Bone Mineral Density as an Individual Prognostic Biomarker in Patients with Surgically-Treated Brain Metastasis from Lung Cancer (NSCLC)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
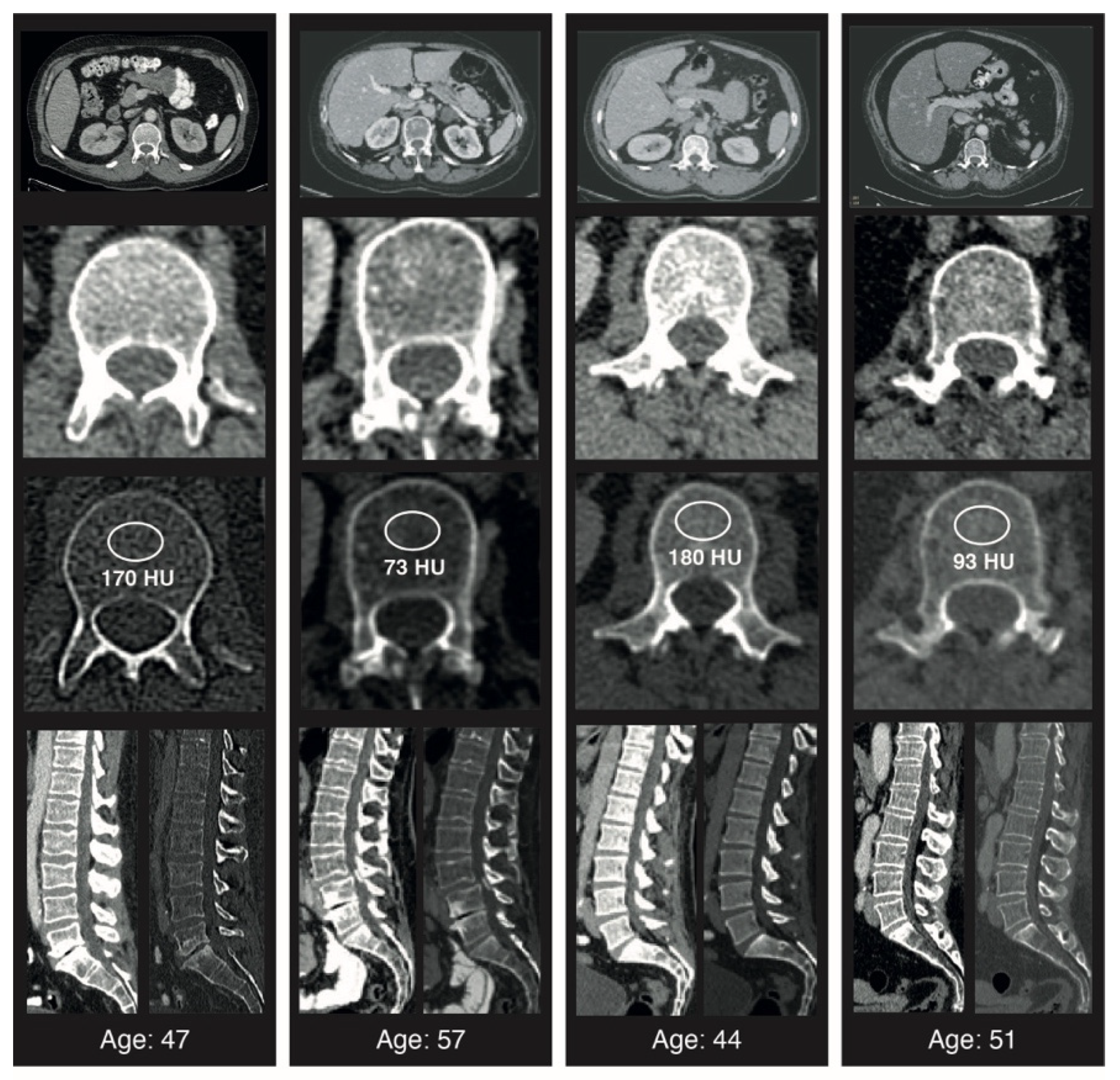
2.2. CT Image Acquisition
2.3. Image Analysis
2.4. Statistics
3. Results
3.1. Patient Baseline Characteristics
3.2. Patient- and Disease-Related Characteristics Dependent on Physiologic and Pathologic Bone Mineral Density-Levels
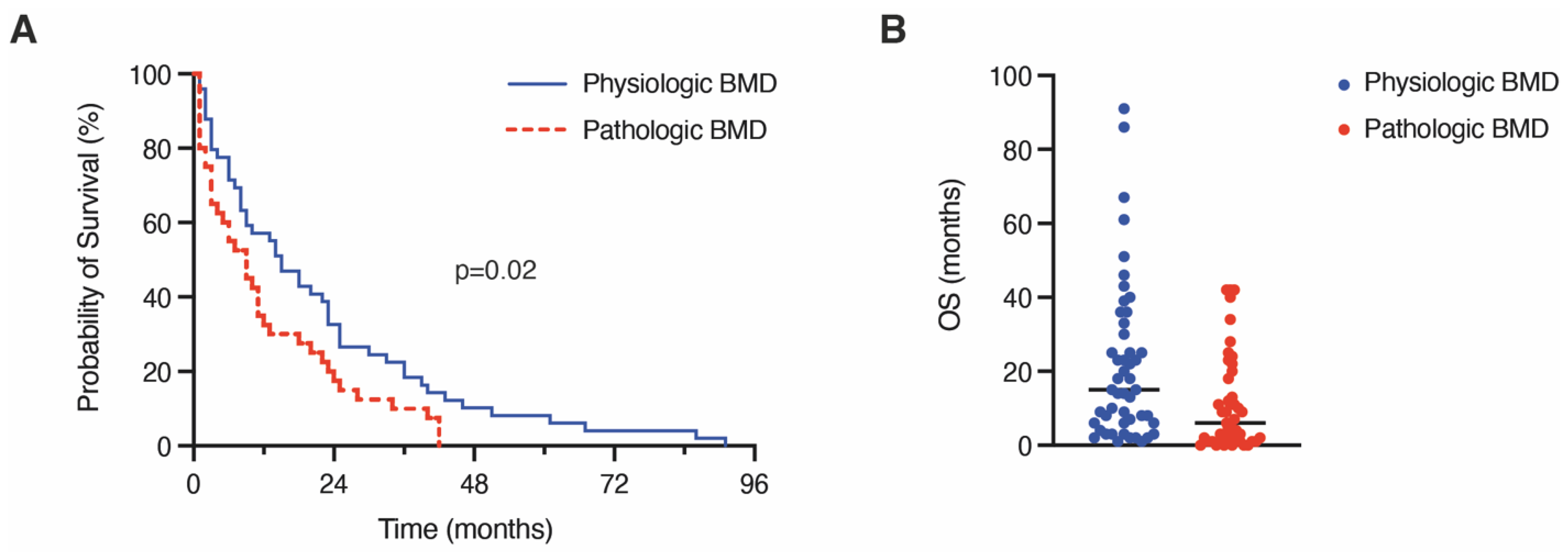
3.3. Influence of Pathologic Bone Mineral Density on 1-Year Mortality and Overall Survival
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ernani, V.; Stinchcombe, T.E. Management of Brain Metastases in Non-Small-Cell Lung Cancer. J. Oncol. Pract. 2019, 15, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Page, S.; Milner-Watts, C.; Perna, M.; Janzic, U.; Vidal, N.; Kaudeer, N.; Ahmed, M.; McDonald, F.; Locke, I.; Minchom, A.; et al. Systemic treatment of brain metastases in non-small cell lung cancer. Eur. J. Cancer 2020, 132, 187–198. [Google Scholar] [CrossRef]
- Ilic, I.; Faron, A.; Heimann, M.; Potthoff, A.L.; Schafer, N.; Bode, C.; Borger, V.; Eichhorn, L.; Giordano, F.A.; Guresir, E.; et al. Combined Assessment of Preoperative Frailty and Sarcopenia Allows the Prediction of Overall Survival in Patients with Lung Cancer (NSCLC) and Surgically Treated Brain Metastasis. Cancers 2021, 13, 3353. [Google Scholar] [CrossRef] [PubMed]
- Troschel, A.S.; Troschel, F.M.; Best, T.D.; Gaissert, H.A.; Torriani, M.; Muniappan, A.; Van Seventer, E.E.; Nipp, R.D.; Roeland, E.J.; Temel, J.S.; et al. Computed Tomography-based Body Composition Analysis and Its Role in Lung Cancer Care. J. Thorac. Imaging 2020, 35, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.P.; Wang, X.L.; Tian, H.; Gao, T.T.; Tang, L.M.; Tian, F.; Wang, J.W.; Zheng, H.J.; Zhang, L.; et al. Computed tomography-quantified body composition predicts short-term outcomes after gastrectomy in gastric cancer. Curr. Oncol. 2018, 25, e411–e422. [Google Scholar] [CrossRef] [PubMed]
- Malietzis, G.; Currie, A.C.; Athanasiou, T.; Johns, N.; Anyamene, N.; Glynne-Jones, R.; Kennedy, R.H.; Fearon, K.C.; Jenkins, J.T. Influence of body composition profile on outcomes following colorectal cancer surgery. Br. J. Surg. 2016, 103, 572–580. [Google Scholar] [CrossRef]
- Furtner, J.; Berghoff, A.S.; Albtoush, O.M.; Woitek, R.; Asenbaum, U.; Prayer, D.; Widhalm, G.; Gatterbauer, B.; Dieckmann, K.; Birner, P.; et al. Survival prediction using temporal muscle thickness measurements on cranial magnetic resonance images in patients with newly diagnosed brain metastases. Eur. Radiol. 2017, 27, 3167–3173. [Google Scholar] [CrossRef]
- Lareida, A.; Terziev, R.; Grossenbacher, B.; Andratschke, N.; Roth, P.; Rohrmann, S.; Stahel, R.; Guckenberger, M.; Le Rhun, E.; Weller, M.; et al. Underweight and weight loss are predictors of poor outcome in patients with brain metastasis. J. Neurooncol. 2019, 145, 339–347. [Google Scholar] [CrossRef]
- Sakin, A.; Sahin, S.; Mustafa Atci, M.; Yasar, N.; Demir, C.; Geredeli, C.; Sakin, A.; Cihan, S. The Effect of Body Mass Index on Treatment Outcomes in Patients with Metastatic Non-Small Cell Lung Cancer Treated with Platinum-Based Therapy. Nutr. Cancer 2021, 73, 1411–1418. [Google Scholar] [CrossRef]
- Schulze-Hagen, M.F.; Roderburg, C.; Wirtz, T.H.; Jordens, M.S.; Bundgens, L.; Abu Jhaisha, S.; Hohlstein, P.; Brozat, J.F.; Bruners, P.; Loberg, C.; et al. Decreased Bone Mineral Density Is a Predictor of Poor Survival in Critically Ill Patients. J. Clin. Med. 2021, 10, 3741. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Kaido, T.; Okumura, S.; Iwamura, S.; Miyachi, Y.; Shirai, H.; Kobayashi, A.; Hamaguchi, Y.; Kamo, N.; Uozumi, R.; et al. Bone mineral density correlates with survival after resection of extrahepatic biliary malignancies. Clin. Nutr. 2019, 38, 2770–2777. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Huang, X.; Jin, F.; Wang, H.; Hao, Y.; Tang, T.; Dai, K. Bone mineral density and all-cause, cardiovascular and stroke mortality: A meta-analysis of prospective cohort studies. Int. J. Cardiol. 2013, 166, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Sharshar, M.; Kaido, T.; Shirai, H.; Okumura, S.; Yao, S.; Miyachi, Y.; Iwamura, S.; Kamo, N.; Yagi, S.; Macshut, M.; et al. Impact of the preoperative bone mineral density on the outcomes after resection of pancreatic cancer. Surg. Today 2020, 50, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Ganry, O.; Lapotre-Ledoux, B.; Fardellone, P.; Dubreuil, A. Bone mass density, subsequent risk of colon cancer and survival in postmenopausal women. Eur. J. Epidemiol. 2008, 23, 467–473. [Google Scholar] [CrossRef]
- Tseng, O.L.; Dawes, M.G.; Spinelli, J.J.; Gotay, C.C.; McBride, M.L. Utilization of bone mineral density testing among breast cancer survivors in British Columbia, Canada. Osteoporos. Int. 2017, 28, 3439–3449. [Google Scholar] [CrossRef]
- Jordens, M.S.; Wittig, L.; Loberg, C.; Heinrichs, L.; Keitel, V.; Schulze-Hagen, M.; Antoch, G.; Knoefel, W.T.; Fluegen, G.; Loosen, S.H.; et al. Bone Mineral Density Is a Predictor of Mortality in Female Patients with Cholangiocellular Carcinoma Undergoing Palliative Treatment. Biomedicines 2022, 10, 1660. [Google Scholar] [CrossRef]
- Sharma, P.; Parikh, N.D.; Yu, J.; Barman, P.; Derstine, B.A.; Sonnenday, C.J.; Wang, S.C.; Su, G.L. Bone mineral density predicts posttransplant survival among hepatocellular carcinoma liver transplant recipients. Liver Transpl. 2016, 22, 1092–1098. [Google Scholar] [CrossRef]
- Schuss, P.; Schafer, N.; Bode, C.; Borger, V.; Eichhorn, L.; Giordano, F.A.; Guresir, E.; Heimann, M.; Ko, Y.D.; Landsberg, J.; et al. The Impact of Prolonged Mechanical Ventilation on Overall Survival in Patients with Surgically Treated Brain Metastases. Front. Oncol. 2021, 11, 658949. [Google Scholar] [CrossRef]
- Schneider, M.; Heimann, M.; Schaub, C.; Eichhorn, L.; Potthoff, A.L.; Giordano, F.A.; Guresir, E.; Ko, Y.D.; Landsberg, J.; Lehmann, F.; et al. Comorbidity Burden and Presence of Multiple Intracranial Lesions Are Associated with Adverse Events after Surgical Treatment of Patients with Brain Metastases. Cancers 2020, 12, 3209. [Google Scholar] [CrossRef]
- Schafer, N.; Bumes, E.; Eberle, F.; Fox, V.; Gessler, F.; Giordano, F.A.; Konczalla, J.; Onken, J.; Ottenhausen, M.; Scherer, M.; et al. Implementation, relevance, and virtual adaptation of neuro-oncological tumor boards during the COVID-19 pandemic: A nationwide provider survey. J. Neuro-Oncol. 2021, 153, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Graffy, P.M.; Ziemlewicz, T.J.; Lee, S.J.; Summers, R.M.; Pickhardt, P.J. Opportunistic Osteoporosis Screening at Routine Abdominal and Thoracic CT: Normative L1 Trabecular Attenuation Values in More than 20 000 Adults. Radiology 2019, 291, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef]
- Peng, Y.C.; Wu, C.H.; Tien, Y.W.; Lu, T.P.; Wang, Y.H.; Chen, B.B. Preoperative sarcopenia is associated with poor overall survival in pancreatic cancer patients following pancreaticoduodenectomy. Eur. Radiol. 2021, 31, 2472–2481. [Google Scholar] [CrossRef]
- Wong, A.; Zhu, D.; Kraus, D.; Tham, T. Radiologically Defined Sarcopenia Affects Survival in Head and Neck Cancer: A Meta-Analysis. Laryngoscope 2021, 131, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Sperduto, P.W.; Mesko, S.; Li, J.; Cagney, D.; Aizer, A.; Lin, N.U.; Nesbit, E.; Kruser, T.J.; Chan, J.; Braunstein, S.; et al. Survival in Patients with Brain Metastases: Summary Report on the Updated Diagnosis-Specific Graded Prognostic Assessment and Definition of the Eligibility Quotient. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 3773–3784. [Google Scholar] [CrossRef] [PubMed]
- Campos-Obando, N.; Castano-Betancourt, M.C.; Oei, L.; Franco, O.H.; Stricker, B.H.; Brusselle, G.G.; Lahousse, L.; Hofman, A.; Tiemeier, H.; Rivadeneira, F.; et al. Bone mineral density and chronic lung disease mortality: The rotterdam study. J. Clin. Endocrinol. Metab. 2014, 99, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, D.P.; Khaw, K.T. Bone mineral density at the hip predicts mortality in elderly men. Osteoporos. Int. 2001, 12, 259–265. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Gridley, G.; Mellemkjaer, L.; Brinton, L.A.; Anderson, K.C.; Caporaso, N.E.; Landgren, O.; Olsen, J.H. Risks of cancer among a cohort of 23,935 men and women with osteoporosis. Int. J. Cancer 2008, 122, 1879–1884. [Google Scholar] [CrossRef]
- Marquez-Garban, D.C.; Chen, H.W.; Fishbein, M.C.; Goodglick, L.; Pietras, R.J. Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids 2007, 72, 135–143. [Google Scholar] [CrossRef]
- Martinez-Alonso, M.; Dusso, A.; Ariza, G.; Nabal, M. The effect on quality of life of vitamin D administration for advanced cancer treatment (VIDAFACT study): Protocol of a randomised controlled trial. BMJ Open 2014, 4, e006128. [Google Scholar] [CrossRef] [PubMed]
- Dreizen, S.; McCredie, K.B.; Keating, M.J.; Andersson, B.S. Nutritional deficiencies in patients receiving cancer chemotherapy. Postgrad. Med. 1990, 87, 163–167, 170. [Google Scholar] [CrossRef]
- Vassbakk-Brovold, K.; Berntsen, S.; Fegran, L.; Lian, H.; Mjaland, O.; Mjaland, S.; Nordin, K.; Seiler, S.; Kersten, C. Lifestyle changes in cancer patients undergoing curative or palliative chemotherapy: Is it feasible? Acta Oncol. 2018, 57, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Bager, C.L.; Bay, F.; Christiansen, C.; Karsdal, M. Low bone turnover levels predict increased risk of cancer. Bone 2019, 127, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Bolland, M.J.; Grey, A.B.; Gamble, G.D.; Reid, I.R. Effect of osteoporosis treatment on mortality: A meta-analysis. J. Clin. Endocrinol. Metab. 2010, 95, 1174–1181. [Google Scholar] [CrossRef]
| No. of Patients | n = 95 |
|---|---|
| Median age (IQR) (in y) | 63 (58–70) |
| Female sex | 47 (49) |
| Multiple BM | 39 (41) |
| Preoperative KPS ≥ 70 | 83 (87) |
| Median CCI-index (IQR) | 9 (8–9) |
| ASA ≥ 3 | 49 (51) |
| Median BMD (in HU) | 125 (95–165) |
| 1-year mortality | 54 (57) |
| Median OS (in months) | 9 (3–24) |
| Patients with Physiologic BMD n= 49 | Patients with Pathologic BMD n= 46 | p-Value | |
|---|---|---|---|
| Median BMD (HU, IQR) | 140 (113–159) | 99 (74–195) | 0.03 |
| Median Age (yrs, IQR) | 64 (58–71) | 62 (57–70) | 0.59 |
| Female sex | 24 (49) | 23 (50) | 1.0 |
| Multiple BM | 13 (26) | 26 (56) | 0.004 |
| Preoperative KPS ≥ 70 | 44 (90) | 39 (85) | 0.54 |
| Median CCI-index (IQR) | 9 (8–9) | 9 (8–10) | 0.46 |
| Preoperative radiotherapy | 1 (2) | 1 (2) | 1.0 |
| 1-year mortality | 21 (43) | 33 (72) | 0.007 |
| Median OS (in months) | 15 (6–32) | 6 (1–19) | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilic, I.; Potthoff, A.-L.; Borger, V.; Heimann, M.; Paech, D.; Giordano, F.A.; Schmeel, L.C.; Radbruch, A.; Schuss, P.; Schäfer, N.; et al. Bone Mineral Density as an Individual Prognostic Biomarker in Patients with Surgically-Treated Brain Metastasis from Lung Cancer (NSCLC). Cancers 2022, 14, 4633. https://doi.org/10.3390/cancers14194633
Ilic I, Potthoff A-L, Borger V, Heimann M, Paech D, Giordano FA, Schmeel LC, Radbruch A, Schuss P, Schäfer N, et al. Bone Mineral Density as an Individual Prognostic Biomarker in Patients with Surgically-Treated Brain Metastasis from Lung Cancer (NSCLC). Cancers. 2022; 14(19):4633. https://doi.org/10.3390/cancers14194633
Chicago/Turabian StyleIlic, Inja, Anna-Laura Potthoff, Valeri Borger, Muriel Heimann, Daniel Paech, Frank Anton Giordano, Leonard Christopher Schmeel, Alexander Radbruch, Patrick Schuss, Niklas Schäfer, and et al. 2022. "Bone Mineral Density as an Individual Prognostic Biomarker in Patients with Surgically-Treated Brain Metastasis from Lung Cancer (NSCLC)" Cancers 14, no. 19: 4633. https://doi.org/10.3390/cancers14194633
APA StyleIlic, I., Potthoff, A.-L., Borger, V., Heimann, M., Paech, D., Giordano, F. A., Schmeel, L. C., Radbruch, A., Schuss, P., Schäfer, N., Herrlinger, U., Vatter, H., Lakghomi, A., & Schneider, M. (2022). Bone Mineral Density as an Individual Prognostic Biomarker in Patients with Surgically-Treated Brain Metastasis from Lung Cancer (NSCLC). Cancers, 14(19), 4633. https://doi.org/10.3390/cancers14194633









