Multiple Myeloma in Patients over 80: A Real World Retrospective Study of First Line Conservative Approach with Bortezomib Dexamethasone Doublet Therapy and Mini-Review of Literature
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment
2.3. Geriatric Assessment
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Response
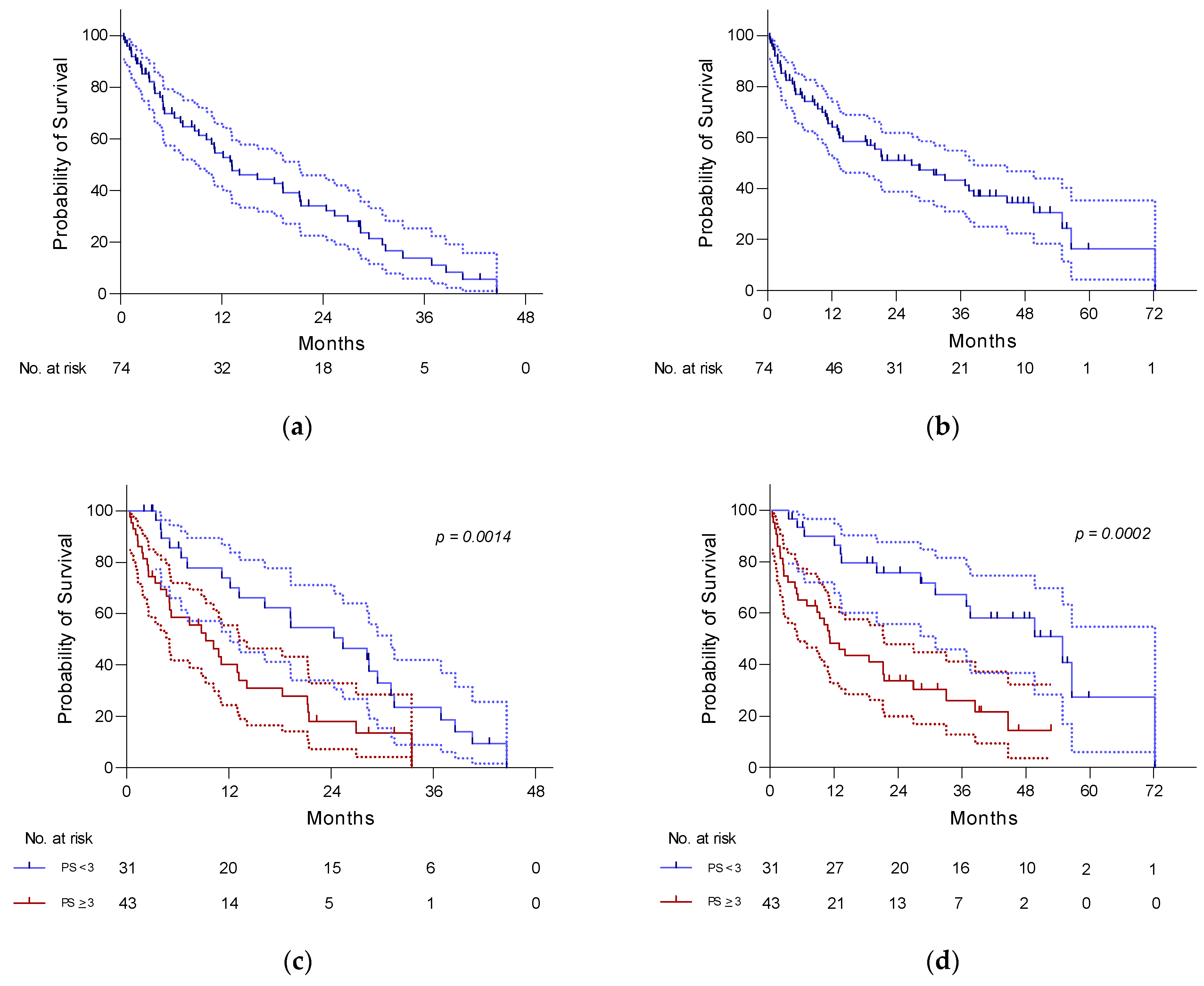
3.2. PFS and OS
3.3. Tolerability and Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mateos, M.-V.; Richardson, P.G.; Schlag, R.; Khuageva, N.K.; Dimopoulos, M.A.; Shpilberg, O.; Kropff, M.; Spicka, I.; Petrucci, M.T.; Palumbo, A.; et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: Updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Facon, T.; Dimopoulos, M.A.; Dispenzieri, A.; Catalano, J.V.; Belch, A.; Cavo, M.; Pinto, A.; Weisel, K.; Ludwig, H.; Bahlis, N.J.; et al. Final analysis of survival outcomes in the phase 3 FIRST trial of up-front treatment for multiple myeloma. Blood 2018, 131, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Durie, B.G.M.; Hoering, A.; Abidi, M.H.; Rajkumar, S.V.; Epstein, J.; Kahanic, S.P.; Thakuri, M.; Reu, F.; Reynolds, C.M.; Sexton, R.; et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): A randomised, open-label, phase 3 trial. Lancet 2017, 389, 519–527. [Google Scholar] [CrossRef]
- Durie, B.G.M.; Hoering, A.; Sexton, R.; Abidi, M.H.; Epstein, J.; Rajkumar, S.V.; Dispenzieri, A.; Kahanic, S.P.; Thakuri, M.C.; Reu, F.J.; et al. Longer term follow-up of the randomized phase III trial SWOG S0777: Bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (Pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT). Blood Cancer J. 2020, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.-V.; Dimopoulos, M.A.; Cavo, M.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lucio, P.; Nagy, Z.; Kaplan, P.; et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N. Engl. J. Med. 2018, 378, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.-V.; Cavo, M.; Blade, J.; Dimopoulos, M.A.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lucio, P.; Nagy, Z.; et al. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): A randomised, open-label, phase 3 trial. Lancet Lond. Engl. 2020, 395, 132–141. [Google Scholar] [CrossRef]
- Facon, T.; Kumar, S.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N. Engl. J. Med. 2019, 380, 2104–2115. [Google Scholar] [CrossRef]
- Facon, T.; Kumar, S.K.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): Overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 1582–1596. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.V.; Zweegman, S.; Cook, G.; Delforge, M.; Hájek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 2021, 32, 309–322. [Google Scholar] [CrossRef]
- Institut National du Cancer. Estimations nationales de l’incidence et de la mortalité par cancer en France métropolitaine entre 1990 et 2018—Volume 2 Hémopathies malignes. Santé Publique France Rapport, 18 September 2019. [Google Scholar]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.-V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Palumbo, A.; Bringhen, S.; Mateos, M.-V.; Larocca, A.; Facon, T.; Kumar, S.K.; Offidani, M.; McCarthy, P.; Evangelista, A.; Lonial, S.; et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: An International Myeloma Working Group report. Blood 2015, 125, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Facon, T.; Dimopoulos, M.A.; Meuleman, N.; Belch, A.; Mohty, M.; Chen, W.-M.; Kim, K.; Zamagni, E.; Rodriguez-Otero, P.; Renwick, W.; et al. A simplified frailty scale predicts outcomes in transplant-ineligible patients with newly diagnosed multiple myeloma treated in the FIRST (MM-020) trial. Leukemia 2020, 34, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Larocca, A.; Bringhen, S.; Petrucci, M.T.; Oliva, S.; Falcone, A.P.; Caravita, T.; Villani, O.; Benevolo, G.; Liberati, A.M.; Morabito, F.; et al. A phase 2 study of three low-dose intensity subcutaneous bortezomib regimens in elderly frail patients with untreated multiple myeloma. Leukemia 2016, 30, 1320–1326. [Google Scholar] [CrossRef]
- O’Donnell, E.K.; Laubach, J.P.; Yee, A.J.; Chen, T.; Huff, C.A.; Basile, F.G.; Wade, P.M.; Paba-Prada, C.E.; Ghobrial, I.M.; Schlossman, R.L.; et al. A phase 2 study of modified lenalidomide, bortezomib and dexamethasone in transplant-ineligible multiple myeloma. Br. J. Haematol. 2018, 182, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Larocca, A.; Bonello, F.; Gaidano, G.; D’Agostino, M.; Offidani, M.; Cascavilla, N.; Capra, A.; Benevolo, G.; Tosi, P.; Galli, M.; et al. Dose/schedule-adjusted Rd-R vs continuous Rd for elderly, intermediate-fit patients with newly diagnosed multiple myeloma. Blood 2021, 137, 3027–3036. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Kastritis, E.; Delimpasi, S.; Katodritou, E.; Hatzimichael, E.; Kyrtsonis, M.-C.; Repousis, P.; Tsirogianni, M.; Kartasis, Z.; Parcharidou, A.; et al. Multiple myeloma in octogenarians: Clinical features and outcome in the novel agent era. Eur. J. Haematol. 2012, 89, 10–15. [Google Scholar] [CrossRef]
- Bang, S.-M.; Kyle, R.; Rajkumar, S.; Kumar, S. Treatment patterns and outcomes in elderly patients with multiple myeloma. Leukemia 2013, 27, 971–974. [Google Scholar] [CrossRef][Green Version]
- Matsue, K.; Matsue, Y.; Fujisawa, M.; Fukumoto, K.; Suehara, Y.; Sugihara, H.; Takeuchi, M. Clinical features and treatment outcome of very elderly patients over 80 years old with multiple myeloma: Comparison with patients in different age groups in the era of novel agents. Leuk. Lymphoma 2016, 57, 110–115. [Google Scholar] [CrossRef]
- Chan, H.; Chai, K.; Shih, S.; Lewsey, R.; Chen, K.; McDiarmid, B.; Jackson, S.; Simpson, D. Frontline treatment of elderly non transplant-eligible multiple myeloma patients using CyBorD with or without thalidomide-based consolidation: A retrospective multi-centre analysis of real-world data. Br. J. Haematol. 2019, 187, 470–477. [Google Scholar] [CrossRef]
- Panitsas, F.; Kothari, J.; Vallance, G.; Djebbari, F.; Ferguson, L.; Sultanova, M.; Ramasamy, K. Treat or palliate: Outcomes of very elderly myeloma patients. Haematologica 2018, 103, e32–e34. [Google Scholar] [CrossRef]
- Gavriatopoulou, M.; Fotiou, D.; Koloventzou, U.; Roussou, M.; Migkou, M.; Ntanasis-Stathopoulos, I.; Kanellias, N.; Ziogas, D.C.; Panagiotidis, I.; Gika, D.; et al. Vulnerability variables among octogenerian myeloma patients: A single-center analysis of 110 patients. Leuk. Lymphoma 2019, 60, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Ediriwickrama, N.; Oyebamiji, T.; Munisamy, S.; Garland, P.; Cuthill, K.; Benjamin, R.; Ceesay, M.M.; Bowcock, S. The combination of an inclusive novel agent treatment strategy, pre-emptive therapy dose reduction, and prolonged therapy results in good outcomes in elderly myeloma patients. Br. J. Haematol. 2019, 186, e11–e13. [Google Scholar] [CrossRef] [PubMed]
- Stege, C.A.M.; Nasserinejad, K.; van der Spek, E.; Bilgin, Y.M.; Kentos, A.; Sohne, M.; van Kampen, R.J.W.; Ludwig, I.; Thielen, N.; Durdu-Rayman, N.; et al. Ixazomib, Daratumumab, and Low-Dose Dexamethasone in Frail Patients With Newly Diagnosed Multiple Myeloma: The Hovon 143 Study. J. Clin. Oncol. 2021, 39, 2758–2767. [Google Scholar] [CrossRef]
- Niesvizky, R.; Flinn, I.W.; Rifkin, R.; Gabrail, N.; Charu, V.; Clowney, B.; Essell, J.; Gaffar, Y.; Warr, T.; Neuwirth, R.; et al. Community-Based Phase IIIB Trial of Three UPFRONT Bortezomib-Based Myeloma Regimens. J. Clin. Oncol. 2015, 33, 3921–3929. [Google Scholar] [CrossRef] [PubMed]
- Medhekar, R.; Ran, T.; Fu, A.Z.; Patel, S.; Kaila, S. Real-world patient characteristics and treatment outcomes among nontransplanted multiple myeloma patients who received Bortezomib in combination with Lenalidomide and Dexamethasone as first line of therapy in the United States. BMC Cancer 2022, 22, 901. [Google Scholar] [CrossRef] [PubMed]
- Smaje, A.; Weston-Clark, M.; Raj, R.; Orlu, M.; Davis, D.; Rawle, M. Factors associated with medication adherence in older patients: A systematic review. AGING Med. 2018, 1, 254–266. [Google Scholar] [CrossRef]
- Mislang, A.R.; Wildes, T.M.; Kanesvaran, R.; Baldini, C.; Holmes, H.M.; Nightingale, G.; Coolbrandt, A.; Biganzoli, L. Adherence to oral cancer therapy in older adults: The International Society of Geriatric Oncology (SIOG) taskforce recommendations. Cancer Treat. Rev. 2017, 57, 58–66. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Cheung, M.C.; Roussel, M.; Liu, T.; Gamberi, B.; Kolb, B.; Derigs, H.G.; Eom, H.; Belhadj, K.; Lenain, P.; et al. Impact of renal impairment on outcomes with lenalidomide and dexamethasone treatment in the FIRST trial, a randomized, open-label phase 3 trial in transplant-ineligible patients with multiple myeloma. Haematologica 2016, 101, 363–370. [Google Scholar] [CrossRef]
- Facon, T.; Mary, J.-Y.; Pégourie, B.; Attal, M.; Renaud, M.; Sadoun, A.; Voillat, L.; Dorvaux, V.; Hulin, C.; Lepeu, G.; et al. Dexamethasone-based regimens versus melphalan-prednisone for elderly multiple myeloma patients ineligible for high-dose therapy. Blood 2006, 107, 1292–1298. [Google Scholar] [CrossRef]
- Drayson, M.T.; Bowcock, S.; Planche, T.; Iqbal, G.; Pratt, G.; Yong, K.; Wood, J.; Raynes, K.; Higgins, H.; Dawkins, B.; et al. Levofloxacin prophylaxis in patients with newly diagnosed myeloma (TEAMM): A multicentre, double-blind, placebo-controlled, randomised, phase 3 trial. Lancet Oncol. 2019, 20, 1760–1772. [Google Scholar] [CrossRef]
- Stahlmann, R.; Lode, H. Safety considerations of fluoroquinolones in the elderly: An update. Drugs Aging 2010, 27, 193–209. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | n = 74 | % |
|---|---|---|
| Median age (years) | 85 (80–95) | - |
| Woman | 40 | 54 |
| Myeloma type | ||
| IgG | 48 | 65 |
| IgA | 10 | 13 |
| Light chains | 16 | 22 |
| Hemoglobin < 10 g/dL | 64 | 86 |
| Calcium > 2.75 mmol/L | 19 | 26 |
| Clearance Cockcroft < 30 mL/min | 34 | 46 |
| GFR CKD EPI < 30 mL/min/1.73 m2 | 25 | 34 |
| Bone damage | 40 | 54 |
| Performance Status | ||
| <3 | 31 | 42 |
| ≥3 | 43 | 58 |
| Arterial hypertension | 49 | 66 |
| Chronic Heart Failure | 6 | 8 |
| Ischemic heart disease | 8 | 11 |
| Atrial fibrillation | 14 | 19 |
| Valvular heart disease | 5 | 7 |
| Diabetes | 12 | 16 |
| Chronic Kidney Failure | 13 | 18 |
| Chronic respiratory deficiencies | 7 | 9 |
| Cognitive disorders | 11 | 15 |
| History of Stroke | 5 | 7 |
| Albumin < 35 g/L | 41 | 55 |
| ADL | ||
| <4.5 | 23 | 31 |
| ≥4.5 | 51 | 69 |
| Walking with assistance | ||
| Without | 45 | 61 |
| With | 29 | 39 |
| Body Mass Index | ||
| <21 kg/m2 | 19 | 25.7 |
| ≥21 kg/m2 | 55 | 74.3 |
| Age-adjusted Charlson Comorbidity Index | ||
| <5 | 18 | 24 |
| ≥5 | 56 | 76 |
| Response Assessment | n = 74 | % |
|---|---|---|
| Therapeutic responses after induction | ||
| VGPR | 14 | 19 |
| PR | 34 | 45.9 |
| MR | 13 | 17.6 |
| SD | 3 | 4 |
| PD | 1 | 1.3 |
| Not evaluated | 9 | 12.2 |
| Overall response rate (CR + VGPR + PR) | 64.9% | |
| Best response during treatment | ||
| VGPR | 25 | 33.8 |
| PR | 29 | 39.2 |
| MR | 4 | 5.4 |
| SD | 6 | 8.1 |
| PD | 1 | 1.3 |
| Not evaluated | 9 | 12.2 |
| Overall response rate (CR + VGRP + PR) | 73% |
| Prognostic Factors | OS | PFS | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Sex (female vs. male) | 0.94 (0.53 to 1.67) | 0.82 | 0722 (0.40 to 1.28) | 0.23 |
| Age-Adjusted CCI (>5 vs. < 5) | 1.12 (0.58 to 2.15) | 0.74 | 1.04 (0.53 to 2.03) | 0.91 |
| ECOG PS (≥3 vs. <2) | 2.88 (1.61 to 5.15) | 0.0002 * | 2.13 (1.24 to 3,66) | 0.0027 * |
| Heart disease | 1.38 (0.76 to 2.51) | 0.26 | 1.54 (0.84 to 2.84) | 0.14 |
| Neurological disease | 0.70 (0.36 to 1.40) | 0.34 | 0.76 (0.38 to 1.53) | 0.48 |
| Lung disease | 1.43 (0.54 to 3.83) | 0.41 | 1.00 (0.43 to 2.36) | 0.99 |
| Comorbidities > 3 vs. <3 | 1.16 (0.65 to 2.05) | 0.62 | 1.08 (0.61 to 1.92) | 0.79 |
| Albumin < 35 g/L vs. >35 g/L | 1.15 (0.65 to 2.04) | 0.62 | 1.32 (0.45 to 2.33) | 0.34 |
| Hemoglobin < 10 g/dL vs. >10 g/dL | 1.33 (0.61 to 2.89) | 0.51 | 1.08 (0.52 to 2.24) | 0.83 |
| Calcium > 2.75 mmol/L vs. <2.75 mmol/L | 0.71 (0.37 to 1.34) | 0.33 | 0.77 (0.40 to 1.47) | 0.46 |
| Creatinine level > 177 µmol/L vs. <177 µmol/L | 1.42 (0.72 to 2.80) | 0.23 | 1.60 (0.79 to 3.24) | 0.13 |
| Clearance Cockcroft < 30 mL/min vs. >30 mL/min | 1.20 (0.67 to 2.13) | 0.53 | 0.78 (0.46 to 1.33) | 0.86 |
| GFR CKD < 30 mL/min vs. >30 mL/min | 1.54 (0.81 to 2.94) | 0.14 | 1.46 (0.79 to 2.69) | 0.16 |
| ADL < 4.5 vs. >4.5 | 3.16 (1.26 to 7.93) | 0.0002 * | 2.0 (1.074 to 3.72) | 0.0328 * |
| BMI < 21 vs. ≥21 | 1.89 (0.95 to 3.73) | 0.028 * | 1.28 (0.70 to 2.33) | 0.39 |
| Walking without assistance vs. with assistance | 2.01 (0.87 to 4.67) | 0.0033 * | 1.61 (0.92 to 2.84) | 0.073 |
| n = 74 | |
|---|---|
| Median number of bortezomib cycle | 9 |
| Total number of bortezomib cycle | - |
| <5 | 21 |
| 5–10 | 28 |
| >10 (maximum 25) | 25 |
| Bortezomib dose adaptation | n = 53 (71.6%) |
| 1 mg/m2 | 53 (71.6%) |
| 0.7 mg/m2 | 34 (46%) |
| Dexamethasone interruption | n = 10 (14.3%) |
| Temporary | 1 (1.3%) |
| Permanent | 9 (12%) |
| Study | No. of Patients | Median Age (Years) | PS ≥ 3 | Treatment | ORR (%) | PFS (Median, Months) | OS (Median, Months) | |
|---|---|---|---|---|---|---|---|---|
| Prospective | Upfront [25] | 502 | 73 | 0 | Vd/VTD/VMP | 73/80/70 | 14.7/15.4/17.3 | 49.8/51.5/53.1 |
| Vista [1] | 682 | 71 | 0 | VMP/MP | 71/35 | 24/11.6 | NA/43 | |
| First [2] | 1623 | 73 | 0.006 | Rd/MPT | 75/62 | 25.5/21.2 | 59.1/49.1 | |
| Larocca [14] | 152 | 78 | 0 | VP/VCP/VMP | 64/67/86 | 14/15.2/17.1 | 60/70/76% at 24 months | |
| O’Donnel [15] | 50 | 73 | 0 | VRD lite | 86 | 35.1 | Not reached | |
| SWOG [3,4] | 525 | 63 | 0 | VRd/Rd | 82/72 | 41/29 | Not Reached/69 | |
| ALCYONE [5,6] | 706 | 71 | 0 | DVMP/VMP | 90.9/73.9 | 36.4/19.3 | Not reached | |
| MAIA [7] | 737 | 74 | 0 | DRd/Rd | 92.9/81.3 | Not Reached/31.9 | 70.6/55.6% at 30 months | |
| Larocca [16] | 199 | 75 | 0 | Rd/Rd-R | 78/68 | 20.2/18.3 | 74/63% at 36 months | |
| Stege [24] | 65 | 81 | 8 | DIxd | 78 | 13.8 | 62% at 22.9 months | |
| Retrospective | Our study | 74 | 85 | 58 | Vd | 73 | 13.2 | 26.9 |
| Matsue [19] | 42 | 85 | 52.4 | Bortezomib +ImiD based | 88.1 | 19.1 | 31.9 | |
| Ediriwickrama [23] | 52 | 79 | 25 | T/Vd/Rd | 70 | NR | 36 | |
| Chan [20] | 155 | 76 | NR | VCD | 79.4 | 21.7 | 45.1 | |
| Gavriatopoulo [22] | 110 | 83 | 66 | VR/V/R | 63 | 7 | 21 | |
| Panistas [21] | 89 | 87 | NR | Bortezomib +ImiD based | NR | 11.7 | 22.2 | |
| Dimopoulos [22] | 155 | 82 | <60 | Bortezomib +ImiD based | 58 | NR | 22 | |
| Bang [18] | 139 | 80 | <32 | Bortezomib +ImiD based | 51 | 20 | 27 | |
| Medhekar [26] | 2342 | 67 | >3.4 | VRd | NR | 26.5 | NR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huynh, L.; Birsen, R.; Mora, L.; Couderc, A.-L.; Mitha, N.; Farcet, A.; Chebib, A.; Chaibi, P. Multiple Myeloma in Patients over 80: A Real World Retrospective Study of First Line Conservative Approach with Bortezomib Dexamethasone Doublet Therapy and Mini-Review of Literature. Cancers 2022, 14, 4741. https://doi.org/10.3390/cancers14194741
Huynh L, Birsen R, Mora L, Couderc A-L, Mitha N, Farcet A, Chebib A, Chaibi P. Multiple Myeloma in Patients over 80: A Real World Retrospective Study of First Line Conservative Approach with Bortezomib Dexamethasone Doublet Therapy and Mini-Review of Literature. Cancers. 2022; 14(19):4741. https://doi.org/10.3390/cancers14194741
Chicago/Turabian StyleHuynh, Laurence, Rudy Birsen, Lucie Mora, Anne-Laure Couderc, Nathalie Mitha, Anaïs Farcet, Amale Chebib, and Pascal Chaibi. 2022. "Multiple Myeloma in Patients over 80: A Real World Retrospective Study of First Line Conservative Approach with Bortezomib Dexamethasone Doublet Therapy and Mini-Review of Literature" Cancers 14, no. 19: 4741. https://doi.org/10.3390/cancers14194741
APA StyleHuynh, L., Birsen, R., Mora, L., Couderc, A.-L., Mitha, N., Farcet, A., Chebib, A., & Chaibi, P. (2022). Multiple Myeloma in Patients over 80: A Real World Retrospective Study of First Line Conservative Approach with Bortezomib Dexamethasone Doublet Therapy and Mini-Review of Literature. Cancers, 14(19), 4741. https://doi.org/10.3390/cancers14194741






