Simple Summary
Cancer pain is often inadequately treated, as shown by several clinical studies. This problem has been confirmed in different clinical settings but the reasons for this phenomenon are unclear. Furthermore, little evidence is available on the adequacy of pharmacological pain management in patients undergoing radiotherapy. Moreover, studies investigating possible predictors of inadequate pain management reported contradictory results. Therefore, in this analysis, we evaluated a large population of cancer patients undergoing radiotherapy. We recorded, similarly to previous studies, a 45% rate of patients with inadequate analgesic therapy. Furthermore, evaluating the characteristics of patients with inadequate analgesic treatment, we noted that the subjects with better general conditions or better prognostic factors are those most frequently receiving inadequate drug therapy.
Abstract
Aim: The frequent inadequacy of pain management in cancer patients is well known. Moreover, the quality of analgesic treatment in patients treated with radiotherapy (RT) has only been rarely assessed. In order to study the latter topic, we conducted a multicenter, observational and prospective study based on the Pain Management Index (PMI) in RT Italian departments. Methods: We collected data on age, gender, tumor site and stage, performance status, treatment aim, and pain (type: CP—cancer pain, NCP—non-cancer pain, MP—mixed pain; intensity: NRS: Numeric Rating Scale). Furthermore, we analyzed the impact on PMI on these parameters, and we defined a pain score with values from 0 (NRS: 0, no pain) to 3 (NRS: 7–10: intense pain) and an analgesic score from 0 (pain medication not taken) to 3 (strong opioids). By subtracting the pain score from the analgesic score, we obtained the PMI value, considering cases with values < 0 as inadequate analgesic prescriptions. The Ethics Committees of the participating centers approved the study (ARISE-1 study). Results: Two thousand one hundred four non-selected outpatients with cancer and aged 18 years or older were enrolled in 13 RT departments. RT had curative and palliative intent in 62.4% and 37.6% patients, respectively. Tumor stage was non-metastatic in 57.3% and metastatic in 42.7% of subjects, respectively. Pain affected 1417 patients (CP: 49.5%, NCP: 32.0%; MP: 18.5%). PMI was < 0 in 45.0% of patients with pain. At multivariable analysis, inadequate pain management was significantly correlated with curative RT aim, ECOG performance status = 1 (versus both ECOG-PS3 and ECOG- PS4), breast cancer, non-cancer pain, and Central and South Italy RT Departments (versus Northern Italy).Conclusions: Pain management was less adequate in patients with more favorable clinical condition and stage. Educational and organizational strategies are needed in RT departments to reduce the non-negligible percentage of patients with inadequate analgesic therapy.
1. Introduction
Pain, depression, and fatigue are common cancer symptoms. They have been identified by the National Cancer Institute as “priority symptoms” needing assessment [1]. Pain is one of the most frequent clinical symptoms in cancer patients, resulting from primary cancer progression, metastases, and treatment adverse effects. In fact, it has been estimated that up to 90% of patients can suffer from nociceptive and/or neuropathic pain during the course of tumor disease [2,3].
Moreover, pain is a multidimensional syndrome, severely worsening a patient’s quality of life (QoL) due to physical and emotional impact [4,5,6,7]. In fact, in cancer patients, lack of pain control is the best predictor of worse QoL as a result of its negative effect on daily activities, mood, and personal independence [8,9].
Therefore, pain relief represents a priority in oncology, and pain evaluation before and during treatment is recommended to treat this symptom effectively [3,10]. Unfortunately, inadequate treatment of pain is frequent despite the availability of guidelines for cancer pain management and of several effective analgesic therapies [11,12,13,14,15,16].
For this reason, many studies evaluated pain management in different cancer settings [17,18,19,20,21,22,23,24,25,26,27,28]. However, only a few reports on this topic are available for patients treated with radiotherapy (RT). Therefore, we planned a multicenter observational study to assess the adequacy of pain management in cancer patients treated with RT in Italian centers.
2. Patients and Methods
2.1. Study Aims
The primary objective of the trial was to evaluate the adequacy of pain management in patients treated in RT departments. The secondary objective was to evaluate any correlation between adequacy of pain management and potential predictors (gender, age, performance status, timing of the visit, RT aim, primary tumor, stage of disease, type of pain and geographical location of the RT center).
2.2. Study Design
It was an observational, prospective, multicenter cohort study. Patients were enrolled after signing the informed consent. The study was approved by the Ethics Committees of participating centers (ARISE 327/2017/O/Oss). All patients who underwent a medical examination in the participating centers were considered for the study enrollment. All patients who met the enrollment criteria and who underwent a clinical visit at least once in the RT departments of participating centers in the period October–November 2019 were included. The evaluation was performed regardless of the visit timing (ongoing RT visits or clinical evaluation at the end of treatment). However, each patient was evaluated only once. The data were recorded through a collection form filled in during the visit. Data on gender, age, Eastern Cooperative Oncology Group Performance Status Scale (ECOG-PS), RT aim, primary cancer, tumor stage, intensity of pain measured with the Numeric Rating Scale (NRS), analgesic score and type of pain (cancer pain: CP, non-cancer pain: NCP, mixed pain: MP) were collected.
2.3. Inclusion Criteria
Inclusion criteria were: (1) cancer patients (regardless of stage, primary tumor, tumor stage, and RT aim), (2) treated in RT departments, (3) aged ≥ 18 years. Patients with comorbidities (psychiatric disorders or neurosensory deficits) preventing data collection or granting of consent were excluded.
2.4. End Points
We assigned a pain score by using the following values: 0 (NRS: 0, no pain), 1 (NRS: 1–4, mild pain), 2 (NRS: 5–6, moderate pain), and 3 (NRS: 7–0, intense pain). In addition, based on the therapy the patients took, we defined an analgesic score as follows: no analgesics: 0, non-opioid analgesics: 1, “weak”opioids: 2, and “strong” opioids: 3. The Pain Management Index (PMI) was calculated by subtracting the pain score from the analgesic score, considering prescriptions with a negative value as inadequate [29,30].
2.5. Statistical Analysis
Gender, age, PS, timing of the visit, RT aim, primary tumor, stage of disease, type of pain, analgesics score, and RT center were explored as potential correlations with PMI. Using SYSTAT (version 11.0, SPSS, Chicago, IL, USA) we evaluated the correlation between PMI and potential predictors with the chi-squared test, considering values < 0.05 as significant. Furthermore, we included in the multivariate analysis (multiple logistic regression) the variables found to be statistically significant at the univariate analysis, in order to confirm the predictive impact of potential predictors of inadequate PMI.
3. Results
3.1. Patient Characteristics
Overall, 2104 patients were enrolled in the study, of which 1417 complained of pain and 1090 were taking analgesic drugs. Patient characteristics are shown in Table 1.

Table 1.
Patient characteristics.
3.2. Pain Management Index (PMI)
Considering all patients enrolled in the study, the rate of subjects with PMI < 0 was 30% (Figure 1). Furthermore, concerning only patients with pain or receiving analgesics, the PMI value was <0 in 639 subjects (45.0%) (Figure 2). Of patients enrolled and undergoing palliative and curative RT, 28% (Figure 3) and 32% (Figure 4) showed PMI < 0, respectively. Instead, considering only patients with pain of the subjects undergoing palliative and curative RT, 30% (Figure 5) and 62% (Figure 6) showed PMI < 0, respectively.
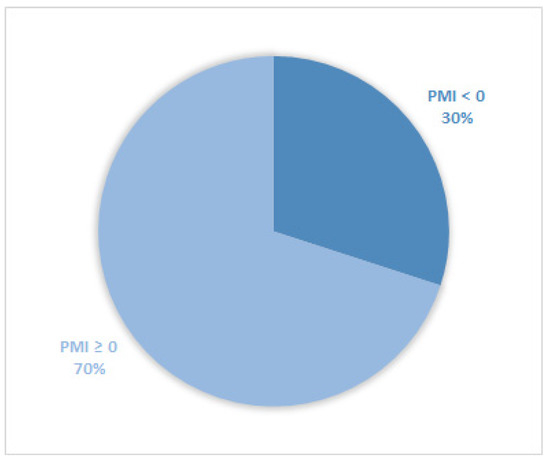
Figure 1.
Pie chart displaying the percentage of patients with PMI < 0 and PMI ≥ 0. All patients were included (2104).
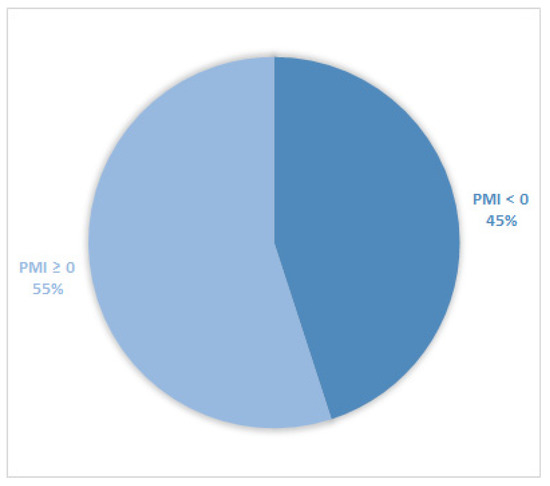
Figure 2.
Pie chart displaying the percentage of patients with PMI < 0 and PMI ≥ 0. Only patients with pain or receiving analgesics were included (1417).

Figure 3.
Pie chart displaying the percentage of patients with PMI < 0 and PMI ≥ 0. Only patients undergoing palliative radiotherapy were included (791).

Figure 4.
Pie chart displaying the percentage of patients with PMI < 0 and PMI ≥ 0. Only patients undergoing curative radiotherapy were included (1313).
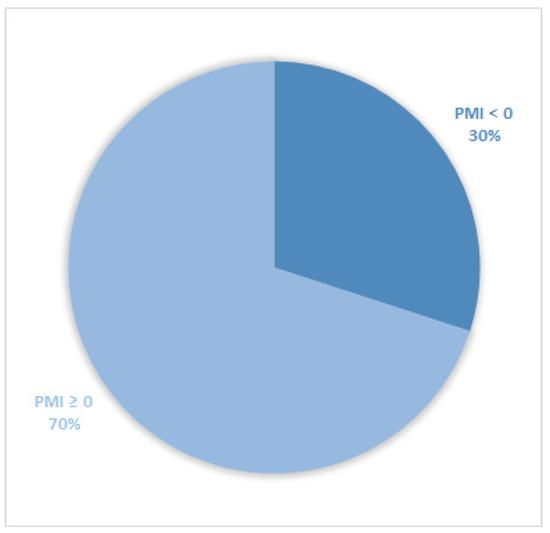
Figure 5.
Pie chart displaying the percentage of patients with PMI < 0 and PMI ≥ 0. Only patients undergoing palliative radiotherapy and with pain or receiving analgesics were included (737).
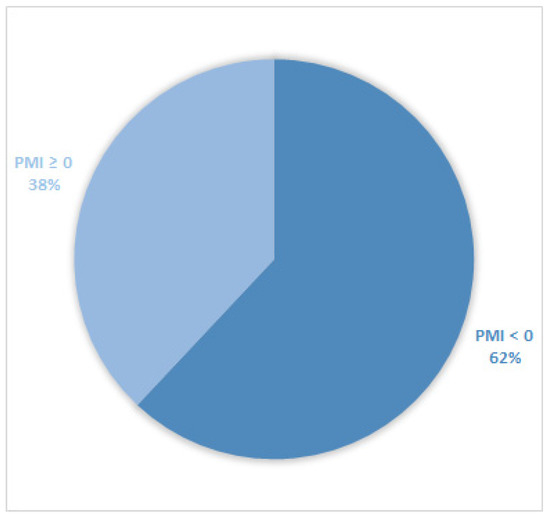
Figure 6.
Pie chart displaying the percentage of patients with PMI < 0 and PMI ≥ 0. Only patients undergoing curative radiotherapy and with pain or receiving analgesics were included (680).
3.3. Predictors of Pain Management Adequacy
At univariate analysis, performed only on patients with pain or taking analgesics, the following parameters were significantly correlated with PMI < 0: female gender, curative treatment aim, lower ECOG-PS score, breast cancer, non-cancer pain, non-metastatic stage, RT department in the center or south of Italy (Table 2). The multivariate analysis, in the same patient population, confirmed the significant correlation with PMI < 0 of the following parameters: ECOG-PS1 (versus both ECOG-PS3 and ECOG-PS4), breast cancer (versus prostate, gastrointestinal, uterine, head and neck, and other cancers), non-cancer pain (versus cancer-related pain), and location of the RT center in the center or south of Italy (versus northern Italy) (Table 3).

Table 2.
Univariate analysis on Pain Management Index (only 1417 patients with pain or under analgesic therapy included).

Table 3.
Multivariable analysis (only 1417 patients with pain or under analgesic therapy included).
4. Discussion
In a multicenter study including over two thousand patients evaluated during RT, the rate of patients with inadequate pain management (PMI < 0) was 45.0%. The inadequacy of analgesic therapy was significantly correlated to different parameters: (i) the patient’s physical condition (more frequent in subjects with better performance status), (ii) the type of tumor treated (more frequent in breast cancer), (iii) the origin of pain (more frequent in non-neoplastic pain), and (iv) the geographic location of the RT department (more frequent in central and southern Italy).
Finally, the lack of pathophysiological classification of pain (nociceptive versus neuropathic versus mixed), in our analysis, did not allow the evaluation of the impact of this parameter on the adequacy of pain management.
Some observations can be made by comparing our results with some previous analyses (Table 4). PMI is more frequently negative in patients undergoing curative treatment than in those undergoing palliative RT. A similar result was previously reported by Fujii et al., who observed a significantly higher rate of patients with PMI < 0 in subjects undergoing adjuvant chemotherapy compared to patients receiving chemotherapy for advanced disease [24]. Furthermore, in our analysis a PMI < 0 was more frequently observed in patients with ECOG-PS 1 than in ECOG-PS 3–4. This result confirms other studies reporting similar correlations [17,24]. Moreover, both the association with a palliative aim of RT and that with worse ECOG-PS suggests greater attention to pain management in patients in worse clinical conditions.

Table 4.
Comparison with other studies on Pain Management Index evaluated in cancer patients.
Furthermore, a negative PMI is more common in breast cancer patients than in all other cancers (60.5% versus 30.9–49.4%). These data cannot be explained simply and in particular, at least in our series, cannot be interpreted just on the basis of the female gender. In fact, our multivariate analysis did not show a significant correlation between gender and PMI. Furthermore, a significantly higher rate of PMI < 0 was recorded also compared to other female cancers (endometrium and uterine cervix). Moreover, the correlation between PMI < 0 and breast cancer was also reported in other studies [17,25,28]. We could reasonably hypothesize that breast cancer patients have several factors predisposing them to poor pain management. In fact, in most cases, they are patients undergoing adjuvant RT after surgery and therefore: (i) they are in good clinical conditions (ECOG-PS 1), (ii) if they suffer from pain this often depends on previous surgery (and therefore the origin of the symptom is non-neoplastic), (iii) they receive an adjuvant treatment (and therefore with curative purposes).
However, these explanations are not convincing given that the same correlation between PMI < 0 and breast cancer was recorded in a study including only metastatic patients undergoing palliative RT [17]. Other authors tried to interpret this finding otherwise and in particular considering the greater sensitivity to pain of female patients [31], their lower compliance with analgesic intake and a tendency to stop therapy early, at the first signs of improvement [14], and, more generally, the complexity of pain management in breast cancer, as this symptom is part of clusters that also include fear of relapse, fatigue, and anxiety [32]. Finally, another possible explanation could concern the high incidence of bone metastases, often painful, in this patient population. However, the lack of registration of the sites of metastatic disease, in our database, precludes a confirmation analysis of this hypothesis.
Furthermore, PMI < 0 is more frequent in case of non-neoplastic pain, i.e., produced by benign comorbidities. This result is similar to the findings of two previously published analyses [19,25]. Finally, negative PMI values are more common in patients treated in southern and central Italy. Geographic variations in the adequacy of analgesic therapy within the same country were previously reported in a study conducted in Taiwan [25].
According to a literature review, PMI < 0 is recorded in about 43% of cancer patients [15], although a trend towards a reduction of this figure has been observed in recent decades [33]. Our result (PMI < 0:45.0%) is similar to that reported in the cited literature review [15] and in other similar PMI-based analyses (PMI < 0:39.7–53.0%) [24,27,28]. Instead, other analyses recorded worse results (PMI <0:77–83%) [19,22]. One study was on patients treated over 10 years ago in a center of southern Italy [19], and the other an analysis on a particularly young population (≤60 years: 75% of patients) and therefore probably in relatively good clinical condition, a status that in our and other analyses correlates with higher negative PMI rates [22]. Conversely, other analyses recorded lower rates (4–33.3%) of inadequate pain management [17,18,20,21,23,25,26]. In some cases [17,18,21] this result can be explained by the enrollment of patients undergoing only palliative treatment, which, from ours and Fujii’s et al. [24] analyses, correlates with better pain management Instead, in other cases, the improved adequacy of pain treatments may be due to patient management in supportive or palliative care departments [20,23,26].
This analysis has several limitations. In fact, the study analyzed only the pain management but not the impact on quality of life. Furthermore, the PMI assessment was performed at different times (during or at the end of RT), with only one evaluation per patient. Therefore, it is difficult to assess how much inadequate pain management is attributable to the physicians who treated the patients prior to RT or to radiation oncologists. However, in patients assessed at the end of RT, the rate of negative PMI was lower compared to patients evaluated during treatment (38.0% versus 47.0%; p: 0.015). Both the progressive adjustment of drug therapy during RT and the analgesic effect of RT in patients undergoing palliative treatment could have led to this difference.
Another weak point of the study is the known limitations of the PMI, the tool we used to assess the adequacy of pain management. In fact, the PMI is based on the obsolete distinction between weak and strong opioids [30]. Furthermore, the correlation of PMI with quality of life is questionable. Indeed, a PMI < 0 is not significantly correlated with patients’ desire to receive greater attention to their pain [28]. However, lower PMI values are generally correlated with a higher percentage of patients complaining of pain interfering with their daily life [26].
Another limitation of the PMI is that this index is generally calculated on the basis of the analgesic therapy prescribed and not of that actually taken by the patients [29,30,34,35]. However, our study was conducted by interviewing patients and then gathering information on the therapy taken and not on the prescribed ones. Nevertheless, the PMI’s main limitation is to consider all patients taking strong opioids as adequately treated. In fact, all these subjects have a PMI value ≥ 0, regardless of the type and dose of the drugs, and especially of the degree of pain relief [30]. However, given its correlation with the quality of pain treatment and the easy calculation and collection, the PMI remains the most frequently used surrogate indicator of the appropriateness of pharmacological pain management [36].
5. Conclusions
In conclusion, the result of our and other analyses suggest that the attention to adequate pain therapy is lower in patients with better clinical conditions (good PS, non-neoplastic pain) and with a more favorable prognosis (RT for curative purposes).
Moreover, the near to 50% rate of patients not receiving adequate analgesic therapy in RT departments deserves attention. Therefore, in this clinical setting, it could be useful: (i) to implement the systematic registration of PMI, in addition to that of pain, to screen patients with inadequate pain management, (ii) to promote educational strategies for medical and nursing staff aimed at improving the awareness of this topic and the ability to adequately identify and treat patients with painful symptoms, (iii) to improve symptom management also through multidisciplinary collaborations (multidisciplinary teams, joint clinics).
Furthermore, considering several points not fully clarified by the published reports, further research seems necessary. Future studies could have the following aims: (i) to prospectively analyze the evolution of pain and its management during the path of patients in RT departments, to identify opportunities for optimization, (ii) to test the impact of educational strategies aimed at improving knowledge and skills of radiation oncologists in non-invasive pharmacological pain management, and (iii) to analyze the characteristics of pain in patients referred to RT to possibly optimize timing and methods of radiation oncologists’ consultations.
Author Contributions
Conceptualization, C.M.D., A.G.M. and M.M.; methodology, A.G.M.; software, C.M.D.; validation, A.G.M., M.M. and F.C.; formal analysis, E.N.; investigation, G.S.; resources, A.Z.; data curation, F.M., A.D.R., M.P., C.D.T., A.S., C.T., R.D.F., S.P., S.C., V.F., A.B., P.Z., L.Z., L.C., G.M., F.F., G.N., R.R.; writing—original draft preparation, M.B., A.G.M. and C.M.D.; writing—review and editing, A.G.M., M.M. and C.M.D.; visualization, M.B.; supervision, F.C., D.G., F.D., M.B., M.M., A.G.M.; project administration, A.G.M., M.M. and C.M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by Ethics Committee of IRCCS Azienda Ospedaliero-Universitaria di Bologna (ARISE: N° 327/2017/O/Oss).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data supporting the reported results will be made available on reasonable request.
Acknowledgments
We would like to express our gratitude to all those who helped us during the writing of this manuscript.
Conflicts of Interest
AGM reports grants from Elekta, personal fees from Astellas and Alfa-Sigma, and grants from Elekta, Tema Sinergie, Janssen, Bayer, and Igea outside the submitted work. The other authors declare no conflict of interest.
References
- Patrick, D.L.; Ferketich, S.L.; Frame, P.S.; Harris, J.J.; Hendricks, C.B.; Levin, B.; Link, M.P.; Lustig, C.; McLaughlin, J.; Douglas Ried, L.; et al. National Institutes of Health State-of-the Science Conference statement: Symptom Management in Cancer: Pain, Depression, and Fatigue. J. Natl. Cancer Inst. 2003, 95, 1110–1117. [Google Scholar] [PubMed]
- Davies, A.N.; Dickman, A.; Reid, C.; Stevens, A.M.; Zeppetella, G. The management of cancer-related breakthrough pain: Recommendations of a task group of the Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. Eur. J. Pain 2009, 13, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.L.; Hamamoto, D.T.; Simone, D.A.; Wilcox, G.L. Mechanism of Cancer Pain. Mol. Interv. 2010, 10, 164–178. [Google Scholar] [CrossRef]
- Viet, C.T.; Schmidt, B.L. Biologic Mechanisms of Oral Cancer Pain and Implications for Clinical Therapy. J. Dent. Res. 2012, 91, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Connelly, S.T.; Schmidt, B.L. Evaluation of pain in patients with oral squamous cell carcinoma. J. Pain 2004, 5, 505–510. [Google Scholar] [CrossRef]
- Cuffari, L.; De Tesseroli, S.J.T.; Nemr, K.; Rapaport, A. Pain complaint as the first symptom of oral cancer: A descriptive study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Black, B.; Herr, K.; Fine, P.; Sanders, S.; Tang, X.; Bergen-Jackson, K.; Titler, M.; Forcucci, C. The relationships among pain, nonpain symptoms, and quality of life measures in older adults with cancer receiving hospice care. Pain Med. 2011, 12, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Sherman, C.A.; Simonton, S.; Adams, D.C.; Vural, E.; Owens, B.; Hanna, E. Assessing quality of life in patients with head and neck cancer. Arch. Otolaryngol. Head Neck Surg. 2000, 126, 459–467. [Google Scholar] [CrossRef]
- Ferrell, B.R.; Dow, K.H.; Grant, M. Measurement of the quality of life in cancer survivors. Qual Life Res. 1995, 4, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, K.G.; Von Zeidler, S.V.; Podestá, J.R.V.; Sena, A.; Souza, E.D.; Lenzi, J.L.; Bissoli, N.S.; Gouvea, S.A. Influence of pain severity on the quality of life in patients with head and neck cancer before antineoplastic therapy. BMC Cancer 2014, 14, 39. [Google Scholar] [CrossRef]
- Linee guida AIOM: Terapia del dolore in oncologia, Edizione 2019. Available online: https://www.aiom.it/wp-content/uploads/2019/10/2019_LG_AIOM_Terapia_dolore.pdf (accessed on 31 October 2019).
- Fallon, M.; Giusti, R.; Aielli, F.; Hoskin, P.; Rolke, R.; Sharma, M.; Ripamonti, C.I. Management of Cancer Pain in Adult Patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, iv166–iv191. [Google Scholar] [CrossRef] [PubMed]
- Caraceni, A.; Hanks, G.; Kaasa, S.; Bennett, M.I.; Brunelli, C.; Cherny, N.; Dale, O.; De Conno, F.; Fallon, M.; Hanna, M.; et al. Use of opioid analgesics in the treatment of cancer pain: Evidence-based recommendations from the EAPC. Lancet Oncol. 2012, 13, e58–e68. [Google Scholar] [CrossRef]
- Fairchild, A. Under-treatment of cancer pain. Curr. Opin. Support Palliat. Care 2010, 4, 11–15. [Google Scholar] [CrossRef]
- Deandrea, S.; Montanari, M.; Moja, L.; Apolone, G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann. Oncol. 2008, 19, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Nersesyan, H.; Slavin, K.V. Current approach to cancer pain management: Availability and implications of different treatment options. Ther. Clin. Risk. Manag. 2007, 3, 381–400. [Google Scholar] [PubMed]
- Mitera, G.; Zeiadin, N.; Kirou-Mauro, A.; DeAngelis, C.; Wong, J.; Sanjeevan, T.; Sinclair, E.; Danjoux, C.; Barnes, E.; Tsao, M.; et al. Retrospective assessment of cancer pain management in an outpatient palliative radiotherapy clinic using the Pain Management Index. J. Pain Symptom Manag. 2010, 39, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Mitera, G.; Fairchild, A.; DeAngelis, C.; Emmenegger, U.; Zurawel-Balaura, L.; Zhang, L.; Bezjak, A.; Levin, W.; Mclean, M.; Zeiadin, N.; et al. A Multicenter Assessment of the Adequacy of Cancer Pain Treatment Using the Pain Management Index. J. Palliat. Med. 2010, 13, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Massaccesi, M.; Deodato, F.; Caravatta, L.; Macchia, G.; Padula, G.D.A.; Di Rito, S.; Woldemariam, A.A.; Rossi, M.; Di Falco, C.; Tambaro, R.; et al. Incidence and management of noncancer pain in cancer patients referred to a radiotherapy center. Clin. J. Pain. 2013, 29, 944–947. [Google Scholar] [CrossRef]
- Gonçalves, F.; Almeida, A.; Antunes, C.; Cardoso, M.; Carvalho, M.; Claro, M.; Coimbra, F.; Diniz, I.; Fonseca, B.; Fradique, E.; et al. A cross-sectional survey of pain in palliative care in Portugal. Support. Care Cancer 2013, 21, 2033–2039. [Google Scholar] [CrossRef] [PubMed]
- Vuong, S.; Pulenzas, N.; DeAngelis, C.; Torabi, S.; Ahrari, S.; Tsao, M.; Danjoux, C.; Barnes, T.; Chow, E. Inadequate pain management in cancer patients attending an outpatient palliative radiotherapy clinic. Support. Care Cancer 2016, 24, 887–892. [Google Scholar] [CrossRef]
- Singh, H.; Singh Banipal, R.P.; Singh, B. Assessment of Adequacy of Pain Management and Analgesic Use in Patients with Advanced Cancer Using the Brief Pain Inventory and Pain Management Index Calculation. J. Glob. Oncol. 2016, 3, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Reis-pina, P.; Lawlor, P.G.; Barbosa, A. Adequacy of cancer-related pain management and predictors of undertreatment at referral to a pain clinic. J. Pain Res. 2017, 10, 2097–2107. [Google Scholar] [CrossRef]
- Fujii, A.; Yamada, Y.; Takayama, K.; Nakano, T.; Kishimoto, J. Longitudinal assessment of pain management with the pain management index in cancer outpatients receiving chemotherapy. Support. Care Cancer 2017, 25, 925–932. [Google Scholar] [CrossRef]
- Shen, W.C.; Chen, J.S.; Shao, Y.Y.; Lee, K.D.; Chiou, T.J.; Sung, Y.C.; Rau, K.M.; Yen, C.J.; Liao, W.M.; Liu, T.C.; et al. Impact of Undertreatment of Cancer Pain with Analgesic Drugs on Patient Outcomes: A Nationwide Survey of Outpatient Cancer Patient Care in Taiwan. J. Pain Symptom Manag. 2017, 54, 55–65.e1. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, N.; Higashi, T.; Yamashita, I.; Yoshimoto, T.; Matoba, M. Negative pain management index scores do not necessarily indicate inade-quate pain management: A cross-sectional study. BMC Palliat. Care 2018, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Tuem, K.B.; Gebremeskel, L.; Hiluf, K.; Arko, K.; Haftom Gebregergs, H. Adequacy of Cancer-Related Pain Treatments and Factors Affecting Proper Management in Ayder Comprehensive Specialized Hospital, Mekelle, Ethiopia. J. Oncol. 2020, 2020, 2903542. [Google Scholar] [CrossRef]
- Thronæs, M.; Balstad, T.R.; Brunelli, C.; Torbjørn Løhre, E.; Klepstad, P.; Vagnildhaug, O.M.; Kaasa, S.; Knudsen, A.K.; Skeidsvoll, S.T. Pain management index (PMI)-does it reflect cancer patients’ wish for focus on pain? Support. Care Cancer 2020, 28, 1675–1684. [Google Scholar] [CrossRef]
- Cleeland, C.S.; Gonin, R.; Hatfield, A.K.; Edmonson, J.H.; Blum, R.H.; Stewart, J.A.; Pandya, K. Pain and its treatment in outpatients with metastatic cancer. N. Engl. J. Med. 1994, 330, 592–596. [Google Scholar] [CrossRef]
- Mercadante, S.; Bruera, E. Good…but Bad News. J. Clin. Oncol. 2015, 33, 2119. [Google Scholar] [CrossRef]
- Fillingim, R.B.; King, C.D.; Ribeiro-Dasilva, M.C.; Rahim-Williams, B.; Riley, J.L. Sex, gender, and pain: A review of recent clinical and experimental findings. J. Pain 2009, 10, 447–485. [Google Scholar] [CrossRef]
- Reich, R.R.; Lengacher, C.A.; Alinat, C.B.; Kip, K.E.; Paterson, C.; Ramesar, S.; Han, H.S.; Ismail-Khan, R.; Johnson-Mallard, V.; Moscoso, M.; et al. Mindfulness based stress reduction in post-treatment breast cancer patients: Immediate and sustained effects across multiple symptom clusters. J. Pain Symptom Manag. 2017, 53, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.T.; Roberto, A.; Corli, O.; Deandrea, S.; Bandieri, E.; Cavuto, S.; Apolone, G. Quality of cancer pain management: An update of a systematic review of undertreatment of patients with cancer. J. Clin. Oncol. 2014, 32, 4149–4154. [Google Scholar] [CrossRef] [PubMed]
- Maltoni, M. Opioids, pain, and fear. Ann. Oncol. 2008, 19, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Sichetti, D.; Bandieri, E.; Romero, M.; Di Biagio, K.; Luppi, M.; Belfiglio, M.; Tognoni, G.; Ripamonti, C.I. Impact of setting of care on pain management in patients with cancer: A multicentre cross-sectional study. Ann. Oncol. 2010, 21, 2088–2093. [Google Scholar] [CrossRef]
- Greco, M.T.; Roberto, A.; Corli, O.; Deandrea, S.; Bandieri, E.; Cavuto, S.; Apolone, G. Reply to S. Mercadante et al. J. Clin. Oncol. 2015, 33, 2119–2120. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).