CXCR6+ Tumor-Associated Macrophages Identify Immunosuppressive Colon Cancer Patients with Poor Prognosis but Favorable Response to Adjuvant Chemotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Immunohistochemistry (IHC) and Immunofluorescence (IF)
2.3. Statistical Analysis
3. Results
3.1. CXCR6+TAMs Infiltrated CC Tissues and Predict Poor Prognosis in All Stages Patients
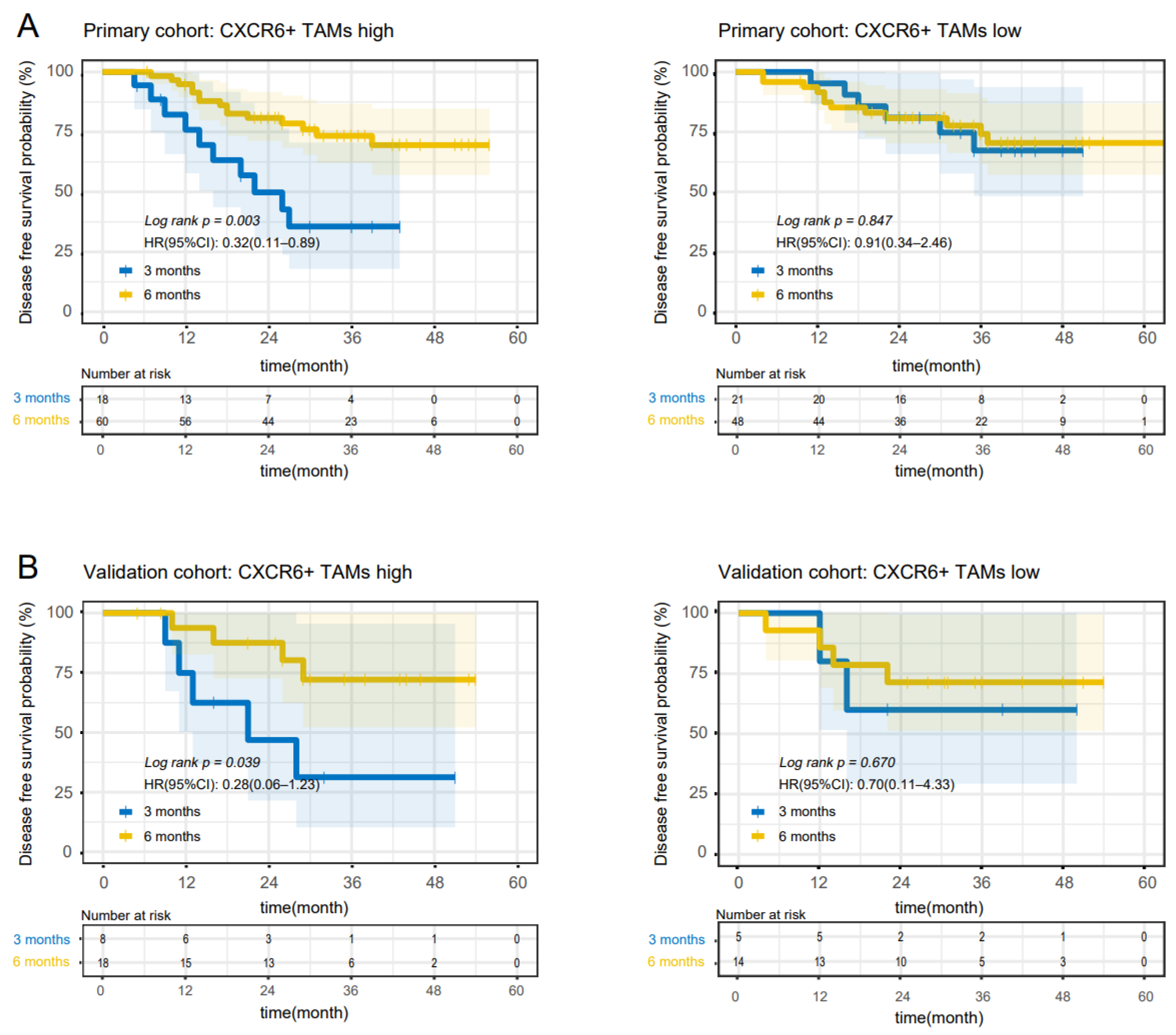
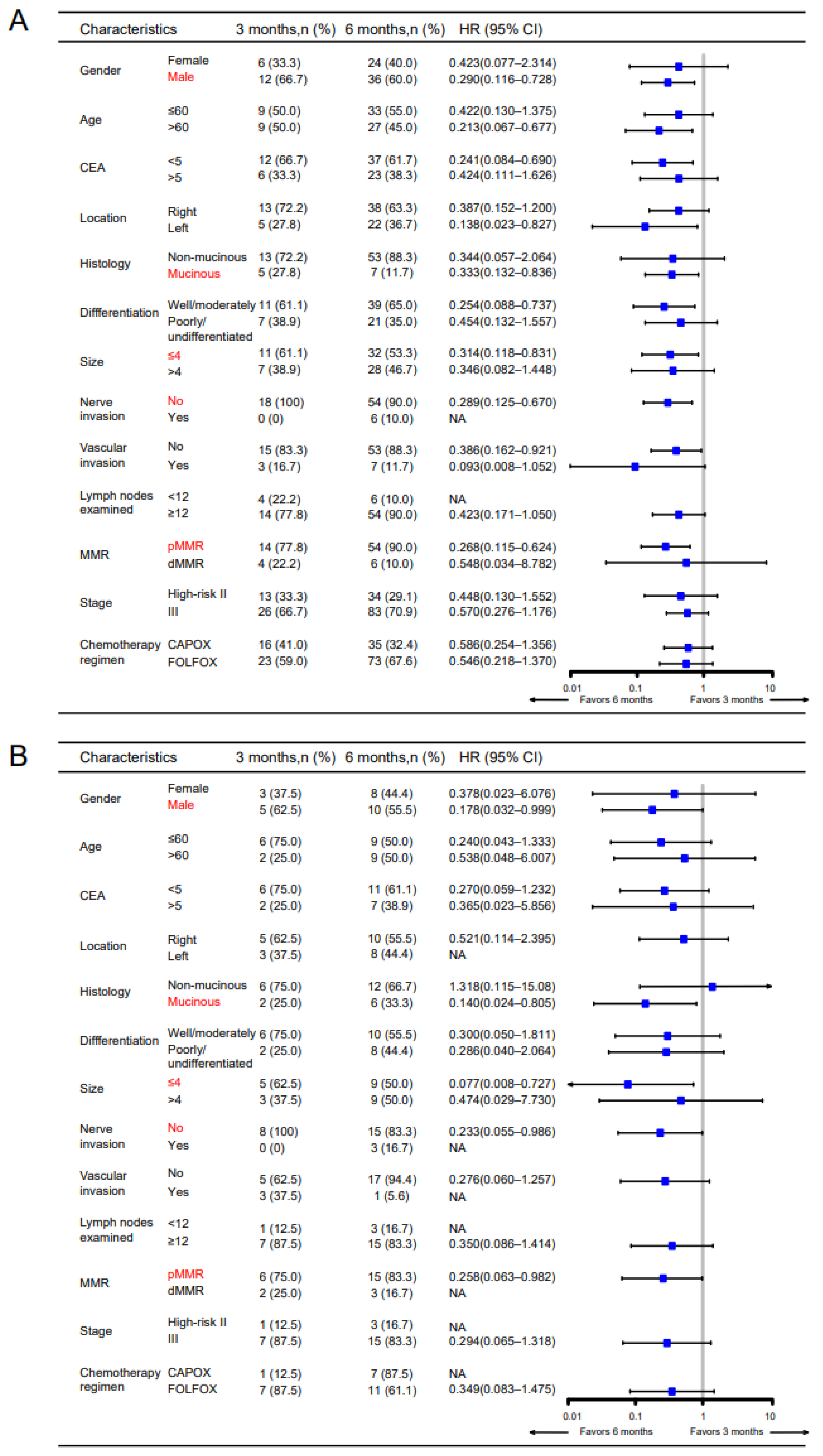
3.2. CXCR6+TAMs Predict the Benefit of 6-Month Adjuvant Chemotherapy in High-Risk Stage II and Stage III Patients
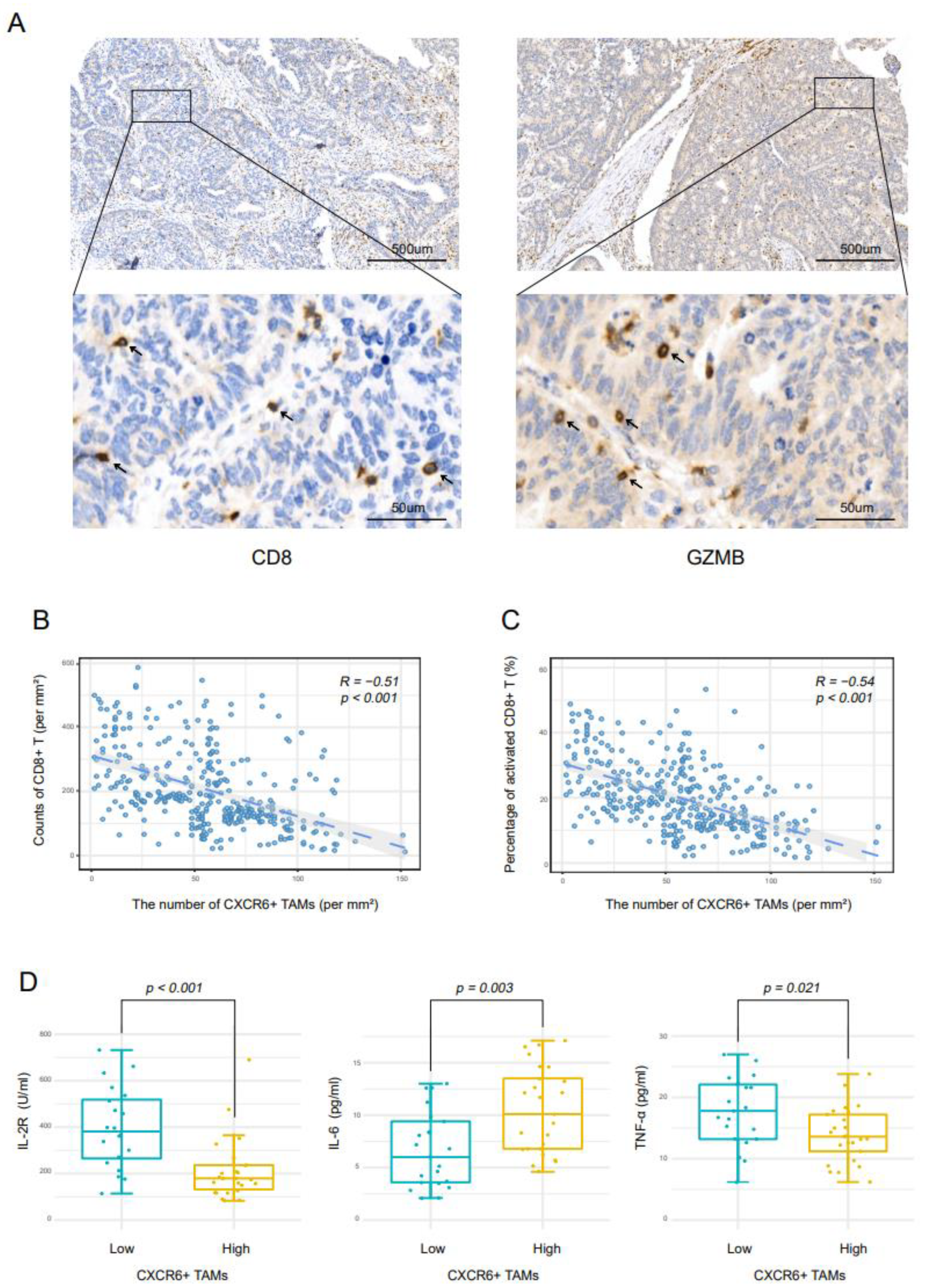
3.3. CXCR6+TAMs Contribute to Immunosuppressive Microenvironment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B., 3rd; Schrag, D.; Somerfield, M.R.; Cohen, A.M.; Figueredo, A.T.; Flynn, P.J.; Krzyzanowska, M.K.; Maroun, J.; McAllister, P.; Van Cutsem, E.; et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J. Clin. Oncol. 2004, 22, 3408–3419. [Google Scholar] [CrossRef] [PubMed]
- Group, Q.C. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet 2007, 370, 2020–2029. [Google Scholar] [CrossRef]
- Schippinger, W.; Samonigg, H.; Schaberl-Moser, R.; Greil, R.; Thodtmann, R.; Tschmelitsch, J.; Jagoditsch, M.; Steger, G.G.; Jakesz, R.; Herbst, F.; et al. A prospective randomised phase III trial of adjuvant chemotherapy with 5-fluorouracil and leucovorin in patients with stage II colon cancer. Br. J. Cancer 2007, 97, 1021–1027. [Google Scholar] [CrossRef]
- Yoshino, T.J.I.A.F.S.T. Duration of Adjuvant Doublet Chemotherapy (3 or 6 months) in Patients With High-Risk Stage II Colorectal Cancer. J. Clin. Oncol. 2021, 39, 631–641. [Google Scholar] [CrossRef]
- André, T.; Meyerhardt, J.; Iveson, T.; Sobrero, A.; Yoshino, T.; Souglakos, I.; Grothey, A.; Niedzwiecki, D.; Saunders, M.; Labianca, R.; et al. Effect of duration of adjuvant chemotherapy for patients with stage III colon cancer (IDEA collaboration): Final results from a prospective, pooled analysis of six randomised, phase 3 trials. Lancet Oncol. 2020, 21, 1620–1629. [Google Scholar] [CrossRef]
- Pagès, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.-S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef]
- Andre, T.; Amonkar, M.; Norquist, J.M.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.J.A.; Smith, D.; Garcia-Carbonero, R.; et al. Health-related quality of life in patients with microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer treated with first-line pembrolizumab versus chemotherapy (KEYNOTE-177): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 665–677. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Noy, R.; Pollard, J.W. Tumor-associated macrophages: From mechanisms to therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Franklin, R.A.; Liao, W.; Sarkar, A.; Kim, M.V.; Bivona, M.R.; Liu, K.; Pamer, E.G.; Li, M.O. The cellular and molecular origin of tumor-associated macrophages. Science 2014, 344, 921–925. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef]
- Feng, Q.; Chang, W.; Mao, Y.; He, G.; Zheng, P.; Tang, W.; Wei, Y.; Ren, L.; Zhu, D.; Ji, M.; et al. Tumor-associated Macrophages as Prognostic and Predictive Biomarkers for Postoperative Adjuvant Chemotherapy in Patients with Stage II Colon Cancer. Clin. Cancer Res. 2019, 25, 3896–3907. [Google Scholar] [CrossRef]
- Komohara, Y.; Fujiwara, Y.; Ohnishi, K.; Takeya, M. Tumor-associated macrophages: Potential therapeutic targets for anti-cancer therapy. Adv. Drug Deliv. Rev. 2016, 99, 180–185. [Google Scholar] [CrossRef]
- Matloubian, M.; David, A.; Engel, S.; Ryan, J.E.; Cyster, J.G. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat. Immunol. 2020, 1, 298–304. [Google Scholar] [CrossRef]
- Liao, F.; Alkhatib, G.; Peden, K.W.; Sharma, G.; Berger, E.A.; Farber, J.M. STRL33, A Novel Chemokine Receptor–like Protein, Functions as a Fusion Cofactor for Both Macrophage-tropic and T Cell Line–tropic HIV-1. J. Exp. Med. 1997, 185, 2015–2023. [Google Scholar] [CrossRef]
- Kim, C.H.; Kunkel, E.J.; Boisvert, J.; Johnston, B.; Campbell, J.J.; Genovese, M.C.; Greenberg, H.B.; Butcher, E.C. Bonzo/CXCR6 expression defines type 1–polarized T-cell subsets with extralymphoid tissue homing potential. J. Clin. Investig. 2001, 107, 595–601. [Google Scholar] [CrossRef]
- Unutmaz, D.; Xiang, W.; Sunshine, M.J.; Campbell, J.; Butcher, E.; Littman, D.R. The primate lentiviral receptor Bonzo/STRL33 is coordinately regulated with CCR5 and its expression pattern is conserved between human and mouse. J. Immunol. 2000, 165, 3284–3292. [Google Scholar] [CrossRef]
- Nakayama, T.; Hieshima, K.; Izawa, D.; Tatsumi, Y.; Kanamaru, A.; Yoshie, O. Cutting Edge: Profile of Chemokine Receptor Expression on Human Plasma Cells Accounts for Their Efficient Recruitment to Target Tissues. J. Immunol. 2003, 170, 1136–1140. [Google Scholar] [CrossRef]
- Korbecki, J.; Bajdak-Rusinek, K.; Kupnicka, P.; Kapczuk, P.; Siminska, D.; Chlubek, D.; Baranowska-Bosiacka, I. The Role of CXCL16 in the Pathogenesis of Cancer and Other Diseases. Int. J. Mol. Sci. 2021, 22, 3490. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, J.; Xu, Y.; Koch, A.E.; Cai, Z.; Chen, X.; Galson, D.L.; Taichman, R.S.; Zhang, J. CXCL16 functions as a novel chemotactic factor for prostate cancer cells in vitro. Mol. Cancer Res. 2008, 6, 546–554. [Google Scholar] [CrossRef]
- Gutwein, P.; Schramme, A.; Sinke, N.; Abdel-Bakky, M.S.; Voss, B.; Obermuller, N.; Doberstein, K.; Koziolek, M.; Fritzsche, F.; Johannsen, M.; et al. Tumoural CXCL16 expression is a novel prognostic marker of longer survival times in renal cell cancer patients. Eur. J. Cancer 2009, 45, 478–489. [Google Scholar] [CrossRef]
- Wågsäter, D.; Dimberg, J. Expression of chemokine receptor CXCR6 in human colorectal adenocarcinomas. Anticancer Res. 2004, 24, 3711–3714. [Google Scholar]
- Wang, X.Q.; Zhou, W.J.; Hou, X.X.; Fu, Q.; Li, D.J. Trophoblast-derived CXCL16 induces M2 macrophage polarization that in turn inactivates NK cells at the maternal-fetal interface. Cell Mol. Immunol. 2018, 15, 1038–1046. [Google Scholar] [CrossRef]
- Hattermann, K.; Sebens, S.; Helm, O.; Schmitt, A.D.; Mentlein, R.; Mehdorn, H.M.; Held-Feindt, J. Chemokine expression profile of freshly isolated human glioblastoma-associated macrophages/microglia. Oncol. Rep. 2014, 32, 270–276. [Google Scholar] [CrossRef]
- Xuan, W.; Qu, Q.; Zheng, B.; Xiong, S.; Fan, G.H. The chemotaxis of M1 and M2 macrophages is regulated by different chemokines. J. Leukoc. Biol. 2015, 97, 61–69. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, N.F.; Goudkade, D.; Jordanova, E.S.; Tops, C.M.; Hes, F.J.; Vasen, H.F.; van Wezel, T.; Morreau, H. Infiltration of Lynch colorectal cancers by activated immune cells associates with early staging of the primary tumor and absence of lymph node metastases. Clin. Cancer Res. 2012, 18, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Di Pilato, M.; Kfuri-Rubens, R.; Pruessmann, J.N.; Ozga, A.J.; Messemaker, M.; Cadilha, B.L.; Sivakumar, R.; Cianciaruso, C.; Warner, R.D.; Marangoni, F.; et al. CXCR6 positions cytotoxic T cells to receive critical survival signals in the tumor microenvironment. Cell 2021, 184, 4512–4530.e22. [Google Scholar] [CrossRef] [PubMed]
- Hattermann, K.; Held-Feindt, J.; Ludwig, A.; Mentlein, R. The CXCL16-CXCR6 chemokine axis in glial tumors. J. Neuroimmunol. 2013, 260, 47–54. [Google Scholar] [CrossRef]
- Shiovitz, S.; Bertagnolli, M.M.; Renfro, L.A.; Nam, E.; Foster, N.R.; Dzieciatkowski, S.; Luo, Y.; Lao, V.V.; Monnat, R.J., Jr.; Emond, M.J.; et al. CpG island methylator phenotype is associated with response to adjuvant irinotecan-based therapy for stage III colon cancer. Gastroenterology 2014, 147, 637–645. [Google Scholar] [CrossRef]
- Prall, F.; Duhrkop, T.; Weirich, V.; Ostwald, C.; Lenz, P.; Nizze, H.; Barten, M. Prognostic role of CD8+ tumor-infiltrating lymphocytes in stage III colorectal cancer with and without microsatellite instability. Hum. Pathol. 2004, 35, 808–816. [Google Scholar] [CrossRef]
- Minami, Y.; Kono, T.; Miyazaki, T.; Taniguchi, T. THE IL-2 RECEPTOR COMPLEX: Its Structure, Function, and Target Genes. Annu. Rev. Immunol. 1993, 11, 245–268. [Google Scholar] [CrossRef]
- Weng, Y.S.; Tseng, H.Y.; Chen, Y.A.; Shen, P.C.; Al Haq, A.T.; Chen, L.M.; Tung, Y.C.; Hsu, H.L. MCT-1/miR-34a/IL-6/IL-6R signaling axis promotes EMT progression, cancer stemness and M2 macrophage polarization in triple-negative breast cancer. Mol. Cancer 2019, 18, 42. [Google Scholar] [CrossRef]
- Balkwill, F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef]
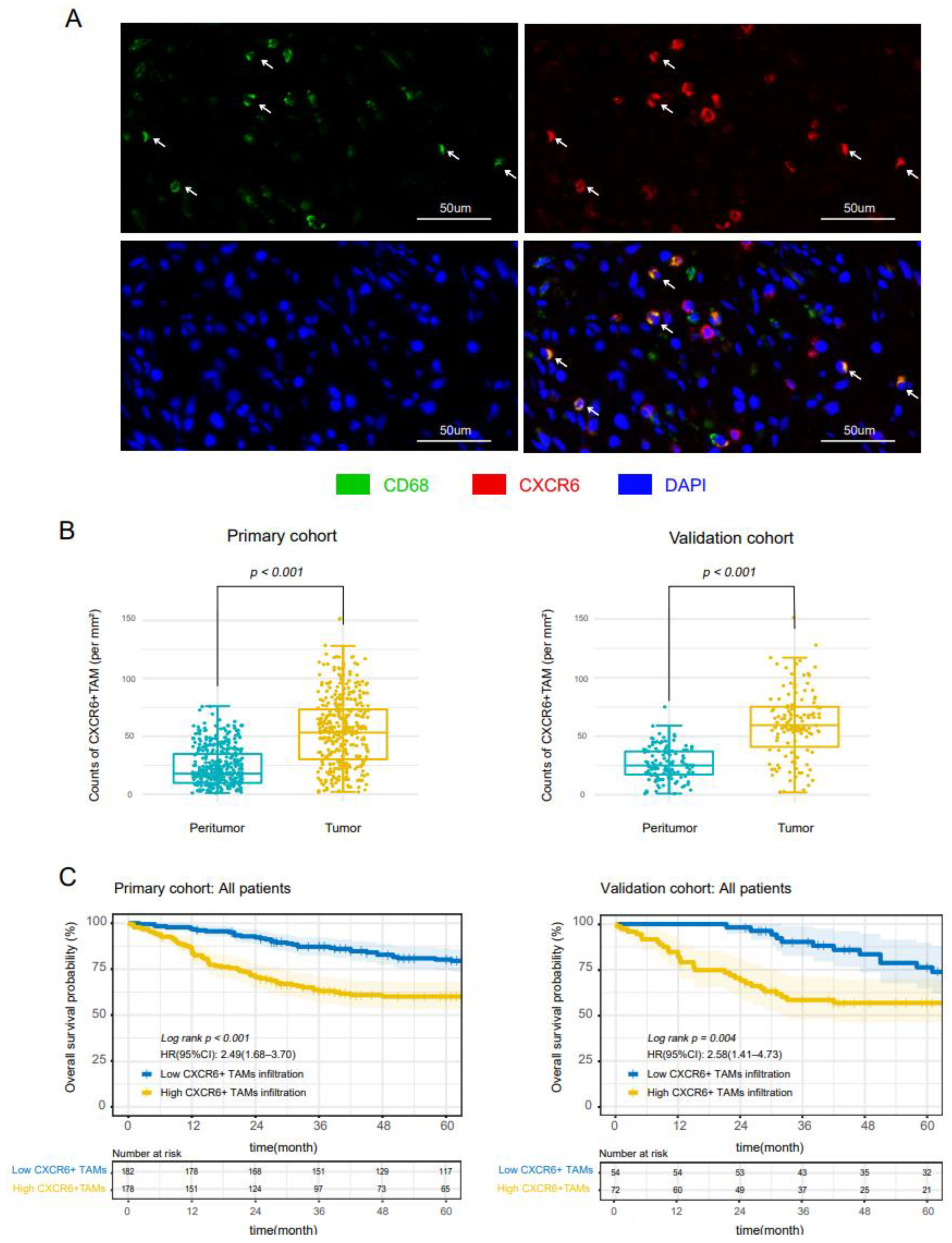
| CXCR6+TAMs Primary Cohort | CXCR6+TAMs Validation Cohort | |||||
|---|---|---|---|---|---|---|
| Low (%) | High (%) | P | Low (%) | High (%) | P | |
| All patients | 182 (50.6) | 178 (49.4) | 54 (42.9) | 72 (57.1) | ||
| Gender | 0.361 | 0.344 | ||||
| Female | 75 (41.2) | 65 (36.5) | 24 (44.4) | 26 (36.1) | ||
| Male | 107 (58.8) | 113 (62.5) | 30 (55.6) | 46 (63.9) | ||
| Age | 0.911 | 0.439 | ||||
| ≤60 | 93 (51.1) | 92 (51.7) | 27 (50.0) | 41 (56.9) | ||
| >60 | 89 (48.9) | 86 (48.3) | 27 (50.0) | 31 (43.1) | ||
| CEA (ng/mL) | 0.021 | 0.797 | ||||
| ≤5 | 109 (59.9) | 85 (47.8) | 29 (53.7) | 37 (51.4) | ||
| >5 | 73 (40.1) | 93 (52.2) | 25 (46.3) | 35 (48.6) | ||
| Tumor location | 0.263 | 0.165 | ||||
| Right-sided colon | 104 (57.1) | 112 (62.9) | 24 (44.4) | 41 (56.9) | ||
| Left-sided colon | 78 (42.9) | 66 (37.1) | 30 (55.6) | 31 (43.1) | ||
| Tumor size (cm) | 0.850 | 0.322 | ||||
| ≤4 | 102 (56.0) | 98 (55.1) | 20 (37.0) | 33 (45.8) | ||
| >4 | 80 (44.0) | 80 (44.9) | 34 (63.0) | 39 (54.2) | ||
| Histology | 0.582 | 0.183 | ||||
| Non-mucinous | 157 (86.3) | 157 (88.2) | 47 (87.0) | 56 (77.8) | ||
| Mucinous | 25 (13.7) | 21 (11.8) | 7 (13.0) | 16 (22.2) | ||
| Differentiation | 0.018 | 0.380 | ||||
| Well/moderately | 141 (77.5) | 118 (66.3) | 42 (77.8) | 51 (70.8) | ||
| Poorly/undifferentiated | 41 (22.5) | 60 (33.7) | 12 (22.2) | 21 (29.2) | ||
| T stage | 0.720 | 0.532 | ||||
| T1–2 | 77 (42.3) | 72 (40.4) | 21 (38.9) | 32 (44.4) | ||
| T3–4 | 105 (57.7) | 106 (59.6) | 33 (61.1) | 40 (55.6) | ||
| N stage | <0.001 | 0.006 | ||||
| N0 | 118 (64.8) | 61 (34.3) | 35 (64.8) | 29 (40.3) | ||
| N1–2 | 64 (35.2) | 117 (65.7) | 19 (35.2) | 43 (59.7) | ||
| M stage | <0.001 | 0.027 | ||||
| M0 | 153 (84.1) | 110 (61.8) | 41 (75.9) | 41 (56.9) | ||
| M1 | 29 (15.9) | 68 (38.2) | 13 (24.1) | 31 (43.1) | ||
| TNM stage | <0.001 | 0.104 | ||||
| I | 41 (22.5) | 14 (7.8) | 11 (20.4) | 9 (12.5) | ||
| II | 67 (36.8) | 32 (18.0) | 15 (27.8) | 12 (16.7) | ||
| III | 45 (24.7) | 64 (36.0) | 15 (27.8) | 20 (27.8) | ||
| IV | 29 (15.9) | 68 (38.2) | 13 (24.1) | 31 (43.1) | ||
| Nerve invasion | 0.156 | 0.998 | ||||
| No | 171 (94.0) | 160 (89.9) | 51 (94.4) | 67 (93.1) | ||
| Yes | 11 (6.0) | 18 (10.1) | 3 (5.6) | 5 (6.9) | ||
| Vascular invasion | 0.017 | 0.643 | ||||
| No | 163 (89.6) | 148 (83.1) | 48 (88.9) | 62 (86.1) | ||
| Yes | 19 (10.4) | 30 (16.9) | 6 (11.1) | 10 (13.9) | ||
| Lymph nodes examined | 0.156 | 0.803 | ||||
| <12 | 11 (6.0) | 18 (10.1) | 3 (5.6) | 6 (8.3) | ||
| ≥12 | 171 (94.0) | 160 (89.9) | 51 (94.4) | 66 (91.7) | ||
| MMR status | 0.357 | 0.122 | ||||
| dMMR | 17 (9.3) | 22 (12.4) | 4 (7.4) | 12 (16.7) | ||
| pMMR | 165 (90.7) | 156 (87.6) | 50 (92.6) | 60 (83.3) | ||
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Gender | ||||
| Female | 1 (reference) | |||
| Male | 1.15 (0.77–1.72) | 0.499 | ||
| Age | ||||
| ≤60 | 1 (reference) | |||
| >60 | 0.94 (0.63–1.31) | 0.749 | ||
| Preoperative serum CEA (ng/mL) | ||||
| ≤5 | 1 (reference) | 1 (reference) | ||
| >5 | 2.91 (1.92–4.40) | <0.001 | 1.80 (1.16–2.81) | 0.009 |
| Primary tumor location | ||||
| Right-sided colon | 1 (reference) | |||
| Left-sided colon | 0.72 (0.48–1.09) | 0.124 | ||
| Primary tumor size (cm) | ||||
| ≤4 | 1 (reference) | |||
| >4 | 1.18 (0.80–1.74) | 0.405 | ||
| Histology | ||||
| Non-mucinous | 1 (reference) | |||
| Mucinous | 0.96 (0.62–1.47) | 0.839 | ||
| Primary Differentiation | ||||
| Well/moderately | 1 (reference) | |||
| Poorly/undifferentiated | 1.40 (0.92–2.11) | 0.113 | ||
| T stage | ||||
| T1–2 | 1 (reference) | 1 (reference) | ||
| T3–4 | 0.70 (0.47–1.03) | 0.070 | 0.78 (0.52–1.16) | 0.212 |
| N stage | ||||
| N0 | 1 (reference) | 1 (reference) | ||
| N1–2 | 2.01 (1.33–3.00) | 0.001 | 1.06 (0.69–1.63) | 0.788 |
| M stage | ||||
| M0 | 1 (reference) | 1 (reference) | ||
| M1 | 4.17 (3.12–5.56) | <0.001 | 3.30 (2.41–4.53) | <0.001 |
| TNM stage | ||||
| I | 1 (reference) | |||
| II | 2.01 (0.74–5.44) | 0.171 | ||
| III | 1.83 (0.67–4.95) | 0.236 | ||
| IV | 12.89 (5.16–32.20) | <0.001 | ||
| Nerve invasion | ||||
| No | 1 (reference) | 1 (reference) | ||
| Yes | 1.79 (0.96–3.35) | 0.068 | 0.96 (0.51–1.81) | 0.894 |
| Vascular invasion | ||||
| No | 1 (reference) | |||
| Yes | 1.34 (0.79–2.25) | 0.274 | ||
| Lymph nodes examined | ||||
| <12 | 1 (reference) | |||
| ≥12 | 0.88 (0.43–1.82) | 0.739 | ||
| MMR status | ||||
| pMMR | 1 (reference) | 1 (reference) | ||
| dMMR | 1.72 (1.01–2.93) | 0.048 | 1.45 (0.85–2.50) | 0.177 |
| The density of CXCR6+TAMs | ||||
| Low | 1 (reference) | 1 (reference) | ||
| High | 2.51 (1.66–3.79) | <0.001 | 1.68 (1.09–2.59) | 0.019 |
| All Patient Stages: High Density vs. Low Density of CXCR6+TAMs | ||||||
|---|---|---|---|---|---|---|
| Cohort | No. of Patients (%) | OS | ||||
| HR (95%) | p Value | |||||
| Primary | 360 (100) | 2.49 (1.68–3.70) | <0.001 | |||
| Validation | 126 (100) | 2.58 (1.41–4.73) | 0.004 | |||
| High-risk stage II and stage III patients: 6 months vs. 3 months of FOLFOX/CAPOX | ||||||
| Cohort | CXCR6+TAM infiltration | No. of patients (%) | DFS | OS | ||
| HR (95%) | p value | HR (95%) | p value | |||
| Primary | High | 78 (53.1) | 0.32 (0.11–0.89) | 0.003 | 0.28 (0.07–1.11) | 0.014 |
| Low | 69 (46.9) | 0.91 (0.34–2.46) | 0.847 | 0.89 (0.26–3.03) | 0.850 | |
| Validation | High | 26 (57.8) | 0.28 (0.06–1.23) | 0.039 | 0.29 (0.07–1.27) | 0.048 |
| Low | 19 (42.2) | 0.70 (0.11–4.33) | 0.670 | 0.83 (0.14–4.80) | 0.826 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, J.; Lin, S.; Mao, Y.; Xu, Y.; Zhang, Z.; Wu, Q.; Chen, Y.; Wei, Y.; Feng, Q.; Xu, J. CXCR6+ Tumor-Associated Macrophages Identify Immunosuppressive Colon Cancer Patients with Poor Prognosis but Favorable Response to Adjuvant Chemotherapy. Cancers 2022, 14, 4646. https://doi.org/10.3390/cancers14194646
Chang J, Lin S, Mao Y, Xu Y, Zhang Z, Wu Q, Chen Y, Wei Y, Feng Q, Xu J. CXCR6+ Tumor-Associated Macrophages Identify Immunosuppressive Colon Cancer Patients with Poor Prognosis but Favorable Response to Adjuvant Chemotherapy. Cancers. 2022; 14(19):4646. https://doi.org/10.3390/cancers14194646
Chicago/Turabian StyleChang, Jiang, Songbin Lin, Yihao Mao, Yuqiu Xu, Zhiyuan Zhang, Qi Wu, Yijiao Chen, Ye Wei, Qingyang Feng, and Jianmin Xu. 2022. "CXCR6+ Tumor-Associated Macrophages Identify Immunosuppressive Colon Cancer Patients with Poor Prognosis but Favorable Response to Adjuvant Chemotherapy" Cancers 14, no. 19: 4646. https://doi.org/10.3390/cancers14194646
APA StyleChang, J., Lin, S., Mao, Y., Xu, Y., Zhang, Z., Wu, Q., Chen, Y., Wei, Y., Feng, Q., & Xu, J. (2022). CXCR6+ Tumor-Associated Macrophages Identify Immunosuppressive Colon Cancer Patients with Poor Prognosis but Favorable Response to Adjuvant Chemotherapy. Cancers, 14(19), 4646. https://doi.org/10.3390/cancers14194646




