Accuracy of Response Assessment FDG PET-CT Post (Chemo)Radiotherapy in HPV Negative Oropharynx Squamous Cell Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Radiotherapy
2.2. Follow Up
2.3. PET-CT Protocol
2.4. Categorisation of PET-CT Response
2.5. Analysis and Statistics
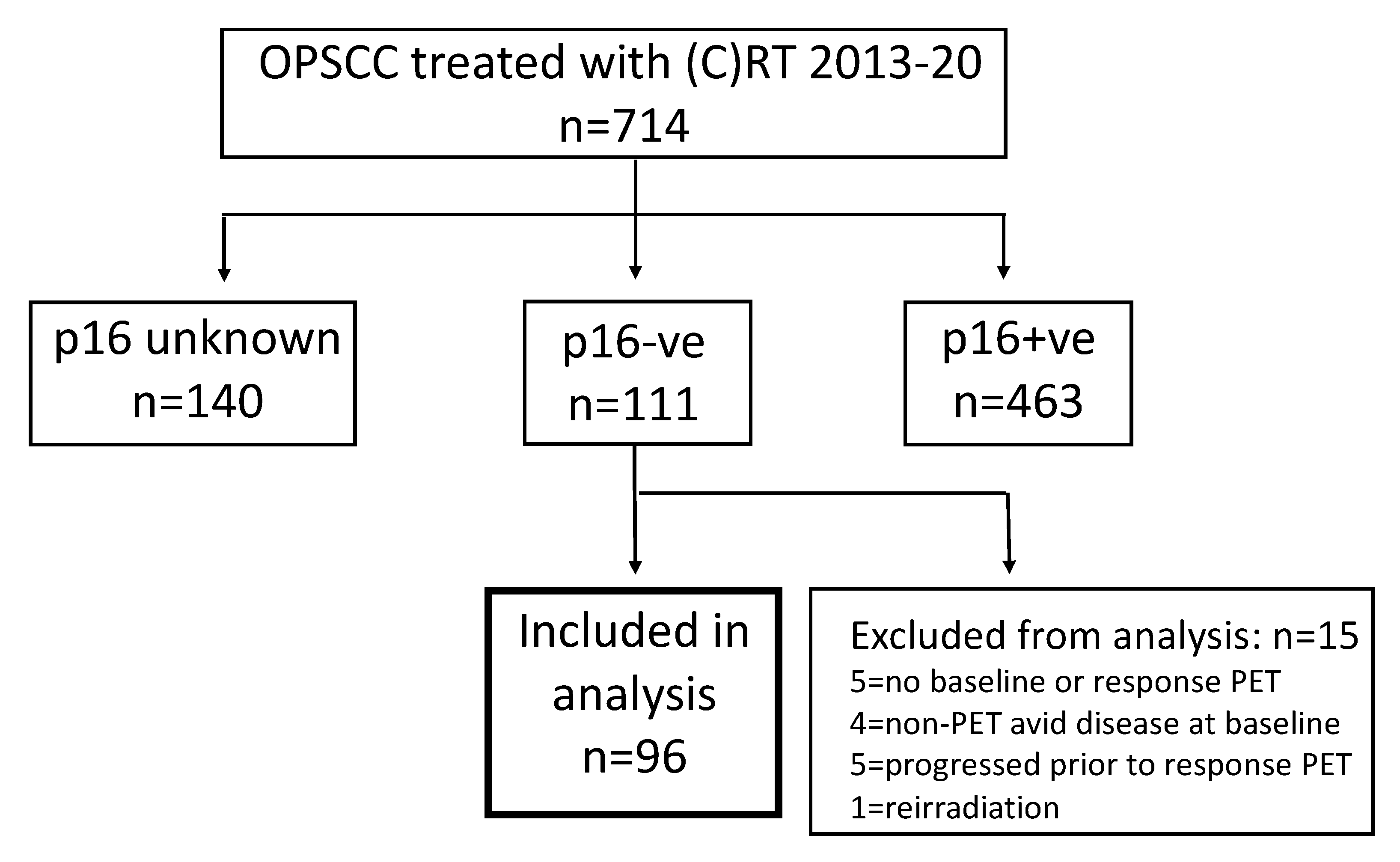
3. Results
3.1. Cohort Characteristics
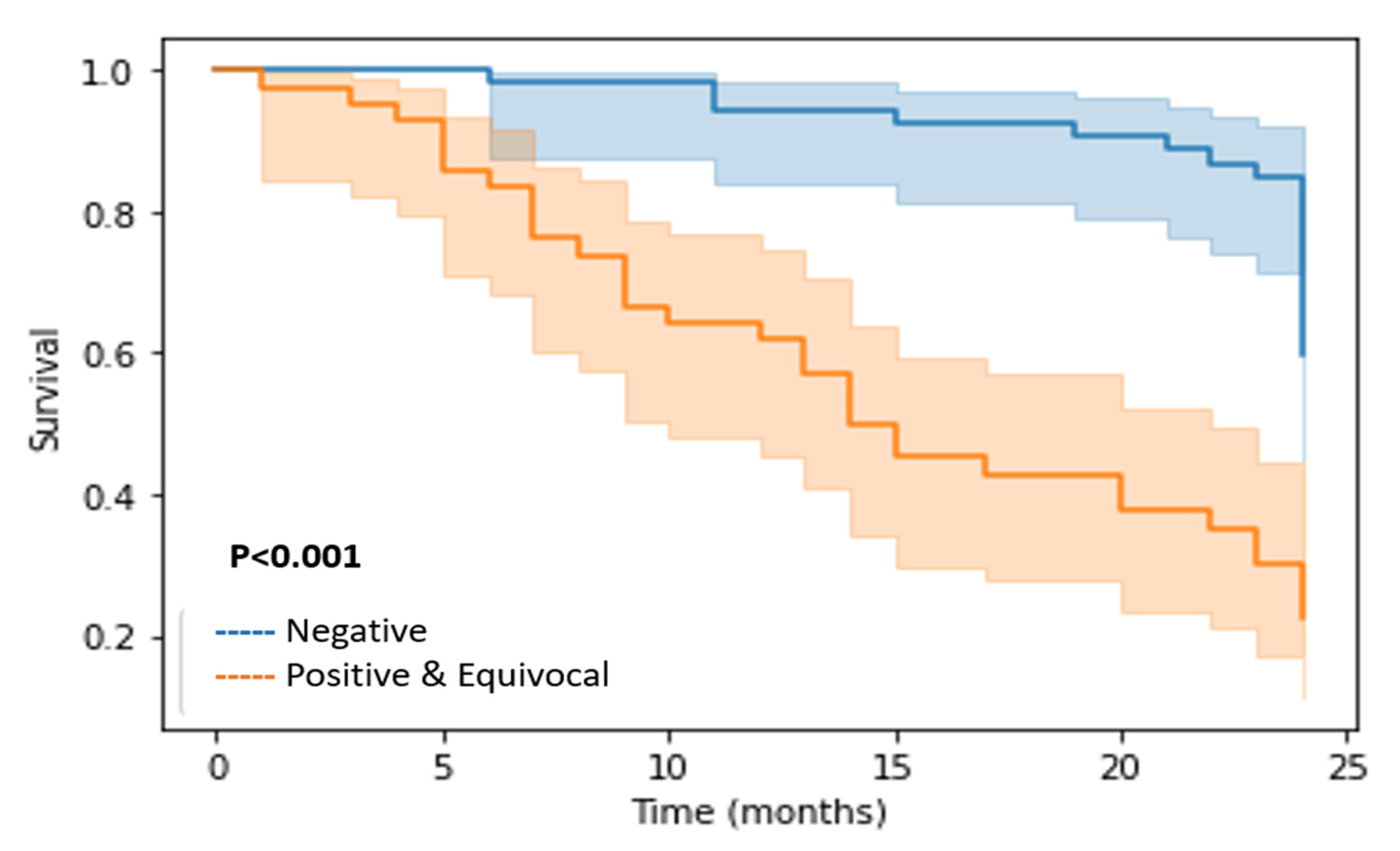
3.2. Outcomes
3.3. Assessment of Response by PET-CT and Correlation with Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tan, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed]
- American Joint Committee on Cancer (AJCC). TNM Classification of Malignant Tumours, 8th ed.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Shi, W.; Kato, H.; Perez-Ordonez, B.; Pintilie, M.; Huang, S.; Hui, A.; O’Sullivan, B.; Waldron, J.; Cummings, B.; Kim, J.; et al. Comparative prognostic value of HPV16 E6 mRNA compared with in situ hybridization for human oropharyngeal squamous carcinoma. J. Clin. Oncol. 2009, 27, 6213–6221. [Google Scholar] [CrossRef]
- Mehanna, H.; Evans, M.; Beasley, M.; Chatterjee, S.; Dilkes, M.; Homer, J.; O’Hara, J.; Robinson, M.; Shaw, R.; Sloan, P. Oropharyngeal cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S90–S96. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Jordan, R.C.K.; Zhao, W.; Sturgis, E.M.; Burtness, B.; et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef]
- Beitler, J.J.; Quon, H.; Jones, C.U.; Salama, J.K.; Busse, P.M.; Cooper, J.S.; Koyfman, S.A.; Ridge, J.A.; Saba, N.F.; Siddiqui, F.; et al. ACR Appropriateness Criteria (R) Locoregional therapy for resectable oropharyngeal squamous cell carcinomas. Head Neck 2016, 38, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, H.; Wong, W.L.; McConkey, C.C.; Rahman, J.K.; Robinson, M.; Hartley, A.G.; Nutting, C.; Powell, N.; Al-Booz, H.; Junor, E.; et al. PET-CT Surveillance versus Neck Dissection in Advanced Head and Neck Cancer. N. Engl. J. Med. 2016, 374, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Royal College of Physicians of Edinburgh; Administration of Radioactive Substances Advisory Committee. Evidence-based indications for the use of PET-CT in the United Kingdom. Clin. Radiol. 2016, 71, e171–e188. [Google Scholar] [CrossRef]
- Jones, T.M.; De, M.; Foran, B.; Harrington, K.; Mortimore, S. Laryngeal cancer: United Kingdom National Multidisciplinary guidelines. J. Laryngol. Otol. 2016, 130, S75–S82. [Google Scholar] [CrossRef]
- Vainshtein, J.M.; Spector, M.E.; Stenmark, M.H.; Bradford, C.R.; Wolf, G.T.; Worden, F.P.; Chepeha, D.B.; McHugh, J.B.; Carey, T.; Wong, K.K.; et al. Reliability of post-chemoradiotherapy F-18-FDG PET/CT for prediction of locoregional failure in human papillomavirus-associated oropharyngeal cancer. Oral Oncol. 2014, 50, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Porceddu, S.V.; Pryor, D.I.; Burmeister, E.; Burmeister, B.H.; Poulsen, M.G.; Foote, M.C.; Panizza, B.; Coman, S.; McFarlane, D.; Coman, W. Results of a prospective study of positron emission tomography-directed management of residual nodal abnormalities in node-positive head and neck cancer after definitive radiotherapy with or without systemic therapy. Head Neck 2011, 33, 1675–1682. [Google Scholar] [CrossRef]
- Slevin, F.; Subesinghe, M.; Ramasamy, S.; Sen, M.; Scarsbrook, A.F.; Prestwich, R.J. Assessment of outcomes with delayed (18) F-FDG PET-CT response assessment in head and neck squamous cell carcinoma. Br. J. Radiol. 2015, 88, 20140592. [Google Scholar] [CrossRef] [PubMed]
- Bird, T.; Barrington, S.; Thavaraj, S.; Jeannon, J.P.; Lyons, A.; Oakley, R.; Simo, R.; Lei, M.; Guerrero Urbano, T. (18) F-FDG PET/CT to assess response and guide risk-stratified follow-up after chemoradiotherapy for oropharyngeal squamous cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.T.; Dmytriw, A.A.; Yu, E.; Waldron, J.; Lu, L.; Fazelzad, R.; de Almeida, J.R.; Veit-Haibach, P.; O’Sullivan, B.; Xu, W.; et al. (18) F-FDG PET/CT for locoregional surveillance following definitive treatment of head and neck cancer: A meta-analysis of reported studies. Head Neck 2019, 41, 551–561. [Google Scholar] [CrossRef]
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-associated oropharyngeal cancer: Epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef]
- Rulach, R.; Zhou, S.; Hendry, F.; Stobo, D.; James, A.; Dempsey, M.F.; Grose, D.; Lamb, C.; Schipani, S.; Rizwanullah, M.; et al. 12 week PET-CT has low positive predictive value for nodal residual disease in human papillomavirus-positive oropharyngeal cancers. Oral Oncol. 2019, 97, 76–81. [Google Scholar] [CrossRef]
- Zhong, J.; Sundersingh, M.; Dyker, K.; Currie, S.; Vaidyanathan, S.; Prestwich, R.; Scarsbrook, A. Post-treatment FDG PET-CT in head and neck carcinoma: Comparative analysis of 4 qualitative interpretative criteria in a large patient cohort. Sci. Rep. 2020, 10, 4086. [Google Scholar] [CrossRef]
- Urban, R.; Godoy, T.; Olson, R.; Wu, J.; Berthelet, E.; Tran, E.; DeVries, K.; Wilson, D.; Hamilton, S. FDG-PET/CT scan assessment of response 12 weeks post radical radiotherapy in oropharynx head and neck cancer: The impact of p16 status. Radiother. Oncol. 2020, 148, 14–20. [Google Scholar] [CrossRef]
- Zhou, S.; Chan, C.; Rulach, R.; Dyab, H.; Hendry, F.; Maxfield, C.; Dempsey, M.F.; James, A.; Grose, D.; Lamb, C.; et al. Long term survival in patients with human papillomavirus-positive oropharyngeal cancer and equivocal response on 12-week PET-CT is not compromised by the omission of neck dissection. Oral Oncol. 2022, 128, 105870. [Google Scholar] [CrossRef]
- Ng, S.P.; Johnson, J.M.; Gunn, G.B.; Rosenthal, D.I.; Skinner, H.D.; Phan, J.; Frank, S.J.; Morrison, W.; Sturgis, E.M.; Mott, F.E.; et al. Significance of Negative Posttreatment 18-FDG PET/CT Imaging in Patients With p16/HPV-Positive Oropharyngeal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1029–1035. [Google Scholar] [CrossRef]
- Singhi, A.D.; Westra, W.H. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer 2010, 116, 2166–2173. [Google Scholar] [CrossRef]
- Gregoire, V.; Evans, M.; Le, Q.T.; Bourhis, J.; Budach, V.; Chen, A.; Eisbruch, A.; Feng, M.; Giralt, J.; Gupta, T.; et al. Delineation of the primary tumour Clinical Target Volumes (CTV-P) in laryngeal, hypopharyngeal, oropharyngeal and oral cavity squamous cell carcinoma: AIRO, CACA, DAHANCA, EORTC, GEORCC, GORTEC, HKNPCSG, HNCIG, IAG-KHT, LPRHHT, NCIC CTG, NCRI, NRG Oncology, PHNS, SBRT, SOMERA, SRO, SSHNO, TROG consensus guidelines. Radiother. Oncol. 2018, 126, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Iyizoba-Ebozue, Z.; Murray, L.J.; Ramasamy, S.; Sen, M.; Murray, P.; Cardale, K.; Dyker, K.; Prestwich, R.J.D. Radiotherapy for Oropharyngeal Carcinoma With an Uninvolved Contralateral Neck: The Safety of Omission of Contralateral High Level II and Retropharyngeal Lymph Nodes From Elective Target Volumes. Clin. Oncol. R Coll. Radiol. 2021, 33, 331–339. [Google Scholar] [CrossRef]
- Ramasamy, S.; Murray, L.J.; Cardale, K.; Dyker, K.E.; Murray, P.; Sen, M.; Prestwich, R.J.D. Quality Assurance Peer Review of Head and Neck Contours in a Large Cancer Centre via a Weekly Meeting Approach. Clin. Oncol. R Coll. Radiol. 2019, 31, 344–351. [Google Scholar] [CrossRef]
- Prestwich, R.J.D.; Arunsingh, M.; Zhong, J.; Dyker, K.E.; Vaidyanathan, S.; Scarsbrook, A.F. Second-look PET-CT following an initial incomplete PET-CT response to (chemo)radiotherapy for head and neck squamous cell carcinoma. Eur. Radiol. 2020, 30, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Arunsingh, M.; Vaidyanathan, S.; Dyker, K.E.; Sen, M.; Scarsbrook, A.F.; Prestwich, R.J.D. Accuracy of Response Assessment Positron Emission Tomography-Computed Tomography Following Definitive Radiotherapy Without Chemotherapy for Head and Neck Squamous Cell Carcinoma. Clin. Oncol. R Coll. Radiol. 2019, 31, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; O’Sullivan, B.; Xu, W.; Zhao, H.; Chen, D.D.; Ringash, J.; Hope, A.; Razak, A.; Gilbert, R.; Irish, J.; et al. Temporal nodal regression and regional control after primary radiation therapy for N2-N3 head-and-neck cancer stratified by HPV status. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 1078–1085. [Google Scholar] [CrossRef]
- Liu, H.Y.; Milne, R.; Lock, G.; Panizza, B.J.; Bernard, A.; Foote, M.; McGrath, M.; Brown, E.; Gandhi, M.; Porceddu, S.V. Utility of a repeat PET/CT scan in HPV-associated Oropharyngeal Cancer following incomplete nodal response from (chemo)radiotherapy. Oral Oncol. 2019, 88, 153–159. [Google Scholar] [CrossRef]
- Awan, M.J.; Lavertu, P.; Zender, C.; Rezaee, R.; Fowler, N.; Karapetyan, L.; Gibson, M.; Wasman, J.; Faulhaber, P.; Machtay, M.; et al. Post-treatment PET/CT and p16 status for predicting treatment outcomes in locally advanced head and neck cancer after definitive radiation. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 988–997. [Google Scholar] [CrossRef]

| n = 96 | % | |
|---|---|---|
| Age (Median, Years) | 60 | |
| Sex | ||
| • Male | 71 | 74% |
| • Female | 25 | 26% |
| Tumour subsite | ||
| • Tonsil | 50 | 27.1% |
| • Base of tongue | 26 | 52.1% |
| • Vallecula | 3 | 3.1% |
| • Posterior pharyngeal wall | 7 | 7.3% |
| • Soft palate | 10 | 10.4% |
| T stage | ||
| • T1 | 8 | 8.3% |
| • T2 | 36 | 37.5% |
| • T3 | 29 | 30.2% |
| • T4 | 23 | 24% |
| N stage | ||
| • N0 | 15 | 15.6% |
| • N1 | 15 | 14.9% |
| • N2a | 2 | 2.1% |
| • N2b | 42 | 43.75% |
| • N2c | 22 | 22.9% |
| • N3 | 0 | 0% |
| Staging | ||
| • I | 1 | 1% |
| • II | 6 | 6.3% |
| • III | 17 | 17.7% |
| • IV | 72 | 75% |
| Smoking | ||
| • Current Smoker | 55 | 57.3% |
| • Ex-smoker | 31 | 32.3% |
| • Never smoked | 10 | 10.4% |
| Histopathology/Grade | ||
| • 1 | 3 | 3.1% |
| • 2 | 39 | 40.6% |
| • 3 | 45 | 46.9% |
| • Unknown | 9 | 9.4% |
| Fractionation | ||
| • 70 Gy/35# | 84 | 87.5% |
| • 65 Gy/30# | 12 | 12.5% |
| Radiotherapy alone | 35 | 36.4% |
| Concurrent chemotherapy | 61 | 63.5% |
| Agent | ||
| • Cisplatin | 44 | 72.1% |
| • Carbo | 8 | 13.1% |
| • Cis/Carbo | 8 | 13.1% |
| • Cetuximab | 1 | 1.6% |
| No of cycles | ||
| • 1 | 2 | 3.33% |
| • 2 | 35 | 58.33% |
| • 3 | 20 | 33.33% |
| • >3 | 3 | 5 |
| Induction chemotherapy | ||
| • TPF | 2 | 3.3% |
| Primary Site n = 93 | Neck Nodes n = 77 | Overall Primary, Neck Nodes and Distant n = 96 | |
|---|---|---|---|
| PET-CT positive (equivocal and positive) | 29 | 18 | 42 |
| PET-CT negative | 64 | 59 | 54 |
| True positive | 19 | 15 | 31 |
| True negative | 60 | 55 | 46 |
| False positive | 10 | 3 | 11 |
| False negative | 4 | 4 | 8 |
| Sensitivity | 82.6% | 78.9% | 79.4% |
| Specificity | 85.7% | 94.8% | 75.4% |
| Positive predictive value | 65.5% | 83.3% | 73.8% |
| Negative predictive value | 93.7% | 93.2% | 85.2% |
| Accuracy | 84.9% | 90.9% | 80.2% |
| PET-CT Response Scan Outcome for FDG-Avid Primary Site | Number | Disease Status on Follow-UpDisease + | Disease Status on Follow-UpDisease − | Post-Test Probability |
|---|---|---|---|---|
| Positive | 8 | 8 | 0 | 0.99 |
| Negative | 64 | 4 | 60 | 0.06 |
| Equivocal | 21 | 11 | 10 | 0.51 |
| Total | 93 | 23 | 70 |
| PET-CT Response Scan Outcome for FDG-Avid Nodal Disease | Number | Disease Status on Follow-Up Disease + | Disease Status on Follow-UpDisease − | Post-Test Probability |
|---|---|---|---|---|
| Positive | 7 | 7 | 0 | 0.99 |
| Negative | 59 | 4 | 55 | 0.07 |
| Equivocal | 11 | 8 | 3 | 0.72 |
| Total | 77 | 19 | 58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iyizoba-Ebozue, Z.; Billingsley, S.; Frood, R.; Vaidyanathan, S.; Scarsbrook, A.; Prestwich, R.J.D. Accuracy of Response Assessment FDG PET-CT Post (Chemo)Radiotherapy in HPV Negative Oropharynx Squamous Cell Carcinoma. Cancers 2022, 14, 4680. https://doi.org/10.3390/cancers14194680
Iyizoba-Ebozue Z, Billingsley S, Frood R, Vaidyanathan S, Scarsbrook A, Prestwich RJD. Accuracy of Response Assessment FDG PET-CT Post (Chemo)Radiotherapy in HPV Negative Oropharynx Squamous Cell Carcinoma. Cancers. 2022; 14(19):4680. https://doi.org/10.3390/cancers14194680
Chicago/Turabian StyleIyizoba-Ebozue, Zsuzsanna, Sarah Billingsley, Russell Frood, Sriram Vaidyanathan, Andrew Scarsbrook, and Robin J. D. Prestwich. 2022. "Accuracy of Response Assessment FDG PET-CT Post (Chemo)Radiotherapy in HPV Negative Oropharynx Squamous Cell Carcinoma" Cancers 14, no. 19: 4680. https://doi.org/10.3390/cancers14194680
APA StyleIyizoba-Ebozue, Z., Billingsley, S., Frood, R., Vaidyanathan, S., Scarsbrook, A., & Prestwich, R. J. D. (2022). Accuracy of Response Assessment FDG PET-CT Post (Chemo)Radiotherapy in HPV Negative Oropharynx Squamous Cell Carcinoma. Cancers, 14(19), 4680. https://doi.org/10.3390/cancers14194680






