Episodic Memory and Recollection Network Disruptions Following Chemotherapy Treatment in Breast Cancer Survivors: A Review of Neuroimaging Findings
Abstract
Simple Summary
Abstract
1. Introduction
2. Physiological Mechanisms Contributing to Chemotherapy-Related Memory Disruption
3. Current Methods for Assessing Chemotherapy-Related Memory Disruption
3.1. Complex Declarative Memory Processing and CRCI
| References | Sample | Age | Tumor Stage | Menopausal Status | Treatment | Timepoints | Neuropsychological Tests | NPT Results | Imaging Results |
|---|---|---|---|---|---|---|---|---|---|
| Inagaki et al. [103] | Ch + MDE (n = 17), Ch+ (n = 51) | 18–55 | 0–III | Post.M. (n = 10 Ch + MDE, n = 27 Ch+) | Chemo, ET, surgery | 6 mo postsurgery (t1) | WMS-R: immediate and delayed verbal and visual memory tasks | =verbal and visual memory for both groups | =left and right HPC volume for Ch + MDE and Ch+ |
| Yoshikawa et al. [104] | Ch+ (n = 44), Ch− (n = 31) | ~48 | 0–I | Post.M. (n = 27 Ch+, n = 8 Ch−) | Chemo (CMF, AC, CAF, CPP, MF, 5FU, HCFU, or doifluridine), ET, RT, surgery | ~3.5 yr postchemo (t1) | WMS-R: immediate and delayed verbal and visual memory tasks | =verbal and visual memory for both groups | =HPC volume between Ch+ and Ch− and between different chemotherapy regimens |
| Ferguson et al. [105] | Ch+ (n = 1), HC (n = 1) mono zygotic twins | 60 | II | - | Chemo (TAC), ET | 22 mo postchemo (t1) | verbal memory: CVLT, Craft stories | =verbal memory for both twins | ↑ WM lesion volumes and hyperintensities for Ch+ than HC |
| Inagaki et al. [106] | Ch+, Ch−, HC (n = 51–55/ group) at t1 | 18–55 | 0–I | Post.M. (n = 40 Ch+, n = 20 Ch−, n = 16 HC) | Chemo (AC, CMF, EC, PTX, 5FU, 5′-DFUR, HCFU, or UFT), ET, RT | 1 yr postsurgery (t1) and 2 yr after t1 (t2) | WMS-R: immediate and delayed verbal and visual memory | - | ↓ GM and ↓ WM in paraHPC, prefrontal, precuneus at t1 for Ch+ than Ch−; = GM and WM at t2 |
| McDonald et al. [107] | Ch+ (n = 17), Ch− (n = 12), HC (n = 18) | ~50 | 0–III | - | Chemo (CPP + DOX, ACT, or AC), ET, surgery | Baseline (t1), 1 mo (t2) and 1 yr postchemo (t3) | - | - | = GM at t1; ↓ GM bilateral paraHPC, STG at t2 than t1 and MTL at t3 than t1 for Ch+ than HC |
| Koppelmans et al. [13] | Ch+ (n = 177), HC (n = 368) | 50–80 | - | - | Chemo (CMF) | ~21 yr postchemo (t1) | - | - | ↓ GM, ↓ TBV, =WM, =left HPC volume for Ch+ and HC |
| Conroy et al. [108] | Ch+ (n = 24), HC (n = 34) | 49–71 | I–III | - | Chemo (AC, ACT, CAF, AT, CMF, CMF + CAF, taxane, ACT+ CAPE, or taxane + CAPE) | ~6.4 yr postchemo (t1) | verbal memory: RAVLT, story recall, BLT | ↓verbal memory for Ch+ than HC | ↓ GMD in left temporal lobe for Ch+ than HC. |
| Kesler et al. [109] | Ch+ (n = 42), HC (n = 35) | ~55 | I–III | Post.M. (n = 33 Ch+, n = 18 HC) | Chemo (DOX + CPP, DOX + PTX, CPP + 5FU + PTX, or CPP + 5FU+ MTX), ET, RT | ~5 yr postchemo | verbal memory: HVLT | ↓ HVLT delayed recall for Ch+ than HC | ↓ bilateral HPC volume for Ch+ than HC |
| Lepage et al. [110] | Ch+ (n = 19), HC (n = 19) | ~50 | I–III | Menstruating, peri.m, post.m (n = 2–9/ group) | Chemo (FECD, CD, or CPP + DOX), surgery | baseline (t1), 20 days (t2) and 1.5 yr postchemo (t3) | verbal memory: HVLT, CNS-VS-Verbal Memory Index; visual memory: BVMT-R, CNS-VS-Visual Memory Index | ↓ NPT scores over time (non- significant) for both groups | ↓ GM volume in temporal regions from t1 to t2 for Ch+ compared to HC, = GM at t3 between both groups |
| Apple et al. [73] | Ch+ (n = 16), HC (n = 18) | 18–45 | I-IV | Pre.M. | Chemo (ACT), ET | 6–18 mo postchemo (t1) | episodic memory: Picture Sequence Memory Test | ↓episodic memory for Ch+ than HC | ↑inward deformation in bilateral HPC, ↓HPC volume for Ch+ than HC |
| References | Sample | Age | Tumor Stage | Menopausal Status | Treatment | Timepoints | Neuropsychological Tests | NPT Results | Imaging Results |
|---|---|---|---|---|---|---|---|---|---|
| Kesler et al. [111] | Ch+ (n = 14), HC (n = 14) | 40–65 | metastatic (n = 8), locally advanced (n = 6) | - | Chemo (CMF, or ACT), ET, RT | >6 mo postchemo (t1) | Verbal declarative memory encoding and recall task in fMRI | =verbal declarative memory for both groups | ↑ right STG activation extending into paraHPC and left HPC during the verbal declarative encoding and recall task for Ch+ than HC |
| de Ruiter et al. [17] | Ch+ (n = 19), Ch− (n = 15) | ~57 | I-III | - | Chemo (FEC, or CTC), ET, RT, and surgery | ~2 (t1) and 9 yr postchemo (t2) | verbal memory: CVLT; visual memory: WMS-R visual reproduction test; episodic memory: PA in fMRI | t1 to t2: ↓ PA for Ch+ than Ch−; ↓ visual and verbal memory for Ch+ than HC | ↓ right PHG and MTG activation during PA task for Ch+ than Ch− |
| Lopez-Zuinini et al. [112] | Ch+ (n = 21), HC (n = 21) | 31–64 | I-III | Peri.M. (n = 2–4/group), Post.M. (n = 9–10/group) | Chemo (CPP + DOX, CPP + DTX, 5FU + CPP+ DTX + hepirubicin+ epirubicin or 5FU, TEC), surgery | baseline (t1), and 1 mo postchemo (t2) | verbal memory: verbal word list learning in fMRI | =verbal word learning for both groups | ↓ activation in right STG, bilateral insula, and left inferior orbitofrontal gyrus during the verbal list learning task for Ch+ than HC |
| Apple et al. [113] | Ch+ (n = 16), HC (n = 18) | 18–45 | - | Pre.M. | Chemo, ET | ~18 mo postchemo (t1) | episodic memory: Picture Sequence Memory Test and RWCR in fMRI | ↓ episodic memory for Ch+ BCP than HC | ↑ HPC FC in the left cuneus, lingual, precuneus, and right middle frontal gyrus during RWCR for Ch+ than HC |
| References | Sample | Age | Tumor Stage | Menopausal Status | Treatment | Timepoints | NPT Tests | NPT Results | Imaging Results |
|---|---|---|---|---|---|---|---|---|---|
| Bruno et al. [114] | Ch+ (n = 34), HC (n = 27) | 40–74 | I-IV | Post and Pre M. | Chemo (ADM + CPP + PTX, CPP + MTX + 5FU and ADM + CPP or, CPP + MTX + 5FU), ET, RT | ~5 yr post-treatment (t1) | verbal memory: HVLT | =HVLT immediate, ↓HVLT delayed for Ch+ than HC | ↓ global and regional network measures in bilateral STG for Ch+ than HC; ↑network hubs in bilateral STG and left HPC for HC than Ch+ |
| Tao et al. [115] | Ch+ (n = 33), HC (n = 31) | 26–52 | I-III | - | Chemo (DOX, CPP, PTX), surgery | - | - | - | ↓ FC in the DMN for Ch+ compared to HC |
| Cheng et al. [116] | Ch+ (n = 34), HC (n = 31) | ~50 | - | - | Chemo (DOX, 5FU, CPP, or PTX) | - | prospective memory: EBPM, TBPM | ↓ EBPM, TBPM for Ch+ than HC; =scores between HC and Ch− | ↑ FC between HPC seed and bilateral vmPFC, dlPFC, inferior and superior parietal lobules, pCC, and precuneus for Ch+ than HC |
| Chen et al. [117] | Ch+ (n = 16), HC (n = 14) | >60 | I-III | - | Chemo (TC or other), surgery | baseline (t1), 1 mo postchemo (t2) | episodic memory: Picture Sequence Memory Test | =NPT scores for Ch+ and HC across t1 and t2 | ↑ ALFF from t1 to t2 in a single cluster including bilateral subcallosal gyri and right anterior cingulate gyrus for Ch+ compared to HC; =rs-fMRI from t1 to t2 for Ch+ and HC |
| Feng et al. [118] | Ch+ (n = 29), HC (n = 25) | 30–50 | I-III | Pre.M.(n = 17–20/group), menopausal (n = 8–9/group) | Chemo (ACT, TEC), surgery | baseline (t1), 1 week postchemo (t2) | verbal memory: AVLT | ↓ AVLT from t1 to t2 for Ch+ than HC | ↑ FC between left anterior HPC and left MTG and STG, and between the right posterior HPC and left STG for Ch+ compared to HC |
| Feng et al. [119] | Ch+ (n = 7), HC (n = 19) | 35–55 | I-III | Pre.M. (n = 11/group), menopausal (n = 6–8/group) | Chemo (ACT, TEC), ET | baseline (t1), 1 week (t2) and 6 mo postchemo (t3) | verbal memory: WDT | ↓ WDT from t1 to t3 for Ch+ than HC | ↓ FC in ADMN, PDMN, LFPN, RFPN, SRN, CN from t1 to t3 for Ch+ than HC |
3.2. Autobiographical Memory and CRCI
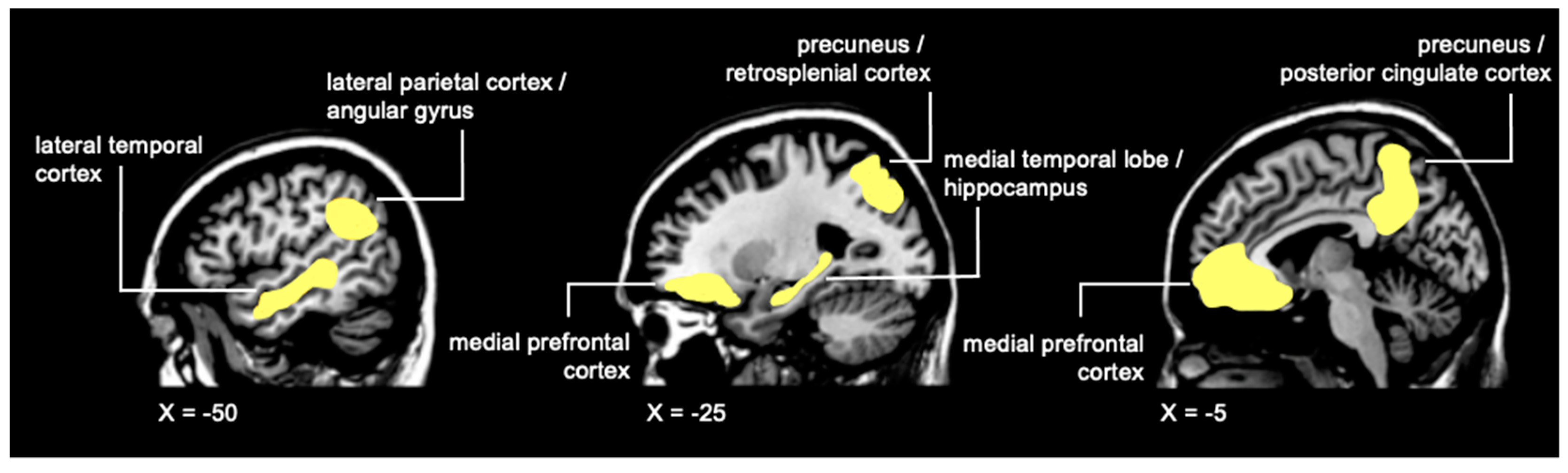
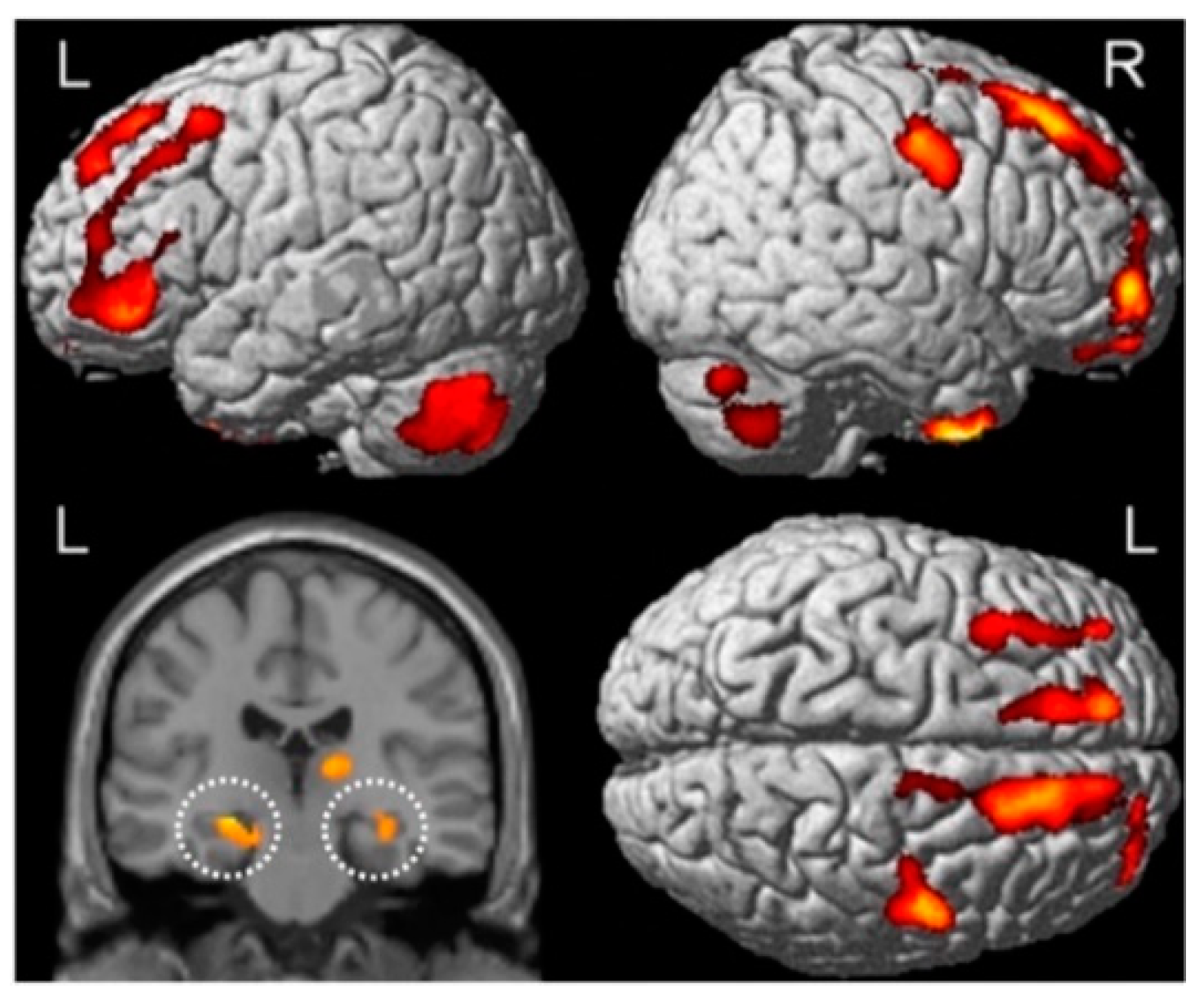
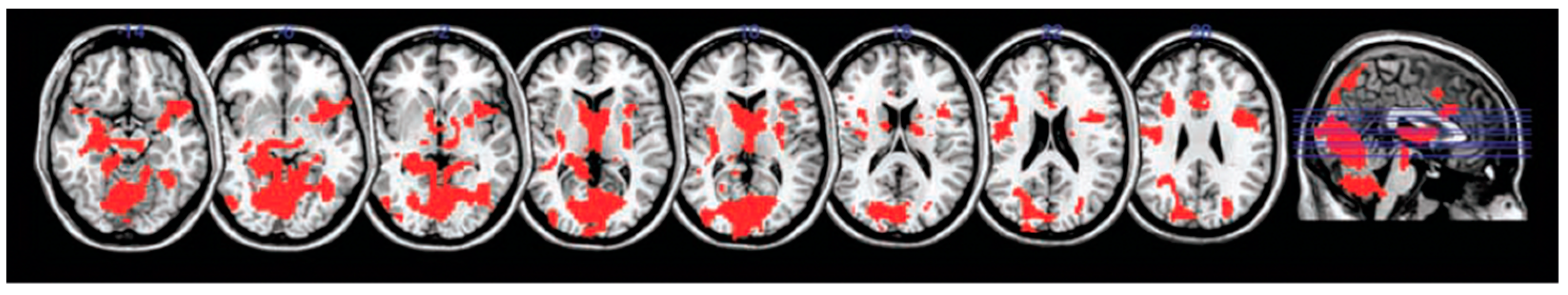
4. Neuroimaging Assessments
4.1. Chemotherapy-Induced Structural Changes to Hippocampus and the Temporal Lobes
4.2. Functional Specialization along the Hippocampal Long-Axis and Implications for Memory Performance following Chemotherapy
4.3. Chemotherapy-Induced Functional Disruptions in the Temporal Lobes and Broader Recollection Network
4.4. Recollection Network and Default Mode Network (DMN) Irregularities: Implications for Chemotherapy-Related Memory Impairments and Deficits in Episodic Future Thinking
5. Other Contributing Psychosocial Factors Affecting Memory Performance
6. Recommendations and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CA1 | cornu Ammonis 1 |
| CA3 | cornu Ammonis 3 |
| Ch− | breast cancer patients that didn’t receive chemotherapy |
| Ch+ | breast cancer patients that were treated with chemotherapy |
| CRCI | chemotherapy-related cognitive impairment |
| dlPFC | dorsolateral prefrontal cortex |
| DMN | default mode network |
| fMRI | functional magnetic resonance imaging |
| IL-1β | interleukin-1β |
| IL-6 | Interleukin 6 |
| mPFC | medial prefrontal cortex |
| MRI | magnetic resonance imaging |
| MTL | medial temporal lobe |
| MVPA | multi-voxel pattern analyses |
| pCC | posterior cingulate cortex |
| TNF-α | Tumor necrosis factor α |
| VBM | voxel-based morphometry |
| vmPFC | ventromedial prefrontal cortex |
Glossary
References
- Wefel, J.S.; Kesler, S.R.; Noll, K.R.; Schagen, S.B. Clinical Characteristics, Pathophysiology, and Management of Noncentral Nervous System Cancer-Related Cognitive Impairment in Adults: Cancer-Related Cognitive Impairment. CA A Cancer J. Clin. 2015, 65, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Pullens, M.J.J.; Vries, J.D.; Warmerdam, L.J.C.V.; Wal, M.A.V.D.; Roukema, J.A. Chemotherapy and Cognitive Complaints in Women with Breast Cancer. Psycho-Oncology 2013, 22, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Ahles, T.A.; Root, J.C.; Ryan, E.L. Cancer- and Cancer Treatment–Associated Cognitive Change: An Update on the State of the Science. JCO 2012, 30, 3675–3686. [Google Scholar] [CrossRef] [PubMed]
- Janelsins, M.C.; Kesler, S.R.; Ahles, T.A.; Morrow, G.R. Prevalence, Mechanisms, and Management of Cancer-Related Cognitive Impairment. Int. Rev. Psychiatry 2014, 26, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Schagen, S.B.; Muller, M.J.; Boogerd, W.; Rosenbrand, R.M.; van Rhijn, D.; Rodenhuis, S.; van Dam, F.S.a.M. Late Effects of Adjuvant Chemotherapy on Cognitive Function: A Follow-up Study in Breast Cancer Patients. Ann. Oncol. 2002, 13, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Ahles, T.A.; Saykin, A.J.; Furstenberg, C.T.; Cole, B.; Mott, L.A.; Skalla, K.; Whedon, M.B.; Bivens, S.; Mitchell, T.; Greenberg, E.R.; et al. Neuropsychologic Impact of Standard-Dose Systemic Chemotherapy in Long-Term Survivors of Breast Cancer and Lymphoma. JCO 2002, 20, 485–493. [Google Scholar] [CrossRef]
- Hutchinson, A.D.; Hosking, J.R.; Kichenadasse, G.; Mattiske, J.K.; Wilson, C. Objective and Subjective Cognitive Impairment Following Chemotherapy for Cancer: A Systematic Review. Cancer Treat. Rev. 2012, 38, 926–934. [Google Scholar] [CrossRef]
- Janelsins, M.C.; Kohli, S.; Mohile, S.G.; Usuki, K.; Ahles, T.A.; Morrow, G.R. An Update on Cancer- and Chemotherapy-Related Cognitive Dysfunction: Current Status. Semin. Oncol. 2011, 38, 431–438. [Google Scholar] [CrossRef]
- Ono, M.; Ogilvie, J.M.; Wilson, J.S.; Green, H.J.; Chambers, S.K.; Ownsworth, T.; Shum, D.H.K. A Meta-Analysis of Cognitive Impairment and Decline Associated with Adjuvant Chemotherapy in Women with Breast Cancer. Front. Oncol. 2015, 5, 59. [Google Scholar] [CrossRef]
- Falleti, M.G.; Sanfilippo, A.; Maruff, P.; Weih, L.; Phillips, K.-A. The Nature and Severity of Cognitive Impairment Associated with Adjuvant Chemotherapy in Women with Breast Cancer: A Meta-Analysis of the Current Literature. Brain Cogn. 2005, 59, 60–70. [Google Scholar] [CrossRef]
- Runowicz, C.D.; Leach, C.R.; Henry, N.L.; Henry, K.S.; Mackey, H.T.; Cowens-Alvarado, R.L.; Cannady, R.S.; Pratt-Chapman, M.L.; Edge, S.B.; Jacobs, L.A.; et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J. Clin. 2016, 66, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Von Ah, D.; Habermann, B.; Carpenter, J.S.; Schneider, B.L. Impact of Perceived Cognitive Impairment in Breast Cancer Survivors. Eur. J. Oncol. Nurs. 2013, 17, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Koppelmans, V.; de Ruiter, M.B.; van der Lijn, F.; Boogerd, W.; Seynaeve, C.; van der Lugt, A.; Vrooman, H.; Niessen, W.J.; Breteler, M.M.B.; Schagen, S.B. Global and Focal Brain Volume in Long-Term Breast Cancer Survivors Exposed to Adjuvant Chemotherapy. Breast Cancer Res. Treat. 2012, 132, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Amidi, A.; Christensen, S.; Mehlsen, M.; Jensen, A.B.; Pedersen, A.D.; Zachariae, R. Long-Term Subjective Cognitive Functioning Following Adjuvant Systemic Treatment: 7-9 Years Follow-up of a Nationwide Cohort of Women Treated for Primary Breast Cancer. Br. J. Cancer 2015, 113, 794–801. [Google Scholar] [CrossRef][Green Version]
- Baxter, M.F.; Dulworth, A.N.; Smith, T.M. Identification of Mild Cognitive Impairments in Cancer Survivors. Occup. Ther. Health Care 2011, 25, 26–37. [Google Scholar] [CrossRef]
- Silverman, D.H.S.; Dy, C.J.; Castellon, S.A.; Lai, J.; Pio, B.S.; Abraham, L.; Waddell, K.; Petersen, L.; Phelps, M.E.; Ganz, P.A. Altered Frontocortical, Cerebellar, and Basal Ganglia Activity in Adjuvant-Treated Breast Cancer Survivors 5–10 Years after Chemotherapy. Breast Cancer Res. Treat. 2007, 103, 303–311. [Google Scholar] [CrossRef]
- De Ruiter, M.B.; Reneman, L.; Boogerd, W.; Veltman, D.J.; van Dam, F.S.A.M.; Nederveen, A.J.; Boven, E.; Schagen, S.B. Cerebral Hyporesponsiveness and Cognitive Impairment 10 Years after Chemotherapy for Breast Cancer. Hum. Brain Mapp. 2011, 32, 1206–1219. [Google Scholar] [CrossRef] [PubMed]
- De Ruiter, M.B.; Reneman, L.; Boogerd, W.; Veltman, D.J.; Caan, M.; Douaud, G.; Lavini, C.; Linn, S.C.; Boven, E.; van Dam, F.S.A.M.; et al. Late Effects of High-Dose Adjuvant Chemotherapy on White and Gray Matter in Breast Cancer Survivors: Converging Results from Multimodal Magnetic Resonance Imaging. Hum. Brain Mapp. 2012, 33, 2971–2983. [Google Scholar] [CrossRef] [PubMed]
- Stouten-Kemperman, M.M.; de Ruiter, M.B.; Koppelmans, V.; Boogerd, W.; Reneman, L.; Schagen, S.B. Neurotoxicity in Breast Cancer Survivors ≥10 Years Post-Treatment Is Dependent on Treatment Type. Brain Imaging Behav. 2015, 9, 275–284. [Google Scholar] [CrossRef]
- Castel, H.; Denouel, A.; Lange, M.; Tonon, M.-C.; Dubois, M.; Joly, F. Biomarkers Associated with Cognitive Impairment in Treated Cancer Patients: Potential Predisposition and Risk Factors. Front. Pharmacol. 2017, 8, 138. [Google Scholar] [CrossRef]
- Jean-Pierre, P.; McDonald, B.C. Neuroepidemiology of Cancer and Treatment-Related Neurocognitive Dysfunction in Adult-Onset Cancer Patients and Survivors. Handb. Clin. Neurol. 2016, 138, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.; Prust, M.; Kaiser, J. Chemotherapy, Cognitive Impairment and Hippocampal Toxicity. Neuroscience 2015, 309, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.T.; Ng, T.; Shwe, M.; Ho, H.K.; Foo, K.M.; Cham, M.T.; Lee, J.A.; Fan, G.; Tan, Y.P.; Yong, W.S.; et al. Association of Proinflammatory Cytokines and Chemotherapy-Associated Cognitive Impairment in Breast Cancer Patients: A Multi-Centered, Prospective, Cohort Study. Ann. Oncol. 2015, 26, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Limoli, C.L.; Giedzinski, E.; Rola, R.; Otsuka, S.; Palmer, T.D.; Fike, J.R. Radiation Response of Neural Precursor Cells: Linking Cellular Sensitivity to Cell Cycle Checkpoints, Apoptosis and Oxidative Stress. Radiat. Res. 2004, 161, 17–27. [Google Scholar] [CrossRef]
- Cardoso, C.V.; de Barros, M.P.; Bachi, A.L.L.; Bernardi, M.M.; Kirsten, T.B.; de Fátima Monteiro Martins, M.; Rocha, P.R.D.; da Silva Rodrigues, P.; Bondan, E.F. Chemobrain in Rats: Behavioral, Morphological, Oxidative and Inflammatory Effects of Doxorubicin Administration. Behav. Brain Res. 2020, 378, 112233. [Google Scholar] [CrossRef]
- Christie, L.-A.; Acharya, M.M.; Parihar, V.K.; Nguyen, A.; Martirosian, V.; Limoli, C.L. Impaired Cognitive Function and Hippocampal Neurogenesis Following Cancer Chemotherapy. Clin. Cancer Res. 2012, 18, 1954–1965. [Google Scholar] [CrossRef]
- Acharya, M.M.; Martirosian, V.; Chmielewski, N.N.; Hanna, N.; Tran, K.K.; Liao, A.C.; Christie, L.-A.; Parihar, V.K.; Limoli, C.L. Stem Cell Transplantation Reverses Chemotherapy-Induced Cognitive Dysfunction. Cancer Res. 2015, 75, 676–686. [Google Scholar] [CrossRef]
- Groves, T.R.; Farris, R.; Anderson, J.E.; Alexander, T.C.; Kiffer, F.; Carter, G.; Wang, J.; Boerma, M.; Allen, A.R. 5-Fluorouracil Chemotherapy Upregulates Cytokines and Alters Hippocampal Dendritic Complexity in Aged Mice. Behav. Brain Res. 2017, 316, 215–224. [Google Scholar] [CrossRef]
- Kang, S.; Lee, S.; Kim, J.; Kim, J.-C.; Kim, S.-H.; Son, Y.; Shin, T.; Youn, B.; Kim, J.-S.; Wang, H.; et al. Chronic Treatment with Combined Chemotherapeutic Agents Affects Hippocampal Micromorphometry and Function in Mice, Independently of Neuroinflammation. Exp. Neurobiol. 2018, 27, 419–436. [Google Scholar] [CrossRef]
- John, J.; Kinra, M.; Mudgal, J.; Viswanatha, G.L.; Nandakumar, K. Animal Models of Chemotherapy-Induced Cognitive Decline in Preclinical Drug Development. Psychopharmacology 2021, 238, 3025–3053. [Google Scholar] [CrossRef]
- Mounier, N.M.; Abdel-Maged, A.E.-S.; Wahdan, S.A.; Gad, A.M.; Azab, S.S. Chemotherapy-Induced Cognitive Impairment (CICI): An Overview of Etiology and Pathogenesis. Life Sci. 2020, 258, 118071. [Google Scholar] [CrossRef] [PubMed]
- Van Praag, H.; Schinder, A.F.; Christie, B.R.; Toni, N.; Palmer, T.D.; Gage, F.H. Functional Neurogenesis in the Adult Hippocampus. Nature 2002, 415, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Kee, N.; Teixeira, C.M.; Wang, A.H.; Frankland, P.W. Preferential Incorporation of Adult-Generated Granule Cells into Spatial Memory Networks in the Dentate Gyrus. Nat. Neurosci. 2007, 10, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Saitoh, Y.; Takashima, N.; Murayama, A.; Niibori, Y.; Ageta, H.; Sekiguchi, M.; Sugiyama, H.; Inokuchi, K. Adult Neurogenesis Modulates the Hippocampus-Dependent Period of Associative Fear Memory. Cell 2009, 139, 814–827. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Rampon, C.; Tang, Y.-P.; Shrom, D.; Jin, J.; Kyin, M.; Sopher, B.; Martin, G.M.; Kim, S.-H.; Langdon, R.B.; et al. Deficient Neurogenesis in Forebrain-Specific Presenilin-1 Knockout Mice Is Associated with Reduced Clearance of Hippocampal Memory Traces. Neuron 2001, 32, 911–926. [Google Scholar] [CrossRef]
- Martinez-Canabal, A.; Akers, K.G.; Josselyn, S.A.; Frankland, P.W. Age-Dependent Effects of Hippocampal Neurogenesis Suppression on Spatial Learning. Hippocampus 2013, 23, 66–74. [Google Scholar] [CrossRef]
- Epp, J.R.; Silva Mera, R.; Köhler, S.; Josselyn, S.A.; Frankland, P.W. Neurogenesis-Mediated Forgetting Minimizes Proactive Interference. Nat. Commun. 2016, 7, 10838. [Google Scholar] [CrossRef]
- Shors, T.J.; Townsend, D.A.; Zhao, M.; Kozorovitskiy, Y.; Gould, E. Neurogenesis May Relate to Some but Not All Types of Hippocampal-Dependent Learning. Hippocampus 2002, 12, 578–584. [Google Scholar] [CrossRef]
- Winocur, G.; Wojtowicz, J.M.; Sekeres, M.; Snyder, J.S.; Wang, S. Inhibition of Neurogenesis Interferes with Hippocampus-Dependent Memory Function. Hippocampus 2006, 16, 296–304. [Google Scholar] [CrossRef]
- Deng, W.; Aimone, J.B.; Gage, F.H. New Neurons and New Memories: How Does Adult Hippocampal Neurogenesis Affect Learning and Memory? Nat. Rev. Neurosci. 2010, 11, 339–350. [Google Scholar] [CrossRef]
- Dupret, D.; Revest, J.-M.; Koehl, M.; Ichas, F.; Giorgi, F.D.; Costet, P.; Abrous, D.N.; Piazza, P.V. Spatial Relational Memory Requires Hippocampal Adult Neurogenesis. PLoS ONE 2008, 3, e1959. [Google Scholar] [CrossRef] [PubMed]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular Advances and Pharmacologic Developments in Antitumor Activity and Cardiotoxicity. Pharm. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [PubMed]
- El-Agamy, S.E.; Abdel-Aziz, A.K.; Esmat, A.; Azab, S.S. Chemotherapy and Cognition: Comprehensive Review on Doxorubicin-Induced Chemobrain. Cancer Chemother. Pharm. 2019, 84, 1–14. [Google Scholar] [CrossRef]
- Monje, M.L.; Toda, H.; Palmer, T.D. Inflammatory Blockade Restores Adult Hippocampal Neurogenesis. Science 2003, 302, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Seigers, R.; Schagen, S.; Beerling, W.; Boogerd, W.; Vantellingen, O.; Vandam, F.; Koolhaas, J.; Buwalda, B. Long-Lasting Suppression of Hippocampal Cell Proliferation and Impaired Cognitive Performance by Methotrexate in the Rat. Behav. Brain Res. 2008, 186, 168–175. [Google Scholar] [CrossRef]
- Seigers, R.; Schagen, S.B.; Coppens, C.M.; van der Most, P.J.; van Dam, F.S.A.M.; Koolhaas, J.M.; Buwalda, B. Methotrexate Decreases Hippocampal Cell Proliferation and Induces Memory Deficits in Rats. Behav. Brain Res. 2009, 201, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Lyons, L.; ElBeltagy, M.; Umka, J.; Markwick, R.; Startin, C.; Bennett, G.; Wigmore, P. Fluoxetine Reverses the Memory Impairment and Reduction in Proliferation and Survival of Hippocampal Cells Caused by Methotrexate Chemotherapy. Psychopharmacology 2011, 215, 105–115. [Google Scholar] [CrossRef]
- Welbat, J.U.; Naewla, S.; Pannangrong, W.; Sirichoat, A.; Aranarochana, A.; Wigmore, P. Neuroprotective Effects of Hesperidin against Methotrexate-Induced Changes in Neurogenesis and Oxidative Stress in the Adult Rat. Biochem. Pharmacol. 2020, 178, 114083. [Google Scholar] [CrossRef]
- Han, R.; Yang, Y.M.; Dietrich, J.; Luebke, A.; Mayer-Pröschel, M.; Noble, M. Systemic 5-Fluorouracil Treatment Causes a Syndrome of Delayed Myelin Destruction in the Central Nervous System. J. Biol. 2008, 7, 12. [Google Scholar] [CrossRef]
- Janelsins, M.C.; Roscoe, J.A.; Berg, M.J.; Thompson, B.D.; Gallagher, M.J.; Morrow, G.R.; Heckler, C.E.; Jean-Pierre, P.; Opanashuk, L.A.; Gross, R.A. IGF-1 Partially Restores Chemotherapy-Induced Reductions in Neural Cell Proliferation in Adult C57BL/6 Mice. Cancer Investig. 2010, 28, 544–553. [Google Scholar] [CrossRef]
- Lyons, L.; ELBeltagy, M.; Bennett, G.; Wigmore, P. Fluoxetine Counteracts the Cognitive and Cellular Effects of 5-Fluorouracil in the Rat Hippocampus by a Mechanism of Prevention Rather than Recovery. PLoS ONE 2012, 7, e30010. [Google Scholar] [CrossRef]
- Sirichoat, A.; Suwannakot, K.; Chaisawang, P.; Pannangrong, W.; Aranarochana, A.; Wigmore, P.; Welbat, J.U. Melatonin Attenuates 5-Fluorouracil-Induced Spatial Memory and Hippocampal Neurogenesis Impairment in Adult Rats. Life Sci. 2020, 248, 117468. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.; Han, R.; Yang, Y.; Mayer-Pröschel, M.; Noble, M. CNS Progenitor Cells and Oligodendrocytes Are Targets of Chemotherapeutic Agents in Vitro and in Vivo. J. Biol. 2006, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Jamesdaniel, S.; Salvi, R. Cisplatin Inhibits Hippocampal Cell Proliferation and Alters the Expression of Apoptotic Genes. Neurotox. Res. 2014, 25, 369–380. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chiu, G.S.; Maj, M.A.; Rizvi, S.; Dantzer, R.; Vichaya, E.G.; Laumet, G.; Kavelaars, A.; Heijnen, C.J. Pifithrin-μ Prevents Cisplatin-Induced Chemobrain by Preserving Neuronal Mitochondrial Function. Cancer Res. 2017, 77, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Hattori, S.; Yoneda, S.; Watanabe, S.; Kanemoto, E.; Sugimoto, M.; Kawai, T.; Machida, A.; Kanzaki, H.; Miyazaki, I.; et al. Doxorubicin and Cyclophosphamide Treatment Produces Anxiety-like Behavior and Spatial Cognition Impairment in Rats: Possible Involvement of Hippocampal Neurogenesis via Brain-Derived Neurotrophic Factor and Cyclin D1 Regulation. Behav. Brain Res. 2015, 292, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Xue, J.; Lee, M.; Sun, M.; Zhao, X.; Zheng, Y.; Sung, C. Compound K Is Able to Ameliorate the Impaired Cognitive Function and Hippocampal Neurogenesis Following Chemotherapy Treatment. Biochem. Biophys. Res. Commun. 2013, 436, 104–109. [Google Scholar] [CrossRef]
- Garthe, A.; Behr, J.; Kempermann, G. Adult-Generated Hippocampal Neurons Allow the Flexible Use of Spatially Precise Learning Strategies. PLoS ONE 2009, 4, e5464. [Google Scholar] [CrossRef]
- Akers, K.G.; Martinez-Canabal, A.; Restivo, L.; Yiu, A.P.; De Cristofaro, A.; Hsiang, H.-L.; Wheeler, A.L.; Guskjolen, A.; Niibori, Y.; Shoji, H.; et al. Hippocampal Neurogenesis Regulates Forgetting During Adulthood and Infancy. Science 2014, 344, 598–602. [Google Scholar] [CrossRef]
- Pereira-Caixeta, A.R.; Guarnieri, L.O.; Medeiros, D.C.; Mendes, E.M.A.M.; Ladeira, L.C.D.; Pereira, M.T.; Moraes, M.F.D.; Pereira, G.S. Inhibiting Constitutive Neurogenesis Compromises Long-Term Social Recognition Memory. Neurobiol. Learn. Mem. 2018, 155, 92–103. [Google Scholar] [CrossRef]
- Niibori, Y.; Yu, T.-S.; Epp, J.R.; Akers, K.G.; Josselyn, S.A.; Frankland, P.W. Suppression of Adult Neurogenesis Impairs Population Coding of Similar Contexts in Hippocampal CA3 Region. Nat. Commun. 2012, 3, 1253. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-S.; Kim, C.-J.; Kwak, H.-B.; No, M.-H.; Heo, J.-W.; Kim, T.-W. Physical Exercise Prevents Cognitive Impairment by Enhancing Hippocampal Neuroplasticity and Mitochondrial Function in Doxorubicin-Induced Chemobrain. Neuropharmacology 2018, 133, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.E.; Choi, B.Y.; Hong, D.K.; Kim, J.H.; Lee, S.H.; Kho, A.R.; Kim, H.; Choi, H.C.; Suh, S.W. The Cancer Chemotherapeutic Agent Paclitaxel (Taxol) Reduces Hippocampal Neurogenesis via down-Regulation of Vesicular Zinc. Sci. Rep. 2017, 7, 11667. [Google Scholar] [CrossRef]
- Panoz-Brown, D.; Carey, L.M.; Smith, A.E.; Gentry, M.; Sluka, C.M.; Corbin, H.E.; Wu, J.-E.; Hohmann, A.G.; Crystal, J.D. The Chemotherapeutic Agent Paclitaxel Selectively Impairs Reversal Learning While Sparing Prior Learning, New Learning and Episodic Memory. Neurobiol. Learn. Mem. 2017, 144, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Huehnchen, P.; Boehmerle, W.; Springer, A.; Freyer, D.; Endres, M. A Novel Preventive Therapy for Paclitaxel-Induced Cognitive Deficits: Preclinical Evidence from C57BL/6 Mice. Transl. Psychiatry 2017, 7, e1185. [Google Scholar] [CrossRef]
- Briones, T.L.; Woods, J. Chemotherapy-Induced Cognitive Impairment Is Associated with Decreases in Cell Proliferation and Histone Modifications. BMC Neurosci. 2011, 12, 124. [Google Scholar] [CrossRef]
- Winocur, G.; Wojtowicz, J.M.; Merkley, C.M.; Tannock, I.F. Environmental Enrichment Protects against Cognitive Impairment Following Chemotherapy in an Animal Model. Behav. Neurosci. 2016, 130, 428–436. [Google Scholar] [CrossRef]
- Winocur, G.; Wojtowicz, J.M.; Huang, J.; Tannock, I.F. Physical Exercise Prevents Suppression of Hippocampal Neurogenesis and Reduces Cognitive Impairment in Chemotherapy-Treated Rats. Psychopharmacology 2014, 231, 2311–2320. [Google Scholar] [CrossRef]
- Winocur, G.; Wojtowicz, J.M.; Tannock, I.F. Memory Loss in Chemotherapy-Treated Rats Is Exacerbated in High-Interference Conditions and Related to Suppression of Hippocampal Neurogenesis. Behav. Brain Res. 2015, 281, 239–244. [Google Scholar] [CrossRef]
- Winocur, G.; Berman, H.; Nguyen, M.; Binns, M.A.; Henkelman, M.; van Eede, M.; Piquette-Miller, M.; Sekeres, M.J.; Wojtowicz, J.M.; Yu, J.; et al. Neurobiological Mechanisms of Chemotherapy-Induced Cognitive Impairment in a Transgenic Model of Breast Cancer. Neuroscience 2018, 369, 51–65. [Google Scholar] [CrossRef]
- Rendeiro, C.; Sheriff, A.; Bhattacharya, T.K.; Gogola, J.V.; Baxter, J.H.; Chen, H.; Helferich, W.G.; Roy, E.J.; Rhodes, J.S. Long-Lasting Impairments in Adult Neurogenesis, Spatial Learning and Memory from a Standard Chemotherapy Regimen Used to Treat Breast Cancer. Behav. Brain Res. 2016, 315, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Sekeres, M.J.; Bradley-Garcia, M.; Martinez-Canabal, A.; Winocur, G. Chemotherapy-Induced Cognitive Impairment and Hippocampal Neurogenesis: A Review of Physiological Mechanisms and Interventions. IJMS 2021, 22, 12697. [Google Scholar] [CrossRef] [PubMed]
- Apple, A.C.; Ryals, A.J.; Alpert, K.I.; Wagner, L.I.; Shih, P.-A.; Dokucu, M.; Cella, D.; Penedo, F.J.; Voss, J.L.; Wang, L. Subtle Hippocampal Deformities in Breast Cancer Survivors with Reduced Episodic Memory and Self-Reported Cognitive Concerns. NeuroImage Clin. 2017, 14, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Monje, M.L.; Vogel, H.; Masek, M.; Ligon, K.L.; Fisher, P.G.; Palmer, T.D. Impaired Human Hippocampal Neurogenesis after Treatment for Central Nervous System Malignancies. Ann. Neurol. 2007, 62, 515–520. [Google Scholar] [CrossRef]
- Eriksson, P.S.; Perfilieva, E.; Björk-Eriksson, T.; Alborn, A.-M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the Adult Human Hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef]
- Knoth, R.; Singec, I.; Ditter, M.; Pantazis, G.; Capetian, P.; Meyer, R.P.; Horvat, V.; Volk, B.; Kempermann, G. Murine Features of Neurogenesis in the Human Hippocampus across the Lifespan from 0 to 100 Years. PLoS ONE 2010, 5, e8809. [Google Scholar] [CrossRef]
- Spalding, K.L.; Bergmann, O.; Alkass, K.; Bernard, S.; Salehpour, M.; Huttner, H.B.; Boström, E.; Westerlund, I.; Vial, C.; Buchholz, B.A.; et al. Dynamics of Hippocampal Neurogenesis in Adult Humans. Cell 2013, 153, 1219–1227. [Google Scholar] [CrossRef]
- Boldrini, M.; Fulmore, C.A.; Tartt, A.N.; Simeon, L.R.; Pavlova, I.; Poposka, V.; Rosoklija, G.B.; Stankov, A.; Arango, V.; Dwork, A.J.; et al. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem. Cell 2018, 22, 589–599.e5. [Google Scholar] [CrossRef]
- Kempermann, G.; Gage, F.H.; Aigner, L.; Song, H.; Curtis, M.A.; Thuret, S.; Kuhn, H.G.; Jessberger, S.; Frankland, P.W.; Cameron, H.A.; et al. Human Adult Neurogenesis: Evidence and Remaining Questions. Cell Stem. Cell 2018, 23, 25–30. [Google Scholar] [CrossRef]
- Dietrich, J.; Monje, M.; Wefel, J.; Meyers, C. Clinical Patterns and Biological Correlates of Cognitive Dysfunction Associated with Cancer Therapy. Oncologist 2008, 13, 1285–1295. [Google Scholar] [CrossRef]
- Monje, M.; Dietrich, J. Cognitive Side Effects of Cancer Therapy Demonstrate a Functional Role for Adult Neurogenesis. Behav. Brain Res. 2012, 227, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.D.; Ehrlich, B.E. Cellular Mechanisms and Treatments for Chemobrain: Insight from Aging and Neurodegenerative Diseases. EMBO Mol. Med. 2020, 12, e12075. [Google Scholar] [CrossRef] [PubMed]
- Greenaway, M.C.; Lacritz, L.H.; Binegar, D.; Weiner, M.F.; Lipton, A.; Munro Cullum, C. Patterns of Verbal Memory Performance in Mild Cognitive Impairment, Alzheimer Disease, and Normal Aging. Cogn. Behav. Neurol. 2006, 19, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Lekeu, F.; Magis, D.; Marique, P.; Delbeuck, X.; Bechet, S.; Guillaume, B.; Adam, S.; Petermans, J.; Moonen, G.; Salmon, E. The California Verbal Learning Test and Other Standard Clinical Neuropsychological Tests to Predict Conversion from Mild Memory Impairment to Dementia. J. Clin. Exp. Neuropsychol. 2010, 32, 164–173. [Google Scholar] [CrossRef]
- Pliskin, J.I.; DeDios Stern, S.; Resch, Z.J.; Saladino, K.F.; Ovsiew, G.P.; Carter, D.A.; Soble, J.R. Comparing the Psychometric Properties of Eight Embedded Performance Validity Tests in the Rey Auditory Verbal Learning Test, Wechsler Memory Scale Logical Memory, and Brief Visuospatial Memory Test–Revised Recognition Trials for Detecting Invalid Neuropsychological Test Performance. Assessment 2021, 28, 1871–1881. [Google Scholar] [CrossRef]
- Bonner-Jackson, A.; Mahmoud, S.; Miller, J.; Banks, S.J. Verbal and Non-Verbal Memory and Hippocampal Volumes in a Memory Clinic Population. Alzheimer Res. Ther. 2015, 7, 61. [Google Scholar] [CrossRef]
- Kelley, W.M.; Miezin, F.M.; McDermott, K.B.; Buckner, R.L.; Raichle, M.E.; Cohen, N.J.; Ollinger, J.M.; Akbudak, E.; Conturo, T.E.; Snyder, A.Z.; et al. Hemispheric Specialization in Human Dorsal Frontal Cortex and Medial Temporal Lobe for Verbal and Nonverbal Memory Encoding. Neuron 1998, 20, 927–936. [Google Scholar] [CrossRef]
- Collins, B.; Mackenzie, J.; Stewart, A.; Bielajew, C.; Verma, S. Cognitive Effects of Hormonal Therapy in Early Stage Breast Cancer Patients: A Prospective Study. Psycho-Oncology 2009, 18, 811–821. [Google Scholar] [CrossRef]
- Collins, B.; MacKenzie, J.; Tasca, G.A.; Scherling, C.; Smith, A. Persistent Cognitive Changes in Breast Cancer Patients 1 Year Following Completion of Chemotherapy. J. Int. Neuropsychol. Soc. 2014, 20, 370–379. [Google Scholar] [CrossRef]
- Ahles, T.A.; Saykin, A.J.; McDonald, B.C.; Li, Y.; Furstenberg, C.T.; Hanscom, B.S.; Mulrooney, T.J.; Schwartz, G.N.; Kaufman, P.A. Longitudinal Assessment of Cognitive Changes Associated With Adjuvant Treatment for Breast Cancer: Impact of Age and Cognitive Reserve. JCO 2010, 28, 4434–4440. [Google Scholar] [CrossRef]
- Menning, S.; de Ruiter, M.B.; Veltman, D.J.; Boogerd, W.; Oldenburg, H.S.A.; Reneman, L.; Schagen, S.B. Changes in Brain Activation in Breast Cancer Patients Depend on Cognitive Domain and Treatment Type. PLoS ONE 2017, 12, e0171724. [Google Scholar] [CrossRef]
- Gilboa, A.; Moscovitch, M. No Consolidation without Representation: Correspondence between Neural and Psychological Representations in Recent and Remote Memory. Neuron 2021, 109, 2239–2255. [Google Scholar] [CrossRef] [PubMed]
- Tulving, E. Episodic and Semantic Memory. In Organization of Memory; Academic Press: Oxford, UK, 1972; pp. xiii, 423–xiii, 423. [Google Scholar]
- Patterson, K.; Nestor, P.J.; Rogers, T.T. Where Do You Know What You Know? The Representation of Semantic Knowledge in the Human Brain. Nat. Rev. Neurosci. 2007, 8, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Sekeres, M.J.; Winocur, G.; Moscovitch, M. The Hippocampus and Related Neocortical Structures in Memory Transformation. Neurosci. Lett. 2018, 680, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Tulving, E. Memory and Consciousness. Can. Psychol. Psychol. Can. 1985, 26, 1–12. [Google Scholar] [CrossRef]
- Rosenbaum, R.S.; Köhler, S.; Schacter, D.L.; Moscovitch, M.; Westmacott, R.; Black, S.E.; Gao, F.; Tulving, E. The Case of K.C.: Contributions of a Memory-Impaired Person to Memory Theory. Neuropsychologia 2005, 43, 989–1021. [Google Scholar] [CrossRef]
- Tulving, E. Episodic Memory: From Mind to Brain. Annu. Rev. Psychol. 2002, 53, 1–25. [Google Scholar] [CrossRef]
- Steinvorth, S.; Levine, B.; Corkin, S. Medial Temporal Lobe Structures Are Needed to Re-Experience Remote Autobiographical Memories: Evidence from H.M. and W.R. Neuropsychologia 2005, 43, 479–496. [Google Scholar] [CrossRef]
- St-Laurent, M.; Moscovitch, M.; Levine, B.; McAndrews, M.P. Determinants of Autobiographical Memory in Patients with Unilateral Temporal Lobe Epilepsy or Excisions. Neuropsychologia 2009, 47, 2211–2221. [Google Scholar] [CrossRef]
- Conway, M.A.; Cohen, G.; Stanhope, N. On the Very Long-Term Retention of Knowledge Acquired through Formal Education: Twelve Years of Cognitive Psychology. J. Exp. Psychol. Gen. 1991, 120, 395–409. [Google Scholar] [CrossRef]
- Berntsen, D. Tunnel Memories for Autobiographical Events: Central Details Are Remembered More Frequently from Shocking than from Happy Experiences. Mem. Cogn. 2002, 30, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M.; Matsuoka, Y.; Sugahara, Y.; Nakano, T.; Akechi, T.; Fujimori, M.; Imoto, S.; Murakami, K.; Uchitomi, Y. Hippocampal Volume and First Major Depressive Episode After Cancer Diagnosis in Breast Cancer Survivors. AJP 2004, 161, 2263–2270. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, E.; Matsuoka, Y.; Inagaki, M.; Nakano, T.; Akechi, T.; Kobayakawa, M.; Fujimori, M.; Nakaya, N.; Akizuki, N.; Imoto, S.; et al. No Adverse Effects of Adjuvant Chemotherapy on Hippocampal Volumein Japanese Breast Cancer Survivors. Breast Cancer Res. Treat 2005, 92, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, R.J.; McDonald, B.C.; Saykin, A.J.; Ahles, T.A. Brain Structure and Function Differences in Monozygotic Twins: Possible Effects of Breast Cancer Chemotherapy. JCO 2007, 25, 3866–3870. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M.; Yoshikawa, E.; Matsuoka, Y.; Sugawara, Y.; Nakano, T.; Akechi, T.; Wada, N.; Imoto, S.; Murakami, K.; Uchitomi, Y. Smaller Regional Volumes of Brain Gray and White Matter Demonstrated in Breast Cancer Survivors Exposed to Adjuvant Chemotherapy. Cancer 2007, 109, 146–156. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.C.; Conroy, S.K.; Ahles, T.A.; West, J.D.; Saykin, A.J. Gray Matter Reduction Associated with Systemic Chemotherapy for Breast Cancer: A Prospective MRI Study. Breast Cancer Res. Treat 2010, 123, 819–828. [Google Scholar] [CrossRef]
- Conroy, S.K.; McDonald, B.C.; Smith, D.J.; Moser, L.R.; West, J.D.; Kamendulis, L.M.; Klaunig, J.E.; Champion, V.L.; Unverzagt, F.W.; Saykin, A.J. Alterations in Brain Structure and Function in Breast Cancer Survivors: Effect of Post-Chemotherapy Interval and Relation to Oxidative DNA Damage. Breast Cancer Res. Treat 2013, 137, 493–502. [Google Scholar] [CrossRef]
- Kesler, S.; Janelsins, M.; Koovakkattu, D.; Palesh, O.; Mustian, K.; Morrow, G.; Dhabhar, F.S. Reduced Hippocampal Volume and Verbal Memory Performance Associated with Interleukin-6 and Tumor Necrosis Factor-Alpha Levels in Chemotherapy-Treated Breast Cancer Survivors. Brain Behav. Immun. 2013, 30, S109–S116. [Google Scholar] [CrossRef]
- Lepage, C.; Smith, A.M.; Moreau, J.; Barlow-Krelina, E.; Wallis, N.; Collins, B.; MacKenzie, J.; Scherling, C. A Prospective Study of Grey Matter and Cognitive Function Alterations in Chemotherapy-Treated Breast Cancer Patients. SpringerPlus 2014, 3, 444. [Google Scholar] [CrossRef]
- Kesler, S.R.; Bennett, F.C.; Mahaffey, M.L.; Spiegel, D. Regional Brain Activation during Verbal Declarative Memory in Metastatic Breast Cancer. Clin. Cancer Res. 2009, 15, 6665–6673. [Google Scholar] [CrossRef]
- López Zunini, R.A.; Scherling, C.; Wallis, N.; Collins, B.; MacKenzie, J.; Bielajew, C.; Smith, A.M. Differences in Verbal Memory Retrieval in Breast Cancer Chemotherapy Patients Compared to Healthy Controls: A Prospective FMRI Study. Brain Imaging Behav. 2013, 7, 460–477. [Google Scholar] [CrossRef] [PubMed]
- Apple, A.C.; Schroeder, M.P.; Ryals, A.J.; Wagner, L.I.; Cella, D.; Shih, P.-A.; Reilly, J.; Penedo, F.J.; Voss, J.L.; Wang, L. Hippocampal Functional Connectivity Is Related to Self-Reported Cognitive Concerns in Breast Cancer Patients Undergoing Adjuvant Therapy. NeuroImage Clin. 2018, 20, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.; Hosseini, S.M.H.; Kesler, S. Altered Resting State Functional Brain Network Topology in Chemotherapy-Treated Breast Cancer Survivors. Neurobiol. Dis. 2012, 48, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Lin, H.; Yan, Y.; Xu, X.; Wang, L.; Zhang, J.; Yu, Y. Impairment of the Executive Function in Breast Cancer Patients Receiving Chemotherapy Treatment: A Functional MRI Study. Eur. J. Cancer Care 2017, 26, e12553. [Google Scholar] [CrossRef]
- Cheng, H.; Li, W.; Gong, L.; Xuan, H.; Huang, Z.; Zhao, H.; Wang, L.S.; Wang, K. Altered Resting-State Hippocampal Functional Networks Associated with Chemotherapy-Induced Prospective Memory Impairment in Breast Cancer Survivors. Sci. Rep. 2017, 7, 45135. [Google Scholar] [CrossRef]
- Chen, Y.; Ou, Y.; Lv, D.; Yang, R.; Li, S.; Jia, C.; Wang, Y.; Meng, X.; Cui, H.; Li, C.; et al. Altered Network Homogeneity of the Default-Mode Network in Drug-Naive Obsessive−compulsive Disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 93, 77–83. [Google Scholar] [CrossRef]
- Feng, Y.; Tuluhong, D.; Shi, Z.; Zheng, L.J.; Chen, T.; Lu, G.M.; Wang, S.; Zhang, L.J. Postchemotherapy Hippocampal Functional Connectivity Patterns in Patients with Breast Cancer: A Longitudinal Resting State Functional MR Imaging Study. Brain Imaging Behav. 2020, 14, 1456–1467. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.F.; Zheng, L.J.; Shi, Z.; Huang, W.; Zhang, L.J. Network-Level Functional Connectivity Alterations in Chemotherapy Treated Breast Cancer Patients: A Longitudinal Resting State Functional MRI Study. Cancer Imaging 2020, 20, 73. [Google Scholar] [CrossRef]
- Bonnici, H.M.; Chadwick, M.J.; Lutti, A.; Hassabis, D.; Weiskopf, N.; Maguire, E.A. Detecting Representations of Recent and Remote Autobiographical Memories in VmPFC and Hippocampus. J. Neurosci. 2012, 32, 16982–16991. [Google Scholar] [CrossRef]
- Bonnici, H.M.; Maguire, E.A. Two Years Later–Revisiting Autobiographical Memory Representations in VmPFC and Hippocampus. Neuropsychologia 2018, 110, 159–169. [Google Scholar] [CrossRef]
- Boccia, M.; Teghil, A.; Guariglia, C. Looking into Recent and Remote Past: Meta-Analytic Evidence for Cortical Re-Organization of Episodic Autobiographical Memories. Neurosci. Biobehav. Rev. 2019, 107, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Saxe, M.D.; Battaglia, F.; Wang, J.-W.; Malleret, G.; David, D.J.; Monckton, J.E.; Garcia, A.D.R.; Sofroniew, M.V.; Kandel, E.R.; Santarelli, L.; et al. Ablation of Hippocampal Neurogenesis Impairs Contextual Fear Conditioning and Synaptic Plasticity in the Dentate Gyrus. Proc. Natl. Acad. Sci. USA 2006, 103, 17501–17506. [Google Scholar] [CrossRef] [PubMed]
- Drew, M.R.; Denny, C.A.; Hen, R. Arrest of Adult Hippocampal Neurogenesis in Mice Impairs Single- but Not Multiple-Trial Contextual Fear Conditioning. Behav. Neurosci. 2010, 124, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Gurguryan, L.; Rioux, M.; Sheldon, S. Reduced Anterior Hippocampal and Ventromedial Prefrontal Activity When Repeatedly Retrieving Autobiographical Memories. Hippocampus 2021, 31, 869–880. [Google Scholar] [CrossRef]
- Mccormick, C.; St-Laurent, M.; Ty, A.; Valiante, T.; Mcandrews, M. Functional and Effective Hippocampal-Neocortical Connectivity During Construction and Elaboration of Autobiographical Memory Retrieval. Cereb. Cortex 2013, 25. [Google Scholar] [CrossRef]
- St-Laurent, M.; Moscovitch, M.; Jadd, R.; McAndrews, M.P. The Perceptual Richness of Complex Memory Episodes Is Compromised by Medial Temporal Lobe Damage. Hippocampus 2014, 24, 560–576. [Google Scholar] [CrossRef]
- Rosenbaum, R.S.; Moscovitch, M.; Foster, J.K.; Schnyer, D.M.; Gao, F.; Kovacevic, N.; Verfaellie, M.; Black, S.E.; Levine, B. Patterns of Autobiographical Memory Loss in Medial-Temporal Lobe Amnesic Patients. J. Cogn. Neurosci. 2008, 20, 1490–1506. [Google Scholar] [CrossRef]
- Nilsson-Ihrfelt, E.; Fjällskog, M.-L.; Liss, A.; Jakobsson, O.; Blomqvist, C.; Andersson, G. Autobiographical Memories in Patients Treated for Breast Cancer. J. Psychosom. Res. 2004, 57, 363–366. [Google Scholar] [CrossRef]
- Conway, M.A.; Pleydell-Pearce, C.W. The Construction of Autobiographical Memories in the Self-Memory System. Psychol. Rev. 2000, 107, 261–288. [Google Scholar] [CrossRef]
- Williams, J.M.; Broadbent, K. Autobiographical Memory in Suicide Attempters. J. Abnorm. Psychol. 1986, 95, 144–149. [Google Scholar] [CrossRef]
- Bergouignan, L.; Lefranc, J.P.; Chupin, M.; Morel, N.; Spano, J.P.; Fossati, P. Breast Cancer Affects Both the Hippocampus Volume and the Episodic Autobiographical Memory Retrieval. PLoS ONE 2011, 6, e25349. [Google Scholar] [CrossRef]
- Sekeres, M.J.; Winocur, G.; Moscovitch, M.; Anderson, J.A.E.; Pishdadian, S.; Martin Wojtowicz, J.; St-Laurent, M.; McAndrews, M.P.; Grady, C.L. Changes in Patterns of Neural Activity Underlie a Time-Dependent Transformation of Memory in Rats and Humans. Hippocampus 2018, 28, 745–764. [Google Scholar] [CrossRef] [PubMed]
- Sekeres, M.J.; Moscovitch, M.; Winocur, G.; Pishdadian, S.; Nichol, D.; Grady, C.L. Reminders Activate the Prefrontal-medial Temporal Cortex and Attenuate Forgetting of Event Memory. Hippocampus 2021, 31, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Kopelman, M.D.; Wilson, B.A.; Baddeley, A.D. The Autobiographical Memory Interview: A New Assessment of Autobiographical and Personal Semantic Memory in Amnesic Patients. J. Clin. Exp. Neuropsychol. 1989, 11, 724–744. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Svoboda, E.; Hay, J.F.; Winocur, G.; Moscovitch, M. Aging and Autobiographical Memory: Dissociating Episodic from Semantic Retrieval. Psychol. Aging 2002, 17, 677–689. [Google Scholar] [CrossRef]
- Addis, D.R.; Wong, A.T.; Schacter, D.L. Remembering the Past and Imagining the Future: Common and Distinct Neural Substrates during Event Construction and Elaboration. Neuropsychologia 2007, 45, 1363–1377. [Google Scholar] [CrossRef]
- Dede, A.J.O.; Frascino, J.C.; Wixted, J.T.; Squire, L.R. Learning and Remembering Real-World Events after Medial Temporal Lobe Damage. Proc. Natl. Acad. Sci. USA 2016, 113, 13480–13485. [Google Scholar] [CrossRef]
- Miller, T.D.; Chong, T.T.-J.; Aimola Davies, A.M.; Johnson, M.R.; Irani, S.R.; Husain, M.; Ng, T.W.; Jacob, S.; Maddison, P.; Kennard, C.; et al. Human Hippocampal CA3 Damage Disrupts Both Recent and Remote Episodic Memories. eLife 2020, 9, e41836. [Google Scholar] [CrossRef]
- Peters, S.; Sheldon, S. Interindividual Differences in Cognitive Functioning Are Associated with Autobiographical Memory Retrieval Specificity in Older Adults. GeroPsych 2020, 33, 15–29. [Google Scholar] [CrossRef]
- Wank, A.A.; Andrews-Hanna, J.R.; Grilli, M.D. Searching for the Past: Exploring the Dynamics of Direct and Generative Autobiographical Memory Reconstruction among Young and Cognitively Normal Older Adults. Mem. Cogn. 2021, 49, 422–437. [Google Scholar] [CrossRef]
- Sekeres, M.J.; Riggs, L.; Decker, A.; de Medeiros, C.B.; Bacopulos, A.; Skocic, J.; Szulc-Lerch, K.; Bouffet, E.; Levine, B.; Grady, C.L.; et al. Impaired Recent, but Preserved Remote, Autobiographical Memory in Pediatric Brain Tumor Patients. J. Neurosci. 2018, 38, 8251–8261. [Google Scholar] [CrossRef] [PubMed]
- Sekeres, M.J.; Bonasia, K.; St-Laurent, M.; Pishdadian, S.; Winocur, G.; Grady, C.; Moscovitch, M. Recovering and Preventing Loss of Detailed Memory: Differential Rates of Forgetting for Detail Types in Episodic Memory. Learn. Mem. 2016, 23, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Wenner, M.; Inagaki, M.; Kugaya, A.; Akechi, T.; Matsuoka, Y.; Sugahara, Y.; Imoto, S.; Murakami, K.; Uchitomi, Y. Relationship Between Distressing Cancer-Related Recollections and Hippocampal Volume in Cancer Survivors. AJP 2002, 159, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Deprez, S.; Amant, F.; Smeets, A.; Peeters, R.; Leemans, A.; Van Hecke, W.; Verhoeven, J.S.; Christiaens, M.-R.; Vandenberghe, J.; Vandenbulcke, M.; et al. Longitudinal Assessment of Chemotherapy-Induced Structural Changes in Cerebral White Matter and Its Correlation With Impaired Cognitive Functioning. JCO 2012, 30, 274–281. [Google Scholar] [CrossRef]
- McDonald, B.C.; Conroy, S.K.; Ahles, T.A.; West, J.D.; Saykin, A.J. Alterations in Brain Activation During Working Memory Processing Associated With Breast Cancer and Treatment: A Prospective Functional Magnetic Resonance Imaging Study. J. Clin. Oncol. 2012, 30, 2500–2508. [Google Scholar] [CrossRef]
- Poppenk, J.; Evensmoen, H.R.; Moscovitch, M.; Nadel, L. Long-Axis Specialization of the Human Hippocampus. Trends Cogn. Sci. 2013, 17, 230–240. [Google Scholar] [CrossRef]
- Fanselow, M.S.; Dong, H.-W. Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef]
- Moscovitch, M.; Cabeza, R.; Winocur, G.; Nadel, L. Episodic Memory and Beyond: The Hippocampus and Neocortex in Transformation. Annu. Rev. Psychol. 2016, 67, 105–134. [Google Scholar] [CrossRef]
- Poppenk, J.; Moscovitch, M. A Hippocampal Marker of Recollection Memory Ability among Healthy Young Adults: Contributions of Posterior and Anterior Segments. Neuron 2011, 72, 931–937. [Google Scholar] [CrossRef]
- Robin, J.; Moscovitch, M. Details, Gist and Schema: Hippocampal–Neocortical Interactions Underlying Recent and Remote Episodic and Spatial Memory. Curr. Opin. Behav. Sci. 2017, 17, 114–123. [Google Scholar] [CrossRef]
- Inman, C.S.; James, G.A.; Vytal, K.; Hamann, S. Dynamic Changes in Large-Scale Functional Network Organization during Autobiographical Memory Retrieval. Neuropsychologia 2018, 110, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Bauer, P.J.; Dikmen, S.S.; Heaton, R.K.; Mungas, D.; Slotkin, J.; Beaumont, J.L. III. NIH Toolbox Cognition Battery (CB): Measuring Episodic Memory: NIH Toolbox Cognition Battery (CB). Monogr. Soc. Res. Child 2013, 78, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Ryals, A.J.; Apple, A.C.; Wang, J.X.; Cella, D.; Penedo, F.J.; Wang, L.; Voss, J.L. Hippocampal Memory Impairment in Breast Cancer Survivors after Chemotherapy Measurement Using Covert Testing. JCO 2015, 33, 1024. [Google Scholar] [CrossRef]
- Schacter, D.L. Implicit Memory: History and Current Status. J. Exp. Psychol. Learn. Mem. Cogn. 1987, 13, 501–518. [Google Scholar] [CrossRef]
- Rugg, M.D.; Mark, R.E.; Walla, P.; Schloerscheidt, A.M.; Birch, C.S.; Allan, K. Dissociation of the Neural Correlates of Implicit and Explicit Memory. Nature 1998, 392, 595–598. [Google Scholar] [CrossRef]
- Buckner, R.L.; Goodman, J.; Burock, M.; Rotte, M.; Koutstaal, W.; Schacter, D.; Rosen, B.; Dale, A.M. Functional-Anatomic Correlates of Object Priming in Humans Revealed by Rapid Presentation Event-Related FMRI. Neuron 1998, 20, 285–296. [Google Scholar] [CrossRef]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A Default Mode of Brain Function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The Brain’s Default Network: Anatomy, Function, and Relevance to Disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Spreng, R.N.; Mar, R.A.; Kim, A.S.N. The Common Neural Basis of Autobiographical Memory, Prospection, Navigation, Theory of Mind, and the Default Mode: A Quantitative Meta-Analysis. J. Cogn. Neurosci. 2009, 21, 489–510. [Google Scholar] [CrossRef]
- Buckner, R.L.; Carroll, D.C. Self-Projection and the Brain. Trends Cogn. Sci. 2007, 11, 49–57. [Google Scholar] [CrossRef]
- Andrews-Hanna, J.R.; Reidler, J.S.; Sepulcre, J.; Poulin, R.; Buckner, R.L. Functional-Anatomic Fractionation of the Brain’s Default Network. Neuron 2010, 65, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Spreng, R.N.; Grady, C.L. Patterns of Brain Activity Supporting Autobiographical Memory, Prospection, and Theory of Mind, and Their Relationship to the Default Mode Network. J. Cogn. Neurosci. 2010, 22, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Okuda, J.; Fujii, T.; Ohtake, H.; Tsukiura, T.; Tanji, K.; Suzuki, K.; Kawashima, R.; Fukuda, H.; Itoh, M.; Yamadori, A. Thinking of the Future and Past: The Roles of the Frontal Pole and the Medial Temporal Lobes. Neuroimage 2003, 19, 1369–1380. [Google Scholar] [CrossRef]
- Addis, D.R.; Schacter, D.L. The Hippocampus and Imagining the Future: Where Do We Stand? Front. Hum. Neurosci. 2011, 5, 173. [Google Scholar] [CrossRef] [PubMed]
- Schacter, D.L.; Addis, D.R.; Buckner, R.L. Remembering the Past to Imagine the Future: The Prospective Brain. Nat. Rev. Neurosci. 2007, 8, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Hassabis, D.; Maguire, E.A. The Construction System of the Brain. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1263–1271. [Google Scholar] [CrossRef]
- Schacter, D.L.; Addis, D.R. The Ghosts of Past and Future. Nature 2007, 445, 27. [Google Scholar] [CrossRef]
- Schacter, D.L. Adaptive Constructive Processes and the Future of Memory. Am. Psychol. 2012, 67, 603–613. [Google Scholar] [CrossRef]
- McDaniel, M.A.; Glisky, E.L.; Guynn, M.J.; Routhieaux, B.C. Prospective Memory: A Neuropsychological Study. Neuropsychology 1999, 13, 103–110. [Google Scholar] [CrossRef]
- Burgess, P.W.; Quayle, A.; Frith, C.D. Brain Regions Involved in Prospective Memory as Determined by Positron Emission Tomography. Neuropsychologia 2001, 39, 545–555. [Google Scholar] [CrossRef]
- Cona, G.; Scarpazza, C.; Sartori, G.; Moscovitch, M.; Bisiacchi, P.S. Neural Bases of Prospective Memory: A Meta-Analysis and the “Attention to Delayed Intention” (AtoDI) Model. Neurosci. Biobehav. Rev. 2015, 52, 21–37. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, M.A.; Einstein, G.O. The Neuropsychology of Prospective Memory in Normal Aging: A Componential Approach. Neuropsychologia 2011, 49, 2147–2155. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Yang, Z.; Dong, B.; Chen, C.; Zhang, M.; Huang, Z.; Chen, Z.; Wang, K. Chemotherapy-Induced Prospective Memory Impairment in Patients with Breast Cancer: Chemotherapy-Induced PM Impairment in Breast Cancer Patients. Psycho-Oncology 2013, 22, 2391–2395. [Google Scholar] [CrossRef] [PubMed]
- Bedard, M.; Verma, S.; Collins, B.; Song, X.; Paquet, L. Prospective Memory Impairment in Chemotherapy-Exposed Early Breast Cancer Survivors: Preliminary Evidence from a Clinical Test. J. Psychosoc. Oncol. 2016, 34, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Atance, C.M.; O’Neill, D.K. Episodic Future Thinking. Trends Cogn. Sci. 2001, 5, 533–539. [Google Scholar] [CrossRef]
- Tulving, E. Origin of Autonoesis in Episodic Memory. In The Nature of Remembering: Essays in Honor of Robert G. Crowder; Roediger, H.L., Nairne, J.S., Neath, I., Surprenant, A.M., Eds.; American Psychological Association: Washington, DC, USA, 2001; pp. 17–34. ISBN 978-1-55798-750-1. [Google Scholar]
- Schacter, D.L.; Benoit, R.G.; Szpunar, K.K. Episodic Future Thinking: Mechanisms and Functions. Curr. Opin. Behav. Sci. 2017, 17, 41–50. [Google Scholar] [CrossRef]
- Gilboa, A.; Winocur, G.; Rosenbaum, R.S.; Poreh, A.; Gao, F.; Black, S.E.; Westmacott, R.; Moscovitch, M. Hippocampal Contributions to Recollection in Retrograde and Anterograde Amnesia. Hippocampus 2006, 16, 966–980. [Google Scholar] [CrossRef]
- Race, E.; Keane, M.M.; Verfaellie, M. Medial Temporal Lobe Damage Causes Deficits in Episodic Memory and Episodic Future Thinking Not Attributable to Deficits in Narrative Construction. J. Neurosci. 2011, 31, 10262–10269. [Google Scholar] [CrossRef]
- De Luca, F.; Benuzzi, F.; Bertossi, E.; Braghittoni, D.; di Pellegrino, G.; Ciaramelli, E. Episodic Future Thinking and Future-Based Decision-Making in a Case of Retrograde Amnesia. Neuropsychologia 2018, 110, 92–103. [Google Scholar] [CrossRef]
- McCormick, C.; Rosenthal, C.R.; Miller, T.D.; Maguire, E.A. Mind-Wandering in People with Hippocampal Damage. J. Neurosci. 2018, 38, 2745–2754. [Google Scholar] [CrossRef]
- Kesler, S.R.; Adams, M.; Packer, M.; Rao, V.; Henneghan, A.M.; Blayney, D.W.; Palesh, O. Disrupted Brain Network Functional Dynamics and Hyper-Correlation of Structural and Functional Connectome Topology in Patients with Breast Cancer Prior to Treatment. Brain Behav. 2017, 7, e00643. [Google Scholar] [CrossRef] [PubMed]
- Kesler, S.R.; Wefel, J.S.; Hosseini, S.M.H.; Cheung, M.; Watson, C.L.; Hoeft, F. Default Mode Network Connectivity Distinguishes Chemotherapy-Treated Breast Cancer Survivors from Controls. Proc. Natl. Acad. Sci. USA 2013, 110, 11600–11605. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.A.; Polyn, S.M.; Detre, G.J.; Haxby, J.V. Beyond Mind-Reading: Multi-Voxel Pattern Analysis of FMRI Data. Trends Cogn. Sci. 2006, 10, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Kesler, S.R. Default Mode Network as a Potential Biomarker of Chemotherapy-Related Brain Injury. Neurobiol. Aging 2014, 35, S11–S19. [Google Scholar] [CrossRef]
- Wang, L.; Yan, Y.; Wang, X.; Tao, L.; Chen, Q.; Bian, Y.; He, X.; Liu, Y.; Ding, W.; Yu, Y.; et al. Executive Function Alternations of Breast Cancer Patients After Chemotherapy. Acad. Radiol. 2016, 23, 1264–1270. [Google Scholar] [CrossRef]
- Miao, H.; Chen, X.; Yan, Y.; He, X.; Hu, S.; Kong, J.; Wu, M.; Wei, Y.; Zhou, Y.; Wang, L.; et al. Functional Connectivity Change of Brain Default Mode Network in Breast Cancer Patients after Chemotherapy. Neuroradiology 2016, 58, 921–928. [Google Scholar] [CrossRef]
- Smith, A.M.; Leeming, A.; Fang, Z.; Hatchard, T.; Mioduszewski, O.; Schneider, M.A.; Ferdossifard, A.; Shergill, Y.; Khoo, E.-L.; Poulin, P. Mindfulness-Based Stress Reduction Alters Brain Activity for Breast Cancer Survivors with Chronic Neuropathic Pain: Preliminary Evidence from Resting-State FMRI. J. Cancer Surviv. 2021, 15, 518–525. [Google Scholar] [CrossRef]
- Menning, S.; de Ruiter, M.B.; Veltman, D.J.; Koppelmans, V.; Kirschbaum, C.; Boogerd, W.; Reneman, L.; Schagen, S.B. Multimodal MRI and Cognitive Function in Patients with Breast Cancer Prior to Adjuvant Treatment—The Role of Fatigue. NeuroImage Clin. 2015, 7, 547–554. [Google Scholar] [CrossRef]
- Scherling, C.; Collins, B.; MacKenzie, J.; Bielajew, C.; Smith, A. Prechemotherapy Differences in Response Inhibition in Breast Cancer Patients Compared to Controls: A Functional Magnetic Resonance Imaging Study. J. Clin. Exp. Neuropsychol. 2012, 34, 543–560. [Google Scholar] [CrossRef]
- Cimprich, B.; Reuter-Lorenz, P.; Nelson, J.; Clark, P.M.; Therrien, B.; Normolle, D.; Berman, M.G.; Hayes, D.F.; Noll, D.C.; Peltier, S.; et al. Prechemotherapy Alterations in Brain Function in Women with Breast Cancer. J. Clin. Exp. Neuropsychol. 2010, 32, 324–331. [Google Scholar] [CrossRef]
- Vardy, J.L.; Stouten-Kemperman, M.M.; Pond, G.; Booth, C.M.; Rourke, S.B.; Dhillon, H.M.; Dodd, A.; Crawley, A.; Tannock, I.F. A Mechanistic Cohort Study Evaluating Cognitive Impairment in Women Treated for Breast Cancer. Brain Imaging Behav. 2019, 13, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; van Marle, H.J.F.; Hermans, E.J.; Fernández, G. Subjective Sense of Memory Strength and the Objective Amount of Information Accurately Remembered Are Related to Distinct Neural Correlates at Encoding. J. Neurosci. 2011, 31, 8920–8927. [Google Scholar] [CrossRef]
- Johnson, M.K.; Kuhl, B.A.; Mitchell, K.J.; Ankudowich, E.; Durbin, K.A. Age-Related Differences in the Neural Basis of the Subjective Vividness of Memories: Evidence from Multivoxel Pattern Classification. Cogn. Affect. Behav. Neurosci. 2015, 15, 644–661. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E. Eyewitness Reliability. Science 1979, 205, 386–387. [Google Scholar] [CrossRef] [PubMed]
- Bender, C.M.; Sereika, S.M.; Berga, S.L.; Vogel, V.G.; Brufsky, A.M.; Paraska, K.K.; Ryan, C.M. Cognitive Impairment Associated with Adjuvant Therapy in Breast Cancer. Psycho-Oncology 2006, 15, 422–430. [Google Scholar] [CrossRef]
- Balash, Y.; Mordechovich, M.; Shabtai, H.; Giladi, N.; Gurevich, T.; Korczyn, A.D. Subjective Memory Complaints in Elders: Depression, Anxiety, or Cognitive Decline? Acta Neurol. Scand. 2013, 127, 344–350. [Google Scholar] [CrossRef]
- Abbey, G.; Thompson, S.B.N.; Hickish, T.; Heathcote, D. A Meta-analysis of Prevalence Rates and Moderating Factors for Cancer-related Post-traumatic Stress Disorder. Psycho-Oncology 2015, 24, 371–381. [Google Scholar] [CrossRef]
- Dimitrov, L.; Moschopoulou, E.; Korszun, A. Interventions for the Treatment of Cancer-related Traumatic Stress Symptoms: A Systematic Review of the Literature. Psycho-Oncology 2019, 28, 970–979. [Google Scholar] [CrossRef]
- Sebri, V.; Triberti, S.; Pravettoni, G. Injured Self: Autobiographical Memory, Self-Concept, and Mental Health Risk in Breast Cancer Survivors. Front. Psychol. 2020, 11, 607514. [Google Scholar] [CrossRef]
- Conway, M.A. Memory and the Self☆. J. Mem. Lang. 2005, 53, 594–628. [Google Scholar] [CrossRef]
- Morel, N.; Dayan, J.; Piolino, P.; Viard, A.; Allouache, D.; Noal, S.; Levy, C.; Joly, F.; Eustache, F.; Giffard, B. Emotional Specificities of Autobiographical Memory after Breast Cancer Diagnosis. Conscious. Cogn. 2015, 35, 42–52. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bradley-Garcia, M.; Winocur, G.; Sekeres, M.J. Episodic Memory and Recollection Network Disruptions Following Chemotherapy Treatment in Breast Cancer Survivors: A Review of Neuroimaging Findings. Cancers 2022, 14, 4752. https://doi.org/10.3390/cancers14194752
Bradley-Garcia M, Winocur G, Sekeres MJ. Episodic Memory and Recollection Network Disruptions Following Chemotherapy Treatment in Breast Cancer Survivors: A Review of Neuroimaging Findings. Cancers. 2022; 14(19):4752. https://doi.org/10.3390/cancers14194752
Chicago/Turabian StyleBradley-Garcia, Meenakshie, Gordon Winocur, and Melanie J. Sekeres. 2022. "Episodic Memory and Recollection Network Disruptions Following Chemotherapy Treatment in Breast Cancer Survivors: A Review of Neuroimaging Findings" Cancers 14, no. 19: 4752. https://doi.org/10.3390/cancers14194752
APA StyleBradley-Garcia, M., Winocur, G., & Sekeres, M. J. (2022). Episodic Memory and Recollection Network Disruptions Following Chemotherapy Treatment in Breast Cancer Survivors: A Review of Neuroimaging Findings. Cancers, 14(19), 4752. https://doi.org/10.3390/cancers14194752





