [Google Scholar] [CrossRef]
- Baindara, P.; Korpole, S.; Grover, V. Bacteriocins: Perspective for the development of novel anticancer drugs. Appl. Microbiol. Biotechnol. 2018, 102, 10393–10408. [Google Scholar] [CrossRef]
- Chauhan, S.; Dhawan, D.K.; Saini, A.; Simran, P. Antimicrobial peptides against colorectal cancer—A focused review. Pharmacol. Res. 2021, 167, 105529. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M.; Hausman, R.E. The Cell, A Molecular Approach, 4th ed.; Sinauer Associates: Sunderland, MA, USA, 2007. [Google Scholar]
- Rossi, F.; Noren, H.; Jove, R.; Beljanski, V.; Grinnemo, K.-H. Differences and similarities between cancer and somatic stem cells: Therapeutic implications’. Cell Res. Ther. 2020, 11, 489. [Google Scholar] [CrossRef] [PubMed]
- Nassar, D.; Blanpain, C. Cancer stem cells: Basic concepts and therapeutic implications. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 47–76. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Frangioni, J.V. New technologies for human cancer imaging. J. Clin. Oncol. 2008, 26, 4012–4021. [Google Scholar] [CrossRef]
- Świderska, M.; Choromańska, B.; Dąbrowska, E.; Konarzewska-Duchnowska, E.; Choromańska, K.; Szczurko, G.; Myśliwiec, P.; Dadan, J.; Ładny, J.R.; Zwierz, K. The diagnostics of colorectal cancer. Contemp. Oncol. 2014, 18, 1–6. [Google Scholar]
- He, L.; Long, L.R.; Antani, S.; Thoma, G.R. Histology image analysis for carcinoma detection and grading. Comput. Methods Programs Biomed. 2012, 107, 538–556. [Google Scholar] [CrossRef]
- Montagne, F.; Guisier, F.; Venissac, N.; Baste, J.-M. The role of surgery in lung cancer treatment: Present indications and future perspectives—State of the art. Cancers 2021, 13, 3711. [Google Scholar] [CrossRef]
- Riis, M. Modern surgical treatment of breast cancer. Ann. Med. Surg. 2020, 56, 95–107. [Google Scholar] [CrossRef]
- Koehler, L.A.; Haddad, T.C.; Hunter, D.W.; Tuttle, T.M. Axillary web syndrome following breast cancer surgery: Symptoms, complications, and management strategies. Breast Cancer Targets Ther. 2018, 11, 13–19. [Google Scholar] [CrossRef]
- Delibegovic, S. Introduction to Total Mesorectal Excision. Med. Arch. 2017, 71, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Campos, S.F.; Alvarez, J.A.; Orozco, C.F.; Camacho, F.J.; Lopez, F.J.; Ley, V.B.; Ojeda, A.G. Dehiscence of anastomosis. What to do and gastrointestinal what not to do. Cir. Gen. 2019, 41, 243–255. [Google Scholar]
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for cancer: A trigger for metastases. Cancer Res. 2017, 77, 1548–1552. [Google Scholar] [CrossRef]
- Acharya, R.S.; Sundareshan, M.K. Development of optimal drug administration strategies for cancer-chemotherapy in the framework of systems theory. Int. J. Bio-Med. Comput. 1983, 19, 71–76. [Google Scholar]
- Amjad, M.T.; Chidharla, A.; Kasi, A. Cancer Chemotherapy; National Center for Biotechnology Information: Bethesda, MD, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK564367/ (accessed on 3 March 2022).
- Rebucci, M.; Michiels, C. Molecular aspects of cancer cell resistance to chemotherapy. Biochem. Pharmacol. 2013, 85, 1219–1226. [Google Scholar] [CrossRef]
- Stewart, J. Zepzelca FDA Approval History. Available online: https://www.drugs.com/history/zepzelca.html (accessed on 27 January 2021).
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef]
- Mohan, G.; Hamma, A.T.; Devi, S.K.; Narayanasamy, A.; Vellingiri, B. Recent advances in radiotherapy and its associated side effects in cancer—A review. J. Basic Appl. Zool. 2019, 80, 14. [Google Scholar] [CrossRef]
- Hatcher, O.; Kumar, A.A. Chemotherapy and radiotherapy for colorectal cancers. Surgery 2014, 32, 179–184. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Ahmadi, S.; Ghollasi, M.; Hoseini, H.M. The apoptotic impact of nisin as a potent bacteriocin on the colon cancer cells. Microb. Pathog. 2017, 111, 193–197. [Google Scholar] [CrossRef]
- Drago, L. Probiotics and colon cancer. Microorganisms 2019, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Burgos, M.J.; Pulido, R.P.; Aguayo, M.D.C.L.; Galvez, A.; Lucas, R. The cyclic antibacterial peptide Enterocin AS-48: Isolation, mode of action, and possible food applications. Int. J. Mol. Sci. 2014, 15, 22706–22727. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Sieiro, P.; Montalbán-López, M.; Mu, D.; Kuipers, O.P. Bacteriocins of lactic acid bacteria: Extending the family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951. [Google Scholar] [CrossRef] [PubMed]
- Joo, N.E.; Ritchie, K.; Kamarajan, P.; Miao, D.; Kapila, Y.L. Nisin, an apoptogenic bacteriocin and food preservative, attenuates HNSCC tumorigenesis via CHAC1. Cancer Med. 2012, 1, 295–305. [Google Scholar] [CrossRef]
- Perez, R.H.; Zendo, T.; Sonomoto, K. Novel bacteriocins from lactic acid bacteria (LAB): Various structures and applications. Microb. Cell Factories 2014, 13, S3. [Google Scholar] [CrossRef]
- Jaye, K.; Li, C.G.; Chang, D.; Bhuyan, D.J. The role of key gut microbial metabolites in the development and treatment of cancer. Gut Microbes 2022, 14, 2038865. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Genet. 2005, 3, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Heilbronner, S.; Krismer, B.; Brötz-Oesterhelt, H.; Peschel, A. The microbiome- shaping roles of bacteriocins. Nat. Rev. Microbiol. 2021, 19, 726–739. [Google Scholar]
- Qiao, Y.; Qiu, Z.; Tian, F.; Yu, L.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Effect of bacteriocin-producing Pediococcus acidilactici strains on the immune system and intestinal flora of normal mice. Food Sci. Hum. Wellness 2021, 11, 238–246. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Adamczak, A. Anticancer activity of bacterial proteins and peptides. Pharmaceutics 2018, 10, 54. [Google Scholar] [CrossRef]
- Kaur, S.; Kaur, S. Bacteriocins as potential anticancer agents. Front. Pharmacol. 2015, 6, 272. [Google Scholar] [CrossRef]
- Meade, E.; Slattery, M.A.; Garvey, M. Bacteriocins, potent antimicrobial peptides and the fight against multi drug resistant species: Resistance is futile? Antibiotics 2020, 9, 32. [Google Scholar] [CrossRef]
- Paiva, A.D.; de Oliviera, M.D.; de Paula, S.O.; Baracat-Pereira, M.C.; Breukink, E.; Mantovani, H.C. Toxicity of bovicin HC5 against mammalian cell lines and the role of cholesterol in bacteriocin activity. Microbiology 2012, 158, 2851–2858. [Google Scholar] [CrossRef]
- De Giani, A.; Bovio, F.; Forcella, M.; Fusi, P.; Sello, G.; Di Gennaro, P. Identification of bacteriocin-like compound from Lactobacillus plantarum with antimicrobial activity and effects on normal and cancerogenic human intestinal cells. AMB Express 2019, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Villarante, K.I.; Elegado, F.B.; Iwatani, S.; Zendo, T.; Sonomoto, K.; Guzman, E. Purification, characterization and in vitro cytotoxicity of the bacteriocin from Pediococcus acidilactici K2a2-3 against human colon adenocarcinoma (HT29) and human cervical carcinoma (HeLa) cells). World J. Microbiol. Biotechnol. 2011, 27, 975–980. [Google Scholar] [CrossRef]
- Varas, M.A.; Munoz-Montecinos, C.; Kallens, V.; Simon, V.; Allende, M.L.; Marcoleta, A.E.; Lagos, R. Exploiting zebrafish xenografts for testing the in vivo antitumorigenic activity of microcin E492 against human colorectal cancer cells. Front. Microbiol. 2020, 11, 405. [Google Scholar] [CrossRef] [PubMed]
- Dodamani, S.; Kaliwal, B.B.; Tendulkar, S.; Hattiholi, A. Evaluation of antimicrobial and anticancer activity of Lactococcus garvieae derived bacteriocin. Arch. Cancer Res. 2021, 9, 1–6. [Google Scholar]
- Saidumohamed, B.E.; Johny, T.K.; Raveendran, A.T.; Sheela, U.B.; Sreeraganathan, M.; Sasidharan, R.S.; Bhat, S.G. 3D structure elucidation and appraisal of mode of action of a bacteriocin BaCf3 with anticancer potential produced by marine Bacillus amyloliquefaciens BTSS3. Re GEN Open 2022, 2, 45–56. [Google Scholar] [CrossRef]
- Rayapu, V.; Revathi, K.; Arunagirinathan, A.; Pragash, S.D.; Mohiddin, K. Isolation, characterization of Lactobacillus delbrueckii derived bacteriocin and assessment of its bioactive potential. Ann. Rom. Soc. Cell Biol. 2021, 25, 7520–7530. [Google Scholar]
- Himeno, K.; Rosengren, K.J.; Inoue, T.; Perez, R.H.; Colgrave, M.L.; Lee, H.S.; Chan, L.Y.; Henriques, S.T.; Fujita, K.; Ishibashi, N.; et al. Identification, characterization, and three-dimensional structure of the novel circular bacteriocin, enterocin NKR-5-3B, from Enterococcus Faecium. Biochemistry 2015, 54, 4863–4876. [Google Scholar] [CrossRef]
- Balgir, P.P.; Bhatia, P.; Kaur, B. Sequence analysis and homology based modeling to assess structure-function relationship of Pediocin Cp2 of Pediococcus acidilactici MTCC 5101. Indian J. Biotechnol. 2010, 9, 431–434. [Google Scholar]
- Kumar, B.; Balgir, P.P.; Kaur, B.; Mittu, B.; Chauhan, A. In vitro cytotoxicity of native and rec-pediocin CP2 against cancer cell lines: A comparative study. Pharm. Anal. Acta 2012, 3, 8. [Google Scholar] [CrossRef]
- Hols, P.; Ledesma-Garcia, L.; Gabant, P.; Mignolet, J. Mobilization of microbiota commensals and their bacteriocins for therapeutics. Trends Microbiol. 2019, 27, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.; Dreyer, L.; Smith, C.; Staden, A.D. A review: The fate of bacteriocins in the human gastro-intestinal tract: Do they cross the gut-blood barrier? Front. Microbiol. 2018, 9, 2297. [Google Scholar] [CrossRef] [PubMed]
| Bacteriocin | Origin Bacteria | Type of Cancer | Type of Cell Line | Effect | Type of Assay | References |
|---|---|---|---|---|---|---|
| Nisin | Lactococcus lactis | Head and neck cancer | HNSCC cells | Reduced tumour volume in mice model by about 50% using dosage of 200 mg/kg | Measurement of tumour volume using mice tumour model | [38] |
| Colorectal cancer | LS180, SW780, HT29 and Caco-2 colorectal cancer cells | Reduced cell proliferation of LS180 (IC50 = 80–400 IU/mL), SW48, HT29 and Caco-2 (IC50 = 350–800 IU/mL) | MTT assay and trypan blue exclusion assay | [2] | ||
| Breast cancer and liver cancer | MCF-7 human breast adenocarcinoma, HepG2 carcinoma cells | Inhibited cell proliferation of MCF-7 cell (IC50 = 105.46 μM) HepG2 cell (IC50 = 112.25 μM) | MTT assay and cell morphology analysis using an inverted optical microscope | [47] | ||
| Enterocin | Enterococcus sp. | Liver cancer | HepG2 carcinoma cell | Inhibited cell proliferation of HepG2 cell (IC50 = 15.643 μM) | Neutral red assay | [10] |
| Plantaricin | Lactobacillus plantarum | Colorectal cancer | E705 colon cancer cells | Inhibitory effect of cell proliferation of nearly 30% at 10 ng/mL | MTT assay | [48] |
| Pediocin | Pediococcus acidilactici | Colorectal cancer and cervical cancer | HT29 colon adenocarcinoma, HeLa cervical adenocarcinoma cells | Inhibited the growth of HT29 cell (Undialysed: 55.0 ± 4.8%, Dialysed: 53.7 ± 7.0%) HeLa cell (Undialysed: 52.3 ± 6.0%, Dialysed: 15.6 ± 4.0%) | MTT assay | [49] |
| Bovicin | Streptococcus bovis | Breast cancer and liver cancer | MCF-7 human breast adenocarcinoma, HepG2 carcinoma cells | Inhibited cell proliferation of MCF-7 cell (IC50 = 279.39 μM) HepG2 cell (IC50 = 289.30 μM) | MTT assay and cell morphology analysis using an inverted optical microscope | [48] |
| Microcins | Klebsiella pneumoniae | Colorectal cancer | HT29 and SW620 colorectal adenocarcinoma cell lines | Decreased in cancer cell viability HT29 cell (treatment with 60 μg/mL reduces growth up to 50%) SW620 cell (treatment with 60 μg/mL reduces growth up to 69%) Significant reduction of SW620 tumour size | Flow cytometry and measurement of tumour size | [50] |
| Others | Lactococcus garvieae | Colorectal cancer | HT29 colon adenocarcinoma cells | Induced cell death at a low dosage of 2 μg/mL. Apoptosis of cancer cells observed through DAPI staining | MTT assay and DAPI staining | [51] |
| Bacillus amyloliquefaciens | Lung cancer | A549 human alveolar epithelial cell line | The proliferation rate of less than 50% from 40 μg/mL to 200 μg/mL after 72 h of incubation | MTT assay, morphology analysis using a fluorescent microscope | [52] | |
| Lactobacillus delbrueckii | Cervical cancer, breast cancer, fibrosarcoma, lung cancer | HeLa cervical adenocarcinoma cells, MCF-7 human breast adenocarcinoma, HT1080 human fibrosarcoma cell line, H1299 non-small lung carcinoma | Cytotoxicity at 10 μM, MCF-7 = 60% cytotoxicity HT1080 and H1299 = 40% cytotoxicity HeLa = no significant Cytotoxicity at 10 μM, All cell line = 50% cytotoxicity | Trypan blue exclusion assay | [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molujin, A.M.; Abbasiliasi, S.; Nurdin, A.; Lee, P.-C.; Gansau, J.A.; Jawan, R. Bacteriocins as Potential Therapeutic Approaches in the Treatment of Various Cancers: A Review of In Vitro Studies. Cancers 2022, 14, 4758. https://doi.org/10.3390/cancers14194758
Molujin AM, Abbasiliasi S, Nurdin A, Lee P-C, Gansau JA, Jawan R. Bacteriocins as Potential Therapeutic Approaches in the Treatment of Various Cancers: A Review of In Vitro Studies. Cancers. 2022; 14(19):4758. https://doi.org/10.3390/cancers14194758
Chicago/Turabian StyleMolujin, Arnold Marshall, Sahar Abbasiliasi, Armania Nurdin, Ping-Chin Lee, Jualang Azlan Gansau, and Roslina Jawan. 2022. "Bacteriocins as Potential Therapeutic Approaches in the Treatment of Various Cancers: A Review of In Vitro Studies" Cancers 14, no. 19: 4758. https://doi.org/10.3390/cancers14194758
APA StyleMolujin, A. M., Abbasiliasi, S., Nurdin, A., Lee, P.-C., Gansau, J. A., & Jawan, R. (2022). Bacteriocins as Potential Therapeutic Approaches in the Treatment of Various Cancers: A Review of In Vitro Studies. Cancers, 14(19), 4758. https://doi.org/10.3390/cancers14194758







